The mutual control of iron and erythropoiesis
Summary
Background
Iron is essential for hemoglobin synthesis during terminal erythropoiesis. To supply adequate iron the carrier transferrin is required together with transferrin receptor endosomal cycle and normal mitochondrial iron utilization. Iron and iron protein deficiencies result in different types of anemia. Iron-deficiency anemia is the commonest anemia worldwide due to increased requirements, malnutrition, chronic blood losses and malabsorption. Mutations of transferrin, transferrin receptor cycle proteins, enzymes of the first step of heme synthesis and iron sulfur cluster biogenesis lead to rare anemias, usually accompanied by iron overload. Hepcidin plays an indirect role in erythropoiesis by controlling plasma iron. Inappropriately high hepcidin levels characterize the rare genetic iron-refractory iron-deficiency anemia (IRIDA) and the common anemia of chronic disease. Iron modulates both effective and ineffective erythropoiesis: iron restriction reduces heme and alpha-globin synthesis that may be of benefit in thalassemia.
Material and Methods
This review relies on the analysis of the most recent literature and personal data.
Results
Erythropoiesis controls iron homeostasis, by releasing erythroferrone that inhibits hepcidin transcription to increase iron acquisition in iron deficiency, hypoxia and EPO treatment. Erythroferrone, produced by EPO-stimulated erythropoiesis, inhibits hepcidin only when the activity of BMP/SMAD pathway is low, suggesting that EPO somehow modulates the latter signaling. Erythroblasts sense circulating iron through the second transferrin receptor (TFR2) that, in animal models, modulates the sensitivity of the erythroid cells to EPO.
Discussion
The advanced knowledge of the regulation of systemic iron homeostasis and erythropoiesis-mediated hepcidin regulation is leading to the development of targeted therapies for anemias and iron disorders.
Introduction
The process of erythropoiesis entails the generation of mature red cells from multipotent stem cells and may be divided into two phases. The first related to the proliferation and maturation of progenitors BFUe and CFU-e is erythropoietin (EPO) dependent; the second the differentiation process from proerythroblasts to red cells is gradually less EPO responsive, but strongly iron dependent. Iron is essential for heme and iron–sulfur cluster synthesis in every cell of the body but is required in larger amounts for hemoglobin synthesis in maturing erythroblasts. To produce about 200 billion erythrocytes every day, approximately 25 mg of iron must be supplied to bone marrow. The vast majority is provided by macrophages, which recycle hemoglobin-derived iron from the breakdown of senescent erythrocytes, while in the absence of anemia, <1–2 mg iron daily derives from intestinal absorption.
Classic iron studies have clarified the molecular mechanisms underlying iron absorption, transport in the circulation, cell utilization and macrophage recycling, and the function of the key intracellular and systemic iron regulators, as iron regulatory proteins (IRPs) and hepcidin, respectively (for review, see Ref. 1). Experimental data, studies of human genetic disorders, and spontaneous or engineered animal models of diseases have clarified the relevance of iron for erythropoiesis and exemplified which iron proteins are needed for erythroid iron trafficking. However, only after the discovery of hepcidin, the reverse problem of how erythropoiesis controls iron acquisition according to its own needs was started to be unraveled.
The essential role of iron is evident considering that lack of iron or mutations in genes encoding iron proteins lead to different forms of anemia. Iron deficiency anemia (IDA) is the most common anemia worldwide that occurs because of increased demands in children and young women, malnutrition in low income countries, dietary iron restriction, or pathological causes, as malabsorption or chronic blood loss. To discuss IDA is outside the scope of this study; readers are referred to a recent review 2.
The Iron Control of Erythropoiesis
Classic and novel iron proteins in erythropoiesis
Iron is transported into the circulation by transferrin and released to erythroblasts by the interaction of di-ferric transferrin (holo-TF) with transferrin receptor (TFR or TFR1). Different transporters may uptake iron according to the cell type, the preferential route being the well-known TF-TFR1 endosomal cycle. This cycle is indispensable for erythropoiesis, as erythroblasts do not have alternative routes for iron import, at variance with hepatocytes or pancreatic cells, that may pick up also non-transferrin-bound-iron (NTBI) through ZIP14 3 or cardiomyocytes that uptake NTBI using still unknown transporters 4.
Mutations that reduce circulating transferrin reduce iron utilization in bone marrow causing both anemia and iron overload. Hypotransferrinemia is a rare recessive disorder in which IDA is associated with severe iron overload with low hepcidin that is manageable by plasma infusions 5. Manipulation of atransferrinemic mice has highlighted a role for transferrin in hepcidin regulation independently from erythropoiesis 6. Mutations of TFR1 are not reported in patients, but Tfr KO mice die of severe anemia during embryonic life 7. Tfr1 haplo-insufficient mice have normal Hb, reduced total body iron, reduced MCV and MCH, suggesting the possibility of analogous defects in humans. More recently, the selective inactivation of Tfr1 in different organs has demonstrated that in mice, Tfr1 is indispensable also for cardiac 8, for muscle 9, for and intestine 10 functions. Mutations of other components of the TFR1 cycle may cause anemias with microcytic hypochromic red cells, suggesting the relevance of this endosomal cycle for erythroblast iron supply. This occurs in mutations of DMT1 11 and of the ferrireductase STEAP3 12. Animal models have shown that other genetic anemia is due to mutations of carriers of iron from cytosol to mitochondria. Other proteins as NcoA4, a cargo of ferritin to phagosomes 13, may cause anemia in zebra fish when deleted, but whether mutations of the homologous proteins cause anemia in humans are unknown.
Intestinal iron absorption requires the co-ordinated function of luminal transporters as DMT1, which is mainly regulated by hypoxia, intracellular unknown carriers, and iron exporters as ferroportin, which works together with the oxidase hephaestin and is mainly regulated through hepcidin-mediated degradation 1.
Iron control of erythropoietin
Iron influences erythropoiesis by contributing to the regulation of EPO production in the kidney, through a co-ordinated function of IRP1 and the hypoxia-inducible factor 2-alpha (HIF-2-alpha). IRPs bind to iron responsive element (IRE), stem loop structures present in RNA UTR of several genes 1. Iron deficiency favors the RNA-binding form of IRP1. In iron repletion, IRP1, acquiring an iron–sulfur cluster, loses its function of iron regulator and becomes aconitase. In the kidney, IRP1 is the most active form while IRP2 is not functioning. IRP1 binds to the 5′ IRE of kidney HIF2-alpha in iron deficiency, thus limiting HIF translation and partially repressing EPO expression. Consistent with this observation, Irp1 KO mice, with a normal phenotype in iron repletion, develop severe polycythemia during growth or in iron deficiency, because of HIF-2alpha and EPO deregulation 14. HIF2-alpha protein is stabilized (less degraded) in hypoxia by prolyl-hydroxylase, an iron-dependent enzyme. This control is an example of the complex cross talk between iron and oxygen, whose co-ordination is indispensable as erythropoiesis is aimed at producing red cells for oxygen transport.
The hepcidin pathway and erythropoiesis
The key regulator hepcidin is suppressed in iron deficiency to allow the compensatory mechanism of iron acquisition from the gut and release by macrophages. Iron-refractory iron deficiency anemia 15 is a rare recessive disorder due to mutations of TMPRSS6/matriptase-2, the hepatocyte serine protease, which inhibits hepcidin by cleaving the BMP co-receptor hemojuvelin from plasma membrane 16. High hepcidin levels block iron absorption. Patients, usually children, have moderate anemia (Hb 8–10 g/dL), severe microcytosis with low iron and transferrin saturation, and normal/high ferritin levels. The inappropriately high hepcidin levels explain the refractoriness to oral iron treatment 17. The essential role of hepcidin for iron absorption is even better illustrated by anemia of chronic disease, a form of anemia commonly associated with autoimmunity, chronic infections, renal failure, and cancer, where high hepcidin levels block iron absorption in patients without gastrointestinal tract disorders and favor iron sequestration in macrophages 2. In acute inflammation, high hepcidin levels associate with increased erythrophagocytosis.
Iron utilization by erythroblasts
In erythroblasts, the vast majority of iron, incorporated into heme, is used for hemoglobin synthesis. However, as in all other cells, it is also utilized for iron–sulfur cluster synthesis within mitochondria. Deficiency of heme synthesis leads to sideroblastic anemia: A form with iron accumulation around the nucleus of erythroblasts, which have the appearance of ringed sideroblasts. The X-linked form is due to delta-aminolevulinic acid synthase 2 deficiency, while the recessive form is due to mutations of the mitochondrial glycine importer SLC25A38 18. The complex molecular mechanisms involved in the production and trafficking of iron–sulfur clusters have been partly clarified in the last years 19. Some rare inherited sideroblastic anemias are due to specific deficiencies of some of these proteins, as ABCB7 or GLRX5 20-22. Genetic disorders associated with dysfunctional iron proteins important for erythropoiesis are summarized in Table 1.
| Gene/protein | Genetic disorder | OMIM number | Inheritance | Phenotype |
|---|---|---|---|---|
| TMPRSS6/Matriptase-2 | IRIDA | #206200 | AR | Microcytic anemia |
| TF/Transferrin | Atransferrinemia | #209300 | AR | Microcytic anemia, iron overload |
| SLC11A2/DMT1 | DMT1 deficiency | #206100 | AR | Microcytic anemia, iron overload |
| ALAS2/delta-aminolevulinic acid synthase 2 | Sideroblastic anemia | #300751 | X-linked | Microcytic anemia, ring sideroblasts, iron overload |
| SLC25A38/mitochondrial aminoacid transporter | Sideroblastic anemia | #205950 | AR | Microcytic anemia, ring sideroblasts, iron overload |
| GLRX5/glutaredoxin 5 | Sideroblastic anemia | #205950 | AR | Microcytic anemia, ring sideroblasts, iron overload |
| HSPA9/heat shock protein 9 | Sideroblastic anemia | Not yet defined | Pseudo-D AR | Normo-microcytic anemia |
| ABCB7/ATP binding cassette 7 | Sideroblastic anemia | #301310 | X-linked | Microcytic anemia, ring sideroblasts, ataxia |
| STEAP3/TSAP6/ferrireductase | Microcytic anemia | #615234 | AD | Congenital hypochromic anemia, iron overload |
- OMIM, online Mendelian inheritance in men; IRIDA, iron-refractory iron deficiency anemia; AR, autosomal recessive; AD, autosomal dominant; DMT1, divalent metal transporter 1.
The Inverse Relationship
The erythroid regulator of iron homeostasis
Erythropoiesis participates to systemic iron homeostasis by regulating hepcidin. It has been shown that EPO by itself cannot inhibit hepcidin production when erythropoiesis is suppressed by cytotoxic drugs 23. Hepcidin is down-regulated in physiologic conditions that enhance iron requirements, such as iron deficiency, hypoxia, and recovery from anemia after bleeding or erythropoiesis increase induced by EPO treatment. Pathologic low hepcidin levels are found in inherited anemias with high degree of ineffective erythropoiesis, the so-called iron-loading anemias, nontransfusion-dependent beta-thalassemia being a paradigmatic example 24. Multiple proteins have been proposed to act as hepcidin inhibitors and erythroid regulators: among them, EPO itself, soluble TFR1, soluble hemojuvelin, HIF1-alpha, and TWGS1 (for detailed discussion, see Ref. 5). Some have been ruled out in humans, other reconsidered 25. GDF15, a cytokine produced by mature erythroblasts, might play some role, as it may partially inhibit hepcidin transcription in primary hepatocytes. In addition, it was found in extremely high concentrations in the sera of beta-thalassemia patients 26, although this finding might simply reflect the release from ineffective erythropoiesis or from hypoxic cells. To make the story more complex, Gdf15 KO mice are able to suppress hepcidin after EPO treatment, as normal mice do 27. The most recent candidate erythroid regulator is erythroferrone (ERFE) 28, previously called Fam132b or myonectin, identified as a skeletal muscle-derived myokine with the function of mTOR activator in the liver 29. ERFE is a member of the C1q-tumor-necrosis factor (TNF)-alpha family, produced by several tissues, but increased by EPO injection only in maturing erythroblasts. Originally proposed as a mediator between skeletal muscle and lipids in liver and adipose tissue, it rapidly suppresses hepcidin to allow iron acquisition from absorption and storage sites, thus favoring recovery from anemia secondary to blood loss 28 and inflammation 30. Erfe KO mice have normal hematologic parameters, but are unable to suppress hepcidin after phlebotomy or EPO injection. ERFE contributes to iron loading in beta-thalassemia mice 31. The mechanisms of ERFE function in the liver, its receptor, and the relative signaling pathways are still unknown. We have shown that the function of ERFE in the liver must be co-ordinated with the function of TMPRSS6. How the effect of ERFE is co-ordinated with the suppression of BMP pathway is unknown 32. Hepcidin is suppressed by other regulators, released by hypoxic cells/tissues. Soluble hemojuvelin, cleaved by furin in hypoxia 33, inhibits hepcidin in hepatoma cells in vitro, but data in vivo are lacking 34. The best-characterized mediator is platelet-derived growth factor-BB (PDGF-BB), expressed by several cell types in hypoxia, as it has been shown to suppress hepcidin in vivo in volunteers 35.
The transferrin–transferrin receptor 2 axis: a link between erythropoiesis and hepcidin
Transferrin receptor 2 is a member of the TFR family, homologous to TFR1, originally reported mutated in hemochromatosis type 3 36. TFR2 in the liver is considered to be an iron sensor and activator of hepcidin, although the molecular mechanisms are unclear. TFR2 is highly expressed in the erythroid tissue, where it is a component of the EPO receptor complex, important for the receptor export and its stability on cell surface 37. We have shown in mice that the deletion of Tfr2 exclusively in erythropoiesis enhances the EPO sensitivity of erythroid cells, leads to decreased apoptosis, erythrocytosis, and increased Erfe production, mimicking events that occur in mild iron deficiency 38.
It is well established that in hepatoma cell lines, TFR2 is stabilized on the cell surface by holo-TF and destabilized in iron deficiency 39. This occurs also in erythroid cells (UT7) and ex vivo in erythroblasts differentiated in culture 40. We have proposed that in iron deficiency, TFR2 releases a soluble isoform from plasma membrane 40, while others have suggested an increased degradation 41. Lack of TFR2 on the erythroblast surface, irrespective of how it is achieved, increases both EPO sensitivity and ERFE expression 38. In this way, TFR2 connects erythropoiesis with hepcidin regulation through the level of transferrin-bound (easily utilizable) iron and modulates the EPO response to adapt erythropoiesis according to available iron. At the same time, it co-ordinates iron requirements through the release of ERFE. We have recently shown that the effect of ERFE requires an attenuation of the BMP-SMAD pathway signaling 32. We speculate that EPO co-ordinates TMPRSS6 protease activity (to attenuate the BMP/SMAD signaling) with ERFE production through mechanisms that are under clarification.
Our global interpretation is that acute erythropoiesis expansion induced by either EPO or hypoxia causes a relative iron deficiency followed by loss of surface TFR2, increased EPO effect, and increased ERFE. This is likely a common mechanism of the EPO effect: In this way, the degree of iron saturation of transferrin (that is influenced by bone marrow activity) influences also hepcidin activation in a homeostatic manner (Figure 1). Loss of TFR2 and likely increased activity of TMPRSS6 (less degraded in iron deficiency) 42 reduce BMP/SMAD pathway activation allowing ERFE to function 32. Further studies will elucidate the molecular pathway in detail.
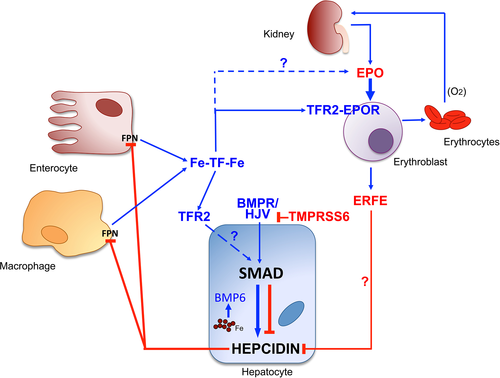
Our knowledge on the mutual control of erythropoiesis and systemic iron regulation has stimulated the development of compounds against molecular targets shown to be efficacious in preclinical studies, as anti-TMPRSS6 short interference RNA 43 or allele-specific oligonucleotides 44 or mini hepcidin modified for oral treatment 45 to increase hepcidin in thalassemia models. These approaches will be potentially useful to interfere with the vicious cycle of ineffective erythropoiesis and iron overload that occurs in pathologic conditions as beta-thalassemia.
Acknowledgement
This manuscript was partially supported by Italian Ministry of Health, Ricerca Finalizzata RF 20102312048 to CC.




