Factors affecting HLA expression: A review
Abstract
The detection and semiquantitative measurement of circulating human leucocyte antigen (HLA)-specific antibodies is essential for the management of patients before and after transplantation. In addition, the pretransplant cross-match to assess the reactivity of recipient HLA antibody against donor lymphocytes has long been the gold standard to prevent hyperacute rejection. Whilst both of these tests assume that recipient HLA-specific antibody is the only variable in the assessment of transplant risk, this is not the case. Transplant immunologists recognize that some HLA antigens are expressed at levels a magnitude lower than others (e.g., HLA-C, HLA-DQ), but within loci, and between different cell types there are many factors that influence HLA expression in both resting and activated cells. HLA is not usually expressed without the specific promoter proteins NLRC5, for HLA class I, and CIITA, for class II. The quantity of HLA protein production is then affected by factors including promoter region polymorphisms, alternative exon splice sites, methylation and microRNA-directed degradation. Different loci are influenced by multiple combinations of these control mechanisms making prediction of HLA regulation difficult, but an ability to measure the cellular expression of each HLA antigen, in conjunction with knowledge of circulating HLA-specific antibody, would lead to a more informed algorithm to assess transplant risk.
1 INTRODUCTION
The major histocompatibility complex (MHC) contains highly polymorphic genes coding for proteins involved in discrimination between self and non-self (Peakman & Vergani, 2009). Known in man as human leucocyte antigens (HLA) and coded on the short arm of chromosome 6, HLA differences between donor and recipient are the primary cause of transplant rejection (Terasaki & Ozawa, 2004; Williams, Opelz, Mcgarvey, Weil, & Chakkera, 2016).
Rejection can be T-cell-mediated (CMR), antibody-mediated (AbMR) or both (Sellarés et al., 2013; Venner et al., 2014). CMR occurs as the result of direct interaction between the T-cell receptor and the foreign HLA–peptide combination leading to activation of the T cell (Boardman, Jacob, Smyth, Lombardi, & Lechler, 2016). AbMR requires an inflammatory response and Th cell costimulation producing antibody which recognizes foreign HLA molecules (Batista & Harwood, 2008; Mitchison, 2004). Once primed, antibody-producing cells require no further T-cell help and can initiate acute AbMR on repeat challenge with an organ possessing the same mismatched HLA molecules as the original sensitizing event.
Both AbMR and CMR are diagnosed by histological staining of a transplant biopsy (Haas et al., 2018). This procedure is invasive and not without risk to the patient, particularly haematoma as a result of the biopsy procedure (Shidham et al., 2005). Part of the Banff classification for AbMR requires the detection of circulating donor-specific HLA antibody (DSA) which is less invasive, utilizing only a small peripheral blood sample, and as there is currently no simple test to predict CMR, the pretransplant work-up must rely predominantly upon the detection and avoidance of DSA alone and minimizing HLA mismatch where this is possible. Post-transplantation both AbMR and CMR can be abrogated by immunosuppression.
Transplant laboratories measure circulating HLA antibodies to assess the risk of hyperacute and accelerated acute rejection in solid organ transplants, or engraftment failure in haematopoietic stem cell transplantation (HSCT) (Leffell, Jones, & Gladstone, 2015; Tait et al., 2013). Post-transplantation, this measurement is an aid to the diagnosis of rejection (Brand, Doxiadis, & Roelen, 2013; Brooks et al., 2015; Piazza et al., 2001).
Great advancements have been made in both the sensitivity and specificity of HLA antibody detection methods, and these techniques can often be used in place of the standard pretransplant cross-match. This is particularly important for those organs where minimization of cold ischaemia time is essential, such as cardiothoracic transplantation, although the use of virtual cross-matching in low-risk renal transplantation is becoming increasingly widespread. (Johnson et al., 2016; Tait, 2016; Taylor, Kosmoliaptsis, Summers, & Bradley, 2009).
Current methods for the analysis of donor-specific antibodies include Luminex X-map technology using HLA-specific single antigen beads (SAB) (BSHI & BTS, 2014; Chaidaroglou et al., 2013). HLA proteins are coated onto the surface of microspheres, and the level of HLA antibody binding to each bead is measured using a fluorescence semiquantification system and recorded as relative median fluorescence intensity (MFI). Detection of DSA above a predetermined MFI threshold has been shown to be correlated with increased risk of AbMR and transplant rejection (Lefaucheur et al., 2010; Tambur et al., 2015).
For patients with circulating HLA antibodies, a pretransplant cross-match is usually performed by mixing serum from the patient with lymphocytes derived from the potential donor (Altermann, Seliger, Sel, Wendt, & Schlaf, 2006). Progression to transplant, or the determination of transplant risk and choice of antirejection therapy, is therefore based on the measurement of the circulating HLA antibody using bead-based assays on a Luminex platform, and the binding of antibody with HLA antigens on the surface of donor T and B cells.
Human leucocyte antigen class I is found on the surface of all nucleated cells, presenting intracellular peptides to the immune system. In addition to class I, HLA class II is present on specialized antigen-presenting cells (APC) (Batista & Harwood, 2008). The level of HLA expression on each cell is, however, not static. Proinflammatory cytokines upregulate basal HLA class I expression in all cells and induce class II presentation on non-APCs such as T cells and endothelial cells (Butler, Valenzuela, Thomas, & Reed, 2017; Van den Elsen, 2011; Van Den Elsen, Holling, Kuipers, & Van Der Stoep, 2004). In bovine endothelial cell culture experiments, for example, Spanel-Borowski and Bein (1993) demonstrated increased overall MHC class I expression by between 7- and 13-fold upon stimulation with interferon-γ (IFNγ).
Using peripheral blood cells, Honger et al. (2015) have demonstrated a threefold variation in the total HLA class I or class II expression between individuals, which will have implications for the overall antigenic burden in a transplant setting. Moreover, whilst Honger et al. (2015) found the total class I difference was threefold, variation in the surface density of the specific antigen HLA-A2 was over 30-fold between just seven individuals.
Using both HLA-A2-specific monoclonal antibody and alloantibody directed solely against HLA-A2, Honger's team have shown donor-specific differences in a complement-dependent cytotoxicity (CDC) assay, where HLA-A2-specific lysis of cells with high HLA-A2 antigen expression was detected at serum dilutions 250 times greater than in low expressing cells. The team excluded cells containing antigens in the HLA-A2 cross-reactive group (CREG) and were able to conclusively show that cell death was directly correlated with HLA-A2 density on the cell surface.
In the stem cell transplant setting, recipients mismatched for HLA-C or HLA-DP have a higher risk of graft-versus-host disease and mortality if the recipient is a genetically high expresser of the mismatched protein from either locus (Petersdorf et al., 2015; Dendrou, Petersen, Rossjohn, & Fugger, 2018; Petersdorf et al., 2014).
Whilst we are beginning to understand the many different mechanisms that govern cellular expression of HLA proteins, we are as yet unable to predict the amount of HLA protein on the cell surface of any one individual, or even on different cell types in the same individual. In addition, we cannot estimate by how much basal HLA expression will increase when cells are activated.
Prediction of transplant risk based on the measurement of HLA antibody alone, without a clear determination of cognate antigen expression on the target cell (in the case of kidney transplantation, for example, renal endothelial cells), is a severe oversimplification of the problem, denying some patients a viable transplant or exposing others to a greater risk of AbMR. This is particularly important for deceased donor transplantation where cold ischaemia has been shown to increase expression of inflammatory cytokines which upregulate HLA expression (Lutz, Thürmel, & Heemann, 2010; Siedlecki, Irish, & Brennan, 2011).
Disease association is not directly covered in this review, however it is clear that over expression of some HLA molecules may lead to an increased immune response, whilst low expression could allow self reactive T cells to escape thymic tolerance and lead to peripheral autoimmunity.
This paper will review our current understanding of the mechanisms that drive surface expression of HLA antigens. Ultimately, an algorithm to combine HLA antibody and antigen levels in each donor–recipient pair could be used to better stratify transplant risk.
2 FACTORS AFFECTING SURFACE EXPRESSION
2.1 CIITA and NLRC5
The principal HLA gene promoter region is in the 5′ untranslated region (5′UTR), up to 300 bp upstream of the transcription initiation sequence (Handunnetthi, Ramagopalan, Ebers, & Knight, 2010; Wong et al., 2014). All MHC genes share this highly conserved regulatory module consisting of a typical “SXY” box known as the enhanceosome (Elsen et al., 1998; Meissner, Liu, et al., 2012; Gobin et al., 2015. see Figure 1). In addition, HLA class I genes contain the cytokine-inducible promoter site EnhA and the IFNγ-stimulated response element, ISRE (Van Den Elsen et al., 2004; Meissner, Liu, et al., 2012; Neerincx et al., 2013). The enhanceosome also permits binding of thyroid-stimulating hormone (TSH) which acts to repress RNA transcription (Howcroft, Raval, Weissman, Gegonne, & Singer, 2003).
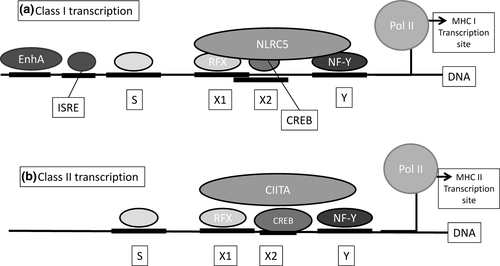
Studies in patients with bare lymphocyte syndrome (BLS) have shown that critical deficiencies in any of the proteins binding to this promoter region will result in failure of HLA expression (Elsen et al., 1998), and the predominant moderators of HLA transcription are the NOD-like receptor (NLR) proteins NLRC5 and CIITA which act through the enhanceosome (Meissner, Li, et al., 2012; Meissner, Liu, et al., 2012).
In studies of BLS, the class II transactivator (CIITA) was identified as an essential component of HLA class II mRNA transcription (Muhlethaler-Mottet, Otten, Steimle, & Mach, 1997). CIITA expression was only detectable in cells expressing HLA class II, and levels of CIITA and HLA class II were correlated. Whilst CIITA appears to have some influence on class I expression, Kobayashi and van den Elsen (2012) were able to show that HLA class I is specifically induced by the protein known as “NOD-, LRR- and CARD-containing 5” (NLRC5) acting through the SXY module in the class I leader sequence in a dose-dependent manner.
Both CIITA (found on chromosome 16p13) and NLRC5 (chromosome 16q13) are themselves highly regulated and different promoter regions control basal and INFɣ-induced upregulation of these proteins (Kobayashi & Elsen, 2012; Wong et al., 2014). It therefore appears that cytokines such as INFɣ not only influence HLA expression directly through the EnhA, ISRE and CRE elements but also act by upregulating the control proteins NLRC5 and CIITA.
HLA class II expression is constitutive in some cell types (APC) and induced via activation and upregulation in others. There are at least five different promoters for CIITA and together these induce alternative splicing of CIITA exon 1 sequences (Devaiah & Singer, 2013; Muhlethaler-Mottet et al., 1997). These splice variants give rise to at least three isoforms which are cell type specific (Devaiah & Singer, 2013; Muczynski, Ekle, Coder, & Anderson, 2003). CIITA isoform pIII binds to the enhanceosome in B cells and activated T cells, whilst isoform pI controls HLA class II expression in dendritic cells. IFNγ initiates splicing to the pIV isoform in non-bone marrow-derived cells such as endothelial cells (Muczynski et al., 2003; Neerincx et al., 2013; Van Den Elsen et al., 2004).
To prevent an uncontrolled inflammatory response following HLA upregulation, CIITA itself is rapidly degraded by the ubiquitin–proteasome pathway giving it a half-life of around 30 min (Devaiah & Singer, 2013). In attempts to control the immune response, some viruses such as EBV have been shown to encode CIITA repressors that block HLA class II upregulation (Dendrou et al., 2018).
Interestingly, extra villous trophoblasts (EVT) do not express NLRC5 or CIITA but do present the classical class I HLA-C proteins in addition to the nonclassical HLA-E and HLA-G. HLA-C possesses a distinct RFX sequence at the NLRC5 binding region which creates a unique recognition site for the ELF3 promotor (see Figure 1). ELF3 is known to be upregulated in trophoblasts, and this alternate RFX binding sequence may be responsible for both the reduced constitutive expression of HLA-C by altered NLRC5 binding in normal cells, and the continued expression of the protein in trophoblasts where NLRC5 is absent (Ferreira, Meissner, Tilburgs, & Strominger, 2017; Johnson, Wright, Li, & Anderson, 2018).
2.2 Promoter polymorphisms
Sequence polymorphisms in the transcription promoter region of HLA genes affect the assembly of the SXY module and overall HLA transcription. Experiments showed that the absence of two bases between the S and X boxes in the 5′UTR for HLA-DQB1*03:01, at position −178, results in significantly higher levels of a reporter molecule than in HLA-DQB1*03:02 where the bases were present (Andersen et al., 1991; Handunnetthi et al., 2010). Ferstl et al. (2004) describe polymorphisms resulting in ten different variations of the SXY promoter region of HLA-DQB1 genes. These variants result in both allele-specific and tissue-specific changes in HLA-DQ expression, with a two-and-a-half-fold increase of HLA-DQB1*03:01 gene products over DQB1*06:02, for example.
Additional polymorphisms in the enhanceosome, particularly in the Y box, affect cytokine-induced upregulation of HLA-DR genes (Sindwani & Singal, 2001), and Belicha-Villanueva and Mcevoy (2009) describe variations in MHC transcription as a result of polymorphisms in the EnhA site immediately upstream of the SXY module in the 5′UTR (Figure 1).
Further upstream from the S box, Fernandez (2003) has used the real-time reverse transcriptase–polymerase chain reaction (real-time RT-PCR) to map increased expression of HLA-DQA1 mRNA to a hypervariable region between 240-bp and 200-bp upstream of exon 1.
Polymorphisms in the transcription factor binding sites for HLA-C are associated with alleles demonstrating low surface expression. Mutations found in the EnhA region affect HLA-C*07 expression, TATA affect HLA-C*03, and insertions and deletions between these sites alter HLA-C*17 protein expression (Ramsuran et al., 2017).
Unlike HLA-A and HLA-C, HLA-B mRNA levels show less variation in expression between alleles, suggesting 5’UTR polymorphism does not significantly affect mRNA production for this locus (Ramsuran et al., 2017). On average, mRNA levels for resting cells varied by around 1.2-fold for different HLA-B alleles compared with 3.8-fold for HLA-A and 2.2-fold for HLA-C.
Additional promoter polymorphisms include a vitamin D response element (VDRE) in the 5′UTR of HLA-DR which is disrupted in many alleles. The VDRE is fully functional in HLA-DRB1*15 alleles, and it is interesting to note that HLA-DRB1*15 and geographical latitude (affecting vitamin D synthesis) are both strongly associated with the disease multiple sclerosis (Handunnetthi et al., 2010).
2.3 Long-range promoters
Bettens et al. (2014) detected an association between HLA-C expression and the extended HLA haplotype, suggesting additional control mechanisms beyond the immediate promoter region. The Bettens group described one particular HLA-B*49:01 – C*07:01 haplotype which consistently demonstrated a markedly elevated HLA-C mRNA level. Similarly for HLA class II, Handunnetthi et al.'s (2010) work describes repeats and inverted repeats of the SXY module several kilobases upstream of HLA class II genes. It is thought that these repeat sequences are involved in histone acetylation, initiating and stabilizing DNA unwinding to allow transcription promoter binding. Histone acetylation prevents the reformation of the DNA helix structure and enables the enzyme DNA polymerase II to initiate gene transcription (see Figure 1) (Venkatesh & Workman, 2015). In addition, Li et al. (2018) describe a region 1,300-bp upstream of the HLA-C start codon which may be involved in enhancing or repressing HLA promoters.
2.4 Splice variants
The HLA protein is transcribed after splicing together up to eight exons from the gene sequence. Alternative splicing of the HLA gene has been shown to result in longer or shorter mRNA sequences and variation in the final protein structure (see Figure 2). Dai et al. (2014) have demonstrated splice variants leading to the deletion of exon 3 from the HLA-A24 mRNA. This deletion resulted in the formation of homodimers and heterodimers with wild-type HLA-A24. The lack of exon 3 would prevent antigen presentation and also disrupt much of the HLA-specific antibody binding, which is commonly directed at protein within the binding cleft, coded for by exons 2 and 3.
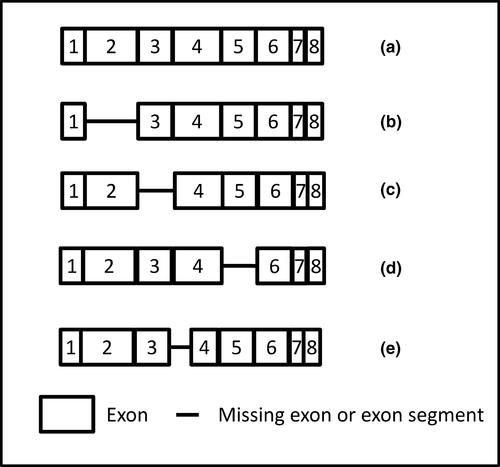
Splice variants have been implicated in HLA null alleles by skipping exon two with no resultant protein (Belicha-villanueva & Mcevoy, 2009) and by skipping exon five as soluble isoforms (Dai et al., 2014). Dunn et al. (2011) describe a silent mutation (G-A) at position 705 in exon four of HLA-A*01 that creates an alternative splice site and a resultant exon which is 87bp shorter than the wild-type. Whilst the splice site does create an in-frame reading sequence, the resultant protein is likely misfolded and therefore not expressed. Interestingly, low levels of full-length protein were still detectable on some cells within this individual, showing, as with Dai's work, that the choice of splice variant is not absolute.
Splice variants affect HLA class I (Belicha-villanueva & Mcevoy, 2009; Tijssen, Sistermans, & Joosten, 2000) and class II gene transcription (Handunnetthi et al., 2010; Hoarau, Cesari, Caillens, Cadet, & Pabion, 2004) and a number of these result in an elongated cytoplasmic tail (Belicha-villanueva & Mcevoy, 2009; Hoarau et al., 2004). Whilst the effect of the longer tail is unknown, in the nonclassical class I molecule HLA-G splice variants create either membrane bound or secreted isoforms that are essential for effective trophoblast implantation (Carosella, Rouas-Freiss, Roux, Moreau, & LeMaoult, 2015).
In most of these cases, it is likely that the resultant protein, if any, will be misfolded and will not present peptide to T cells, reducing the ability for these cells to contribute to immune surveillance. In the transplant setting, these variants will almost invariably result in a reduction in HLA mismatched antigen presentation to the recipient immune system. The effect of soluble HLA, for example through exon five splicing, has yet to be elucidated in transplantation.
2.5 Alternative start codons
Li et al. (2018) have found alternative mRNA transcripts due to polymorphisms in exon one of the HLA-C allele. These result in the use of alternative start codons and the generation of an exon of varying length, sometimes even including part of the intron one sequence. The selection of transcript depends on both the HLA-C allele and the tissue in which it is expressed and ultimately influences surface expression of the protein. This is most notable in NK cell maturation, with higher HLA-C levels on educated NK cells. In total, seven start codons were identified including one within the start region of exon two, which could result in the production of intracellular HLA-C (Johnson et al., 2018; Li et al., 2018).
2.6 Alternate polyadenylation sites
Up to 70% of human genes use alternative polyadenylation sites (PAS) at the 3′ end of the gene to create a longer or shorter product, with immune genes in particular switching to the shorter form on infection and upregulation (Kulkarni et al., 2017). Variation in HLA-A expression has been shown to be dependent on two such PAS, the nearer being conserved but the more distant is disrupted by sequence variation in some alleles.
Mutations that disrupt this distal PAS result in a shorter mRNA transcript as only the nearer polyadenylation site is used, but if both sites are active the cell can utilize either. Whilst similar amounts of mRNA are produced regardless of the PAS selected, there appears to be a translation inhibitory region between the two sites resulting in lower amounts of HLA protein from the longer sequence.
Different HLA-A genes are under control of the proximal PAS only, the distal PAS or both. In resting cells, HLA-A*03 uses the distal PAS resulting in lower expression, but switches to the proximal PAS on upregulation by some pathogens, enabling greater surface expression of the protein and increased presentation of pathogenic peptides. HLA-A*01 and A*11 are only able to utilize the proximal PAS and do not switch to the resultant low expression PAS when not activated (Kulkarni et al., 2017). This alternate PAS usage does not occur for HLA-B and HLA-C which only possess the more conserved distal polyadenylation site, suggesting that HLA-A upregulation is more greatly influenced by infection or other stimulation.
A combination of alternate splicing and the use of multiple polyadenylation sites has also been noted for HLA class II transcription where two possible splice sites and three polyadenylation sites were found in the 3′UTR of HLA-DQA1 (Hoarau et al., 2004). The authors detected up to four different mRNA transcripts for each of the HLA-DQA1*01 alleles and six transcripts, using all combinations of splice and polyadenylation sites, for DQA1*02, 03, 04 and 05.
2.7 DNA methylation
DNA methylation, mediated by the DNA methyltransferase enzymes, allows permanent or temporary silencing of genes, and in humans, this commonly occurs across cytosine–guanine dinucleotide (CpG) methylation sites. Point mutations or single nucleotide polymorphisms (SNPs) that create or remove a CpG site can therefore lead to variations in DNA methylation between subjects (Bird, 2002; Morimoto et al., 2004; Nie, 2001; Rakyan et al., 2004). Indeed, HLA expression has been shown to be altered by DNA methylation of CpG sites in the promoter region, within exons and introns, and also across the CIITA promoter itself.
For class I HLA genes, methylation can be locus and allele specific. Despite similar numbers of potential methylation sites, only HLA-A expression is affected by methylation. Ramsuran et al. (2015) demonstrated an inverse correlation between the amount of DNA methylation in the HLA-A gene and mRNA levels. Methylation was concentrated around the promoter region despite numerous possible methylation sites within exons two and three. The team showed that for the high expression allele HLA-A*24, only one CpG site was methylated, whereas the low expresser HLA-A*03 was methylated at more than one site. Ramsuran's group demonstrated that up to 6% of sequencing reads were methylated at some CpG sites for HLA-A*03, whereas <0.5% of reads were methylated for the one CpG site for HLA-A*24.
Using 5′-Aza-CdR treatment, which reverses DNA methylation, Ramsuran's group demonstrated that mRNA expression increased more when 5′-Aza-CdR was used in genes which tended to have more initial methylation (e.g., HLA-A*03). Interestingly, the team also found that despite possessing similar numbers of possible methylation sites, HLA-B and HLA-C were not methylated, and the addition of 5′-Aza-CdR had little effect on mRNA levels for these loci. The authors postulate that upstream regulatory elements and long-range determinants of chromosome structure may affect locus-specific methylation (Ramsuran et al., 2015).
Unlike HLA class I, where only the promoter region is normally affected by methylation, differentially methylated regions (DMR) within exon two of the HLA-DRB1 gene may be an additional risk factor in the disease multiple sclerosis (MS; Kular et al., 2018). Exon two of the DRB1*15:01 allele is hypomethylated in MS sufferers compared to DRB1*15:01-positive individuals without MS. Kular's team suggest that DRB1 protein expression is downregulated by methylation of up to 19 CpG sites in exon two, affecting expression between nonactivated and activated cells, but the hypomethylation of DRB1*15:01 in affected individuals permits increased expression of this antigen even when cells are not activated.
Following a similar pathway shown in HLA-A, reduced methylation within the promoter region is the prime switch for HLA-G expression in human embryonic stem cells (with further post-transcriptional modification affected by microRNA binding). Methylation of the HLA-G promoter will permit permanent silencing in other cells where its expression does not normally occur (Verloes et al., 2017).
Mutations and cancers can cause unusual DNA methylation, and this has been shown to be one reason for the lack of HLA-DR expression in both T-cell and myeloid leukaemia patients (Morimoto et al., 2004). Methylation of the CIITA-PIV splice variant prevents IFNγ-induced expression of HLA-DR and therefore abrogates a normal antitumour response. Neither addition of the methyltransferase inhibitor 5’-Aza-CdR, nor IFNγ alone enabled a normal HLA class II antitumour response, but these cells required both components to restore HLA-DR upregulation.
DNA methylation has therefore been shown to have an impact in the promoter region for HLA-A and HLA-G, intraexon for HLA class II and across CIITA in some cancers.
2.8 Post-transcriptional regulation
MicroRNAs (miRNA) are involved in the post-transcriptional regulation of many genes, including accelerated degradation of HLA-specific mRNA (Handunnetthi et al., 2010; Kulkarni et al., 2011). Increased levels of HLA-C are associated with better HIV control and reduced serum viral load, and polymorphisms in the 3’UTR result in differential expression of HLA-C on the cell surface (Kulkarni et al., 2011). This agrees with McCutcheon et al. (1995) who had previously suggested that low expression of HLA-C is correlated with increased mRNA degradation associated with DNA polymorphisms in this same region.
HLA polymorphisms in the 3′UTR affect a binding site for the specific miRNA known as has-miR-148. mRNA transcripts containing this has-miR-148 binding site undergo increased post-transcriptional degradation resulting in significantly lower surface expression of HLA-C protein (Kaur et al., 2017; Kulkarni et al., 2011). Whilst HLA-A also possesses the has-miR-148 binding site, it is nonpolymorphic in this locus. However, polymorphisms in the has-miR-148 gene itself lead to higher or lower expression of this miRNA which may then influence HLA-A and HLA-C regulation and alter overall HLA levels in some individuals (Petersdorf & O'hUigin, 2018).
In the 3′UTR for the class II gene HLA-DP, a single A/G polymorphism at position 496 results in an almost twofold higher expression of HLA-DP in the G/G homozygous group, than all others (Thomas et al., 2012). This higher expression correlated with greater persistence of HBV infection, whereas lower expression resulted in a faster recovery. The polymorphism is also linked to an increased risk of GVHD in HLA-DPB1 mismatched haematopoietic stem cell transplants where the recipient carries the higher expressed allele (Klasberg et al., 2019; Petersdorf et al., 2015).
Using in silico predictions, Shieh et al. (2018) have shown the low expressing variant (A at position 496) to be associated with potential binding sites for a higher number of miRNAs than the 496-G polymorphism. Interestingly, the 496 polymorphism can be used to separate HLA-DP into two highly divergent clades. These have different miRNA binding sites within the first and second introns and have been shown to contain 174 fixed polymorphisms in the 3′UTR that define the two clades, although other than 496 A/G, how these affect expression or function is not yet known (Klasberg et al., 2019; Shieh et al., 2018).
Similarly, a 14-bp indel in the 3′UTR of the nonclassical class I HLA-G gene has been shown to affect stability of the HLA-G mRNA, with the insertion resulting in a lower amount of mRNA. HLA-G possesses variations in the 3′UTR which affects the binding site for miR-148 and additionally miR-152 (Carosella et al., 2015; Verloes et al., 2017).
2.9 Physical stability and protein expression
The conformational stability of HLA molecules is in part determined by the strength of binding of peptide as it is loaded in the endoplasmic reticulum. “Empty” HLA is rapidly internalized, and HLA molecules which bind peptide strongly are more stable and are thought to remain longer on the cell surface, leading to accumulation of this protein as new molecules are synthesized (see Figure 3). During infection, stable HLA could lead to greater antigen presentation to T cells, but may also contribute to autoimmunity such as coeliac disease and narcolepsy (Dendrou et al., 2018; Rizvi et al., 2014).
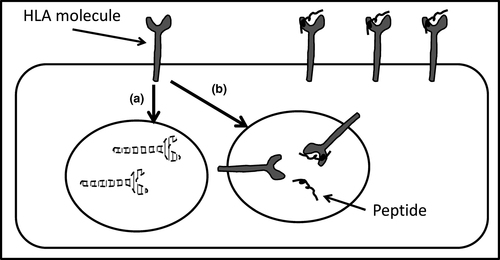
Rizvi et al. (2014) show how a SNP in the peptide binding groove of the HLA-B*44 gene results in a molecule where peptide loading is either Tapasin dependent (HLA-B*44:02) or independent (B*44:05). Tapasin-independent loading tends to result in faster shuttling of loaded HLA to the cell surface, whereas HLA molecules requiring Tapasin-mediated peptide loading can result in delayed, but potentially far more stable surface expression.
The passive binding of peptide to the HLA-B*44:05 molecule results in a lower affinity peptide: HLA association and therefore a greater likelihood of peptide dissociation on the cell surface. Loss of peptide initiates HLA internalization and unless reloaded with new peptide leads to degradation (ubiquitination) within the cell (see Figure 3). Tapasin mediates binding of peptides with a much stronger affinity for the HLA molecule and significantly reduced spontaneous peptide dissociation. The prolonged surface expression of stable HLA-B*44:02 molecules with continued shuttling of new molecules to the cell surface enables higher overall cell surface expression of this B*44:02 molecule compared to B*44:05.
Rizvi et al. (2014) noted a general trend of HLA-B molecules carrying the Bw6 motif to have less dependence on Tapasin loading than those expressing Bw4, although whether HLA-Bw6 proteins are generally less populous on the surface of cells requires more investigation.
Similarly, HLA-B molecules which are less promiscuous in their peptide repertoire (e.g., HLA-B*57, B*27:05) usually bind those peptides more strongly and are more dependent on Tapasin-mediated loading. They also appear to be more stable and are therefore expressed at higher densities than those with a less stringent peptide requirement (e.g., B*35:01; Chappell et al., 2015).
Conversely, Kaur et al. (2017) suggest that differences in the binding cleft for HLA-C affect its surface stability through greater promiscuity for peptide. This permits a greater availability of possible peptides, leading to faster loading and shuttling to the cell surface, resulting in higher surface expression. They show that HLA-C*05 alleles, with a wide and easily accessible binding cleft, can hold approximately three times as many distinct peptides and are found in greater abundance on the surface, compared with HLA-C*07 which has a narrow cleft. These differences between HLA-B and HLA-C stability may be locus specific.
2.10 Active internalization of HLA
Total cell surface HLA expression is governed by the interplay between new protein synthesis and the active rate of HLA endocytosis (Muller, Schumacher, & Kyewski, 1993; Tse & Pernis, 1984). When HLA is eventually internalized, there may be specific control over whether ubiquitination or recycling back to the surface occurs (Cho & Roche, 2013; Muller et al., 1993; Reid & Watts, 1990).
In a mouse model, MHC class I and class II internalization is a selective process which varies between cell types (Tse & Pernis, 1984), and the rate of recycling within antigen-presenting cells is organ specific (Muller et al., 1993). Similarly in a human lymphoblastoid cell line, the majority of internalized HLA class I and class II is rapidly recycled back to the cell surface, although this may not reflect normal cell function (Reid & Watts, 1990).
Accelerated endocytosis of intact HLA–peptide appears to be an active and specific process which for HLA class I is determined by the amino acid structure of the cytoplasmic tail (Vega & Strominger, 1989). The amino acid motif YSQA (residues 320–323 of exon seven) is similar to tyrosine-based endocytosis mechanisms described in other cell expression systems (Piguet, Schwartz, Le Gall, & Trono, 1999). Once internalized, the protein is either degraded or recycled back to the cell surface, for example in dendritic cells where recycling enables a rapid response to stimuli and also the opportunity to load alternative peptides in sites of local inflammation (Villadangos, Schnorrer, & Wilson, 2005).
Piguet et al. (1999) and Lin et al. (2007) have shown an increase in HLA endocytosis and degradation in lentiviral and cytomegalovirus (CMV) infections, reducing the half-life of HLA class I on the surface from 60 min to as little as 10. Similarly, in antigen-presenting cells, CMV infection increases HLA class II ubiquitination where it is degraded in lysosomes (Lin et al., 2007).
The HLA class I viral pathway may hijack normal cell machinery as virus-driven endocytosis is still dependent on specific amino acids within the cytoplasmic tail, particularly the tyrosine at position 320 described above. This motif is present in all HLA-A and HLA-B molecules, but HLA-C possesses a cysteine at position 320 instead of tyrosine. HLA-C is unaffected by viral-induced endocytosis, and this may explain the crucial role that HLA-C plays in protection from HIV and other viral infections (Apps et al., 2013; Thomas et al., 2009).
In immature dendritic cells, newly synthesized HLA class II proteins are held within endosomes. Dendritic cell maturation allows rapid movement of this HLA class II to the cell surface, and there is evidence that the class II cytoplasmic tail is modified to reduce or prevent ubiquitination, a unique process to control surface expression (Shin et al., 2006; Villadangos et al., 2005). Cho and Roche (2013) suggest that whilst this may not reduce HLA class II internalization, it allows the protein to recycle back to the cell surface rather than be directed to degradation in the lysosomes. The different cytoplasmic tail amino acid structure appears to direct the class II molecules deeper into the lysosomes for degradation, or remains near the cell surface in multivesicular bodies for peptide reloading and re-expression, resulting in a highly variable half-life of between 15 and 50 hr (Thibodeau, Moulefera, & Balthazard, 2018).
So whilst there are a host of mechanisms governing the production of mRNA and subsequent processing into intact peptide-loaded HLA molecules, the amount of surface HLA is a balance between de novo production, HLA locus-specific internalization and whether the HLA molecule is recycled back to the cell surface or directed to lysosomes for destruction. Cell activation appears to reduce the availability of proteins involved in HLA ubiquitination, allowing more HLA to recycle back to the surface, adding to newly produced HLA protein and increasing overall surface density.
In endothelial cells, HLA class II expression as a result of cell activation is short lived in the absence of continual cytokine stimulation (Cross et al., 2016), and these cytokines may be required to promote recycling back to the cell surface rather than degradation (Cho & Roche, 2013). HLA cycling is therefore locus specific and dependent on the cytoplasmic tail. Polymorphisms in this region will affect the rate of endocytosis and whether internalization leads to recycling or ubiquitination.
In a review of the relationship between HLA and disease, Dendrou et al. (2018) suggest variations in HLA expression caused by many of the mechanisms described above lead to either autoimmunity through insufficient HLA expression and ineffective deletion of autoreactive T cells in the thymus (in type I diabetes for example), or resistance to disease by variation in the presentation of foreign peptides or a greater density of HLA proteins.
3 IMPLICATIONS FOR TRANSPLANTATION
Antibody binding to HLA proteins can lead to cell damage via complement-dependent cell lysis, and in general, this cell death is correlated with antibody concentration (Honger et al., 2015; Zhang & Reed, 2009).
In addition to cell destruction at higher concentrations, antibody ligation of HLA class I induces proinflammatory changes within the cell, upregulation of HLA class II and recruitment of leucocytes through release of proinflammatory cytokines, chemokines and adhesion molecules (Xu et al., 2015; Zhang & Reed, 2009). Cross et al. (2016) demonstrated physiological levels of inflammatory cytokines are required to maintain the presentation of HLA-DQ and HLA-DP proteins on renal microvascular endothelial cells, and this suggests a requirement for a minimal and continual level of HLA ligation for endothelial expression of HLA class II proteins.
Cold ischaemia and reperfusion injury will create a proinflammatory cytokine milieu which has been shown to upregulate HLA class I and will likely induce HLA class II expression (Lutz et al., 2010; Menke, Sollinger, Schamberger, Heemann, & Lutz, 2014; Siedlecki et al., 2011). Understanding of not only the basal HLA expression level, but the significantly increased presence of HLA proteins immediately after deceased donor transplantation could help refine transplant risk.
BSHI/BST guidelines (BSHI & BTS, 2014) state that antibody levels above a specific threshold, as measured using in vitro methods, may be a contraindication in cardiothoracic transplantation. Yet whilst antibody levels may be high, lower expression of cognate antigen could represent a reduced risk of graft rejection. Evidence for this exists in hematopoietic stem cell transplantation where there is a direct correlation between the expression level of mismatched HLA-C, and HLA-DP antigen with both GVHD and mortality (Petersdorf et al., 2014; Petersdorf et al., 2015).
The normal resting expression of HLA protein varies many-fold in a locus-specific manner, and this is likely a result of many of the factors listed above: There are large locus-specific variations in HLA class I expression, with HLA-A and HLA-B being expressed at similar levels, but HLA-C at about 15 times lower (Apps et al., 2015). Similarly in healthy renal microvascular endothelial cells, HLA-DR expression is found at much higher levels than HLA-DQ or HLA-DP (Cross et al., 2016). Despite comparable MFIs between HLA loci as measured by fluorescence intensities on Luminex SAB in vitro, the effect of antibody in vivo is therefore likely to be governed by these large locus variations in surface density as well as intralocus differences.
Knowledge of the basal and induced expression of each HLA antigen could allow a more informed stratification of transplant risk for each patient/donor combination. For example, transplantation may be acceptable in the presence of higher antibody levels against HLA DQB1*03:02 than DQB1*03:01, where in the latter protein expression has been shown to be considerably higher (Andersen et al., 1991; Handunnetthi et al., 2010). Zachary et al. (2009) note the poor correlation between SAB data and cellular cross-match results which may reflect these differences.
There are also more immediate implications from the works of Neerincx et al. (2013) and Honger et al. (2015). Pretransplant cross-matching is based on antibodies directed against HLA antigens on the surface of T and B cells, yet Neerincx has shown differential CIITA control of HLA expression between cell types. Cross-matching using lymphocytes (HLA expression controlled by CIITA isoform PIII) may not therefore represent the true risk to transplant endothelium (under CIITA isoform PIV control) as HLA protein levels are very likely to vary between the two.
Finally, little has been done to investigate the activation state of peripheral blood cells at the time of cross-match, which is likely to transiently affect surface HLA expression. HLA levels have been shown to vary between living and deceased donor lymphocytes which may well affect the pretransplant cross-match result (Badders, Jones, Jeresano, Schillinger, & Jackson, 2015). Islam et al. (1995), whilst not investigating HLA directly, have shown variations in the surface density of CD3 and CD4 molecules on T cells depending on the method of lymphocyte storage and preparation, and it is therefore highly likely that HLA proteins on cells will be affected by storage, transport and separation techniques.
4 IMPLICATIONS FOR THE LABORATORY
In a recent audit of relevant scientific journal articles over the past 10 years, 67% of the publications were concerned with HLA antibody but only 7% investigated HLA antigen expression (data not shown). Scientists therefore have a greater understanding of the production and effects of HLA-specific antibodies, including route of sensitization, antibody duration and antibody affinity, avidity and measurement. In relation to expression of the cognate HLA antigen, we are less clear and, other than specific case reports, generally make an assumption that HLA expression is uniform. Many transplant units treat all HLA sensitization of equal risk despite the increasing evidence of locus-specific and allele-specific variations.
HLA expression is not uniform and there are cell-specific, HLA locus-specific and activation state differences in HLA regulation within and between individuals. There is control on DNA transcription, RNA translation and protein expression. Once expressed, there are additional factors affecting protein stability, recycling and degradation.
Honger et al. (2015) have shown less HLA antibody “target” will result in less cell damage in vitro, and some transplant centres employ algorithms where different locus-specific Luminex MFI cut-off values are selected for listing of unacceptable antigens for transplantation, although there are little published data on this practice (e.g., a higher cut-off may be used when HLA-C, HLA-DQ or HLA-DP antibodies are detected). BSHI/BTS guidelines for cardiothoracic transplantation recognize the importance of antigen density where a calculation of transplant risk should include a doubling of the Luminex MFI for any homozygous allele (BSHI & BTS, 2014).
Transplant risk can be estimated by calculating the sum of all median fluorescent intensities of donor-directed HLA-specific antibody as measured by Luminex SAB (BSHI & BTS, 2014), but there is much debate concerning what level of antibody that is clinically significant (Gebel & Bray, 2014; Picascia et al., 2014; Roelen, Doxiadis, & Claas, 2012). Differences in HLA antigen expression will confound data concerning HLA antibody variations between transplant recipients and will contribute to the confusion concerning SAB data and clinical outcome.
HLA protein is initially regulated by the control elements NLRC5 and CIITA. Both are under cytokine control and CIITA forms splice variants which will result in cell-specific differences in HLA expression. CIITA clearly induces HLA class II transcription in non-antigen-presenting cells, but differences in CIITA exon one will alter HLA surface expression in different cell types. This may question the reliability of using peripheral blood lymphocytes in a cross-match test to predict the risk of antibody binding to renal endothelial cells.
Evidence from the IMGT database (http://www.ebi.ac.uk/ipd/imgt/hla/allele.html) shows less than 30% of the full-length HLA gene is transcribed into protein (only 7% for HLA DRB1). The remaining 70%–93% of the gene contains sites for transcription promoters, inhibitors, alternative splice sites, methylation sites, binding sites for post-translational miRNA degradation and many other functions as yet undetermined. The vast quantity of information contained within noncoding regions reinforces the biological importance of this portion of the DNA.
Evidence is growing to show how polymorphisms in the 5’UTR will affect subsequent RNA translation, and indeed, there are data to show how polymorphisms even further upstream can promote DNA unwinding to allow RNA polymerase access to the transcription sites. Once transcribed, polymorphisms in the 3’UTR may allow miRNA binding, rapid RNA degradation and reduced protein synthesis. HLA transcription is also subject to alternative splicing, although it is not clear whether this happens in isolated cases or is a common occurrence, or whether external stimuli such as infection are always required.
When protein reaches the cell surface, the overall expression is a combination of de novo production and locus-specific endocytosis. Once internalized, recycling back to the surface is increased in activated cells and HLA degradation is increased when quiescent, or in the presence of viral infections such as CMV. Recycling is also influenced by the amino acid structure of HLA exon seven where variation can increase the rate of HLA internalization. There is evidence that where specific alleles require Tapasin-mediated peptide loading the surface the HLA protein: peptide combination is more stable and therefore more likely to be expressed in greater abundance, with “empty” HLA being cycled back as soon as peptide is displaced.
Control of HLA expression is therefore multifactorial (see Table 1 for summary). Positive and negative feedback mechanisms exist at every stage, and these vary in importance between individuals, cell types and HLA alleles. This is essential for population health as it allows some individuals to mount stronger responses against pathogens, yet these same individuals may be at greater risk of autoimmunity.
| HLA locus | Known factors |
|---|---|
| HLA-A |
Methylation of promoter sites. Alternative polyadenylation around positions 300 and 400 of the 3′UTR. MicroRNA polymorphism (3′UTR binding site is nonpolymorphic). Splice variants (deletion or shortening of exons). Polymorphisms in 5′UTR affecting promoter binding sites. Ubiquitination via exon seven polymorphism (YSQA motif at amino acids 320–323) |
| HLA-B |
Peptide-groove stability (e.g., Tapasin-dependent B*44:02 vs. Tapasin-independent B*44:05). Ubiquitination via exon seven polymorphism (YSQA motif at amino acids 320–323) |
| HLA-C |
MicroRNA degradation (e.g., positions 256–266 of the 3′UTR binding miR-148a). Polymorphisms in 5′UTR affecting promoter binding sites. Long-range 5′ polymorphisms affecting histone acetylation. Alternative start codons resulting in cell and maturation state-specific mRNA transcripts. Peptide-groove promiscuity allowing greater more rapid binding (e.g., HLA-C*05). “Trophoblast pathway”: HLA-C induction despite absence of NLRC5 or CIITA |
| All class I | NLRC5 upregulation via IFNγ |
| HLA-DRB1 |
Methylation of exons (e.g., exon two of HLA-DRB1). Polymorphisms in the Y box of 5′UTR. Polymorphisms in 5′UTR affecting promoter binding sites. Vitamin D response element in 5′UTR of HLA-DRB1, disrupted in many alleles. Modification of cytoplasmic tail in dendritic cells |
| HLA-DQA1 |
Alternative polyadenylation at multiple sites in the 3′UTR. Alternative splicing of 3′UTR (up to six alternative mRNA transcripts). Polymorphisms in 5′UTR 240−200 bp upstream of exon one. |
| HLA-DQB1 |
Polymorphisms at position −178 of 5′UTR affecting DQB1*03:01 and *03:02 expression. Polymorphisms across the SXY box of the 5′UTR affecting HLA expression |
| HLA-DPB1 |
Up to 174 known polymorphisms in the 3′UTR segregating two HLA-DP clades. A/G dimorphism at position 496 of the 3′UTR |
| All class II |
Methylation of CIITA. CIITA upregulation via IFNγ. Alternative CIITA transcripts via alternative exon one splicing, leading to at least three variants with cell-specific functions. Variation in cytoplasmic tail (10–18 bp) leading to recycling with new peptide as opposed to ubiquitination: Dependence on presence of Tyrosine for internalization |
| Nonclassical HLA-G |
Methylation of promoter region preventing expression on nontrophoblast cells. MicroRNA downregulation via a 14-bp indel in the 3′UTR. Splice variants creating either membrane bound or secreted molecules. “Trophoblast pathway.” Specific activation in trophoblasts by a mechanism as yet unconfirmed |
5 TRANSPLANTATION IN THE FUTURE
With the advent of highly cost-effective next-generation sequencing (NGS), we are now in an era where routine HLA typing can be performed at the allelic level. Robust automated platforms are available for sequencing of the entire HLA gene, including both 5′ and 3′ UTRs and intronic sequences (Hosomichi, Shiina, Tajima, & Inoue, 2015; Monos & Drake, 2019; Petersdorf & O'hUigin, 2018). Whilst this is creating a huge increase in the number of described alleles, it is also allowing the opportunity to link DNA sequence and function in both the disease association and transplantation settings (Petersdorf & O'hUigin, 2018).
Current sequencing methods take some days to complete and selection of potentially “low expressing” donors would be confined to living donors in conjunction with kidney sharing such as the United Kingdom Living Donor Kidney Sharing Scheme. With the advent of novel sequencing methods however, such as the Oxford Nanopore Minion, complete gene HLA sequencing is a possibility in the future (Lu, Giordano, & Ning, 2016).
This review sought to highlight many of the factors which affect HLA expression. These influences may be overarching, such as through NLRC5 or CIITA, or may be locus, allele and even cell specific. Many of the issues here are the result of isolated studies and may not be applicable with other cell types or activation states, for example at times of transplant rejection.
With the advent of next-generation sequencing at prices comparable to other DNA-based HLA typing methodologies, the scientific community now has the opportunity to compare full-length gene data with experiments measuring specific HLA expression across various cell types, and even with transplant outcomes. Ultimately, even small advances in our understanding may allow some alleles to be considered “safer” in terms of transplant mismatch, as has already been shown with HLA-C and HLA-DP in the stem cell transplant setting.
CONFLICT OF INTEREST
The authors declare no conflict of interest in this review.




