Fiix-prothrombin time monitoring improves warfarin anticoagulation outcome in atrial fibrillation: a systematic review of randomized trials comparing Fiix-warfarin or direct oral anticoagulants to standard PT-warfarin
Summary
Background
Monitoring warfarin with Fiix-prothrombin time (Fiix-PT), which is only affected by coagulation factors II and X, stabilizes anticoagulation and reduces thromboembolism compared to PT/INR monitoring. We compared outcome in nonvalvular atrial fibrillation (NVAF) patients treated with Fiix-warfarin, direct oral anticoagulants (DOACs), or PT-warfarin.
Methods
A systematic efficacy and safety assessment by retrieving data from the Fiix trial and the four major phase III DOAC trials in NVAF. Prespecified outcomes included stroke and systemic embolism (SSE), SSE and myocardial infarction (MI), major bleeding (MB), composite major vascular events (SSEMI and MB; CMVE), and deaths. We calculated relative risk, 95% CI, and 95% confidence limits (CL) for each outcome and performed meta-analysis using fixed- and random-effects modeling.
Results
There were 613 and 628 observation years with Fiix-warfarin and PT-warfarin in the Fiix trial, and 70 628 and 57 962 with DOACs and PT-warfarin in DOAC trials. Populations were comparable although death rates were lower in the Fiix trial. Compared to pooled PT-warfarin, Fiix-warfarin reduced SSE (RR 0.54;95% CI 0.26–1.10/95% CL <1.00), SSEMI (0.51;0.26–0.99/<0.90), MB (RR 0.63;0.37–1.07/<0.99), and CMVE (RR 0.66;0.43–1.00/<0.94). Vascular death was lower (RR 0.13;0.04–0.47/<0.42). Compared to pooled DOACs, Fiix-warfarin consistently had lower point estimates for the RR for efficacy and safety, but only significant for lower death rates (vascular death RR 0.14;0.04–0.49/<0.43). Meta-analysis comparing Fiix-warfarin and DOACs with PT-warfarin consistently found Fiix-warfarin to have the lowest point estimates for efficacy.
Conclusion
Monitoring warfarin with Fiix-PT reduces risk of vascular events in NVAF patients as much as DOACs. Warfarin monitored with Fiix-PT is an improved anticoagulant.
Introduction
Warfarin and other vitamin K antagonists (VKA) inhibit liver gamma-carboxylation of the four vitamin K-dependent coagulation factors (F) II, VII, IX, and X 1. The Quick prothrombin time (PT) 2, and its later modification prothrombin–proconvertin time (P&P test) 3, allowed controlling the anticoagulant effect of VKAs and their use in humans. Consequently, VKAs became the cornerstone of long-term anticoagulation until the direct oral anticoagulants (DOACs) became available after year 2009 1.
It is common wisdom that deficiencies in any of FVII, FII, or FX have an identical influence on the PT 4. However, despite in vitro and animal experiments, it is less appreciated that the antithrombotic effect of VKAs is mainly generated by reducing FII and FX 4-6. Nevertheless, PT results have been considered to adequately reflect the antithrombotic effect for over 65 years and its appropriateness as a monitoring test for VKA has been questioned seriously by only a few investigators 7, 8. The Fiix-PT is a new PT derivative intended for monitoring VKA that is affected only by reduced FII or FX activity. This is achieved by mixing a specially made FII and FX immunodepleted plasma into the test plasma which corrects for any deficiency other than that of FII or FX in the patient sample before adding thromboplastin and calcium chloride 4. The results can be reported as a standardized Fiix-PT ratio, that is, a Fiix normalized ratio (Fiix-INR/Fiix-NR), similar to the international normalized ratio (INR, PT-INR) 4, 9. The Fiix-PT was tested in the randomized clinical noninferiority Fiix trial which demonstrated that compared to high time in target range (TTR) standard PT monitoring, the Fiix-PT stabilized warfarin anticoagulation, increased TTR further, and reduced dose adjustment needs. Moreover, the annual total thromboembolism (TE) rate was reduced from 2.3% to 1.2% with Fiix-NR monitoring (statistically noninferior for the whole study period, significantly superior beyond the first 6 months) without an increase in bleeding which occurred at a low annual rate in both study arms; 2.5% vs. 2.2%, respectively 10.
The magnitude of apparent clinical improvement in the Fiix trial led us to reclassify Fiix-warfarin (warfarin monitored with Fiix-PT) for the purpose of the current analysis as a ‘new’ oral anticoagulant (NOAC), different from traditional PT-warfarin, that is, warfarin monitored with traditional Quick PT-INR. Based on this reclassification, we undertook a systematic comparison and meta-analysis of clinical outcome with Fiix-warfarin compared to outcomes observed with DOACs and PT-warfarin in the recent major phase III noninferiority DOAC trials in nonvalvular atrial fibrillation (NVAF) patients, the most common indication for long-term anticoagulation.
Methods
Terminology
In this article, warfarin managed by monitoring with the Fiix-PT (Fiix-INR, Fiix-NR) is called Fiix-warfarin. Likewise, warfarin managed with the standard PT (INR, PT-INR) is called PT-warfarin. The data is presented here as if they were two separate drugs. We use the term NOAC for any oral anticoagulant that is an alternative to PT-warfarin and the term DOAC for directly inhibiting oral anticoagulants.
Trial selection
We compared the clinical outcome of NVAF patients in the Fiix trial 10 and in those trial arms of the four major phase III RCTs in NVAF that used doses of DOACs that were shown to be at least noninferior to PT-warfarin and were later approved for general use. The Fiix trial was a single-center trial comparing the outcome of Fiix-warfarin and PT-warfarin. The included DOAC trials are the RE-LY trial (Randomized evaluation of long-term anticoagulation therapy trial) of dabigatran 110 mg b.i.d. or dabigatran 150 mg b.i.d 11, 12, the ROCKET-AF trial (Rivaroxaban once daily oral direct factor Xa inhibition compared with Vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation) 13, the ARISTOTLE trial (apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation) 14, and the ENGAGE AF-TIMI 48 trial (Effective anticoagulation with factor Xa next generation in atrial fibrillation trial) comparing edoxaban 30 and 60 mg q.d. and PT-warfarin 15. In the current analysis, the RE-LY trial's two separate dabigatran dosing arms are treated as two separate controlled trials and the single PT-warfarin control arm is split into two equal-sized arms to prevent double counts of the same patients. We excluded the edoxaban 30 mg trial arm because this dose has not been approved for clinical use as it was found to be inferior to PT-warfarin. Hence, we compared the Fiix trial to outcome in five noninferior DOAC trial arms and to the corresponding PT-warfarin controls.
All the RCT's compared outcome in the active NOAC arm with that of controls that were treated with PT-warfarin dose-adjusted to a target INR of 2-3. As the Fiix trial was much smaller, we only statistically compared major outcomes, for example, overall efficacy analyzed as combined ischemic stroke, hemorrhagic stroke and systemic embolism (SSE), combined SSE and MI (SSEMI), safety analyzed as total major bleeding by ISTH criteria (MB) 16 or gastrointestinal bleeding (GIB), and composite major vascular events (all ischemic events combined with MB; CMVE). Transient ischemic attacks (TIAs) were excluded from analysis as not all trials reported them. Finally, we assessed death rates from any cause and death from vascular causes (fatal events in the composite vascular events group).
Data extraction
Two of the investigators (PTO, DOA) separately extracted outcomes from the published trials, supplements, or regulatory drug agency documents if needed followed by a consensus meeting. We extracted from each trial for each trial arm the number of patients, number of events, mean observation time (patient years), age, sex, exclusion criteria, CHADS2 score, and quality of control group treatment (time in range; TTR). The total observation time was calculated by multiplying patients’ number with mean observation time when not supplied in publication. The following data on fatal and nonfatal major clinical event occurrence during the studies were extracted: ischemic stroke (IS), systemic arterial embolism (SE), acute myocardial infarction (MI), major bleeding by ISTH criteria (MB), all intracranial bleeding (ICH), hemorrhagic stroke (intracerebral bleeding, ICBH), and major gastrointestinal bleeding (GI bleeding). We use the term SSE as synonymous for any stroke (ischemic or hemorrhagic) or systemic embolism. Fatal events were evaluated as total death from any cause and death from vascular causes as mortality from ischemic events or major bleeding. Finally, composite major vascular events were calculated as combined IS, SE, MI, and MB. Efficacy was assessed as reported in each trial (intention to treat or per protocol as described in the relevant tables) and safety was assessed per protocol.
Statistical analysis
Statistical analyses were carried out using graphpad Prism version 6.0 and r version 3.2.3. We did two separate types of analyses. In the first analysis, we evaluated the comparability of the trial populations by comparing outcomes of PT-warfarin patients in the Fiix trial with pooled outcomes with PT-warfarin in DOAC trials. Then, we compared outcomes of Fiix-warfarin patients to pooled outcomes in PT-warfarin patients in DOAC trials and outcome with Fiix-warfarin to pooled DOACs. For these comparisons, we calculated absolute rates and absolute rate differences based on number of events and total calculated observation years in each trial arm and number needed to treat to prevent one event (NNT). When not available in the publications, we calculated event numbers and observation years based on reported event rates, participant number, and mean duration of follow-up. We calculated relative risk (RR) and two-sided 95% confidence intervals (CI). We also calculated one-sided 95% confidence limits (CL). In the second analysis, we performed a meta-analysis of clinical outcome with NOACs (Fiix-warfarin and DOACs) compared to that with standard PT-warfarin using both fixed-effects and random-effects (DerSimonian-Laird) analysis with the package meta in R version 3.2.3 (Guido Schwarzer (2015) General Package for Meta-Analysis. r package version 4.3-2. http://CRAN.R-project.org/package=meta). The Cochrane Q statistic and I2 test for heterogeneity of the data were calculated to give a measure of comparability of the pooled data, and significant study differences (heterogeneity) are suggested by a Cochrane's Q P < 0.10 and I2 >50%. Results of the meta-analyses are shown as Forest plots.
Role of the funding source
This analysis was initiated by the investigators and carried out without external funding.
Results
Trial selection
In the Fiix trial, 406 and 427 patients received Fiix-warfarin or PT-warfarin, respectively, and in the pooled DOAC trials 42 411 and 29 272 received DOAC or PT-warfarin. The presented results are based on 613 observation years with Fiix-warfarin in the Fiix trial and 70 628 with DOACs combined. Likewise, there were 628 and 57 962 observation years in the ITT analysis with PT-warfarin in the Fiix and DOAC trials, respectively. The mean follow-up varied from 1.5 to 2.2 years, the shortest in the Fiix and the longest in the ENGAGE trial (Table 1).
| Fiix-trial NVAF patients 10 2015 | RE-LY 11, 12 2009/2010 | ROCKET-AF 13 2011 | ARISTOTLE 14 2011 | ENGAGE TIMI 48 15 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental drugs | Fiix-warfarin | PT-warfarin, | Dabigatran 110 mg b.i.d | Dabigatran 150 mg b.i.d. | PT-warfarin | Rivaroxaban 20 mg q.d. | PT-warfarin | Apixaban 5.0/2.5 mg b.i.d. | PT-warfarin | Edoxaban 60 mg q.d. | PT-warfarin |
| Trial type | Single center, double blind | Multicenter, open-label | Multicenter, double blind | Multicenter, double blind | Multicenter, double blind | ||||||
| Analysis method efficacy/safety | ITT/PP | ITT/PP | PP/PP | ITT/PP | ITT/PP | ||||||
| Participant number | 406 | 427 | 6015 | 6076 | 6022 | 7131 | 7133 | 9120 | 9081 | 7035 | 7036 |
| Females - % | 33 | 31 | 37 | 36 | 37 | 40 | 40 | 36 | 35 | 38 | 38 |
| Age in years – median (IQR) or mean (SD) | 73 (66–79) | 74 (66–80) | 71 (9) | 72 (9) | 72 (9) | 73 (65–78) | 73 (65–78) | 70 (63–76) | 70 (63–76) | 72 (64–78) | 72 (64–78) |
| Observation years – mean | 1.5 | 1.5 | 2.0 | 2.0 | 2.0 | 1.8 | 1.9 | 1.8 | 1.8 | 2.2 | 2.2 |
| Heart failure | 30 | 30 | 32 | 32 | 32 | 63 | 62 | 36 | 35 | 58 | 58 |
| Hypertension | 64 | 65 | 79 | 79 | 79 | 90 | 91 | 87 | 88 | 94 | 94 |
| Age ≥75 year – % | 45% | 48% | 38 | 40 | 39 | 43 | 43 | 31 | 31 | 41 | 40 |
| Diabetes | 15 | 12 | 23 | 23 | 23 | 40 | 40 | 25 | 25 | 36 | 36 |
| Prior stroke – % | 24 | 23 | 20 | 20 | 20 | 55 | 55 | 19 | 18 | 28 | 28 |
| CHADS2 score ≥2* – % | 63 | 60 | 68 | 68 | 69 | 100 | 100 | 66 | 66 | 100 | 100 |
| Warfarin experienced – % | 87 | 84 | 50 | 50 | 49 | 62 | 63 | 57 | 57 | 59 | 59 |
| Aspirin on recruitment – % | 24 | 23 | 40 | 39 | 41 | 36 | 37 | 31 | 31 | 29 | 29 |
| Percent TTR median (IQR) | 83 (73–90) | 81 (70–88) | – | – | 67 (54–78) | – | 58 (43–71) | – | 66 (52–77) | – | 68 (57–77) |
| Blinded adjudication | Yes | Yes | Yes | Yes | Yes | ||||||
| Patient selection | Little selection | Selected | Selected | Selected | Selected | ||||||
| Inclusion criteria | Age ≥18 years, target INR 2-3, nonvalvular atrial fibrillation on warfarin subgroup | Age ≥75 years or 65–74 years with type 2 DM, HTN, or CAD. AF documented on EKG within 6 months of screening. One of the following: Prior stroke or TIA, LVEF <40%, NYHA class II-IV within 6 months of screening. | Age ≥18 years, nonvalvular atrial fibrillation, CHADS2 score ≥2. | Atrial fibrillation or atrial flutter on 2 EKGs at least 2 weeks apart in the prior year and ≥1 stroke risk factor, that is, age ≥75 years, prior stroke, TIA, or embolism, symptomatic heart failure in prior 3 months or LVEF <40%, DM, hypertension requiring medications. | Patients >21 years, documented AF within 12 months prior to randomization, CHADS2 score >2, anticoagulation therapy planned for the duration of the trial. | ||||||
| Patient selection; exclusion criteria | Nursing home residents. Cardioversion patients with fixed INR intervals. | Including but not limited to: any stroke within 14d or disabling stroke within 6 months, poorly controlled hypertension, co-conditions increasing bleeding risk, creatinine clearance ≤30 mL/min, liver disease, Hb ≤100 g/L, platelets ≤100 x109/L, noncompliant patients, expected lifespan ≤ trial duration, cancer with ≤3 year expected survival, substance abuse. | Including but not limited to: any stroke within 14 days or disabling stroke within 3 months, TIA within 3 days, major surgery or trauma within 1 month, GI bleeding within 6 months, history of intracranial, intraocular, spinal, or | Including but not limited to: Stroke within 7 days, planned major surgery, platelets <100 x109/L, poorly controlled hypertension, creatinine clearance ≤25 mL/min, abnormal liver function tests, | Secondary AF, creatinine clearance <30 mL/min, high bleeding risk, dual anti-platelet therapy. Other indications for anticoagulation therapy. Acute coronary syndromes and coronary | ||||||
| intra-articular bleeding; known intracranial neoplasm, arteriovenous malformation or aneurysm; planned invasive procedure with potential for uncontrolled bleeding; anemia or platelet count <90 x109/L at screening; uncontrolled hypertension; malignancy or radiation therapy within 6 months and not expected to survive 3 years. Planned cardioversion, long-term NSAID, left ventricular thrombus, known liver disease, life expectancy <2 years, substance abuse, psychosocial disorder. | inability to comply with INR monitoring, life expectancy <1 year, substance abuse. | revascularization. Stroke within 30 days before randomization. Inability to adhere to study procedures. | |||||||||
- Fiix-warfarin and PT-warfarin describe warfarin monitored with Fiix-PT/Fiix-NR or PT/INR, respectively. Data is mean (SD), median (IQR), or percent unless otherwise indicated. ITT, intention-to-treat, PP, per-protocol, *CHADS2 score=stroke risk factor score (one point for history of congestive heart failure, hypertension, age ≥75 years, and diabetes; two points for history of stroke or transient ischemic attack), TTR, time in therapeutic range.
Comparability of study populations
Table 1 shows similarities and differences of the compared trials. The Fiix trial used little patient selection whereas both the ROCKET-AF and ENGAGE included mainly selected patients, leading to a 100% CHADS2 score ≥2 in the latter two trials compared to 60%, 68%, and 66% in the Fiix trial, RE-LY, and ARISTOTLE, respectively. The Fiix-trial controls had a median TTR of 80% whereas the other trials had lower median TTR, ranging from 58% in ROCKET-AF to 68% in ENGAGE. The Fiix trial and ROCKET-AF enrolled the oldest patients. The prior stroke percentage was particularly high in the ROCKET-AF. Table 2 shows the absolute annual major vascular event rates in patients in PT-warfarin control groups in the Fiix trial vs. pooled DOAC trials and demonstrates that there are no significant differences in efficacy and safety. However, there is significantly lower total mortality in the Fiix-trial controls than in the pooled DOAC trials’ control patients, 0.8% vs. 2.3% (RR 0.32, 95% CO 0.14-0.74), that is all explained by a lower vascular death rate in the Fiix-trial control patients.
| PT-warfarin in Fiix trial | Pooled PT-warfarin in DOAC trials | Calculations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Observation years | Annual rate % ppy | Events | Observation years | Annual rate % ppy | Absolute rate difference %ppy | NNT | RR | Two- sided 95% CI | One-sided 95% CL | |
| Efficacy: | |||||||||||
| SSE (ISSE and hemorrhagic stroke) | 15 | 628 | 2.4 | 1233 | 57962 | 2.1 | +0.3 | 333 | 1.12 | 0.68–1.84 | ns |
| SSE and MI | 17 | 628 | 2.7 | 1495 | 57962 | 2.6 | +0.1 | 1000 | 1.05 | 0.66–1.67 | ns |
| Safety: | |||||||||||
| Total major bleeding | 15 | 586 | 2.6 | 1770 | 48833 | 3.6 | −1.0 | 100 | 0.71 | 0.43–2.06 | ns |
| GI bleeding | 7 | 586 | 1.2 | 583 | 48833 | 1.2 | 0.0 | n.a. | 1.00 | 0.48–2.06 | ns |
| Composite Events | |||||||||||
| Composite major vascular events | 30 | 628 | 4.8 | 3001 | 57962 | 5.2 | −0.4 | 250 | 0.92 | 0.65–1.30 | ns |
| Death from any cause | 12 | 628 | 1.9 | 2245 | 57962 | 3.9 | −2.0 | 50 | 0.49 | 0.28–0.86 | <0.79 |
| Death from vascular causes | 5 | 628 | 0.8 | 1458 | 57962 | 2.5 | −1.7 | 143 | 0.32 | 0.14–0.74 | <0.66 |
- Fiix-warfarin and PT-warfarin describe warfarin monitored with Fiix-PT/Fiix-NR or PT/INR, respectively; ISSE= ischemic stroke or systemic embolism; SSE, hemorrhagic stroke (intracerebral bleeding) and ISSE; MI, acute myocardial infarction; GI, gastrointestinal; NNT, number needed to treat to prevent one event; RR, relative risk; 95% CI, two-sided confidence interval; 95% CL, one-sided confidence limit; PPY, rate per patient year.
Fiix-warfarin vs. pooled PT-warfarin
Table 3 compares outcome with Fiix-warfarin to that of treatment with pooled PT-warfarin in the DOAC trials. It demonstrates a lower absolute annual rate of stroke and systemic embolism (SSE), SSE and MI, and composite major vascular events with Fiix-warfarin. Only the reduction in SSE and MI was significant in the two-sided analysis (RR 0.51;0.26–0.99). However, the one-sided 95% confidence limit (CL) was significant for all. Likewise, the MB rate was nonsignficantly reduced (RR 0.63;0.37–1.07), but the 95% CL suggests significance. Finally, death from any cause was significantly lower (RR 0.38;0.20–0.72), all driven by a reduction in vascular death (RR 0.13;0.04–0.47).
| Fiix-warfarin | Pooled PT-warfarin in DOAC trials | Calculations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Observation years | Annual rate % ppy | Events | Observation years | Annual rate % ppy | Absolute rate difference %ppy | NNT | RR | Two-sided 95% CI | One-sided 95% CL | |
| Efficacy: | |||||||||||
| SSE (ISSE and hemorrhagic stroke) | 7 | 613 | 1.1 | 1233 | 57962 | 2.1 | −1.0 | 100 | 0.54 | 0.26–1.10 | <1.00 |
| SSE and MI | 8 | 613 | 1.3 | 1495 | 57962 | 2.6 | −1.3 | 77 | 0.51 | 0.26–0.99 | <0.90 |
| Safety: | |||||||||||
| Total major bleeding | 13 | 570 | 2.3 | 1770 | 48833 | 3.6 | −1.3 | 77 | 0.63 | 0.37−1.07 | <0.99 |
| GI bleeding | 8 | 570 | 1.4 | 583 | 48833 | 1.2 | +0.2 | 500 | 1.18 | 0.59–2.31 | ns |
| Composite events: | |||||||||||
| Composite major vascular events | 21 | 613 | 3.4 | 3001 | 57962 | 5.2 | −1.8 | 56 | 0.66 | 0.43–1.00 | <0.94 |
| Death from any cause | 9 | 613 | 1.5 | 2245 | 57962 | 3.9 | −2.4 | 42 | 0.38 | 0.20–0.72 | <0.65 |
| Death from vascular causes | 2 | 613 | 0.3 | 1458 | 57962 | 2.5 | −2.2 | 45 | 0.13 | 0.04–0.47 | <0.41 |
- Fiix-warfarin and PT-warfarin describe warfarin monitored with Fiix-PT/Fiix-NR or PT/INR, respectively; ISSE, ischemic stroke or systemic embolism; SSE, hemorrhagic stroke (intracerebral bleeding) and ISSE; MI, acute myocardial infarction; GI, gastrointestinal; NNT, number needed to treat to prevent one event; RR, relative risk; 95% CI, two-sided confidence interval; 95% CL, one-sided confidence limit; PPY, rate per patient year.
Fiix-warfarin vs. pooled DOACs
Table 4 shows that although the absolute event rates with Fiix-warfarin were consistently lower than those observed with pooled DOACs except for the GI bleeding rates, this difference was not statistically significant. This can also be observed visually in the meta-anaysis shown in Figure 1 where it is evident that 95% CI's are always wide with Fiix-warfarin vs. PT-warfarin due the Fiix trial's smaller size. Again, the total death rates were significantly lower with Fiix-warfarin, explained mainly by reduced vascular death rates (RR 0.13; 0.03–0.51).
| Fiix-warfarin | Pooled DOACs | Calculations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Observation years | Annual rate % ppy | Events | Observation years | Annual rate % ppy | Absolute rate Difference %ppy | NNT | RR | Two-sided 95% CI | One-sided 95% CL | |
| Efficacy: | |||||||||||
| SSE (ISSE and hemorrhagic stroke) | 7 | 613 | 1.1 | 1026 | 70628 | 1.5 | −0.4 | 250 | 0.79 | 0.38–1.61 | ns |
| SSE and MI | 8 | 613 | 1.3 | 1525 | 70628 | 2.2 | −0.9 | 111 | 0.61 | 0.31–1.18 | ns |
| Safety: | |||||||||||
| Total major bleeding | 13 | 570 | 2.3 | 1837 | 61255 | 3.0 | −0.7 | 143 | 0.76 | 0.45–1.29 | ns |
| GI bleeding | 8 | 570 | 1.4 | 876 | 61255 | 1.4 | 0.0 | n.a. | 0.98 | 0.50–1.93 | ns |
| Composite events: | ns | ||||||||||
| Composite major vascular events | 21 | 613 | 3.4 | 3218 | 70628 | 4.6 | −1.2 | 83 | 0.75 | 0.49–1.14 | ns |
| Death from any cause | 9 | 613 | 1.5 | 2468 | 70628 | 3.5 | −2.0 | 50 | 0.42 | 0.22–0.79 | <0.68 |
| Death from vascular causes | 2 | 613 | 0.3 | 1562 | 70628 | 2.2 | −1.9 | 53 | 0.15 | 0.04–0.59 | <0.47 |
- Fiix-warfarin and PT-warfarin describe warfarin monitored with Fiix-PT/Fiix-NR or PT/INR, respectively; ISSE, ischemic stroke or systemic embolism; SSE, hemorrhagic stroke (intracerebral bleeding) and ISSE; MI, acute myocardial infarction; GI, gastrointestinal; NNT, number needed to treat to prevent one event; RR, relative risk; 95% CI, two-sided confidence interval; 95% CL, one-sided confidence limit; PPY, rate per patient year.
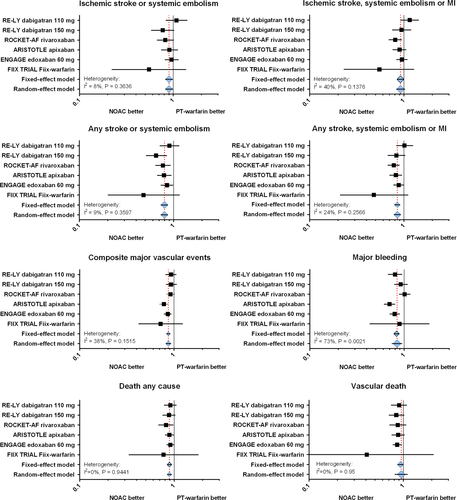
Meta-analysis
The meta-analysis estimates the average treatment effect of pooled NOACs (Fiix-warfarin and DOACs) to that in controls. Due to small study size, the Fiix trial accounts for only 1–1.5% of the observation time and, thus, did not influence the pooled effect to a significant deal. The Forest plots based on multiple meta-analyses are shown in Figure 1 and demonstrate that the selected studies were not significantly heterogeneous except for MB and vascular death where the studies appear to be significantly heterogeneous. The pooled meta-analyses suggest that the average treatment effect of pooled NOACs leads to an improvement in most efficacy outcome parameters by 10–20% compared to PT-warfarin. Nevertheless, Fiix-warfarin consistently had the lowest point estimates, varying from 35–60% reduction in the same events compared to standard warfarin (n.s.).
Intracranial and intracerebral hemorrhage rates
Intracranial hemorrhage/intracerebral hemorrhage occurred in 2/0 patients during 570 observation years (0.35%/0% ppy) with Fiix-warfarin and in 4/2 during 586 observation years (0.68%/0.34% ppy) with PT-warfarin in NVAF patients in the Fiix trial. ICH/ICBH occurred in 426/268 during 48 833 observation years (0.87%/0.55%) with pooled PT-warfarin arms in the DOAC trials and in 231/152 during 61 255 observation years (0.38%/0.25%) in pooled DOAC patients in the DOAC trials. The few ICHs in the Fiix trial do not allow statistical analysis.
Discussion
Our results show that in NVAF patients on Fiix-warfarin, thromboembolic and hemorrhagic events were consistenly reduced compared to pooled standard PT-warfarin treatment in the DOAC trials. Likewise, Fiix-warfarin was consistently equivalent to DOACs in reducing the same events. Importantly, the absolute rate of major bleeding with Fiix-warfarin was low and similar to the lowest hemorrhagic rate in the DOAC trials 14.
It should be noted that with Fiix-warfarin, the magnitude of improvement achieved was considerable as the PT-warfarin control group in the Fiix trial had high-quality PT-warfarin treatment (median TTR about 80%). The median quality of PT-warfarin treatment (TTR) in the DOAC trials’ patients was at best moderate or 58–68%, so their expected event rates are higher if the population is identical 17. Also, although the CI is wide due to small numbers in the Fiix trial, the point estimates of the meta-analysis consistently trend in the way that Fiix-warfarin is at least equivalent to the new DOACs although no definitive conclusion can be made. Overall, this analysis in NVAF patients supports the results of the prior mixed population results of the Fiix trial that Fiix-warfarin is an improved anticoagulant over standard PT-warfarin.
The improved efficacy of Fiix-warfarin over PT-warfarin is most likely due to improved stability of the anticoagulant effect achieved using a monitoring test that ignores the activity of the short half-life FVII. FVII reductions have major influence on the PT/INR, but none on the Fiix-PT/Fiix-NR 4. The new Fiix-PT was developed based on the premise that mainly factors II and X are responsible for the antithrombotic effect of VKA and that monitoring FVII is not important during VKA treatment, but confounds dose management.
During monitoring of VKA with the traditional PT (Quick PT), undiluted homogenized animal or human brain thromboplastin and calcium chloride are added to a citrated plasma sample from a patient and the clotting time is measured 2. Initially, only deficiency in factors I (fibrinogen) or II (prothrombin) were considered to prolong the PT as no other factor deficiencies were known. Indeed, when the first VKA (dicoumarol) was discovered in Wisconsin in the early 1940s, its potency in animals causing bleeding and prolongation of the PT was considered to be from an acquired deficiency of FII. Later in the 1940s, other factors that influence the PT became known, namely factor V 18 and FVII 19 that both influenced the PT. In the 1950s, FIX 20 and FX 21 were discovered, reductions in the latter, but not the former affecting the PT.
It is really for historical but not biological reasons that over the past 65 years VKAs have been monitored with the PT, either the Quick PT that is affected by three of the vitamin K-dependent factors (FII, FVII, and FX, but not IX) as well as by I and V 2 or its variant, Owren's PT is only affected by reductions in II, VII or X 3. It is, crucial to realize that the PT (Quick or Owren's) is equally influenced by a reduction in the activity of each of FII, FVII, or FX whereas the antithrombotic effect depends mainly on a reduction in FII and FX which both may provide a biological effect of similar magnitude 4, 6. Notably, FVII has only minor influence on thrombin generation or fibrin formation unless its concentration is far below that expected during controlled VKA treatment 4-6.
Our experimental conditions provided data to suggest that measuring FII and X together would lead to an improvement in anticoagulation control based on a biologically similar effect of FII and FX that differs from that of FVII and FIX 4. Factor VII also has a very short half-life (4 h) 1 and therefore is rapidly influenced by VKA drugs or food and drugs that influence VKA action. FII and FX have much longer half-lives than FVII (24–48 and 72 h, respectively) and fluctuate less in the short term. Therefore, large fluctuations that occur in FVII in the short term can cause major changes in the PT/INR without much change having occurred in FII or FX, especially during initiation of VKAs or with frequent dose changing. An elevated INR reported at such timepoints will be prolonged due to low FVII, but is not reflective of a change having occurred in the true antithrombotic effect. Such elevations will confound the dosing staff and may lead to inappropriate dose adjustments and further unwarranted testing. As FII and FX were found to be interchangeable in our experiments 4, but with different half-lives, we hypothesized that their simultaneous measurement together (‘both and only’) was important as opposed to measuring one or the other. In contrast, measuring FVII was hypothesized not to be important during VKA treatment and that its influence on the monitoring test could actually mislead the caretaker. Based on these considerations, we invented a method to monitor only reductions in FII and FX, that is, the Fiix-PT. We then hypothesized that monitoring VKA with the new test would increase the stability of anticoagulation and would lead to at least noninferior efficacy and safety. This was then tested in the investigator-initiated randomized clinical noninferiority Fiix trial 10.
Four recent enormous pharmaceutical industry initiated multicentric phase III clinical trials testing efficacy and safety of dabigatran 11, 12, rivaroxaban 13, apixaban 14, or edoxaban 15 in comparison with standard PT-warfarin based on noninferiority assessment have concluded at least equivalence and in some aspects superiority of the unmonitored DOACs over standard PT-warfarin treatment. Meta-analyses of the same trials’ data suggest the same 22, 23. Interestingly, our current systematic analysis shows improvements in both efficacy and safety end-points for Fiix-warfarin that are consistently in the same direction as that observed with the DOACs. The analysis may even suggest that the extra benefit of the DOACs over standard warfarin is annulled with Fiix-warfarin compared with standard PT-warfarin which is not totally surprising considering the magnitude of TE reduction (almost 50%) observed with Fiix-warfarin in the Fiix trial. This conclusion, however, is inferred and has not been directly tested. Also, the confidence intervals are wide precluding firm conclusions.
It is somewhat peculiar that although both TE and major bleeding events occurred at similar rates in controls in the Fiix trial and in the pooled DOAC trials, the total death rate was significantly lower in the Fiix-trial controls, apparently all explained by a lower vascular death rate in the Fiix trial. One explanation for this could be that about 2/3 of patients in the DOAC trials were managed at geographical sites where the baseline mortality and cardiovascular mortality in the elderly is higher than in Iceland. The TTR was markedly lower at those sites as well, likely leading to worse outcome of strokes and hemorrhage with PT-warfarin at those sites 12, 24. Additionally, a faulty point-of-care PT device used in warfarin-treated controls in the ROCKET-AF trial may have caused low overall TTR and poor clinical outcome with warfarin in that trial skewing the results in favor of rivaroxaban 25. Low TTRs, whatever the cause, in DOAC trials, inevitably leads to worse outcome with warfarin which favors the DOACs, but may not reflect outcome at management sites practicing modern high-quality warfarin management. Finally, although no statistical analysis is possible, it is worth mentioning that the intracranial hemorrhage/ICH) and intracerebral hemorrhage/ICBH rates in the Fiix trial were low and consistent with rates observed with DOACs in the DOAC trials.
The Fiix trial, a single-center noninferiority RCT, was limited by not being powered for evaluations of outcome in subgroups. However, by comparing results from the Fiix trial to the much larger group of pooled PT-warfarin controls in the enormous DOAC trials, we were able achieve statistical power to compare major outcomes although confidence intervals remain wide. We chose to do two separate statistical analyses. First, we compared relative risks using both two-sided 95% confidence intervals (CI) and one-sided 95% confidence limits (CL). The one-sided 95% CL identifies a critical value where 95% of expected RR estimates will fall on one side of the critical value and 5% on the opposite side. This reduces the risk of making type 2 statistical errors of interpretation and clarifies expected RR distributions. Secondly, we did fixed- and random-effect meta-analyses comparing individual NOACs (including Fiix-warfarin) to PT-warfarin, separately and pooled. It should be kept in mind that there are differences in all the trial's designs and populations. Thus, although the populations of the Fiix trial, RE-LY, and ARISTOTLE appear to be quite similar, the ROCKET-AF and ENGAGE patients may differ more due to complex inclusion and exclusion criteria. This has, however, not prevented others from performing meta-analyses combining results in three 22 or all four phase III DOAC trials 23. Importantly, in our meta-analysis, heterogeneity did not differ significantly except for major bleeding where trials differed. Results of a separate meta-analysis (not shown) of the most similar trial arms only (Fiix trial, RE-LY, and ARISTOTLE) did not differ from the full meta-analysis.
Finally, some additional limitations of the current systematic review should be addressed. First, in the Fiix trial, there was little patient selection compared to the DOAC trials, so the DOAC trials may actually not represent general anticoagulation practice. Secondly, the proportional improvement in efficacy with Fiix-PT due to improved stability suggested by the Fiix trial might actually be even more in areas of poor INR control, but this we could not test in a single-center trial at a site practicing high-quality warfarin management. Third, it would have been interesting to compare outcome with Fiix-PT to outcome with PT monitoring at high-quality sites only in the DOAC trials, but such data was not readily available to us.
In conclusion, considering limitations of this kind of an analysis, stabilizing anticoagulation by monitoring warfarin with the new Fiix-PT (Fiix-INR) instead of the traditional PT (INR) in NVAF patients reduces the risk of vascular events at least as much as do DOACs compared to traditional PT-INR monitoring. Warfarin monitored with the new Fiix-PT test, is an improved new oral anticoagulant.




