Influence of dental bleaching on the pulp tissue: A systematic review of in vivo studies
Abstract
Background
Although several studies indicate the harmful effects of bleaching on pulp tissue, the demand for this procedure using high concentrations of hydrogen peroxide (HP) is high.
Objectives
To investigate the influence of bleaching on the pulp tissue.
Methods
Electronic searches were conducted (PubMed/MEDLINE, Scopus, Cochrane Library and grey literature) until February 2021. Only in vivo studies that evaluated the effects of HP and/or carbamide peroxide (CP) bleaching gels on the inflammatory response in the pulp tissue compared with a non-bleached group were included. Risk of bias was performed according to a modified Methodological Index for Non-Randomized Studies scale for human studies and the Systematic Review Centre for Laboratory Animal Experimentation's RoB tool for animal studies. Meta-analysis was unfeasible.
Results
Of the 1311 studies, 30 were eligible. Of these, 18 studies evaluated the inflammatory response in animal models. All these studies reported a moderate-to-strong inflammatory response in the superficial regions of pulp, characterized by cell disorganization and necrotic areas, particularly during the initial periods following exposure to 35%–38% HP, for 30–40 min. In the evaluation of human teeth across 11 studies, seven investigated inflammatory responses, with five observing significant inflammation in the pulp of bleached teeth. In terms of tertiary dentine deposition, 11 out of 12 studies noted its occurrence after bleaching with 35%–38% HP in long-term assessments. Additionally, three studies reported significant levels of osteocalcin/osteopontin at 2 or 10 days post-treatment. Other studies indicated an increase in pro-inflammatory cytokines ranging from immediately up to 10 days after bleaching. Studies using humans' teeth had a low risk of bias, whereas animal studies had a high risk of bias.
Discussion
Despite the heterogeneity in bleaching protocols among studies, High-concentrations of HP shows the potential to induce significant pulp damage.
Conclusions
High-concentrations of bleaching gel increases inflammatory response and necrosis in the pulp tissue at short periods after bleaching, mainly in rat molars and in human incisors, in addition to greater hard tissue deposition over time. However, further well-described histological studies with long-term follow-up are encouraged due to the methodological limitations of these studies.
Registration
PROSPERO (CRD42021230937).
INTRODUCTION
Dental bleaching has become the most popular treatment for achieving visibly aesthetic teeth (Briso et al., 2015; Santos et al., 2018). The procedure can be performed either as ‘in-office bleaching’, in which higher concentrations of bleaching agents are used, or as ‘at-home bleaching’, in which multiple sessions with lower concentrations of bleaching agents are required. Both in-office and at-home bleaching should be conducted under the supervision of an oral health practitioner (Kwon & Wertz, 2015; Li & Greenwall, 2013). In both protocols, patients often experience tooth sensitivity, especially when high concentrations of bleaching gel are used (Faria-E-Silva et al., 2015; Moncada et al., 2013).
Hydrogen peroxide (HP), the active compound in bleaching gels, induces bleaching by oxidizing organic structures through reactive oxygen species (ROS). ROS target the long-chain dark-coloured chromophore molecules, breaking them down into smaller and less coloured molecules (Cintra et al., 2017; Dahl & Pallesen, 2003). In addition to causing morphological changes in the enamel, such as increased roughness and reduced hardness (Benetti, Briso, et al., 2018; Briso et al., 2015; Chen et al., 2008), ROS derived from HP can also affect dentine by breaking the double bonds in both organic and inorganic compounds within the dentinal tubules (Karaarslan et al., 2019). Furthermore, the low molecular weight of ROS enables rapid diffusion into mineralized tissues, potentially leading to damage in pulp tissue and cells (Barbosa et al., 2019; Benetti et al., 2017; Cintra, Benetti, Ferreira, Gomes-Filho, et al., 2016; Cintra, Benetti, Ferreira, Rahal, et al., 2016;Costa et al., 2010; Roderjan et al., 2015).
While there has been an increase in the demand for bleaching, their effects on pulp tissue remain unclear (Cintra et al., 2013, 2017; Costa et al., 2010). The literature has reported changes such as severe inflammation and areas of necrosis in human mandibular incisors (Costa et al., 2010; Roderjan et al., 2015), dogs' teeth (Seale et al., 1981) and rats' molars (Benetti et al., 2017; Benetti, Briso, et al., 2018; Benetti, Briso, Carminatti, et al., 2019; Cintra et al., 2013, 2017; Cintra, Benetti, Ferreira, Gomes-Filho, et al., 2016) that have been subjected to HP-based bleaching agents. Additionally, in vivo studies have shown that bleaching accelerates pulp aging, leading to the deposition of tertiary dentine and a reduction of pulp chamber area (Benetti, Briso, Carminatti, et al., 2019; Benetti, Briso, de Araújo Lopes, et al., 2019; Cintra et al., 2017). Clinical side effects, such as tooth sensitivity during bleaching, have been described in 47% of patients undergoing treatment (Browning et al., 2007). These effects persist even after treatment is completed. On the other hand, when human premolars were bleached, no pulp changes were observed (Costa et al., 2010; Kina et al., 2010).
Thus, several in vivo studies assessing pulp tissue response after bleaching have been conducted (Benetti et al., 2017), and there is a need for a systematic evaluation of these studies to understand the effects of bleaching gel on pulp tissue. In vivo studies enable the evaluation of alterations such as inflammation, the presence of necrosis, tissue disorganization, the assessment of collagen and the formation of mineralized tissue. Therefore, the purpose of this study was to conduct a systematic review to investigate the inflammatory response in pulp tissue induced by bleaching.
METHODS
The reporting of the present systematic review was carried out in full accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 checklist (Page et al., 2021). A research protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42021230937).
Eligibility criteria
The following studies were included: (1) studies that evaluated the effects of bleaching gels on pulp tissue compared with a control group that was not bleached; (2) in vivo studies using animal teeth; (3) in vivo studies using human teeth. The exclusion criteria were as follows: (1) studies assessing agents other than HP and/or carbamide peroxide (CP); (2) studies for which the full text was unavailable. No restrictions on language or date of publication were imposed.
The Population, Intervention, Comparison and Outcome (PICO) approach was used to address the following question: ‘Does bleaching gel (HP and/or CP) induce changes in pulp tissue?’ The study population (P) included animals or humans. The intervention (I) under investigation was dental bleaching using HP and/or CP agents. The comparison (C) involved a placebo gel or teeth that had not undergone bleaching gels. The primary outcome (O) assessed was the inflammatory response. The secondary outcomes included pulp tissue disorganization, evaluation of collagen fibres, induction of mineralization and expression of other proteins.
Search strategy
Electronic searches were conducted in PubMed/MEDLINE, Scopus, Cochrane Library and Web of Science databases up to February 2021. Grey literature was consulted through OpenGrey, and manual searches were performed on the reference list of eligible articles. The search strategy utilized a combination of keywords and Medical Subject Heading (MeSH) terms, combined with the Boolean operators ‘AND’ and ‘OR’, as detailed in Data S1.
Study selection
Study selection was carried out by two independent authors (M.V.D. and H.G.S.C.) in a two-step process. In Step 1, the authors evaluated the titles and abstracts of the studies retrieved from the searches. To remove duplicates, the articles were sorted alphabetically by title, and duplicates were manually removed. Studies with titles and abstracts that met the eligibility criteria were included immediately. For studies with titles and abstracts that provided insufficient information to make a decision, the full texts were obtained. In Step 2, a full text assessment of the remaining records was conducted. Studies whose full text met the eligibility criteria were also included. Disagreements were resolved through discussion, and when necessary, a third author (F.B.) was consulted. Cohen's kappa coefficient was calculated to measure inter-investigator agreement during the studies selection process (Landis & Koch, 1977).
Data extraction and analyses
Two authors (M.V.D. and L.C.A.) collected the following data from the included studies: the last name of the first author, year of publication, experimental model, groups, dental bleaching protocol, sample size, analysis period, disclosure of funding sources and the results of the assessment of bleaching on primary and secondary outcomes. Data collection was carried out using a piloted data extraction form within an Excel spreadsheet. If data were unavailable, they were categorized as not applicable. Subsequently, a third author (A.H.R.P.) reviewed the data. In cases where data were missing, the authors were contacted twice via email messages.
Risk of bias assessment
Two investigators (L.C.A. and A.H.R.P.) independently assessed the risk of bias in the selected studies, focusing on the primary outcome (inflammatory response of the pulp tissue). For in vivo analyses of studies involving humans, the Methodological Index for Non-Randomized Studies (MINORS) scale with adaptations was utilized. These adaptations mainly pertained to the reporting quality of the experimental protocol and the presence of blinded evaluators (Benetti, Briso, et al., 2018; Slim et al., 2003). The items on the scale included a clear statement on the aim, contemporary groups, a clear description of the bleaching protocol, justification of sample size (the authors either provided the sample size and power calculation methods or justified the sample size used), adequate statistical analysis, equivalence of groups at baseline and blinded analysis of the outcomes. Each item on the MINORS scale was scored as follows: 0, content not reported; 1, content reported inadequately; and 2, content reported sufficiently (Tsirogiannis et al., 2016).
For studies involving animals, the tool ‘Systematic Review Centre for Laboratory Animal Experimentation’ (SYRCLE's RoB tool) with modifications was employed (Hooijmans et al., 2014). The items included adequate generation of allocation sequence, similarity of groups at baseline or adjustment of confounder due to differences between groups, adequate allocation concealment, random housing, blinded intervention/outcome assessment, sample randomization for outcome assessment, incomplete outcome data, selective outcome reporting and the presence of other biases. Sample size justification was also assessed to provide further characterization of reporting in the included animal studies. A judgement of ‘no’ indicated a high risk of bias, ‘yes’ indicated a low risk of bias, and ‘unclear’ indicated either a lack of information or uncertainty regarding bias. Doubts and discrepancies were discussed until a consensus was reached. If not resolved, a third examiner (L.G.A.) was consulted.
Synthesis of results
The selected studies were analysed in terms of methodological homogeneity to determine whether a meta-analysis could be performed. However, a meta-analysis was not performed due to considerable heterogeneity among studies regarding the experimental models, groups, concentrations of bleaching agents and observation periods. Additionally, the eligible reports failed to report the dispersion measure (standard deviation) of the measure of effect (mean difference). Thus, a qualitative synthesis of the results of the included studies was provided instead.
RESULTS
Study selection
The PRISMA flowchart illustrating the search process is shown in Figure 1. A total of 1311 records were screened, and 83 studies were selected for full-text assessment (Step 2). Subsequently, 53 studies were excluded, and the reasons are provided in Figure 1 and Data S2. Thirty studies that met the inclusion criteria were chosen for qualitative analysis (Data S3).
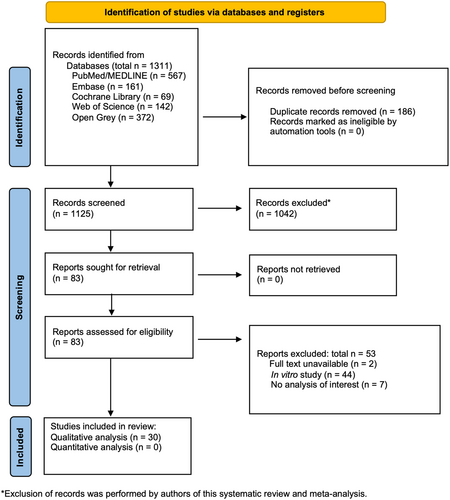
The inter-examiner kappa scores assessed to measure agreement between reviewers were 0.960 for references retrieved from PubMed/MEDLINE, 1.000 for references retrieved from Embase, 1.000 for references in the Cochrane Library, 1.000 for references in Web of Science and 1.000 for references in OpenGrey. These values indicated an almost perfect level of agreement between reviewers (Landis & Koch, 1977). No additional records were identified through manual search in the references lists and Google Scholar.
Characteristics of the included studies
The characteristics of the studies are described in Table 1. Among the 19 studies that used experimental rat models, 16 of them used upper molars (Barbosa et al., 2019; Benetti et al., 2017; Benetti, Briso, et al., 2018; Benetti, Briso, Carminatti, et al., 2019; Benetti, Briso, de Araújo Lopes, et al., 2019; Benetti, Gomes-Filho, et al., 2018; Carminatti et al., 2020; Cintra et al., 2013, 2017; Cintra, Benetti, Ferreira, Gomes-Filho, et al., 2016; Cintra, Benetti, Ferreira, Rahal, et al., 2016; da Silva et al., 2020; Ferreira et al., 2018; Gallinari et al., 2019; Louzada et al., 2019; Terayama et al., 2020). Twelve studies were conducted using human teeth, including molars (n = 2), premolars (n = 8) and incisors (n = 2).
| Author | Experimental model | Groups and bleaching protocol | Analysis | n | Period of analysis | Disclosure of funding sources |
|---|---|---|---|---|---|---|
| Studies with animal teeth | ||||||
| Carminatti et al. (2020) | Upper molars of rats | G1: placebo gel; G2: 30 min 35% HP; G3: 30 min 35% HP + 20 min BS-based gel; G4: 20 min BS-based gel +30 min 35% HP; G5: 20 min BS-based gel for 7 days + 30 min 35% HP at 7 days; G6: 30 min 35% HP mixed with BS-based gel | Inflammation and reduction of the pulp chamber | 10 | 2 and 30 days after bleaching | Yes |
| da Silva et al. (2020) | Upper molars of rats | G1: placebo gel; G2: 40 min 38% HP; G3: 0.2 mL Ibu; G4: 0.2 mL Ibu before and after 38% HP and every 12 h; G5: 10 min 2% Des KF; G6: 10 min 2% Des KF + 38% HP | Inflammation and Immunostaining of SP and CGRP | 8 | 0, 24 and 48 h after bleaching | Yes |
| Terayama et al. (2020) | Upper molars of rats | G1: nonbleached control; G2: 30 min 35% HP; G3: 35% HP + 1 S IRL; G4: 35% HP + 3 S IRL; G5: 35% HP + 1 S RL; G6: 35% HP + 3 S RL; G7: 3 S IRL; G8: 3 S RL | Inflammation, collagen fibres maturation and reactionary dentine deposition | 10 | 2 and 30 days after bleaching | Yes |
| Barbosa et al. (2019) | Upper molars of rats | G1: nonbleached control; G2: 30 min 35% HP; G3: 30 min 35% HP + 30 min MI Paste Plus; G4: MI Paste Plus +35% HP; G5: MI Paste Plus before and after 35% HP; G6: 35% HP mixed MI Paste Plus | Inflammation and reduction of the pulp chamber | 10 | 2 and 30 days after bleaching | Yes |
| Benetti, Briso, Carminatti, et al. (2019) | Upper molars of rats | G1: placebo gel; G2: 30 min 35% HP | Inflammation, immunolabelling of HO-1 and Jun-D, and cell counting (CD90+/CD73+/CD105+/CD45−) | 10 | 2, 3, 7, 15 and 30 days after bleaching | Yes |
| Benetti, Briso, de Araújo Lopes, et al. (2019) | Upper molars of rats | G1: placebo gel; G2: 30 min 35% HP | Inflammation, reactionary dentine deposition, immunolabelling of OCN and OPN, and ROS-positive cells counting | 10 | 0 h, 2 days, 7 days, 15 days and 30 days after bleaching | Yes |
| Louzada et al. (2019) | Upper molars of rats | G1: nonbleached control, G2: 30 min 35% HP; G3: 30 min 35% HP + 2.5% carvedilol gel | Inflammation and reduction of the pulp chamber | 7 | 2 and 30 days after bleaching | Yes |
| Gallinari et al. (2019) | Upper molars of rats | G1: placebo gel; G2: 40 min 35% HP; G3: placebo gel +10 min otosporin; G4: 35% HP + otosporin; G5: placebo gel + tylenol; G6: 35% HP + tylenol | Inflammation and Immunolabelling of SP and CGRP | 7 | 0, 24 and 48 h after bleaching | Yes |
| Benetti, Briso, et al. (2018) | Upper molars of rats | G1: placebo gel; G2: 30 min 35% HP; G3: 35% HP + 10 min 0.01 mL Otosporin | Inflammation and immunolabelling of TNF-α, IL-6 and IL-17 | 10 | 2 and 30 days after bleaching | Yes |
| Benetti, Gomes-Filho, et al. (2018) | Upper molars of rats | G1: placebo gel; G2: 20% HP (1 × 50 min); G3: 35% HP (3 × 15 min) | Inflammation, immunolabelling of IL-6 and IL-7, and CD5-positive cells | 10 | 2 and 30 days after bleaching | Yes |
| Silva-Costa et al. (2018) | Upper and lower incisors of rats | G1: nonbleached control; G2: 38% HP (2 × 15 min) | Inflammation, Immunolabelling of lL-1β, TNF-β, FGF-2 and GPX, and immunofluorescence of OCN | 20 | 24 h and 10 days after bleaching | No |
| Ferreira et al. (2018) | Upper molars of rats | G1: normoglycaemic nonbleached control; G2: NBle 30 min 35% HP; G3: diabetic nonbleached control; G4: DBle 30 min 35% HP | Inflammation and immunolabelling of IL-6, TNF-α and IL-17 | 7 | 2 and 30 days after bleaching | Yes |
| Benetti et al. (2017) | Upper molars of rats | G1: placebo gel; G2: 20% HP (1 × 50 min); G3: 35% HP (3 × 15 min) | Inflammation, immunolabelling for PCNA and C3C | 10 | 2 and 30 days after bleaching | Yes |
| Cintra et al. (2017) | Maxillary molars of rats | G1: normoglycaemic nonbleached control; G2: NBle 30 min 35% HP; G3: diabetic nonbleached control; G4: DBle 30 min 35% | Inflammation, reduction of the pulp chamber and collagen fibres maturation | 7 | 2 and 30 days after bleaching | Yes |
| Cintra, Benetti, Ferreira, Gomes-Filho, et al. (2016) | Upper molars of rats | G1: control; G2: 20% HP (1 × 50 min); G3: 35% HP (3 × 15 min) | Inflammation | 10 | 2 days after bleaching | Yes |
| Lima et al. (2016) | Mandibular molars of rats | G1: nonbleached control; G2: DW + 35% HP (2 × 5 min); G3: AA+ 35% HP (2 × 5 min) | Inflammation and tissue disorganization | 5 | 6 h, 24 h, 3 days, 7 days | Yes |
| Ferreira et al. (2013) | Incisors of rats | G1: nonbleached control; G2: 15 min 25% HP; G3: 30 min 25% HP; G4: 45 min 25% HP; G5: 15 min 35% HP; G6: 30 min 35% HP; G7: 45 min 35% HP | Vascular permeability | 7 (bleached), 4 (control) | 1 h | No |
| Cintra et al. (2013) | Upper molars of rats | G1: nonbleached control, G2: 1 S 35% HP (3 × 15 min); G3: 2 S 35% HP; G4: 3 S 35% HP; G5: 4 S 35% HP; G6: 5 S 35% HP | Inflammation | 10 | 2 days after bleaching | Yes |
| Seale et al. (1981) | Canine of dogs | G1: nonbleached control; G2: 30 min 35% HP; G3: 30 min 35% HP + heat; G4: sterile water + heat | Inflammation | 6 | 3, 15 and 60 days after bleaching | No |
| Studies with human teeth | ||||||
| Lima et al. (2019) | Human third molars | G1: placebo gel; G2: 2 h 1% CP for 30 days; G3: 2 h 2% CP for 30 days; G4: 2 h 5% CP for 30 days | Inflammation | 10 | 30 days after bleaching | Yes |
| Karaarslan et al. (2019) | Human premolars | G1: 46% HP + diode laser (940 nm); G2: 35% HP + halogen light; G3: 15 min 35% HP; G4: control (untreated) | Cathepsin B, MMPs and ROS activities | 10 | 24 h after bleaching | Yes |
| Vaz et al. (2016) | Human third molars | G1: nonbleached control; G2: 2 h 15% CP for 16 days; G3: 38% HP (45 min/three visits) | Inflammation, pulp tissue disorganization and collagen evaluation | G1 = 7, G2 = 10 and G3: = 12 | 7 days after bleaching | Yes |
| Roderjan et al. (2015) | Mandibular human incisors | G1: nonbleached control; G2: 35% HP (3 × 15 min); G3: 35% HP (1 × 45 min); G4: 35% HP + thickener (1 × 45 min) | Inflammation, tissue disorganization and reactionary dentine formation | n.a. | 2 days after bleaching | Yes |
| Kina et al. (2010) | Human premolars | G1: nonbleached control; G2: 38% HP (3 × 10 min); G3: 38% HP + halogen light | Inflammation, tissue disorganization, reactionary dentine formation | 10 (bleached), 4 (control) | 2 and 15 days after bleaching | Yes |
| Costa et al. (2010) | Human lower incisors and premolars | G1: nonbleached premolars; G2: nonbleached incisors, G3: 38% HP (3 × 15 min) premolars, G4: 38% HP (3 × 15 min) incisors | Inflammation, tissue disorganization, reactionary dentine formation | 3 (controls), 6 (bleached premolars), 4 (bleached incisors) | 2 days after bleaching | Yes |
| Caviedes-Bucheli et al. (2008) | Human premolars | G1: nonbleached control; G2: 15 min 38% HP, G3: 3 min 38% HP + diode laser, G4: 20 min 25% HP light-activated | SP expression | 10 | 10 min after bleaching | No |
| Fugaro et al. (2004) | Human premolars | G1: nonbleached control; G2: 6 h 10% HP for 4 days; G3: 6 h 10% HP for 2 weeks; G4: 2 weeks 10% HP + 2 weeks recovery | Inflammation | 15 (bleached), 14 (control) | n.a. | No |
| Anderson et al. (1999) | Human premolars | G1: nonbleached control, G2: 4 h 10% CP | Immunostaining of HO-1 | 14 (control), 17 (bleached) | Immediate after bleaching | Yes |
| Robertson and Melfi (1980) | Human premolars | G1: nonbleached control; G2: 35% HP + heat (2 × 5 min); G3: saline solution +heat; G4: 35% HP (2 × 5 min) | Inflammation | 7 | 4 d after bleaching | No |
| Cohen and Chase (1979) | Human premolars | G1: control; G2: 35% HP + heat (3 × 30 min) | Inflammation and cell morphology | 51 (bleached), 19 (control) | 1 h, 3 days, 15 days and 30 days | No |
- Abbreviations: AA, ascorbic acid; BS, Biosilicate; C3C, caspase3-cleaved; CGRP, calcitonin gene-related peptide; CP, carbamide peroxide; DBle, diabetic bleached; Des, desensitizing agent; DW, distilled water; FGF, fibroblast growth factor; G, group; GPX, glutathione peroxidase; HO-1, heme oxygenase-1; HP, hydrogen peroxide; Ibu, ibuprofen; IL, interleukin; IRL, infrared laser; min, minute; MMP, matrix metalloproteinase; n.a., not applicable; NBle, normoglycaemic bleached; OCN, osteocalcin; OPN, osteopontin; PCNA, proliferation of cell nuclear antigen; RL, red laser; ROS, reactive oxygen species; S, session; SP, substance P; TNF, tumour necrosis factor.
The concentration of 35% HP was the most used agent in the articles (n = 20), with varying application times employed. Additionally, some studies explored the combination of bleaching agents with other substances, such as carvedilol (Louzada et al., 2019), MI Paste Plus remineralizer (Barbosa et al., 2019), otosporin (Benetti, Briso, et al., 2018; Gallinari et al., 2019) and tylenol (Gallinari et al., 2019). Low-level light therapy was also included in some studies. The analysis periods ranged from 0 to 60 days after bleaching, with the 2-day period being the most studied.
Regarding financial support, most of the included studies disclosed their sources of funding, primarily from scholarship and research assistance. Two articles also received donations of bleaching products from dental companies (Kina et al., 2010; Roderjan et al., 2015). In contrast, seven articles did not report their sources of funding (Caviedes-Bucheli et al., 2008; Cohen & Chase, 1979; Ferreira et al., 2013; Fugaro et al., 2004; Robertson & Melfi, 1980; Seale et al., 1981; Silva-Costa et al., 2018).
Table 2 summarizes the results of the analyses conducted in each selected study in this systematic review. The findings related to the inflammatory response and the secondary outcomes are described below.
| Author | Inflammatory response and vascular permeability | Pulp tissue disorganization | Collagen fibres evaluation | Induction of mineralization | Other proteins expression | Outcomes |
|---|---|---|---|---|---|---|
| Studies with animal teeth | ||||||
| Carminatti et al. (2020) | HE, scores. 2 days (Occlusal; G1: no inflammation =; G2: mostly necrosis #; G3: mostly severe #; G4: mostly moderate = #; G5: mostly moderate = #; G6: mostly moderate =. Middle; G1: no inflammation =; G2: mostly severe #; G3: mostly moderate = §; G4: mostly mild = §; G5: mostly mild = §; G6: mostly mild = §. Cervical; G1: no inflammation =; G2: mostly moderate #; G3: mostly mild # §; G4: mostly no inflammation = §; G5: mostly no inflammation = §; G6: mostly no inflammation = §). 30 days; (G1–G5: no inflammation) | n.a. | n.a. | HE, %. 2 days (G1–G5: no mineralization). 30 days (G1: 0 #; G2: 69.2 =; G3: 45.9 = §; G4: 37 = #; G5: 35.2 = #; G6: 30.3 # §) | n.a. | HP increased inflammation in the pulp thirds at 2 days, in addition to showing higher tertiary dentine deposition than placebo at 30 days |
| da Silva et al. (2020) | HE, scores. Crown [0 h; Occlusal (G1, G2 and G5: no inflammation =; G2 and G4: necrosis #; G6: necrosis = #); Medial (G1, G3 and G5: no inflammation =; G2, G4 and G6: necrosis #); Cervical (G1, G3 and G5: no inflammation =; G2, G4 and G6: severe to necrosis #). 24 h; Occlusal (G1, G3 and G5: no inflammation =; G2 and G4: moderate to severe inflammation #; G6: moderate = #); Medial (G1, G3 and G5: no inflammation =; G2, G4 and G6: mild to moderate #); Cervical (G1, G3 and G5: no inflammation =; G2 and G4 mild #;G6: no inflammation = #). 48 h; Occlusal (G1, G3 and G5: no inflammation =; G2 and G6: moderate #; G4: moderate inflammation = #); Medial (G1, G3 and G5: no inflammation =; G2, G4 and G6: mild #); Cervical (G1, G3 and G5: no inflammation =; G2: mild #; G4 and G6: no inflammation #)]. Root (0 h; Coronal; G1, G3 and G5: no inflammation =; G2, G4 and G6: mild to moderate inflammation #); Medial (G1, G3 and G5: no inflammation =; G2: mild #; G4 and G6: no inflammation #); Apical (G1–G6: no inflammation =). 24 h, 48 h; Coronal to apical (G1–G6: no inflammation =) | n.a. | n.a. | n.a. | IHC, scores. SP; Crown [0 h; Occlusal to cervical (G1, G3 and G5: low =; G2, G4 and G6: necrosis #). 24 h; Occlusal (G1, G3 and G5: low =; G2 and G4 high #; G6: high # =); Medial (G1, G3 and G5: low =; G2 and G4 high #; G6: moderate # =); Cervical (G1, G3 and G5: low =; G2: high =; G4 and G6: moderate = #). 48 h; Occlusal (G1, G3 and G5: low =; G2 and G4 high #; G6: moderate # =); Medial (G1, G3 and G5: low =; G2: high #; G4: moderate #; G6: moderate = #); Cervical (G1, G3 and G5: low =; G2: moderate #; G4: moderate = #, G6: low = #)]. Root [0 h; Coronal (G1, G3 and G5: low =; G2: necrosis #; G4: high #; G6: high = #); Medial (G1, G3 and G5: low =; G2 and G4: high #; G6: high = #); Apical (G1, G3 and G5: low =; G2: moderate =; G4: moderate #; G6: moderate = #). 24 h (G1, G3 and G5: low =; G2: moderate #; G4 and G6: moderate = #); Medial (G1, G3 and G5: low =; G2: moderate #; G4: low = #; G6: low =); Apical (G1–G6: low =). 48 h; Coronal to apical (G1–G6: low =)]. CGRP; Crown [0 h; Occlusal to cervical (G1, G3 and G5: low =; G2, G4 and G6: necrosis #). 24 h; Occlusal (G1, G3 and G5: low =; G2 and G4: high #; G6: moderate #); Medial and cervical (G1, G3 and G5: low =; G2: high #; G4: high = #; G6: moderate = #). 48 h; Occlusal to medial (G1, G3 and G5: low =; G2 and G4: high #; G6: moderate =); Apical (G1, G3, G5 and G6: low =; G2 and G4: moderate #)]. Root [0 h; Coronal; G1: (G1, G3 and G5: low =; G2 and G4: necrosis #; G6: high = #); Medial (G1, G3 and G5: low =; G2 and G4: high #; G6: high = #); Apical (G1, G3 and G5: low =; G2: high #; G4 and G6: moderate = #). 24 h; Coronal (G1, G3 and G5: low =; G2 and G4: moderate #; G6: moderate = #); Medial (G1, G3 and G5: low =; G2: moderate #; G4: moderate = #; G6: low = #); Apical (G1–G6: low =). 48 h; Coronal to apical (G1–G6: low =)] | Ble groups had a significant inflammation and expression of SP and CGRP at the initial periods compared with controls, which decreased over time |
| Terayama et al. (2020) | HE, scores. 2 days (Occlusal; G1, G7 and G8: no inflammation =; G2 and G5: necrosis #; G3 and G6: moderate = #; G4: mostly moderate #. Middle; G1, G7 and G8: no inflammation =; G2 and G5: severe #; G3, G4 and G6: mostly moderate = #. Cervical; G1, G7 and G8: no inflammation =; G2 and G5: mostly moderate #; G3, G4 and G6: mostly mild = #). 30 days (G1 and G2: no inflammation =) | n.a. | PSR, %. 2 days (G1: 86.30 ± 8.70 immature =; G2: 52.80 ± 12.42 immature #; G3: 54.75 ± 6.30 immature # §; G4: 69.15 ± 12.22 immature §; G5: 55.18 ± 13.58 mature #; G6: 56.56 ± 15.86 mature #; G7: 74.52 ± 9.91 immature =; G8: 85.66 ± 5.94 immature =). 30 days (G1: 51.12 ± 11.70 mature =; G2: 58.38 ± 12.6 mature =; G3: 55.07 ± 14.30 immature =; G4: 57.89 ± 11.62 mature =; G5: 51.00 ± 17.76 immature =; G6: 55.32 ± 18.60 mature =; G7: 58.70 ± 8.73 mature =; G8: 51.68 ± 10.47 immature =) | Microtomography, reduction in mm3. 30 days. All Ble groups showed a reduction in the volume of the pulp chamber. G1: 0.74 ± 0.07 =; G2: 0.32 ± 0.06 #; G3: 0.25 ± 0.14 #; G4: 0.25 ± 0.08 #; G5: 0.34 ± 0.09 #; G6: 0.30 ± 0.05 #; G7: 0.72 ± 0.07 =; G8: 0.80 ± 0.15 = | n.a. | The Ble groups showed significant inflammation and necrosis at 2 days, with a significant amount of mature fibres. There was a reduction in the pulp chamber area at 30 days There was no influence of bleaching on inflammation and on collagen fibres maturation at 30 days |
| Barbosa et al. (2019) | HE, scores. 2 days (Occlusal; G1: no inflammation =; G2: necrosis #; G3: severe to necrosis #; G4 and G5: severe #; G6: moderate#. Middle; G1: no inflammation =; G2: severe §; G3: severe § #, G4 and G5: moderate § #, G6: mild = #. Cervical; G1: no inflammation =; G2 and G3: moderate #, G4 and G5: mild = #, G6: mild =). 30 days (G1–G6: no inflammation =) | n.a | n.a | HE, reduction of pulp chamber (%). 2 days (G1–G5: no mineralization). 30 days (G1: 0 =; G2: 46.64 #; G3: 49.57 #; G4: 39.36 #; G5: 40.29 #; G6: 30.78 = #) |
n.a |
HP showed significant inflammation at 2 days compared with the control, and Ble groups had a significant deposition of tertiary dentine |
| Benetti, Briso, Carminatti, et al. (2019) | HE, scores. 2 days (Occlusal; G1: no inflammation =; G2: necrosis #. Middle; G1: no inflammation =; G2: severe #. Cervical; G1: no inflammation =; G2: moderate #). 3 days (Occlusal; G1: no inflammation =; G2: severe #. Middle; G1: no inflammation =; G2: moderate #. Cervical; G1: no inflammation =; G2: moderate #). 7, 15 and 30 days (G1–G8: no inflammation =) | n.a. | n.a. | n.a. | IHC, positive cells (mm2). HO-1; Occlusal (2 days; G1: ≈ 15 =; G2: 20 =. 3 days; G1: ≈ 15 =; G2: > 20 =. 7 days; G1: ≈ 15 =; G2: > 60 #. 15 days; G1: ≈ 15 =; G2: ≈ 30 #. 30 days; G1: ≈ 15 =; G2: 0 #). Middle (2 days; G1: ≈ 10 =; G2: > 60 #. 3 days: G1: ≈ 10 =; G2: ≈ 60 #. 7 days; G1: ≈ 10 =; G2: 40 #. 15 days; G1: ≈ 10 =; G2: ≈ 15 =. 30 days; G1: ≈ 10 =; G2: 0 =). Cervical (2 days; G1: ≈ 15 =; G2: ≈ 60 #. 3 days; G1: ≈ 15 =; G2: 40 #. 7 days; G1: ≈ 15 =; G2: < 30 #. 15 days; G1: ≈ 15 =; G2: ≈ 15. 30 days; G1: ≈ 15 =; G2: > 15 =). Jun-D; Occlusal (2, 3, 15 and 30 days; G1: 1.5 =; G2: 0 #. 7 days; 1.5 =; G2: 4.5 #). Middle (2 days; G1: 1.5 =; G2: ≈ 0.5. 3 days; G1: 1.5 =; G2: ≈ 1.5 =. 7 days; G1: 1.5 =; G2: ≈ 6. 15 days; G1: 1.5 =; G2: ≈ 3 =. 30 days; G1: 1.5 =; G2: 0 #). Cervical (2 days; G1: > 2 =; G2: ≈ 2 =. 3 days; G1: > 2 =; G2: > 3 =. 7 days; G1: > 2 =; G2: > 4.5 #. 15 days; G1: > 2 =; G2: > 4.5 #. 30 days; G1: > 2 =; G2: ≈ 1 #). Immunofluorescence, CD90+/CD73+/CD105+/CD45− cell counts (mm2). 2 days; G1: ≈ 1=, G2: ≈ 0.75 =. 3 days; G1: ≈ 1 =, G2: 1.5 =. 7 days; G1: ≈ 1 =, G2: ≈ 1.2 =. 15 days; G1: ≈ 1 =, G2: ≈ 0.8. 30 days; G1: ≈ 1 =, G2: ≈ 1 = | HP showed significant inflammation at 2 and 3 days, in addition to greater HO-1 immunolabelling in the middle and cervical thirds at 2 and 3 days, in all thirds at 7 days and occlusal third at 15 days. Regarding Jun-D immunolabelling, it was higher at 7 days in all thirds and at 15 days in the cervical third of the Ble group; however, there was a reduction in the other periods in the occlusal third, 2 and 30 days in the middle and 30 days in the cervical third. Low cell counting without differences between the control and Ble groups |
| Benetti, Briso, de Araújo Lopes, et al. (2019) | HE, scores. Occlusal (0 h; G1: no inflammation =; G2: necrosis #. 2 days; G1: no inflammation =; G2: mostly necrosis #. 7, 15 and 30 days; G1 and G2: no inflammation =). Middle (0 h; G1: no inflammation =; G2: necrosis #. 2 days; G1: no inflammation =; G2: mostly severe #. 7, 15 and 30 days; G1 and G2: no inflammation =). Cervical (0 h; G1: no inflammation=; G2: necrosis #. 2 days; G1: no inflammation =; G2: mostly moderate #. 7, 15 and 30 days; G1 and G2: no inflammation =) | n.a. | n.a. | HE, pulp chamber area (105). 0 h; G1: 21.89 ± 1.18 =; G2: 21.78 ± 2.64 =. 2 days; G1: 21.89 ± 1.18 =; G2: 23.79 ± 4.19 =. 7 days; G1: 21.89 ± 1.18 =; G2: 14.18 ± 9.90 #. 15 days; G1: 21.89 ± 1.18 =; G2: 12.30 ± 5.25 #. 30 days; G1: 21.89 ± 1.18 =; G2: 6.65 ± 2.47 #. IHC, scores. OCN; 0 h; G1: low =; G2: mostly missing #. 2 days; G1: low =; G2: moderate #. 7 days; G1: low =; G2: moderate #; 15 days; G2: severe #. 30 days; G2: very severe #. OPN; 0 h; G1: low =; G2: mostly missing =; 2 days; G1: low =; G2: moderate #. 7 days; G1: low =; G2: very severe #; 15 days; G1: low =; G2: severe #. 30 days; G1: low =; G2: severe # | IHC, ROS-positive cells (mm2). 0 h (All thirds; G1 and G2: 0). 2 days (Occlusal; G1: 0 =; G2 < 5 =. Middle; G1: 0 =; G2: ≈ 10 #. Cervical; G1: 0 =; G2: ≈ 15 #). 7 days (Occlusal; G1: 0 =; G2: ≈ 45 #. Middle; G1: 0 =; G2: ≈ 35 #. Cervical; G1: 0 =; G2: > 40 #). 15 days (Occlusal; G1: 0 =; G2: > 30 #. Middle and Cervical; G1: 0 =; G2: > 40 #). 30 days (Occlusal and Middle; G1 and G2: 0. Cervical; G1: 0 =; G2: 10 #) | HP increased inflammation in the coronal pulp at 0 and 2 days. There was a significant presence of tertiary dentine after 7 days. OCN was absent at 0 h, and increased after 2 days; in addition, OPN was high after 2 days of bleaching. The Ble group had more ROS-positive cells at 7 and 15 days |
| Louzada et al. (2019) | HE, scores. 2 days (Occlusal; G1: no inflammation =; G2: mostly necrosis #; G3: mostly severe #. Middle; G1: no inflammation =; G2: mostly severe #; G3: mostly moderate = #. Cervical; G1: no inflammation =; G2: moderate #; G3: mostly mild = #). 30 days (G1 and G2: no inflammation =) | n.a. | n.a. | HE, reduction of pulp chamber (%); G1: 0 =; G2: 48.5 #; G3: 48.3 # | n.a. | Ble groups significantly increased inflammation in the occlusal third at 2 days; however, only bleaching had more inflammation than control in the other thirds. There was no inflammation at 30 days, but there was an increased amount of tertiary dentine in Ble teeth |
| Gallinari et al. (2019) | HE, scores. Crown [Occlusal (0 h; G1, G3 and G5: no inflammation =; G2 and G6: necrosis #; G4: moderate = #. 24 h; G1, G3 and G5: no inflammation =; G2 and G6: severe #; G4: moderate = #. 48 h; G1, G3–G5: no inflammation =; G2 and G6: severe #). Medium (0 h; G1, G3 and G5: no inflammation =; G2: necrosis #; G3: mild = #; G6: severe #. 24 h; G1: no inflammation =; G2 and G6: moderate #; G4: mild = #. 48 h; G1, G3–G5: no inflammation =; G2 and G6: moderate #). Cervical (0 h; G1, G3 and G5: no inflammation =; G2: necrosis #; G4: mild = #; G6: severe #. 24 h; G1, G3 and G5: no inflammation =; G2, G4 and G6: mild #. 48 h; G1, G3–G5: no inflammation =; G2 and G6: mild #)]. Root (Coronary; 0 h; G1, G3 and G5: no inflammation =; G2: severe #; G4: mild = #; G6: moderate #. 24 and 48 h; G1–G6: no inflammation =). Medium [0 h; G1, G3–G5: no inflammation =; G2: mild #; G6: severe #. 24 and 48 h; G1–G6: no inflammation =. Apical (0, 24 and 48 h; G1–G6: no inflammation =)] | n.a. | n.a. | n.a. | IHC, scores. SP; Crown [Occlusal (0 h; G1, G3 and G5: minimal =; G2 and G6: necrosis #; G4: high = #. 24 h; G1: minimal=; G2: high #; G4 and G6: average = #. 48 h; G1, G3–G6: minimal =; G2: average #)]. Medium [0 h; G1, G3 and G5: minimal =; G2: necrosis #; G4 and G6: high = #. 24 h; G1, G3–G5: minimal =; G2: high #; G6: average = #. 48 h; G1, G3–G6: minimal =; G2: minimal #. Cervical (0 h; G1, G3 and G5: minimal =; G2: necrosis #; G4: average =; G6: high = #. 24 h; G1, G3–G5: minimal =; G2: average #. 48 h; G1–G6: minimal=)]. Root [Coronary (0 h; G1, G3 and G5: minimal =; G2: high #; G4: average =; G4: minimal #. 24 h; G1, G3–G6: minimal =; G2: average #. 48 h; G1, G3–G6: minimal =; G2: average=)]. Medium: 0 h; G1, G3–G6: minimal =; G2: high #. 24 and 48 h; G1–G6: minimal =. Apical; 0, 24 and 48 h; G1–G6: minimal=. CGRP; Crown [Occlusal (0 h; G1, G3 and G5: minimal =; G2: necrosis #; G4 and G6: high = #. 24 h; G1, G3 and G5: minimal =; G2: high #; G4 and G6: average = #. 48 h; G1, G3–G6: minimal =; G2: average #). Medium; 0 h; G1, G3 and G5: minimal =; G2: necrosis #; G4 and G6: high = #. 24 h; G1, G3 and G5: minimal=; G2: high #; G4 and G6: average = #. 48 h; G1–G6: minimal=. Cervical (0 h; G1, G3 and G5: minimal #; G2: necrosis #; G4: average #; G6: high = #. 24 h; G1, G3–G5: minimal =; G2: high #; G6: minimal = #. 48 h; G1, G3–G6: minimal =; G2: minimal #)]. Root [Coronary (0 h; G1, G3–G5: minimal to average =; G2: high #; G6: average = #. 24 h; G1, G3–G5: minimal =; G2: average #; G6: minimal = #. 48 h; G1, G3–G6: minimal =; G2: average #). Medium (0 h; G1, G3–G5: minimal =; G2: high #. 24 and 48 h; G1–G6: minimal =). Apical (0, 24 and 48 h, G1–G6: minimal =)] | The Ble group had significant areas of necrosis, whereas reduced damage was seen in the Otosporin group. The Ble groups had stronger immunolabelling of SP and CGRP than controls, showing a decrease over time in the Ble groups |
| Benetti, Briso, et al. (2018) | HE, scores. Occlusal (G1: no inflammation =; G2: mostly severe to necrosis #; G3: mostly mild =). Middle (G1: no inflammation =; G2: mostly moderate #; G3: no inflammation =). Cervical (G1: no inflammation =; G2: mild =; G3: no inflammation =) | n.a. | n.a. | n.a. | IHC, scores. TNF-α; G1: low =; G2: strong #; G3: moderate =. IL-6; G1: low =; G2: low to moderate #; G3: moderate = #. IL-17; G1: low =; G2: moderate #; G3: moderate = # | HP alone had higher inflammation than control in the occlusal and middle thirds. Bleaching alone had higher immunolabelling of TNF-α, IL-6 and IL-17 than control |
| Benetti, Gomes-Filho, et al. (2018) | HE, scores. Occlusal (2 days; G1: no inflammation; G2: moderate =; G3: necrosis #). Middle (G1: no inflammation; G2: mild =; G3: moderate #). Cervical (G1: no inflammation; G2 and G3: mild =. 30 days; G1–G3: no inflammation) | n.a. | n.a. | HE, pulp chamber area (105); G1: 19.86 ± 2.35 =; G2: 9.25 ± 0.59 #; G3: 7.00 ± 0.25 § | IHC, scores. IL-17 (2 days; G1 and G2: low to moderate =; G3: mostly moderate #. 30 days; G1–G3: low =). IL-6 (2 days; G1: low =; G2: moderate #; G3: moderate to severe #. 30 days; G1: low =; G2: low to moderate =; G3: mostly moderate =); CD5-positive cells (mm2). 2 days (Occlusal; G1: ≈ 10 =; G2: > 45 #; G3: ≈ 15 =. Middle: G1: > 5 =; G2: > 30 #; G3: ≈ 40 #. Cervical; G1: ≈ 5 =; G2: > 35 #; G3: > 45 #). 30 days (Cervical; G1: < 5 =; G2: 30 #; G3: 40 #) | HP had higher inflammation in the occlusal and middle thirds at 2 days, and superior IL-17 immunolabelling than other groups. 20% and 35% HP had moderate to severe IL-6 immunolabelling. At 30 days, bleaching did not influence inflammation, IL-17 and IL6, but increased tertiary dentine formation and lymphocytes marker |
| Silva-Costa et al. (2018) | HE, scores. 24 h; G1: no inflammation =; G2: moderate #. 10 days; G1: no inflammation =; G2: severe/necrosis # | n.a. | n.a. | Immunofluorescence, n.a. OCN. 24 h; G1: 2.043 ± 0.23 =; G2: 2.137 ± 0.64 =. 10 days; G1: 2.373 ± 0.63 =; G2: 7.767 ± 0.95 # | IHC, scores. lL-1β (24 h and 10 days; G1: weak =; G2: strong #). TNF-β (24 h and 10 days; G1: weak =; G2: strong #). FGF-2 (24 h; G1 and G2: weak =. 10 days; G1: weak =; G2: strong #). GPX (24 h and 10 days; G1: weak =; G2: strong #) | HP increased inflammation, lL-1β, TNF-β and GPX at 24 h and 10 days. At 10 days, there was strong staining of FGF-2 and a superior amount of OCN |
| Ferreira et al. (2018) | HE, scores. 2 days; G1 and G3: no inflammation =; G2: mostly mild #; G4: mostly severe/necrosis §. 30 days; G1–G4: no inflammation = | n.a. | n.a. | n.a. | IHC, scores. IL-6 (2 days; G1 and G3: low =; G2: moderate #; G4: moderate to high #. 30 days; G1–G3: low =; G4: moderate =). TNF-α (2 days; G1 and G3: low =; G2 and G4: high #. 30 days; G1 and G3: mostly low =; G2: moderate #; G4: moderate to high #). IL-17 (2 days; G1, G3 and G4: mostly low =; G2: mostly moderate #. 30 days; G1–G4: mostly low =) | Ble groups showed more inflammation compared with controls at 2 days. No inflammation was observed at 30 days. Bleaching influenced the increase of IL-6, TNF-α and IL-17 cytokines at 2 days and at 30 days for TNF-α |
| Benetti et al. (2017) | HE, scores. 2 days. Crown (Occlusal; G1: no inflammation =; G2: moderate #; G3: necrosis §. Middle; G1: no inflammation =; G2: mild #; G3: moderate §. Cervical; G1: no inflammation =; G2 and G3: mostly mild #). Root (Cervical; G1–G3: no inflammation=). 30 days. Crown and root (All thirds; G1–G3: no inflammation =) | n.a. | n.a. | n.a. | IHC, cell number (mm2). PCNA; 2 days (Occlusal; G1: 1.5 ± 0.3 =; G2: 121.5 ± 40.2 #; G3: 26.2 ± 7.4 =. Middle; G1: 2.1 ± 0.7 #; G2: 172.5 ± 39.5 #; G3: 65.0 ± 17.3 §. Cervical; G1: 0 ± 0 =; G2: 113.0 ± 32.7 #; G3: 167.0 ± 33.6 §). 30 days. (Cervical; G1: 3 ± 1.0 =; G2: 20.7 ± 6.8 =; G3: 7.1 ± 2.2 =). C3C; 2 days (Occlusal; G1: 3.8 ± 1.2 =; G2: 20.8 ± 6.6 #; G3: 6.9 ± 2.1 =. Middle; G1: 6.3 ± 1.9 =; G2: 20.4 ± 5.9 #; G3: 27.5 ± 7.7 #. Cervical; G1: 9.0 ± 3.0 =; G2: 40.5 ± 12.3 #; G3: 37.5 ± 10.9 #). 30 days (Cervical; G1: 6.5 ± 2.1 =; G2: 24.4 ± 7.6 #; G3: 45.9 ± 15.1 §) | HP increased inflammation in the coronal pulp at 2 days, with necrosis in the 35% HP group. No inflammation was seen at 30 days. Cell proliferation was higher in the occlusal and middle thirds of the 20% HP and cervical of the 35% HP at 2 days, decreasing at 30 days. Apoptosis was significantly present in the Ble groups, mainly in the cervical third at 2 and 30 days, reducing to 20% HP at 30 days |
| Cintra et al. (2017) | HE, scores. 2 days (G1 and G3: no inflammation =; G2: mild #; G4: severe or necrosis §). 30 days (G1–G4: no inflammation =) | n.a. | PSR, %. 2 days (G1: 83.57 ± 11.98 immature =; G2: 54.71 ± 14.85 immature §; G3: 58.43 ± 08.40 mature #; G4: 57.15 ± 05.78 mature # §). 30 days (G1: 55.00 ± 26.01 mature =; G2: 68.29 ± 12.24 mature = #; G3: 53.85 ± 20.42 immature =; G4: 54.28 ± 09.65 mature = §) | HE, %. G1 and G3: 0 =; G2: 52.18 #; G4: 79.43 § | n.a. | Ble groups had increased inflammation and hard tissue deposition compared with the controls. At 2 days, Ble groups had more immature collagen fibres |
| Cintra, Benetti, Ferreira, Gomes-Filho, et al. (2016) | HE, scores. 2 days. Crown (Occlusal; G1: no inflammation =; G2: mostly moderate #; G3: severe/necrosis §. Middle; G1: no inflammation =; G2: mild to moderate #; G3: mostly moderate §. Cervical; G1: no inflammation =; G2 and G3: mostly mild #). Root (Cervical to apical; G1–G3: no inflammation =) | n.a. | n.a. | n.a. | n.a. | Ble groups showed increased inflammation in the coronal pulp, without differences in the radicular pulp compared with the control |
| Lima et al. (2016) | HE, scores. 6 h (G1: no inflammation =; G2 and G3: moderate #); 24 h (G1: no inflammation =; G1: mostly moderate =; G3: mostly mild =); 3 days (G1: no inflammation =; G2 and G3: mostly mild =); 7 days (G1–G3: no inflammation =) | HE, scores. 6 h (G1: normal tissue =; G2 and G3: necrosis #); 24 h (G1: normal tissue =; G2: necrosis #; G3: mostly odontoblastic layer disorganized =); 3 days (G1: normal tissue =; G2 and G3: mostly odontoblastic layer disorganized =); 7 days (G1–G3: normal tissue =). | n.a. | n.a. | n.a. | Ble groups had significant inflammation and tissue necrosis at 6 h. At 24 h, DW + 35% HP showed moderate inflammation and tissue necrosis compared with the control, without significant differences in the other periods |
| Ferreira et al. (2013) | Dye penetration, pixels. G1: 0 =, G2: MC =, G3: < 250 #, G4: > 500 #; G5: MC =, G6: ≈ 250 =; G7: ≈ 2000 # | n.a. | n.a. | n.a. | n.a. | 45 min 25% HP and 35% HP for 30 or 45 min increased vascular permeability compared with the control group |
| Cintra et al. (2013) | HE, scores. Crown (Occlusal; G1: no inflammation =; G2–G6: severe or necrosis #. Middle; G1: no inflammation =; G2: moderate #; G3–G6: severe or necrosis §. Cervical; G1 and G2: no inflammation =; G3: moderate #; G4–G6: severe or necrosis §). Root (Cervical; G1 and G2: no inflammation =; G3: mild =; G4: moderate #; G5 and G6: severe or necrosis §. Middle; G1–G3: no inflammation =; G4: mild =; G5: moderate #; G6: severe or necrosis §. Apical; G1–G5: no inflammation =; G6: moderate #) | n.a. | n.a. | n.a. | n.a. | There was increased inflammation in the coronal pulp of the Ble groups, and higher inflammation when increasing the number of bleaching sessions in the radicular pulp compared with the control |
| Seale et al. (1981) | HE, 3 days. G1 and G3: normal tissue with slightly dilated blood vessels; G2 and G4: dense inflammatory infiltrate. 15 days. G1 and G3: normal tissue with slightly dilated blood vessels; G2 and G4: presence of inflammation. 60 days. G1: teeth lost; G2–G4: no inflammation | n.a. | n.a. | n.a. | n.a. | HP increased inflammation in the at 3 and 15 days compared with the control |
| Studies with human teeth | ||||||
| Lima et al. (2019) | Inflammation. G1–G4: no pulp damage | n.a. | n.a. | n.a. | n.a. | The Ble groups were similar to placebo without pulp damage or inflammation in human teeth |
| Karaarslan et al. (2019) | n.a. | n.a. | n.a. | n.a. | Spectrofluorometry (AUF/μg), 24 h. Cathepsin B. G1: 5.8 ± 0.83 =; G2: 6.66 ± 1.08 =; G3: 6.06 ± 0.95 =; G4: 5.92 ± 1.09 =. MMPs. G1: 9.01 ± 1.68 =; G2: 10.26 ± 3.02 =; G3: 9.31 ± 1.77 =; G4: 10.43 ± 2.04 =. ROS. G1: 9.89 ± 4.08 =; G2: 10.73 ± 3.62 =; G3: 10.79 ± 4.07 =; G4: 13.41 ± 8.18 = | HP did not influence proteolytic activities and ROS production compared with untreated teeth in human teeth |
| Vaz et al. (2016) | HE, scores. G1: no inflammation =; G2: mostly no inflammation =; G3: mostly mild # | HE, scores. G1: mostly normal tissue =; G2 and G3: mostly partly disorganized = | HE, scores. G1 and G2: mostly preserved =; G3: mostly degraded = | n.a. | IHC, density (mm2). CD68+; G1: 13.58 ± 10.44 =; G2: 16.99 ± 8.91 =; G3: 38.14 ± 19.73 #. CD31+; G1: 61.39 ± 20.03 =; G2: 52.29 ± 27.62 =; G3: 57.43 ± 8.69 = | The inflammation and number of macrophages were great after bleaching with 38% HP. There were no differences in pulp tissue disorganization, collagen degradation and number of blood vessels among the groups |
| Roderjan et al. (2015) | HE, scores. G1: no inflammation =; G2–G4: mild inflammation # | HE, scores. G1: normal tissue =; G2–G4: mostly total disorganization # | n.a. | MT, scores. G1: absence #; G2–G4: moderate hard tissue deposition # | n.a. | Ble groups showed moderate inflammation, total tissue disorganization and moderate hard tissue deposition compared with the control group |
| Kina et al. (2010) | HE, scores. 2 and 15 days; G1–G3: no inflammation = | HE and MT, scores. 2 and 15 days; G1–G3: normal tissue = | n.a. | HE and MT, scores. G1: absence =; G3: mostly absence = | n.a. | Bleaching did not influence inflammation, tissue disorganization and hard tissue formation compared with the control |
| Costa et al. (2010) | HE, scores. G1–G3: no inflammation =; G4: moderate #; | HE, scores; G1–G3: normal tissue =; G4: necrosis # | n.a. | HE, scores. G1–G3: absence =; G4: intense hard tissue deposition # | n.a. | Ble incisors with 38% HP showed increased inflammation, areas of necrosis and hard tissue deposition compared with the control |
| Caviedes-Bucheli et al. (2008) | n.a. | n.a. | n.a. | n.a. | Radioimmunoassay, pmol/mg. G1: 756.94 ± 62.85 =; G2: 760.23 ± 141.71 =; G3: 1054.66 ± 155.55 #; G4: 1649.52 ± 341.97 § | SP levels were increased in the 38% HP activated with an infrared laser diode and light-activated 25% HP compared with the non-Ble control |
| Fugaro et al. (2004) | HE, n° of teeth without reaction. G1: 14 =; G2: 9 #; G3: 8 #; G4: 12 = # | n.a. | n.a. | n.a. | n.a. | 4 days and 2 w treatments with 10% HP increased inflammation compared with the control group |
| Anderson et al. (1999) | n.a. | n.a. | n.a. | n.a. | IHC, number of teeth with positive staining. HO-1; G1: 5 =; G2: 14 = | There was no significant difference in the presence of HO-1 between total Ble versus total unBle teeth |
| Robertson & Melfi (1980) | HE, scores. Superficial tissue (G1, G3 and G4: mostly no inflammation =; G2: mostly slight #); Deep tissue (G1 and G4: mostly no inflammation =; G2 and G3: no inflammation =) | HE, scores; G1–G4: no displacement = | n.a. | n.a. | n.a. | 35% HP associated with heat increased inflammation in the superficial tissue; no influence of bleaching was observed in cellular displacement |
| Cohen and Chase (1979) | HE. G1 and G2: no inflammation | HE. G1 and G2: normal tissue with some aspiration of odontoblastic nuclei into the dentinal tubules | n.a. | n.a. | n.a. | 35% HP did not influence inflammatory reactions and tissue disorganization compared with the control |
- Note: Different symbols (=, #, §) indicate a significant difference between groups; ≈ indicates approximately; > indicates ‘greater than’; < indicates ‘less than’.
- Abbreviations: Ble, bleached; C3C, caspase3-cleaved; CGRP, calcitonin gene-related peptide; CP, carbamide peroxide; DBle, diabetic bleached; FGF, fibroblast growth factor; G, group; GPX, glutathione peroxidase; HE, haematoxylin and eosin; HO-1, heme oxygenase-1; HP, hydrogen peroxide; IHC, immunohistochemistry; IL, interleukin; MC, minimal concentration; min, minute; MMP, matrix metalloproteinase; MT, Masson's trichrome; n.a., not applicable; NBle, normoglycaemic bleached; PCNA, proliferation of cell nuclear antigen; pmol/mg, picomolars per milligrams; ROS, reactive oxygen species; SP, substance P; TNF, tumour necrosis factor.
Inflammatory response
The inflammatory response in pulp tissues was evaluated in 27 in vivo studies, primarily assessed based on the intensity of inflammatory infiltrate in different thirds of pulp tissue (Benetti et al., 2017; Benetti, Briso, et al., 2018; Benetti, Briso, Carminatti, et al., 2019; Benetti, Briso, de Araújo Lopes, et al., 2019; Benetti, Gomes-Filho, et al., 2018; Carminatti et al., 2020; Cintra et al., 2013; Cintra et al., 2017; Cintra, Benetti, Ferreira, Gomes-Filho, et al., 2016; Costa et al., 2010; da Silva et al., 2020; Ferreira et al., 2018; Gallinari et al., 2019; Kina et al., 2010; Lima et al., 2016; Louzada et al., 2019; Robertson & Melfi, 1980; Roderjan et al., 2015; Silva-Costa et al., 2018; Terayama et al., 2020; Vaz et al., 2016), or by counting inflammatory cells (Cintra, Benetti, Ferreira, Rahal, et al., 2016).
In animal studies (19 studies), 18 of them assessed the inflammatory response, and all demonstrated significant inflammation in the most superficial regions of coronal pulp, often accompanied by necrotic areas. This was particularly evident after bleaching with 35% or 38% HP and during the initial periods (Barbosa et al., 2019; Benetti et al., 2017; Benetti, Briso, et al., 2018; Benetti, Briso, Carminatti, et al., 2019; Benetti, Briso, de Araújo Lopes, et al., 2019; Benetti, Gomes-Filho, et al., 2018; Carminatti et al., 2020; Cintra et al., 2013, 2017; Cintra, Benetti, Ferreira, Gomes-Filho, et al., 2016; da Silva et al., 2020; Ferreira et al., 2018; Gallinari et al., 2019; Lima et al., 2016; Louzada et al., 2019; Seale et al., 1981; Silva-Costa et al., 2018; Terayama et al., 2020).
Regarding studies involving human teeth (11 studies), seven of them assessed the inflammatory response, and four of these studies reported significant inflammation or necrosis area in the pulp of the bleached teeth (Costa et al., 2010; Fugaro et al., 2004; Roderjan et al., 2015; Vaz et al., 2016). Among these studies, one evaluated the pulp of premolars (Fugaro et al., 2004), two evaluated the pulp of mandibular incisors (Costa et al., 2010; Roderjan et al., 2015) and one study evaluated third molar (Vaz et al., 2016).
Pulp tissue disorganization, collagen fibres evaluation and induction of mineralization
Among the eight studies that assessed pulp organization, most of them (four involving human teeth and two involving animal teeth) reported significant alterations, ranging from a disorganized odontoblastic layer to pulp necrosis. These alterations were primarily observed after the application of high concentrations and longer exposure times to bleaching gels. Three studies evaluated collagen fibres (Cintra et al., 2017; Terayama et al., 2020; Vaz et al., 2016). The use of 35% HP for 30 min was associated with significant collagen maturation at 2 days (Terayama et al., 2020), while another study reported more immature collagen fibres during the same period (Cintra et al., 2017). The study that evaluated collagen in the pulp of human teeth found no influence of 38% HP and 15% CP on collagen in the pulp tissue (Vaz et al., 2016).
Among the 12 studies that assessed mineralization induction, 11 reported the deposition of tertiary dentine following bleaching with 35%–38% HP, particularly during longer analysis periods. Two articles also note significant immunolabelling of osteocalcin (OCN) (Benetti, Briso, de Araújo Lopes, et al., 2019; Silva-Costa et al., 2018).
Proteins immunolabelling
The results of IHC revealed increased immunolabelling of inflammatory markers, including substance P (SP) (n = 3), calcitonin gene-related peptide (CGRP) (n = 2), interleukin (IL)-6 (n = 2), IL-17 (n = 2), lL-1β (n = 1) and tumour necrosis factor (TNF) (n = 2), primarily during initial periods. Other different proteins were separately assessed using IHC, including glutathione peroxidase (GPX), CD68+ cells, Proliferation of Cell Nuclear Antigen (PCNA), caspase-3-cleaved (C3C), heme oxygenase (HO)-1, Jun-D and fibroblast growth factor (FGF)-2 (Table 2).
Risk of bias assessment
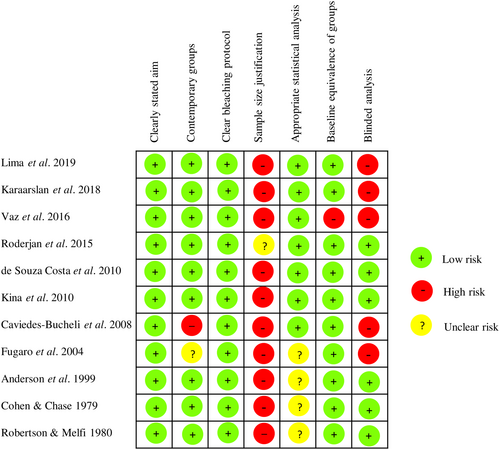
Risk-of-bias analyses can be consulted in Data S4 and S5, Figures 2 and 3. The critical appraisal of in vivo studies involving humans was conducted using the MINORS tool (Data S4 and Figure 2). A high risk of bias was identified only for sample size justification, which was not reported in most of the selected records. One study lacked clear reporting for this item. All studies exhibited a clearly stated aim and a clear description of the bleaching protocol. Overall, the risk of bias in most domains was deemed to be low for all the included in vivo studies with humans.
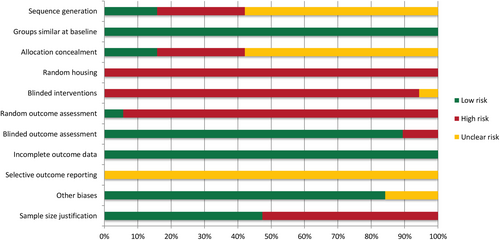
Regarding the risk of bias in the animal studies, there was insufficient information to assess most of the evaluated items. A low risk of bias was observed for groups similar at baseline, blinded outcome assessment, incomplete outcome data and other sources of bias. The critical appraisal of the animal studies using the SYRCLE tool can be found in Data S5 and Figure 3. Overall, the risk of bias in the animal studies ranged from unclear to high.
DISCUSSION
This systematic review primarily analysed whether bleaching can induce an inflammatory response in the pulp tissue of both animal and human teeth. Additionally, this study evaluated pulp tissue disorganization, collagen fibres assessment, mineralization induction and the expression of other proteins after bleaching. This analysis used data from 30 selected in vivo studies involving animals or humans. Overall, the data from animal studies suggested that high concentrations of bleaching gels increased the inflammatory response and areas of necrosis in the coronal pulp, though these effects diminished over time. In studies involving human teeth (11 in total), significant changes were observed in all evaluated bleached incisors (Costa et al., 2010; Roderjan et al., 2015), in third molars of one study (Vaz et al., 2016) and in premolars, regardless of whether sources of heat or light were used (Caviedes-Bucheli et al., 2008; Robertson & Melfi, 1980) or not (Fugaro et al., 2004).
To the best of our knowledge, this is the first systematic review of in vivo studies that comprehensively assessed the primary changes in pulp tissue following bleaching with HP or CP, as well as the mechanisms underlying the inflammatory response that occurs in the pulp tissue after this procedure. Investigating the effects of bleaching on the pulp tissue is crucial given the rising demand for aesthetic bleaching among patients and the availability of HP-based gels on the market (Kury et al., 2022). This importance is further emphasized by studies revealing inflammatory events and alterations in the pulp–dentine complex (Cartagena et al., 2015; Costa et al., 2010; Kiyuna et al., 2021; Kury et al., 2022; Terayama et al., 2020), as well as reports of tooth sensitivity from patients (Faria-E-Silva et al., 2015; Moncada et al., 2013; Rezende et al., 2016).
A recent systematic review of in vitro studies that evaluated the effects of HP on mineralization in pulp cells highlighted the necessity for a systematic analysis of in vivo outcomes related to the effects of bleaching on pulp tissue (Reis-Prado et al., 2021). Therefore, this review was proposed to investigate the influence of peroxide-containing bleaching gels on the pulp tissue. The choice of an in vivo model instead of in vitro evaluations allows for the observation of pulp tissue reactions to aggressive agents in a manner more similar to what occurs in clinical settings, particularly during bleaching. This is due to the presence of physical barriers in in vivo models, such as dentine, dentinal fluid and cytoplasmatic extensions of odontoblasts (Cintra, Benetti, Ferreira, Gomes-Filho, et al., 2016; Cintra, Benetti, Ferreira, Rahal, et al., 2016), as well as HP-degrading enzymes (Benetti et al., 2017).
In this systematic review, most studies involving animals used rat molars to assess the damage induced by bleaching pulp tissue. This is an acceptable and previously standardized model (Cintra, Benetti, Ferreira, Gomes-Filho, et al., 2016; Cintra, Benetti, Ferreira, Rahal, et al., 2016) since rats exhibit similar biological responses and repair processes compared with other mammals. Additionally, rat molars share histological characteristics similar to those of humans (Dammaschke, 2010; Sasaki & Kawamata-Kido, 1995; Terayama et al., 2020), including a comparable proportion of enamel and dentine, albeit with smaller thickness (Cintra et al., 2013; Cintra, Benetti, Ferreira, Rahal, et al., 2016; Dammaschke, 2010). Moreover, the limited number of in vivo evaluations involving other animal species or human teeth in this review may be attributed to the difficulty of obtaining an adequate sample size and ethical considerations. The use of rats enables reproducibility and the possibility of studying different variables (Cintra, Benetti, Ferreira, Rahal, et al., 2016). However, it is essential to propose the validation of these findings in humans with smaller groups while adhering to ethical principles (Cintra, Benetti, Ferreira, Rahal, et al., 2016).
In this review, some studies evaluated bleached third molars due to the challenges associated with obtaining human samples. The tooth selection should exclude those that are vital, healthy and indicated for extraction. It is important to understand that teeth with periodontal problems might also have underlying endodontic issues. Furthermore, the periodontal pocket could extend apically and continue progressing, potentially affecting the apical tissues, accessory canals, or the apical foramen. These issues could lead to pulp alterations and potentially interfere with the study's results (Simon et al., 2013). Typically, the most suitable teeth for extraction are third molars and premolars indicated for orthodontic reasons. Two studies used human third molars; however, in one study (Lima et al., 2019), the specific tooth surface that was bleached remains unclear. In the other study (Vaz et al., 2016), the teeth used were fully erupted, and while not explicitly mentioned, it's presumed that the bleaching gel was applied on the buccal surface, as is standard practice. In this latter study, significant inflammation was observed, accompanied by an increase in the number of macrophages in the pulp of molars bleached with 38% HP 7 days post-bleaching (Vaz et al., 2016). In contrast, Lima et al. (2019) did not observe this alteration. However, it is worth noting that the gel and concentration used in their study were CP up to 5%, and the analysis extended to 30 days.
An important factor that should be mentioned is that the thickness of the mineralized tissues of the tooth influences the penetration of HP into the pulp chamber. Incisors are notably more susceptible to extensive damage (Costa et al., 2010; Roderjan et al., 2015). In contrast to these findings, most studies assessing the pulp of bleached human premolars did not observe inflammation (Anderson et al., 1999; Caviedes-Bucheli et al., 2008; Cohen & Chase, 1979; Costa et al., 2010; Karaarslan et al., 2019; Kina et al., 2010). However, among the eight studies that evaluated premolars, three studies did indicate significant changes in the dental pulp (Caviedes-Bucheli et al., 2008; Fugaro et al., 2004; Robertson & Melfi, 1980).
The effects of bleaching gels are influenced by several factors: HP concentration, the application of heat, the composition of the bleaching gel and the duration of its application. Higher HP concentrations are associated with greater pulpal damage, as evidenced by the results of this systematic review. Only three studies in this review had groups of teeth bleached with CP-based gels, which resulted in reduced HP release (Anderson et al., 1999; Lima et al., 2019; Vaz et al., 2016). These groups did not exhibit significant changes in pulp tissue, emphasizing the potential benefits of less concentrated bleaching gels. Activation of the bleaching gel using light sources, for instance, may lead to heating of the gel and a subsequent rise in the pulp chamber's temperature (Klaric et al., 2015; Mondelli et al., 2016). Only one study in this review compared a control group with a heated bleached group, without including a non-heated bleached group for comparison (Cohen & Chase, 1979). Nevertheless, the study did not find significant changes in the pulp tissue, consistent with studies showing that such heating does not necessarily lead to damage to the pulp tissue because the increase in temperature does not reach critical values (Benetti, Briso, et al., 2018; Moncada et al., 2013). Regarding the composition of the bleaching gel, the presence of calcium is responsible for reducing the penetration of HP into the pulp chamber, subsequently reducing clinical sensitivity (Mena-Serrano et al., 2015), in addition to maintaining the bleaching gel at a neutral pH. One study assessing 26 commercial bleaching gel brands found that their pH values ranged from 3.67 to 11.13 (Price et al., 2000). However, most of the studies in this systematic review did not measure the pH of the bleaching gel or specify its composition.
Also, it must be considered that damage to the pulp tissue is also time-dependent (Cardoso et al., 2010; Cintra et al., 2013), and most of the animal studies applied HP for 30 min, while studies involving humans showed a wide variation in the duration of bleaching gel application. Robertson and Melfi (1980) used 10 min of HP and found no alteration in the pulp; Karaarslan et al. (2019) and Caviedes-Bucheli et al. (2008) used 15 min of HP and found no pulp alterations; similar results were observed in a study by Kina et al. (2010), which used 30 min of HP application. However, Vaz et al. (2016), Roderjan et al. (2015) and Costa et al. (2010) applied HP for 45 min and found significant pulp alterations.
Thus, there is a high level of methodological heterogeneity among the selected records. The main sources of heterogeneity were the various bleaching protocols with different concentrations of HP and the number of bleaching sessions used, as well as the use of heat or light activation in somestudies (Benetti, Lemos, Gallinari, et al., 2018). The significant inflammatory response and necrotic areas were mainly observed in the superficial regions of coronal pulp, especially during the initial periods and with highly concentrated HP or an increased number of sessions. These findings indicate that underlying tissue receives a lower concentration of HP compared with the most superficial regions. Additionally, studies show that the accumulation of ROS during oxidative stress is a reversible event, attributed to the effects of the enzymatic components of the antioxidant system (Silva-Costa et al., 2018; Sinha et al., 2013). Furthermore, enhanced immunolabelling of pro-inflammatory markers, including IL-6, IL-17, IL-1β, TNF and substance P, has been observed, highlighting a pronounced immunoinflammatory response in pulp tissue and cells, particularly in the initial periods after bleaching.
These findings align with studies indicating that higher concentrations of bleaching gel increase post-operative sensitivity observed in clinical trials (Mounika et al., 2018; Rezende et al., 2016). In addition to severe inflammation, bleached groups exhibited dentine deposition after bleaching with 35%–38% HP over extended observation periods. They also exhibited elevated levels of OCN (Benetti, Briso, de Araújo Lopes, et al., 2019; Silva-Costa et al., 2018) and OPN within 2–10 days post-bleaching (Benetti, Briso, de Araújo Lopes, et al., 2019). This increased hard tissue deposition is a response to the injury caused by ROS in pulp tissue. The magnitude of this event corresponds to the inflammation level during the initial periods (Ferreira et al., 2018; Terayama et al., 2020), indicating pulp aging and the tissue's reparative capacity following damage caused by the bleaching gel. This chemical aggressor is highly unstable, reacting rapidly and diminishing in effect over time (Benetti, Briso, de Araújo Lopes, et al., 2019; Cintra et al., 2013; Dahl & Pallesen, 2003). The oxidative stress induced by HP on pulp cells results in the expression of mineralization markers, prompting calcium phosphate production within these cells (Matsui et al., 2009). These observations contrast with findings from a recent systematic review of in vitro studies (Reis-Prado et al., 2021), in which high concentrations of bleaching gels reduced the mineralization potential of pulp cells. These differences may be related to the cell monolayer traditional model used in those in vitro evaluations, which does not properly resemble in vivo cell environments, and the shorter analysis periods.
Moreover, an investigation of collagen content in pulp tissue is essential in these in vivo studies since it plays a significant role in the mineralization process. Unfortunately, the few articles that assessed collagen maturation and degradation provided inconclusive results, which hindered more in-depth discussions (Cintra et al., 2017; Terayama et al., 2020; Vaz et al., 2016). Therefore, additional in vivo evaluations of collagen are necessary to draw more accurate conclusions from these outcomes.
Blinded intervention can be challenging to perform, especially in animal experimentation. Consequently, a high risk of bias for this item was identified in the current review. In such cases, it is important for researchers to establish an adequate allocation sequence for the animals to minimize bias, especially when blinding among investigators and/or caregivers is impossible (du Sert et al., 2020). Similarly, assessing reporting bias in animal studies is difficult because the registration of study protocols, including all expected outcomes, is an uncommon procedure. As a result, an unclear risk of bias is expected in most articles due to poor reporting. In addition, there has not been a publicly accessible and focused database for the registration of protocols for animal studies. However, the registration of animal experiments should become a common practice to enhance transparency, as there is a current attention towards open science pathways and standardization of research in animal science.
The critical appraisal of the animal studies was assessed using a modified version of SYRCLE's RoB tool. A potential source of bias is related to sample size, which can impact the statistically significant effect of a study and lead to an unethical waste of resources when not properly performed. Therefore, to further characterize reporting in the included animal studies, the current study also assessed whether the articles had adequately described sample size calculations. The animal studies exhibited an unclear-to-high risk of bias, mostly involving randomization procedures, blinded intervention and selective outcome assessment. Despite the relatively low external validity of animal experiments, it is essential to conduct randomization and blinding because they strongly affect the internal validity and reliability of measurements (Hooijmans et al., 2014). For instance, randomization of experimental groups and random housing can influence outcomes, and their absence might result in problems with the distribution of characteristics between the groups and introduce performance bias (Hooijmans et al., 2014).
In the in vivo studies involving humans, a modified version of the MINORS scale was used to assess the methodological quality of the articles. An evaluation of the reporting quality of bleaching procedures and the presence of blinding in outcome assessment was performed due to their significant impact on the transparency and validity of the study, respectively. Moreover, certain items related to prospective data collection and follow-up of participants present in the original instrument were excluded due to the characteristics of the in vivo design. For these studies, a low risk of bias was observed for most items; however, there was no sample size justification in all studies involving human samples. Hence, an appropriate sample size calculation with participants randomly distributed across groups is required, in addition to considering different age ranges of participants, bleaching protocols and products. This approach can enhance the predictability and accuracy of the conclusions, allowing for more precise health-related decisions.
Information about the funding sources was collected from the articles included. While most studies were funded by national or local research grants without any influence on their design or methods, two studies (Kina et al., 2010; Roderjan et al., 2015) also received sponsored bleaching products from dental companies. Moreover, seven articles (Caviedes-Bucheli et al., 2008; Cohen & Chase, 1979; Ferreira et al., 2013; Fugaro et al., 2004; Robertson & Melfi, 1980; Seale et al., 1981; Silva-Costa et al., 2018) did not provide details about their funding, which raises concerns about potential conflicts of interest (Buckley, 2022).
In clinical settings, dentists frequently utilize a broad spectrum of bleaching protocols tailored to their patients' varied needs. Similarly, methodological heterogeneity concerning the bleaching protocols and observation periods was observed, especially in the eligible animal studies. To discern the effects of HP on pulp tissue, these studies need well-standardized protocols. Standardization increases the possibility for meaningful comparisons and subsequently bolsters the evidence base on this subject. However, this heterogeneity is a limitation of this study, as well as the fact that only in vivo studies were considered. These in vivo studies have constraints when extrapolating their findings to a clinical context, especially those employing animal models. The decision to include in vivo studies in this systematic review stems from the fundamental understanding that this study design can shed light on the biological processes underlying the effects of bleaching gels on pulp tissue. This information is crucial for the development of future therapeutic strategies aimed at minimizing these side effects. Thus, the comprehensive evaluation of all in vivo studies that analysed the effects of bleaching on pulp tissue represents a significant strength of this review. However, it's worth noting that a limitation of this systematic review is the comparatively limited number of studies on human teeth that were identified.
The findings of this systematic review consistently indicate that bleaching with high concentrations of HP can cause significant inflammation or necrosis in the pulp tissue over short observation periods, which is a relevant finding of this review. Some studies reviewed also examined therapies applied before, during, or after using bleaching gel on animal teeth (Barbosa et al., 2019; Benetti, Briso, et al., 2018; Carminatti et al., 2020; Terayama et al., 2020). Although certain treatments, such as the application of bioglasses or remineralizers (Barbosa et al., 2019; Carminatti et al., 2020) and the use of photobiomodulation post-bleaching (Terayama et al., 2020), demonstrated potential in mitigating the inflammatory process, none have been conclusively effective, and their effects on humans remain unexamined. Thus, lower concentrations of HP are often the preferred choice due to their reduced trans-enamel/dentinal penetration (Cintra, Benetti, Ferreira, Gomes-Filho, et al., 2016; Cintra, Benetti, Ferreira, Rahal, et al., 2016), decreased pulp damage and minimized cytotoxic effects on pulp cells (Benetti et al., 2017; Benetti, Gomes-Filho, et al., 2018; Reis-Prado et al., 2021), all while not compromising the aesthetic outcome (Cintra, Benetti, Ferreira, Rahal, et al., 2016). Dentist supervision is crucial for both at-home and in-office bleaching treatments, given the potential risks these products pose to dental pulp. It is essential to adhere to the directive that limits dentists to using a maximum concentration of 6% HP for bleaching protocols (Kahler, 2022; The Council of The European Union, 2011). However, only four studies in this systematic review referred to this directive (Benetti, Briso, de Araújo Lopes, et al., 2019; Carminatti et al., 2020; Cintra et al., 2017; Ferreira et al., 2018), highlighting the need for broader adoption of this regulation. Additionally, this systematic review underscores the need for continued research on the development of bleaching gels that can achieve a bleaching effect without causing harm to pulp tissue or inducing postoperative sensitivity.
CONCLUSION
This systematic review indicates that high concentrations of bleaching gel increase inflammatory response and necrosis in the pulp tissue at short periods after bleaching, mainly in rat molars and human incisors, in addition to causing greater hard tissue deposition over time. However, further well-described histological studies with long-term follow-up are encouraged due to the methodological limitations of eligible studies, mainly involving proper sample randomization, blinding and justification of sample size.
AUTHOR CONTRIBUTIONS
Conceptualization: F Benetti, IFC Peixoto, LTA Cintra; Study selection: MV Donato, AH dos Reis-Prado, J Goto, ALF Briso; Data collection: MV Donato, AH dos Reis-Prado, LC Arantes, HGS Chaves, J Goto; Quality assessment: F Benetti, AH dos Reis-Prado, LG Abreu, LTA Cintra; Methodology: IFC Peixoto, AH dos Reis-Prado, LG Abreu, LC Arantes, HGS Chaves; Project administration: F Benetti, IFC Peixoto, ALF Briso; Resources: F Benetti, LG Abreu, ALF Briso; Supervision: F Benetti, IFC Peixoto, LTA Cintra; Visualization: MV Donato, LC Arantes, HGS Chaves; Writing (original draft): MV Donato, AH dos Reis-Prado, J Goto, LC Arantes, HGS Chaves; Writing (review/editing): IFC Peixoto, LG Abreu, LTA Cintra, F Benetti. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
This research was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES (n. 88887.489995/2020-00, n. 88887.649870/2021-00, n. 001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (n. 310683/2022-0).
FUNDING INFORMATION
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (88887.489995/2020-00 and 88887.649870/2021-00).
CONFLICT OF INTEREST STATEMENT
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
ETHICS STATEMENT
Ethical approval was not necessary as this article is not a research study that involved any human or animal experiments.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




