The role of electrochemotherapy in the treatment of locally advanced or recurrent eyelid-periocular basal cell carcinoma: long-term results
Conflict of interest: None.
Funding source: None.
Abstract
Background
While electrochemotherapy (ECT) is increasingly utilized as a highly effective method in the treatment of tumors in the head and neck region, there is significantly less data available for eyelid-periocular skin tumors. Our group reported the first extensive case series of eyelid-periocular basal cell carcinoma (BCC) patients with short-term follow-up treatment with ECT. The present study aims to report our long-term results of eyelid-periocular BCC cases treated with ECT.
Methods
The treatments were performed according to the ESOPE (European Standard Operating Procedures on Electrochemotherapy) guidelines using the Cliniporator™ device. All patients received bleomycin-based ECT, administered intratumorally or intravenously. Tumor response was evaluated using the RECIST 1.1 criteria.
Results
The results of 19 patients treated with ECT are presented. Four patients had locally advanced primary tumors, while 15 patients had recurrent tumors. Bleomycin was administered intratumorally in four patients and intravenously in 15 patients. The overall response was 100%, while the complete response rate proved to be 95%. In three cases (15.8%), recurrence was observed during the mean follow-up period of 78.9 months.
Conclusions
ECT can effectively treat locally advanced or recurrent BCC in the eyelid-periocular skin region. Excellent tumor control can be achieved with good functional and cosmetic results without systemic adverse events with long interval follow-up.
Introduction
Basal cell carcinoma (BCC) is the most common human malignancy. The incidence of affected individuals has continuously increased for many years, with epidemiological data indicating an annual incidence growth rate of 1–3%. Chronic sun exposure, in addition to skin phototype, stands out as the primary risk factor for the development of BCC. Since the head and neck, especially the periocular skin region, receive the most intense sun exposure, BCC predominantly manifests in these areas.1, 2
The gold standard for the treatment of BCC in the head and neck area is surgical removal of the tumor in a proper manner, preferably with intraoperative histological control of the excision margins if possible (Mohs surgery). In cases of inoperable tumors (e.g., where bone involvement occurred), incompletely removed malignancies, or as adjuvant therapy in the presence of perineural invasion, irradiation can be highly effective. Patients with locally advanced, recurrent, or metastatic BCCs can benefit from systemic treatment with hedgehog or programmed death-1 inhibitors.3-5 Despite all these therapeutic options, treating locally advanced/recurrent BCCs remains challenging. However, these difficulties are particularly pronounced in the periocular-eyelid area. While primarily considering oncological aspects and patient safety in determining the therapeutic plan, other factors should also be considered when treating advanced BCC in the periocular region.6 For elderly patients with comorbidities, the surgical removal of large tumors and the reconstruction of tissue defects often pose a substantial surgical burden. In surgeries of the periocular skin area, it is crucial to consider that more extensive excisions requiring significant reconstruction may alter the patient's self-image, potentially leading to the development of severe psychological disorders. Beyond oncological, patient safety, and cosmetic factors, preserving and restoring eyelid function are also of fundamental importance after extended surgery. The homeostasis of the ocular surface relies on the eyelid's proper functioning. If there is significant disruption in the kinetics of eyelid closure and opening, the integrity of the ocular surface may be compromised. In severe cases, the cornea may lose its transparency, threatening vision loss.7
A novel option for the treatment of primary malignant and metastatic solid tumors of many histotypes located on the skin is electrochemotherapy (ECT), which has gradually become a widely used therapeutic procedure over the past 20 years. In addition to cutaneous malignancies, this procedure is now successfully applied to treat deep-seated tumors and visceral cancers as well.8-12 The basic principle of ECT is electroporation, when short-duration, high-voltage electric pulses temporarily create pores in the cell membrane. The transient permeation of the cell membrane allows the entry of large, non-soluble, or poorly permeant, water-soluble chemotherapeutic agents into the cytosol.13 After the cessation of the electric pulses, the pores close, restoring the integrity of the cell membrane. Consequently, any cytotoxic substances that enter the cytosol become trapped inside the cell. In this manner, the cytotoxic agent that has entered the cell can exert its cytolytic effect, amplifying the cell-killing capacity. Bleomycin and cisplatin have proven to be the most effective chemotherapeutic agents in ECT, leading to the widespread use of these two drugs in clinical practice.14, 15 During ECT, the cytotoxicity of bleomycin increases several hundredfold, while that of cisplatin rises to a lesser but still significant extent.16
While ECT is increasingly utilized as a highly effective method in the treatment of tumors in the head and neck region, there is significantly less data available on eyelid-periocular skin tumors.17-20 The first extensive case series of eyelid-periocular BCC patients treated with ECT was reported by our group in 2019.21 The follow-up period in that study (median: 19 months) was not long enough to assess the recurrence-related efficacy. Therefore, only moderate consequences could be deduced. In the present retrospective observational study, we report the long-term results of our locally advanced/recurrent eyelid-periocular BCC cases treated with ECT.
Patients and Methods
Patients
To assess the long-term efficacy of ECT treatment, the present study included all patients with locally advanced or recurrent eyelid-periocular BCC who underwent an ECT procedure at the Department of Dermatology and Allergology at the University of Szeged between May 2014 and October 2018 and had a minimum of 5 years (60 months) or longer follow-up period.
The study was approved by the Institutional Review Board of the University of Szeged (ECT-REPRO-002, 9/2016-SZTE) and conducted in accordance with the principles of the Declaration of Helsinki. All patients gave their written informed consent prior to treatments. Additionally, informed consent was obtained from all patients for the publication of identifying information/images in an online open-access publication.
Methods
All patients underwent detailed dermatological and ophthalmological examinations. In cases where clinical examination suggested the presence of bone involvement, orbital computed tomography was performed. In the case of bone involvement, the patient was not suitable for ECT treatment.
The clinical characteristics of the tumors, such as the size, number, localization, and type (primary or recurrent) of the lesions, were meticulously recorded before the ECT procedure. Biopsy of the tumor tissue was performed in all cases prior to ECT treatment. Treatments were performed according to the ESOPE guidelines using the Cliniporator™ (IGEA Ltd, Modena, Italy) device.16 Every patient received bleomycin-based ECT; the route of administration was intratumoral or intravenous. The technical parameters of the treatment protocol were described earlier by our group.21 In brief, electric pulses were applied by standard needle electrodes after 1 or 8 min following intratumoral or systemic bleomycin administration, respectively. Needle electrodes with linear (N-20-4B) or hexagonal (N20-HG and N-30-HG) configurations or plate electrodes (P-30-8B) were used.
The electrical parameters of treatments with row needle electrodes were 8 square wave pulses 1000 V/cm for 100 ms at 5 kHz, with hexagonal electrodes, 4 square wave pulses 910 V/cm for 100 ms at 5 kHz. Depending on the clinical tumor status (number, location, and size of the lesions), general anesthesia with endotracheal intubation or laryngeal mask was used. Following the treatment, all patients were observed in the hospital for 1 day to closely monitor potential adverse events such as nausea and flu-like symptoms. Simple non-adhesive dressings were applied to the wounds, and antibiotic eye drops (ofloxacine) were prescribed for 6–10 days.
Tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.22 Complete response (CR) was diagnosed in case of the disappearance of the target lesion, while partial response (PR) was defined when at least a 30% decrease in the baseline sum of the longest diameter of the target lesion was observed. The treatment strategy for each patient was personalized, determined primarily by the tumor response to the initial treatment.
According to our protocol, the criteria for ECT re-treatment were based on the observation of PR 6 weeks after the initial ECT treatment, with a two-month time interval between the first and second ECT sessions.
Patients were closely monitored after the treatments: They were examined and photo-documented twice in the first month, monthly in the following five months, bimonthly in the first year, quarterly in the second year, and every six months after that.
Only BCCs in the eyelid-periocular localizations were included in the statistical analysis.
Statistical analysis
SPSS software version 17.0 (SPSS, Chicago, IL, USA) was used for statistical analysis.
Results
Between May 2014 and October 2018, 19 patients with eyelid-periocular BCCs were treated with ECT and had a clinical follow-up of at least 60 months. All patients included in the study were of Caucasian descent (12 male and 7 female, mean patient age: 75.9 years, median: 72 years, range: 11–86 years). The follow-up interval was between 61 and 112 months (mean: 78.9 months, median: 72 months). Detailed characteristics of the treated cases can be found in Table 1.
| Age (year)/gender | Periorbital localization | Other localization | Size of periocular tumor/s (mm) | Primary/rec./(previous treatments) | Route of BL | Type of electrode /average current(A) | Follow-up (month) | Number of ECT treatment (dates of treatment) | Results | Rec.: localization and time interval after the first session(s) of ECT | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 83/F | Lower eyelid, eyebrow | Both hands, nose | 10 × 13 | Recurrent (surgery) | iv | N-20-HG, P-30-8B | 98 | 1 | CR | No rec. |
| 2 | 72/M | Lower eyelid | Fronto-temporal region, cheek | 43 × 27 | Recurrent/multiple surgeries) vismodegib | IV | N-20-4B, N-20-HG | 94 | 3 (2 + 1) (2 months interval) | CR | Rec.: 27 months after first sessions of ECT lower eyelid, fronto-temporal area (treatment of recurrence: ECT) |
| 3 | 44/F | Eyebrow, upper eyelid | — | 10 × 25 | Recurrent/surgery | N-20-HG | 112 | 2 (2 months interval) | CR | No rec. | |
| 4 | 81/M | Medial canthal region | Head, face area | 7 × 9 | Recurrent/surgery | IT | N-20-HG | 74 | 1 | CR | No rec. |
| 5 | 77/F | Lower eyelid | Both hands, nose | 13 × 10 | Recurrent after surgery | iv | N-20-HG | 70 | 1 | CR | No rec. |
| 6 | 35/F | Eyebrow, upper eyelid | — | 25 × 10 | Recurrent/surgery | it | N-20-HG | 97 | 2 (2 months interval) | CR | No rec. |
| 7 | 11/F | Upper eyelid, lower eyelid, both canthal regions |
Nose, perioral skin, frontotemporal area, entire skin surface xeroderma pigmentosum |
6 × 8 12 × 5 20 × 15 |
Recurrent/surgery/pembrolizumab | iv | P-30-8B, N-20-4B | 110 | 6 in the periocular region | PR | Continous rec. |
| 8 | 45/M | Upper eyelid | — | 35 × 12 | Recurrent/surgery | it | N-20-4B, P-30-8B | 84 | 1 | CR | No rec. |
| 9 | 81/M | Both lower eyelids, medial canthi | Head-neck, back |
15 × 110 8 × 15 |
Recurrent/surgery | iv | N-20-4B, N-20-HG | 87 | 1 | CR | No rec. |
| 10 | 82/M | Lower eyelid, medial canthus | Nose | 13 × 27 | Recurrent/surgery | iv | N-30-HG, N-20-4B | 74 | 2 (2 months interval) | CR | No rec. |
| 11 | 80/F | Lower eyelid, medial canthus | Head, neck, trunk |
10 × 15 5 × 8 |
Recurrent/surgery | iv | N-20-4B, P-30-8B | 69 | 2 (2 months interval) | CR | No rec. |
| 12 | 66/M | Lateral canthus, upper eyelid | Head-neck, trunk, arm, labial area Gorlin-Goltz syndrome |
8 × 6 5 × 4 |
Recurrent/surgery | iv | N-20-HG | 71 |
2 in the periocular region with 2 months interval 2 in other localization (trunk, arm, labial area) |
CR | No rec. |
| 13 | 72/M | Lower eyelid, medial canthal region | Head-neck, chest, upper extremities |
7 × 5 2 × 3 |
Recurrent/surgery PDT, pembrolizumab |
iv | N-20-4B | 72 | 1 in the periocular region, 2 in other localization | CR |
No rec. in the periocular skin area Chest: rec. (treatment: one ECT session) |
| 14 | 86/M | Lateral canthal region | Trunk, head-neck, extremities | 25 × 25 | Periocular: primary trunk: surgery | iv | N-20-4B | 70 |
1 in the periocular area 1 retreatment in other localization |
CR |
No rec. in the periocular skin area Head: rec. (treatment: one ECT session) |
| 15 | 64/M | Lower eyelid, medial canthus | Trunk, head-neck, extremities | 14 × 14 | Primary | iv | N-20-4B | 68 | 2 (2 months interval) | CR | No rec. |
| 16 | 31/M | Lower eyelid, medial canthal area, nose | — | 40 × 40 | Primary | iv | N-20-4B | 66 |
3 (2 + 1) (2 months interval) |
CR | Rec.: 24 months after first sessions of ECT (treatment: ECT) |
| 17 | 70/M | Lower eyelid | Scalp, neck | 20 × 15 | Primary | iv | N-20-4B | 62 | 1 | CR | No rec. |
| 18 | 72/F | Lateral canthus | Trunk, head, multiplex | 15 × 12 | Recurrent/surgery | iv | N-20-4B | 61 | 1 | CR | No rec. |
| 19 | 74/M | Lower eyelid, medial canthus | Head, neck, multiplex | 14 × 20 4 × 4 | Recurrent/surgery | iv | N-20-4B, N-20-HG | 61 | 2 (2 months interval) | CR | No rec. |
- Bl, bleomycin; CR, complete response; it, intratumoral; iv, intravenous; N-20-4B, needle electrodes with linear configuration; N20-HG, N-30-HG, needle electrodes with hexagonal configurations; P-30-8B, plate electrodes; PR, partial response; rec, recurrence.
Bleomycin was administered intratumorally in four patients and intravenously in 15 patients. For intratumoral administration of bleomycin, 1000 IU/ml concentration was used. The volume was calculated based on the size of the lesion (250-1000 IU/cm3), while the systemic dose was 15,000 IU/m2. The tumor volume was calculated as follows: ab2π/6, where a = longest diameter, b = longest diameter perpendicular to a. After tumor volume calculations, the given volume and concentrations of bleomycin were defined according to the updated ESOPE protocol. After performing intratumoral bleomycin injection, blanching of the lesion and the surrounding tissue up to the planned safety margin was carefully checked to ensure the entire tumor volume was covered. Safety margins were determined according to surgical margins recommended in the guidelines.
Intravenous administration was indicated in patients with multiple tumors, including those with extraperiocular skin localization.
The summary of the results of the treated cases can be found in Table 2.
| Characteristics of the treated cases | No of patients (%) |
|---|---|
| Primary BCC | 4 (21) |
| Recurrent BCC | 15 (79) |
| Localization | |
| Only on the periocular skin | 4 (21%) |
| Multiple localizations | 15 (79%) |
| Sessions of ECT in periocular localization: number of treated patients | 1: 9 (47%) |
| 2: 7 (37%) | |
| 3: 2 (10.5%) | |
| 6: 1 (5%) | |
| Route of bleomycin administration: | |
| Intratumoral | 4 (21%) |
| Intravenous | 15 (79%) |
| Response | |
| Objective response | 19 (100%) |
| Complete remission | 18 (95.0%) |
| Partial remission | 1 (5.0%) |
| Recurrences | 3 (15.8%) |
| Excluding xeroderma pigmentosum case | 2 (10.5%) |
| Follow-up time: mean 78.9 months overall tumor control (no recurrences) | 84.2% |
| Excluding xeroderma pigmentosum case | 89.5% |
Six months after the treatment, CR was achieved in 18 patients (95%); we observed PR in one patient. Among 19 patients who met the criteria for long-term follow-up, recurrence was observed in 3 cases (15.8%). In one case, a child with xeroderma pigmentosum, the continuous appearance of BCCs and squamous cell carcinomas throughout the body was noted. When the xeroderma pigmentosum case was excluded from the statistical analysis, a 5-year recurrence-free tumor control rate was achieved in 17 patients (89.5%). The rationale for the exclusion is that xeroderma pigmentosum is a genetically determined disorder in which the applied surgical treatment barely influences the development and continuous appearance of malignant skin tumors. Therefore, the recurrence does not reflect the effectiveness of the therapy.
For the other two patients who experienced recurrence (10.5% of all patients), tumor relapse occurred 24 and 27 months after ECT treatment. After re-treatment with ECT, complete remission was achieved in both patients, and this state signifies lasting recurrence-free status for both of them, as they have been in a tumor-free condition for 42 and 67 months, respectively.
In two other patients, recurrence was observed only in tumors located in the extraperiocular region and not in those located in the periocular region.
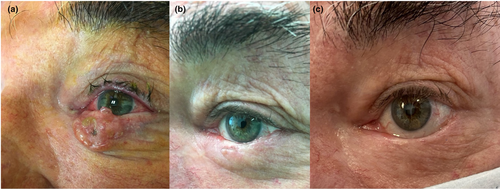
The highest number of treatment sessions was linked to genetic susceptibility for developing various types of skin malignancies: our patient with xeroderma pigmentosum has undergone six applications in the periocular skin area thus far. In the case of 3 patients with lower eyelid BCC, the contracting post-ECT scar caused ectropion, which was surgically corrected 6–8 months after the ECT. No systemic side effects from bleomycin were observed in any of the cases. Furthermore, up to the first 2 months following the treatment, skin ulceration accompanied by mild edema developed on the treated skin surface. However, it was ultimately resolved in all cases. Figure 1 demonstrates a lower eyelid BCC patient before (A) the ECT treatment, 3 months (B), and 3 years after the procedure (C).
Discussion
Approximately 75% of BCCs develop in the head and neck region, with around 20% involving the periocular area.23 Close to two-thirds of malignant tumors in the head and neck region are diagnosed in locally advanced stages. Despite aggressive and often multimodal treatment protocols, there is a high risk of local recurrence.24-26
The treatment of locally advanced or recurrent periocular BCCs is a complex task. In spite of the expanding therapeutic options, the treatment of large or recurrent periocular skin malignancies remains a significant challenge, highlighting the importance of the emergence and integration of new therapeutic modalities into clinical practice.6, 27, 28 In the modern oncotherapeutic toolkit, the role and significance of ECT are becoming increasingly well-defined. The range of tumor types effectively treated with good results is exponentially expanding thanks to rapid developments.
In the field of ophthalmology, ECT has recently gained attention as a potential therapeutic option. Our group has routinely applied the procedure in patients with challenging periocular BCC since 2014. In our previous examination, none of the 12 patients treated with 12 ECT sessions experienced tumor recurrence during the median 19-month follow-up period.21 Summarizing our long-term results, it can be concluded that the achieved overall response of 100% and the CR rate of 95% represent an excellent tumor response and reasonable long-term tumor control. During the mean follow-up period of 78.9 months, 10.5% of the patients experienced local recurrence. Considering that the treated tumors were high-risk BCCs, known to pose a higher risk of recurrence, these results are even more remarkable. Comparing our results with large cohorts of ECT treatments can be beneficial. The InspECT registry is the largest cohort of BCC patients with tumors of various locations treated with ECT.29 The reported results, based on the statistical analysis of 623 BCC lesions from 330 patients in this registry, revealed an overall response of 96% and a CR of 85%. At a 17-month follow-up, 28 (9.3%) patients experienced local recurrence/progression.
While rigorous comparisons with other treatment options are challenging due to the general heterogeneity of published results, considering surgery as the gold standard of BCC treatment, some comparisons can help advance clinical practice. The 5-year recurrence rate after excision of primary BCC was reported to be 1–8%, while it ranged from 11.6% to 17.4% for recurrent lesions.30, 31 Comparing all these results, it can be concluded that for eyelid-periocular BCCs, ECT can be considered a curative-intent procedure even in cases of locally advanced and/or recurrent tumors.
In light of all these positive, encouraging results, the range of patients with periocular BCC treated with ECT has expanded in our recent clinical practice.
Previously, we primarily employed the procedure in cases of locally advanced primary tumors and recurrent lesions after surgical excision and/or irradiation. At present, we often apply ECT treatment even in cases of primary tumors where surgical reconstruction might not necessarily result in optimal functional or cosmetic outcomes. For patients with widespread, multiple tumors throughout the body, ECT can be considered a frontline procedure.
The 2023-updated Consensus-Based Interdisciplinary Guideline for the Diagnosis and Treatment of BCC states that ECT can be utilized in treating locally advanced or recurrent BCC when standard treatments are not feasible.32 Based on our experience in the periocular area, this therapeutic option can be utilized as a palliative measure and with curative intent. ECT can be an excellent option in cases where surgical excision would entail a significant surgical burden or when there is a higher risk of potentially impaired wound healing after surgical excision. The previously detailed thoughts are opinions based on the experiences of our center and are not therapeutic recommendations. Further studies are needed to support these notions.
While the number of our patients is sufficient to draw significant conclusions, considering all these aspects, we believe that ECT plays a substantial role in personalized treatment plans for eyelid-periocular BCC patients. It attains high oncological effectiveness while ensuring excellent function preservation and cosmetic results. ECT should always be considered a viable treatment alternative in cases requiring extended surgery.
Conclusion
ECT is an efficient and safe procedure compared to other methods, with noteworthy advantages being its repeatability and the high intratumoral chemotherapeutic concentration achieved with low systemic load. High rates of complete remissions and good long-term tumor-free outcomes can be achieved in the treated patients, demonstrating the efficacy of the procedure. Further increases in efficacy can be expected based on the continuously growing experience, optimizing intervention conditions, and delineating the patient subpopulation to be most effectively treated.33-35
Patient consent
All patients gave their written informed consent prior to treatment. Additionally, informed consent was obtained from all patients for the publication of identifying information/images in this online open-access publication.
Open Research
Data availability statement
All data generated and analyzed during this study are included in the article as information files.




