Treatment of pulpal and apical disease: The European Society of Endodontology (ESE) S3-level clinical practice guideline
ESE Workshop Participants and Methodological Consultant are presented in Appendix 1.
Abstract
Background
The ESE previously published quality guidelines for endodontic treatment in 2006; however, there have been significant changes since not only in clinical endodontics but also in consensus and guideline development processes. In the development of the inaugural S3-level clinical practice guidelines (CPG), a comprehensive systematic and methodologically robust guideline consultation process was followed in order to produce evidence-based recommendations for the management of patients presenting with pulpal and apical disease.
Aim
To develop an S3-level CPG for the treatment of pulpal and apical disease, focusing on diagnosis and the implementation of the treatment approaches required to manage patients presenting with pulpitis and apical periodontitis (AP) with the ultimate goal of preventing tooth loss.
Methods
This S3-level CPG was developed by the ESE, with the assistance of independent methodological guidance provided by the Association of Scientific Medical Societies in Germany and utilizing the GRADE process. A robust, rigorous and transparent process included the analysis of relevant comparative research in 14 specifically commissioned systematic reviews, prior to evaluation of the quality and strength of evidence, the formulation of specific evidence and expert-based recommendations in a structured consensus process with leading endodontic experts and a broad base of external stakeholders.
Results
The S3-level CPG for the treatment of pulpal and apical disease describes in a series of clinical recommendations the effectiveness of diagnosing pulpitis and AP, prior to investigating the effectiveness of endodontic treatments in managing those diseases. Therapeutic strategies include the effectiveness of deep caries management in cases with, and without, spontaneous pain and pulp exposure, vital versus nonvital teeth, the effectiveness of root canal instrumentation, irrigation, dressing, root canal filling materials and adjunct intracanal procedures in the management of AP. Prior to treatment planning, the critical importance of history and case evaluation, aseptic techniques, appropriate training and re-evaluations during and after treatment is stressed.
Conclusion
The first S3-level CPG in endodontics informs clinical practice, health systems, policymakers, other stakeholders and patients on the available and most effective treatments to manage patients with pulpitis and AP in order to preserve teeth over a patient's lifetime, according to the best comparative evidence currently available.
Clinical relevance
Scientific rationale for guideline
Patients with pulpitis and apical periodontitis (AP) suffer a range of signs, symptoms and disease severities. This Clinical Practice Guideline (CPG) aimed to replace and update the ESE 2006 treatment guidelines (ESE, 2006) using modern techniques of guideline development to provide guidance on the necessary treatment required to manage compromised teeth with pulpal and apical disease. The interventions described in these guidelines should be derived following a rigorous evidence-based and patient-centred decision-making process.
Principal findings
This guideline informs on the best available evidence on the effectiveness of the interventions considered, and provides the most appropriate clinical recommendations for diagnosing and treating pulpitis and apical periodontitis. The guideline was developed using strict and validated methodologies and based on a structured consensus process, including a panel of experts and representatives from key stakeholder groups including patients.
Practical implications
The application of this ESE S3-Level CPG will allow a consistent, interdisciplinary and evidence-based approach to the management of pulpitis and AP for the benefit of endodontists, general dentists, patients and other stakeholders.
INTRODUCTION
Pulpitis and apical periodontitis
Definitions
Endodontology is concerned with the study of the form, function and health of, injuries to and diseases of the dental pulp and periradicular region, their prevention and treatment; the principal diseases being pulpitis and apical periodontitis (AP), which are caused by infection (ESE, 2006). Pulpitis is inflammation of the dental pulp due to injury or infection, whilst AP is inflammation and destruction of the periradicular tissues caused by aetiological agents of endodontic origin (Nair, 2004) often as a result of pulp necrosis.
Prevalence of pulpal and apical disease
Globally, the diseases with the greatest age-standardized prevalence have been identified as oral disorders, with caries in permanent teeth having the highest prevalence of all oral disorders measured (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018); however, the survey did not include pulpitis or AP. A recent systematic review investigated the prevalence of AP globally and included 114 studies for meta-analysis, with 39% of teeth that had been root canal treated and 3% of nontreated teeth exhibiting AP (Tibúrcio-Machado et al., 2021). The prevalence of AP was higher in dental care services and hospitals than those individuals from the general population and it was concluded that half the world's adult population has at least one tooth with AP, which highlights the huge, often hidden, burden of endodontic disease (Tibúrcio-Machado et al., 2021). These findings reflect previous primary research which has highlighted that the global prevalence of people with AP in at least one tooth ranges from 15% to 85% (Al-Zahrani et al., 2017; Skudutyte-Rysstad & Eriksen, 2006) with differences attributed to age (Kirkevang et al., 2007), systemic disease (Al-Zahrani et al., 2017), level of education and access to dental care (Aleksejuniene et al., 2000). In terms of pulpitis, it is more challenging to assess the true prevalence of the disease, as up to 40% of pulps become inflamed and can even progress to necrosis in the absence of symptoms (Michaelson & Holland, 2022). However, the prevalence of symptomatic pulpitis leading to ‘toothache’ is considered high (Santos et al., 2022) with painful pulpitis the most common cause of orofacial pain (Lipton et al., 1993) and the most likely reason for presentation of a dental emergency visit (Rechenberg et al., 2016).
Treatment and consequences of failure to treat
Pulpitis and AP are inflammatory conditions principally caused by microbial infection. Dental caries, pulpitis and AP are biofilm-induced diseases, with caries perpetuated by an oral source of fermentable carbohydrates (Nyvad et al., 2013; Pitts et al., 2017). Untreated caries will create a cariogenic niche, which breaks down enamel and dentine eventually forming a tooth cavity (Dorozhkin & Epple, 2002; ESE, 2019; Schwendicke et al., 2016). Although a pulpitic response is evident in the early carious process, it is not until the carious infected dentine is close to the pulp and invades the tertiary dentine structure that the pulpitis becomes severe and if left untreated the bacteria will enter the pulp tissue (Demant et al., 2021; Reeves & Stanley, 1966) leading to localized inflammation, necrosis and microabscess. However, in experimental animal models, the pulp has demonstrated the capacity to repair as long as the microbial irritation is removed and the tooth restored with a sealing restoration that prevents further contamination (Mjör & Tronstad, 1974; Warfvinge & Bergenholtz, 1986). Maintaining pulp vitality when possible limits further intervention and is a biologically based therapy as it maintains the pulp's defensive, developmental and mechanoreceptor features (Bjørndal et al., 2019; Paphangkorakit & Osborn, 1998). If the infection is permitted to develop in the pulp, the inflammatory response intensifies and spreads to the root canal system (Ricucci et al., 2014); the pulp becomes necrotic and potentially leading to abscess, discomfort and reduced quality of life (Liu et al., 2014). Root canal treatment is a successful and established treatment aimed at chemo-mechanical debridement of the infected root canal system, with resolution of apical disease, before filling the space and restoring the tooth to function (ESE, 2006). If, however, the infection is allowed to fester untreated or the treatment is carried out inadequately, persistent infection, potential systematic complications (Sebring et al., 2022) and tooth loss are likely consequences. Extraction rather than root canal treatment has been shown to result in a reduced quality of life for patients (Wigsten et al., 2020). Notably for a preventable disease, dental caries, alongside advanced periodontitis, are responsible for more years lost to disability than any other human disease (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018).
Economic aspects
Dental care provision is a large contributor to the cost of general healthcare costs with the management of dental diseases estimated to generate cost of approximately USD $357 billion per year globally (Righolt et al., 2018), and according to the American Association of Endodontists (AAE), 15 million teeth are endodontically treated per annum (AAE, 2023). Currently, the global endodontic devices' market size is valued at USD $1.75 billion and is expected (due to an increase in caries and apical disease) to expand at an annual growth rate of 4.3% from 2022 to 2030 (Grand View Research, 2023). A recent systematic review investigating the prevalence of root canal treatment throughout the world highlighted root canal treatment to be very common procedure with more than half the studied population having at least one endodontically treated tooth (León-López et al., 2022). From a health economics perspective, although tooth loss may be financially preferable to the patient in the short-term, retaining teeth using root canal treatment is usually more cost-effective than the option of removing them and replacing them prosthodontically (Pennington et al., 2009; Schwendicke & Herbst, 2023). Notably, this reported financial advantage does not even consider the psychological, social and quality-of-life aspects accompanying tooth loss (Block et al., 2022).
GUIDELINE AIM
This S3-level guideline aims to develop a Clinical Practice Guideline (CPG) for the treatment of pulpal and apical disease, focusing not only on the effectiveness of current treatment approaches employed to manage patients presenting with pulpitis and AP but also on the diagnosis of endodontic disease. The guideline highlights the importance, and need for robust comparative evidence, to support clinical decision-making for patients presenting with pulpal and apical disease. The principal objective is to inform, in a series of clear expert and evidence-based recommendations, the best current therapeutic strategies that are supported by scientific evidence, whilst also highlighting gaps in knowledge and focus areas for future research in the discipline. The ultimate aim is to improve the quality of dental care provided to patients in Europe and worldwide who present with endodontic disease, by reducing the sequelae of disease including pain, infection and reduced quality of life, whilst ultimately preventing tooth loss.
Target users
Dental professionals, together with a range of medical and dental external stakeholders related to oral health care provision, including dental students and patients. In addition, this CPG aims to inform health systems, policymakers, dental industry and the public.
Target environments
Hospital, university and other academic environments as well as specialist practice, general practice and other community-based practices.
Target patient population
- deep carious lesions or deep restorations;
- pulpitis (symptomatic or asymptomatic) and apical periodontitis (symptomatic or asymptomatic);
- traumatized immature teeth;
- failed previous endodontic treatment and evidence of pulpal or apical disease.
Exceptions from guideline
Due to geographical variations and paucity of evidence in a similar manner to previous S3-level guidelines (Herrera et al., 2022), this CPG does not consider detailed economic aspects or the detailed cost–benefit ratio of the proposed management strategies. This guideline does not consider the treatment of vertical root fractures, periodontal–endodontic problems or chronic pain, which were considered beyond the scope of this current guidelines process, but could potentially be included in future iterations. Other multidisciplinary areas such as trauma to permanent teeth (ESE, Krast, et al., 2021) and restoration of the endodontically treated tooth (ESE, Mannocci, et al., 2021) have recently been the subject of ESE-commissioned position statements. Finally, this CPG does not address the management of diseased primary teeth as this was considered the primary responsibility of paediatric dental groups.
METHODOLOGY
General framework
This guideline was developed following methodological guidance published by the Standing Guideline Commission of the Association of Scientific Medical Societies in Germany (AWMF) (https://www.awmf.org/leitlinien/awmf-regelwerk/awmf-guidance.html) and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group (https://www.gradeworkinggroup.org/).
- WG1: The treatment of pulpitis (including diagnosis)—Chairs: Ikhlas El-Karim (I.E.K.) and Gabriel Krastl (G.K.)
- WG2: The nonsurgical treatment of apical periodontitis (including diagnosis)—Chairs: Lise-Lotte Kirkevang (L.L.K.) and Ove Peters (O.P.)
- WG3: The surgical treatment of apical periodontitis—Chairs: Bun San Chong (B.S.) and Massimo Del Fabbro (M.D.F.)
- WG4: The regenerative treatment of apical periodontitis—Chairs: Kerstin Galler (K.G.) and Juan Segura Egea (J.S.E.)
Each WG had two group leaders from different countries who did not have prior experience of working together. The leaders were selected by the guideline leads, Henry Duncan (H.D.) and Moritz Kebschull (M.K.), and approved by the ESE board in order to reflect prominent leaders in endodontics, whilst also reflecting diversity in relation to gender, age and country of work. Key members from Europe formed the basis for this, with the inclusion of members from North America and Australia. The eight WG leads formed the GSG alongside the guideline leads. The CSG met periodically online, separate from the guideline panel, to discuss management and ongoing work associated with the guideline process. Online GSG meetings occurred in December 2020, April 2021 and April 2022.
To ensure broad and representative stakeholder involvement, the GSG discussed, nominated and invited a wide range of dental organizations, student bodies, patient representatives and other stakeholders to be part of the guideline process (Table 1). In the process, these individuals were supplemented with two senior reviewers from each SR. Each external stakeholder was first invited to participate and if they agreed were asked to nominate one representative that would engage in the guideline process. That individual was allocated to one of the four working groups and invited to attend the online methodological sessions relevant to that group as well as the plenary sessions and the consensus summit (Table 2). Due to the absence of pan-European patient groups, one patient representative was selected from different parts of Europe and allocated to each of the four WGs. Continued efforts will be undertaken in the future to further include the perspectives of patients (Brocklehurst et al., 2018), and national societies will be encouraged to involve patient groups within individual countries, as key stakeholders for the Adaptation, Adoption, De Novo Development—‘ADOLOPMENT’ of this GPG (Schunemann et al., 2017).
| Institution | Acronym | Answera | Agreed/Declined | Representative |
|---|---|---|---|---|
| Association for Dental Education in Europe | ADEE | Answered | Agreed | Barry Quinn |
| Council of European Chief Dental Officers | CECDO | Answered | Agreed | Kenneth Eaton |
| Council of European Dentists | CED | Answered | Agreed | Paulo Melo |
| European Academy of Paediatric Dentistry | EAPD | No answer | — | No representative |
| European Association of Craniomaxillofacial Surgery | EACMFS | Answered | Declined | No representative |
| European Association for Osseointegration | EAO | Answered | Agreed | Daniel Soazig |
| European Association of Dentomaxillofacial Radiology | EADMFR | Answered | Agreed | Reinier Hoogeveen |
| European Association of Dental Public Health | EADPH | No answer | — | No representative |
| European Association of Oral Medicine | EAOM | Answered | Declined | No representative |
| European College of Gerodontology | ECG | Answered | Agreed | Anastasia Kossioni |
| European Dental Hygienists Federation | EDHF | Answered | Agreed | Gitana Rederiene |
| European Dental Students Association | EDSA | Answered | Agreed |
Christa Serban Marta Adam |
| European Federation of Conservative Dentistry | EFCD | Answered | Agreed | Sebastian Paris |
| European Federation of Periodontology | EFP | Answered | Agreed | Nicola West |
| European Forum for Primary Care | EFPC | No answer | — | No representative |
| European Organization for Caries Research | ORCA | Answered | Agreed | Christian Splieth |
| European Prosthodontic Association | EPA | Answered | Agreed | Marco Ferrari |
| International Association of Dental Research (Pan European Region) | PER-IADR | Answered | Agreed | Sema Belli |
| International Association of Dental Traumatology | IADT | Answered | Agreed | Cecilia Bourguignon |
| Platform for Better Oral Health | PFBOH | No answer | — | No representative |
- a Sent message 15 January 2022 and reminder on 15 February 2022.
| Scientific society or organization | Delegate(s) |
|---|---|
| Responsible scientific society | |
| European Society of Endodontology |
Guideline leads: Henry Duncan, Moritz Kebschull Working Group Chairs (in alphabetical order): Bun San Chong; Massimo Del Fabbro; Ikhlas El-Karim; Kerstin Galler; Lise-Lotte Kirkevang; Gabriel Krastl; Ove Peters; Juan J. Segura Egea Methodologist: Ina Kopp Clinical experts (in alphabetical order): Francesc Abella Sans; Carsten Appell; Ana Arias; Lars Bjørndal; Christos Boutsioukis; Cristina Bucchi; Sebastian Bürklein; Daniel Cabanillas-Balsera; Josette Camilleri; Antonis Chaniotis; Stefano Corbella; Valerie Chevalier; Elisabetta Cotti; Till Dammaschke; Roeland de Moor; Paul Dummer; Fernando Durán-Sindreu; Vittorio Franco; Helena Fransson; Johnah Galicia; Gianluca Gambarini; Antonio Ginjeira; Brenda Gomes; Aleksandar Jakoviljevic; Casper Kruse Claus Lost; Maarten Meire; Nastaran Meschi; Venkateshbabu Nagendrababu; Yuan-Ling Ng; Dag Ørstavik; Shanon Patel; Chiara Pirani; Gianluca Plotino; Tina Rödig; Eyal Rosen; Giampiero Rossi Fedele; Edgar Schafer; Hagay Shemesh; Jale Tanalp; Silvio Taschieri; Leo Tjäderhane; Phil Tomson; Igor Tsesis; Clemens Walter; John Whitworth; Matthias Widbiller |
| Scientific societies involved in the guideline development | |
| Association for Dental Education in Europe | Barry Quinn |
| European Association for Osseointegration | Daniel Soazig |
| European Association of Dentomaxillofacial Radiology | Reinier Hoogeveen |
| European College of Gerodontology | Anastasia Kossioni |
| European Federation of Conservative Dentistry | Sebastian Paris |
| European Federation of Periodontology | Nicola West |
| European Organization for Caries Research | Christian Splieth |
| European Prosthodontic Association | Marco Ferrari |
| IADR (Pan-European Region) | Sema Belli |
| International Association of Dental Traumatology | Cecilia Bourguignon |
| Other organizations | |
| Council of European Chief Dental Officers | Kenneth Eaton |
| Council of European Dentists | Paulo Melo |
| European Dental Hygienists Federation | Gitana Rederiene |
| European Dental Students' Association | Marta Adam; Christa Serban |
| Patient representatives | |
| No organization | Cathy Dillon; Amanda Jackson; Massimo Guffanti; Thomas Schratzenstaller |
The ESE also engaged an independent guideline methodologist to advise the panel and facilitate the consensus process throughout the process (Ina Kopp [I.K.]). The guideline methodologist had no voting rights.
Evidence synthesis
Search for previous guidelines in endodontology
- Guideline International Network (GIN)
- Guidelinecentral.com
- The National Institute for Health and Clinical Excellence (NICE)
- Canadian Health Technology Assessment (CADTH)
- European Society of Endodontology (ESE)
- American Association of Endodontists (AAE)
- American Dental Association (ADA)
- American Academy of Pediatric Dentistry (AAPD)
- British Endodontic Society (BES)
- German Association of Endodontology and Dental Traumatology (DGET)
The last search was performed on 24th January 2023. Search terms used were as follows: ‘Endodontic’, ‘Endodontology’, ‘Guidelines’, ‘Pulpitis’, ‘Apical Periodontitis’ and ‘Clinical Practice Guidelines’. In addition, content was screened by hand searches.
Only guidelines published in English and with full texts available were included. The methodological quality of these guideline texts was critically appraised using the AGREE II framework and instrument (https://www.agreetrust.org/agree-ii/).
At the end of the search, it was noted that no guidelines/documents directly relevant to the current guideline development process were discovered due to: (i) their publication time, (ii) their methodological approach or (iii) their stated inclusion criteria or scope (Table 3).
| Database | Potentially relevant guidelines identified | Critical appraisal |
|---|---|---|
| Guideline International Network (GIN) International Guidelines Librarya | Therapie des dentalen Traumas bleibender Zähne—DGZMK, DGMKG (2022)—German | In German, outside scope. Not applicable |
| Guidelines for endodontics—Dubai Health Authority (2021) | Published after start of ESE process. Unclear methodology (guideline group). Not applicable | |
| Guidelines for surgical endodontics—Royal College of Surgeons of England (2012) | Over 10 years old, unclear methodology. Not applicable | |
| Guidelines for root canal treatment—University of Singapore (2004) | 19 years old. Unclear methodology. Not applicable | |
| Clinical practice guidelines for the surgical treatment of post-treatment periapical disease—Colombia University and Government (2019) | Unclear methodology (follow-up, study selection/type and recommendation process). Not applicable | |
| Guidelines central.com ‘Dentistry’ categoryb | Thematic hit only related to the American Dental Association (see below) | Not applicable |
| The National Institute for Health and Clinical Excellence (NICE)c | No thematically relevant guidelines identified | Not applicable |
| Canadian Health Technology Assessment (CADTH)d |
Vital Pulp Therapy for Endodontic Treatment of Mature Teeth: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines (2017) Endodontic Therapy Interventions for Root Canal Failure in Permanent Dentition: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines |
Unclear methodology (follow-up, study selection, outcome variables, recommendations and guideline group). Not applicable 6 years old. Unclear methodology (follow-up, study selection, outcome variables, recommendations and guideline group). Not applicable |
| European Society of Endodontology (ESE)e | Quality guidelines for endodontic treatment: consensus report of the European Society of Endodontology (2006) |
17-year-old narrative style guideline. The current S3-level guidelines were commissioned in order to update and replace these guidelines. Not applicable |
| American Dental Associationf | Evidenced-based clinical practice guidelines on restorative treatments for caries lesions (2023) | Out of scope. Indirectly applicable. High quality |
| American Association of Endodontists (AAE)g |
AAE Position Statement on Vital Pulp Therapy (2021) Guideline to Clinical Endodontics (2013) |
Unclear methodology (follow-up, outcome variables, recommendations and guideline group). Not applicable 10 years old, recommendations not based on systematic evaluation of evidence. Not applicable |
| American Academy of Pediatric Dentistry (AAPD)h |
Use of nonvital therapies in primary teeth (2020) Pulp therapy for primary and immature permanent teeth |
Current ESE guideline limited to permanent teeth (out of scope). Not applicable Unclear methodology in relation to study inclusion (follow-up, outcome variables, recommendations and guideline group). Not applicable |
| British Endodontic Society (BES)i | A guide to good endodontic practice (2022) | Different methodology (follow-up, outcome variables and recommendations not based on systematic evaluation of evidence). Not applicable |
| German Association of Endodontology and Dental Traumatology (DGET)j | No equivalent guidelines identified | Not applicable |
- a https://guidelines.ebmportal.com/.
- b https://www.guidelinecentral.com/.
- c https://www.nice.org.uk/guidance/published.
- d https://www.cadth.ca/.
- e https://www.e-s-e.eu/for-professionals/resources-for-clinicians/.
- f https://www.aae.org/specialty/clinical-resources/guide-clinical-endodontics/.
- g https://www.ada.org/topic/Clinical-Guidelines.
- h https://www.aapd.org/.
- i https://britishendodonticsociety.org.uk/professionals/endodontic_publications.aspx.
- j https://www.dget.de/.
Systematic search and critical appraisal of the literature
For this guideline, a total of 14 systematic reviews (SRs) were conducted to support the guideline development process (Bucchi et al., 2022; Bürklein & Arias, 2022; Corbella et al., 2022; Donnermeyer et al., 2022; Hilmi et al., 2023; Jakovljevic et al., 2022; Meire et al., 2022; Meschi et al., 2022; Pirani & Camilleri, 2022; Plotino et al., 2022; Rossi-Fedele & Ng, 2022; Rossi-Fedele & Rödig, 2022; Tomson et al., 2022; Widbiller et al., 2022). Each SR has two designated senior reviewers, who were from different countries and institutions and were not established collaborators, to work together on each review. They were encouraged to ask other members of their institution or other institutions to help with the review process. The completed reviews were reviewed first by the WG leads and guideline leads and thereafter, through a formal submission and double-blind review process in the International Endodontic Journal; corresponding manuscripts are published within this special issue of the International Endodontic Journal.
All SRs were conducted following the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ (PRISMA) framework (Moher et al., 2009), and were prospectively registered in PROSPERO.
Relevance of outcomes
In order to ensure a homogenous systematic review process in the development of the clinical practice guidelines, it was considered essential that the core outcomes for all endodontic treatments were standardized, and recommendations were made regarding minimum follow-up time specific to each outcome measure. In the absence of a recognized core outcome set in endodontics (El-Karim et al., 2023), a separate project linked to the S3 process established and ranked by consensus the most appropriate clinician and patient-reported outcomes (Duncan, Nagendrababu, et al., 2021b). As part of the project, recommendations were agreed regarding an acceptable minimum follow-up period for studies by literature review and group discussion (Duncan, Nagendrababu, et al., 2021a). The selected outcome measures and follow-up periods were subsequently used in the systematic analyses of the literature to investigate the effectiveness of endodontic treatment to alleviate pulpitis and AP. Within this process, previous reviews, ESE Guidelines and Position Statements were searched in order to compile a list of potentially important outcome measures for the treatment of pulpitis (WG1), the nonsurgical treatment of apical periodontitis (WG2), the surgical treatment of apical periodontitis (WG3) and the regenerative treatment of apical periodontitis (WG4) as it was accepted that there would be differences between the WGs. Forty-two members of the Guideline development group then ranked by importance the outcomes using a 9-point Likert scale as described by GRADE (Guyatt et al., 2011): 1–3 limited importance; 4–6 important; and 7–9 critical importance over a series of online surveys. Finally, the selected outcomes were discussed during an online meeting of the GDG. Four tables were constructed, one for each WG in which the minimum and maximum follow-up periods was designated for each outcome as well as the outcomes being separated into ‘most critical’, ‘critical’ and ‘important’ (see Section ‘Focussed PICOTS questions’). The most critical outcome was tooth survival (Duncan, Nagendrababu, et al., 2021a).
Focussed PICOTS questions
In all 14 commissioned SRs, focussed questions in PICOTS format (P = Population; I = Intervention; C = Comparison; O = Outcome; T = Time; S = Study type) (Methley et al., 2014; Riva et al., 2012) were proposed by the SR authors in May 2021 to the GSG and the methodological consultant; these were reviewed, modified (if necessary) and subsequently approved. Particular care was taken to limit overlap, repetition and thematic exclusion in order to ensure the main therapeutic interventions in the treatment of pulpal and apical disease were adequately covered. The PICO questions were as written in the SRs and listed in Table 4. The time (T) and study type (S) to be included were standardized in a consensus process that included the members of the GSG (Duncan, Chong, et al., 2021). This varied for diagnostic and treatment reviews and also between WGs as detailed below.
| Reference | Systematic review title | Final PICOTS questions (as written in manuscript) |
|---|---|---|
| WG1 | ||
| Donnermeyer et al. (2022) | Effectiveness of diagnosing pulpitis: a systematic review |
1. In patients suspected of pulpitis with no pain (P), what is the effectiveness of pre- or intraoperative diagnosis of the pulpal condition with respect to if it is possible to maintain pulp vitality by means of clinical findings such as symptoms, depth of caries lesion, pulp exposure, bleeding or any other method and evaluation of the presence of inflammatory mediators (biomarkers) (I) in comparison to follow-up results in terms of (i) pulp survival, when teeth with suspicion of pulpitis were treated with any type of vital pulp therapy, (ii) histological evaluation of the pulp tissue after extraction and (iii) quantification of inflammatory mediators (e.g. interleukin-8, matrix metalloproteinase 9 and tumour necrosis factor-α) obtained from dentinal fluid or pulp tissue of teeth suspected of pulpitis in comparison of teeth with normal (healthy) pulp tissue (C) regarding sensitivity and specificity of pre- or intraoperative diagnosis of the level of pulp inflammation compared with levels of inflammatory mediators and/or histological findings (O)? 2. In patients suspected of pulpitis with nonspontaneous pain (P), what is the effectiveness of pre- or intraoperative diagnosis of the pulpal condition with respect to if it is possible to maintain pulp vitality by means of clinical findings such as symptoms, depth of caries lesion, pulp exposure, bleeding or any other method including the evaluation of the presence of inflammatory mediators (biomarkers) (I) in comparison to follow-up results in terms of pulp survival, when teeth with suspicion of pulpitis were treated with any type of vital pulp treatment and histological evaluation after extraction, quantification of inflammatory mediators (e.g. interleukin-8, matrix metalloproteinase 9 and tumour necrosis factor-α) obtained from dentinal fluid, pulpal blood or pulp tissue of teeth with normal (healthy) pulp tissue (C) regarding sensitivity and specificity of pre- or intraoperative diagnosis of the pulpal condition compared with follow-up results, in terms of pulp survival, where teeth with caries are treated with any type of vital pulp treatment and the level of pulp inflammation compared with levels of inflammatory mediators and/or histological findings (O)? 3. In patients suspected of pulpitis with spontaneous pain (P), what is the effectiveness of the diagnosis of the tooth as vital and being the cause of the pain by any method including the presence of inflammatory mediators (biomarkers) (I) in comparison to the ocular inspection of pulp tissue status after exposure (e.g. pulp bleeding, pus, necrotic tissue), the histological evaluation after extraction and relieve of pain as a result of an operative procedure in the tooth (excavation, medication, pulpotomy, pulpectomy) including quantification of inflammatory mediators (e.g. interleukin-8, matrix metalloproteinase 9, tumour necrosis factor-α) obtained from dentinal fluid, pulpal blood or pulp tissue of teeth suspected of pulpitis with spontaneous pain in comparison to teeth with normal (healthy) pulp tissue (C) in terms of sensitivity and specificity of preoperative diagnosis of pulpitis compared with evaluation of pulp condition after pulp exposure or histological examination, or relieve of pain after an operative procedure (O)? |
| Jakovljevic et al. (2022) | Effectiveness of vital pulp treatment in managing nontraumatic pulpitis associated with no or nonspontaneous pain: a systematic review |
1. In patients with nontraumatic pulpitis associated with no or nonspontaneous pain in immature and mature permanent teeth (P), is direct pulp capping or pulpotomy (partial/full) (I) as effective as selective or stepwise caries removal (C), in terms of a combination of clinical outcomes (O), with ‘tooth survival’ as the most critical outcome? 2. In patients with nontraumatic pulpitis associated with no or nonspontaneous pain in immature and mature teeth (P), is pulpotomy (partial/full) (I) as effective as direct pulp capping (C), in terms of a combination of clinical outcomes (O), with ‘tooth survival’ as the most critical outcome? 3. In patients with nontraumatic pulpitis associated with no or nonspontaneous pain in mature permanent teeth (P), is pulpotomy (partial/full) (I) as effective as a pulpectomy (C), in terms of a combination of patient- and clinical reported outcomes (O), with ‘tooth survival’ as the most critical outcome? |
| Tomson et al. (2022) | Effectiveness of pulpotomy compared with root canal treatment in managing nontraumatic pulpitis associated with spontaneous pain: a systematic review and meta-analysis | Do pulpotomy (partial or full) (I) results in better patient- and clinical reported outcomes (O), compared with RCTx (C) in permanent teeth with pulpitis characterized by spontaneous pain (P) evaluated at various time intervals (T)? Time interval (T) = Defined as a minimum of 1 year and a maximum of as long as possible for all outcome measures, except ‘pain, tenderness, swelling, need for medication (analgesics)’, which is a minimum of 7 days and maximum of 3 months and oral health-related quality of life (OHRQoL) which is minimum of 6 months and a maximum of as long as possible |
| Rossi-Fedele and Ng (2022) | Effectiveness of root canal treatment for vital pulps compared with necrotic pulps in the presence or absence of signs of periradicular pathosis: a systematic review and meta-analysis | Participants/population (P) were patients undergoing nonsurgical root canal treatment. Intervention(s)/exposure(s) (I) group were teeth with a vital pulp. Comparator(s)/control (C) group were teeth with pulp necrosis (nonvital) with or without signs of periradicular pathosis. Outcomes (O) included a combination of patient- and clinician-reported outcomes measures were assessed |
| WG2 | ||
| Hilmi et al. (2023) | Efficacy of imaging techniques for the diagnosis of apical periodontitis: a systematic review | In the adult human permanent dentition (P), what is the efficacy of diagnostic imaging of the periapical tissues (I) using histopathology as a reference standard (C) in the diagnosis of apical periodontitis, in terms of diagnostic accuracy (O)? Eligible studies must have a primary objective to evaluate the accuracy of a diagnostic imaging technique to detect signs of apical periodontitis and a histopathological reference standard. |
| Bürklein and Arias (2022) | Effectiveness of root canal instrumentation for the treatment of apical periodontitis: a systematic review and meta-analysis |
1. In patients with apical periodontitis (P), what is the effectiveness of root canal instrumentation performed with contemporary techniques (I) in comparison with ‘traditional’ (conventional stainless-steel instruments) technique as described above (Sjogren et al., 1990) (C) in terms of clinical and patient-related outcomes (O)? 2. In patients with apical periodontitis (P), what is the effectiveness of root canal instrumentation performed with contemporary engine-driven NiTi instruments (I) compared with other types of contemporary engine-driven NiTi instruments (with different design and/or technology) (C) in terms of clinical and patient-related outcomes (O)? |
| Rossi-Fedele and Rödig (2022) | Effectiveness of root canal irrigation and dressing for the treatment of apical periodontitis: a systematic review and meta-analysis of clinical trials |
1. In patients with asymptomatic AP in permanent teeth (P), what is the effectiveness of instrumentation and irrigation performed with any root canal irrigant(s) and sequence (I) in comparison with instrumentation and irrigation with NaOCl and EDTA (C) in terms of clinical and patient-related outcomes (O)? 2. In patients with asymptomatic AP in permanent teeth (P), what is the effectiveness of intracanal dressing with any root canal dressing(s) or calcium hydroxide mixed with other vehicles or dressings or no dressing (I) in comparison with calcium hydroxide (mixed with glycol, glycerine, saline, distilled water or unmixed) (C) in terms of clinical- and patient-related outcomes (O)? |
| Pirani and Camilleri (2022) | Effectiveness of root canal filling materials and techniques for treatment of apical periodontitis: a systematic review |
1. In patients with apical periodontitis in permanent teeth (P), what is the effectiveness of chemo-mechanical preparation and root canal filling with any type of nonlateral compaction technique (I) in comparison with chemo-mechanical preparation and cold lateral compaction technique using Gutta–percha (C) in terms of clinical- and patient-related outcomes (O)? 2. In patients with apical periodontitis in permanent teeth (P), what is the effectiveness of chemo-mechanical preparation and root canal filling with any other type of sealer (I) in comparison with chemo-mechanical preparation and root canal filling with epoxy resin (AH Plus/AH 26) using Gutta–percha (C) in terms of clinical- and patient-related outcomes (O)? |
| Meire et al. (2022) | Effectiveness of adjunct therapy for the treatment of apical periodontitis: a systematic review and meta-analysis | General population, adult patients undergoing primary or secondary root canal treatment of a tooth with radiographic evidence of AP (P). (I) Adjunct therapy: any type of intracanal procedure going beyond chemo-mechanical preparation with instruments and traditionally delivered irrigants and carried out within the same visit. It includes irrigant activation methods/devices, light-mediated disinfection (photo-activated disinfection and direct laser irradiation) and the use of ozone. Chemo-mechanical preparation with instruments and traditionally (syringe needle based) delivered irrigants alone (excluding the use of intracanal medication) (C). The most critical outcome (O) is ‘tooth survival’. Other critical outcomes are ‘pain, tenderness, swelling, need for medication (analgesics, antibiotics)’, ‘radiographic evidence of reduction of apical lesion size (loose criteria)’ and ‘radiographic evidence of normal periodontal ligament space (strict criteria)’. Secondary outcomes include the following: ‘tooth function (fracture, restoration longevity)’, ‘need for further intervention’, ‘adverse effects (including exacerbation, restoration integrity, allergy)’, ‘oral health-related quality of life (OHRQoL)’ and ‘presence of sinus tract’ |
| WG3 | ||
| Bucchi et al. (2022) | Nonsurgical root canal treatment and retreatment versus apical surgery in treating apical periodontitis: a systematic review | (P) Patients with teeth showing evidence of apical periodontitis. Studies where guided tissue regeneration was carried out or consisted of teeth with endodontic–periodontal lesions, vertical root fractures or root perforations or patients with severe systemic disorders (e.g. poorly controlled diabetes, immunological disease and malignant neoplastic conditions) were excluded. (I) Patients undergoing surgical treatment of, clinically and radiologically confirmed apical periodontitis. (C) Patients undergoing nonsurgical root canal treatment or retreatment of, clinically and radiologically confirmed, apical periodontitis. (O) The main outcome measure was ‘tooth survival’. Other critical outcome measures include pain, tenderness, swelling, need for medication (analgesics, antibiotics), presence of sinus tract, satisfactory soft-tissue healing, radiological evidence of reduction in apical lesion size (loose criteria) and radiological evidence of normal periodontal ligament space (strict criteria), need for further intervention, adverse effects (including exacerbation, tooth restoration integrity and allergic reaction), oral health-related quality of life (OHRQoL) and tooth mobility |
| Corbella et al. (2022) | Effectiveness of root resection techniques compared with root canal retreatment or apical surgery for the treatment of apical periodontitis and tooth survival: a systematic review | (P) General population with evidence of apical periodontitis (AP), code DA09.6 and DA09.7 according to the International Classification of Diseases (ICD 11) of the World Health Organization (2022). (I) Root resection techniques (root resection in general, crown resection with complete separation of root and crown and root amputation, namely the surgical removal of the root leaving the crown). (C) Nonsurgical root canal retreatment or apical surgery (including root-end preparation and filling). (O) The main outcome was a combination of clinician- and patient-reported outcomes measures |
| Plotino et al. (2022) | Effectiveness of intentional replantation in managing teeth with apical periodontitis: a systematic review | What is the effectiveness of intentional replantation (I) in comparison with nonsurgical root canal treatment/retreatment or apical surgery (C) in terms of clinical- and patient-related outcomes (O) in managing permanent teeth with AP (P)′ |
| WG4 | ||
| Meschi et al. (2022) | Effectiveness of revitalization in treating apical periodontitis: a systematic review and meta-analysis | (P) Patients with permanent immature or mature teeth and pulp necrosis with or without signs of AP. (I) Individuals undergoing revitalization (regenerative endodontic procedures) in teeth with pulp necrosis with or without signs of AP. (C) Individuals undergoing calcium hydroxide apexification, apical plug or root canal treatment in teeth with pulp necrosis with or without signs of AP. (O) Most critical: tooth survival. Critical: pain, tenderness, swelling, need for medication (analgesics and antibiotics), radiographic evidence of reduction of apical lesion size, radiographic evidence of normal periodontal ligament space, radiographic evidence of increased root thickness and length (not for mature teeth); additional: tooth function (fracture and restoration longevity), need for further intervention, adverse effects (including exacerbation, restoration integrity, allergy and discolouration), OHRQoL, presence of sinus tract and response to sensibility testing |
| Widbiller et al. (2022) | Effectiveness of endodontic tissue engineering in treatment of apical periodontitis: a systematic review | (P) Patients with permanent immature or mature teeth and pulp necrosis with or without signs of apical periodontitis. (I) Clinical approaches based on the introduction of scaffolds or biomaterials (natural or synthetic, allogenic or xenogenic and cell based or cell free) into the root canal to facilitate tissue formation. (C) Calcium hydroxide apexification, apical plug or root canal treatment. (O) The main critical outcome was tooth survival and further critical outcomes were pain, tenderness, swelling, need for medication (analgesics and antibiotics), radiographic evidence of reduction of apical lesion size and radiographic evidence of normal periodontal ligament space. Other important outcomes were tooth function (fracture and restoration longevity), the need for further intervention, adverse effects (including exacerbation, restoration integrity, allergy and discolouration), oral health-related quality of life (OHRQoL), presence of sinus tract and response to sensibility testing |
Diagnostic SRs
WG1: The diagnosis of pulpitis
Outcomes (all written as in protocol): Main outcome(s): A combination of outcome measures will be investigated for diagnostic accuracy with data used to calculate the pooled sensitivity, specificity, diagnostic odds ratio, positive predictive value (PPV) and negative predictive value (NPV) as probabilities for a correct test result and perhaps a receiver operating characteristic (ROC) analysis. For comparative studies and diagnostic nonrandomized and randomized clinical trials designed to combine diagnostic tests and therapeutic interventions, the outcomes of treatment will be primary measures. Additional outcome(s): (a) Pulp survival when teeth with caries are treated with any type of vital pulp treatment. (b) Relieve of pain after an operative procedure.
Time: For the review questions focusing on diagnostic accuracy, there is no time limitation. All other included comparative clinical trials must have a minimum of 1-year follow-up and a maximum of as long as possible.
Study type: Diagnostic accuracy studies examining the accuracy of the method in detecting pulp vitality, level of pulpal inflammation and pulpal condition with respect to whether it is possible to maintain pulpal vitality and cause of tooth pain in permanent teeth in humans. The study must have a gold standard reference, for example, histologic examination or pulpal examination (in vivo). Articles in which the primary objective was to evaluate the accuracy (sensitivity and specificity) of any type of diagnostic tool, radiological technique included, in humans will be selected. Diagnostic studies based on the ability to determine change in outcome or diagnostic decision-making are not the primary outcome but may be included (however, sensitivity/specificity will still be calculated wherever possible).
WG2: The diagnosis of apical periodontitis
Outcomes (as written in protocol): Main outcome(s): A combination of outcome measures will be investigated for diagnostic accuracy with data used to calculate the pooled sensitivity, specificity, diagnostic odds ratio, positive predictive value (PPV) and negative predictive value (NPV) as probabilities for a correct test result and perhaps a receiver operating characteristic (ROC) analysis. Additional outcome(s): For comparative studies and diagnostic nonrandomized and randomized clinical trials designed to combine diagnostic tests and therapeutic interventions, the outcomes of treatment will be primary measures and similar to those described for effectiveness of treatment.
Time: There is no defined duration for diagnostic accuracy and diagnostic thinking studies; however, comparative clinical trials will need to be followed up with a minimum time of 1 year and a maximum of as long as possible.
Study type: Diagnostic accuracy studies examining the accuracy of the method in detecting pulpitis/apical periodontitis (AP) on permanent teeth in humans. The study must have a gold standard reference, such as histologic examination for actual AP or pulpitis, pulpal examination (in vivo) or in situ visualization of bone defects (in vitro). Articles in which the primary objective was to evaluate the accuracy (sensitivity and specificity) of any type of diagnostic tool or radiographic technique in humans will be selected. Diagnostic studies may also be based on the ability to determine change in outcome, diagnostic decision-making or thinking and accuracy may not be the primary outcome (however, sensitivity/specificity can still be calculated). This will require other types of prospective comparative study design including before and after studies and trials.
Treatment SRs
WG1: The treatment of pulpitis
Outcomes: Main outcome(s): A combination of patient and clinician-reported outcome measures. The most critical outcome is ‘tooth survival’. Other critical outcomes are ‘pain, tenderness, swelling, need for medication (analgesics)’, ‘evidence of emerging apical radiolucency’ and ‘response to pulp sensibility test (not for full pulpotomy or pulpectomy)’. Additional outcome(s): Important outcomes are as follows: ‘tooth function (fracture, restoration longevity)’, ‘need for further intervention’, ‘adverse effects (including exacerbation, restoration integrity, allergy)’, ‘oral health-related quality of life (OHRQoL)’, ‘presence of sinus tract’ and ‘radiological evidence of continued root formation’.
Time: Defined a minimum of 1 year and maximum of as long as possible for all outcome measures, except ‘pain, tenderness, swelling, need for medication (analgesics)’, which is a minimum of 7 days and a maximum of 3 months, and OHRQoL, which is minimum of 6 months and a maximum of as long as possible.
Study type: Human clinical trials studies (randomized control trials, comparative clinical trials [CCTs]—nonrandomized, longitudinal observational studies [retrospective and prospective comparative cohort and case–control studies]). The number of patients needs to be at least 20 (10 in each arm) at the end of the study.
WG2: The treatment of apical periodontitis
Outcomes: Main outcome(s): A combination of patient- and clinician-reported outcome measures. The most critical outcome is ‘tooth survival’. Other critical outcomes are ‘pain, tenderness, swelling, need for medication (analgesics, antibiotics)’, ‘radiographic evidence of reduction of apical lesion size (loose criteria)’ and ‘radiographic evidence of normal periodontal ligament space (strict criteria)’. Additional outcome(s): Important outcomes are as follows: ‘tooth function (fracture, restoration longevity)’, ‘need for further intervention’, ‘adverse effects (including exacerbation, restoration integrity, allergy)’, ‘OHRQoL’ and ‘presence of sinus tract’.
Time: Defined a minimum of 1 year and a maximum of as long as possible for all outcome measures, except ‘pain, tenderness, swelling, need for medication (analgesics)’, which is a minimum of 7 days and a maximum of 3 months, and OHRQoL, which is minimum of 6 months and maximum of as long as possible.
Study type: Human clinical trials studies (randomized control trials, comparative clinical trials [CCTs]—nonrandomized, longitudinal observational studies [retrospective and prospective comparative cohort and case–control studies]). The number of patients needs to be at least 20 (10 in each arm) at the end of the study.
WG3: The surgical treatment of apical periodontitis
Outcomes: Main outcome(s): Most critical outcome ‘tooth survival’. Other critical outcomes: ‘pain, tenderness, swelling, need for medication (analgesics, antibiotics)’, ‘presence of sinus tract, satisfactory soft tissue healing’, ‘radiographic evidence of reduction of apical lesion size (loose criteria)’ and ‘radiographic evidence of normal periodontal ligament space (strict criteria)’. Additional outcome(s): Important outcomes are as follows: ‘need for further intervention’, ‘adverse effects (including exacerbation, restoration integrity, allergy)’, ‘OHRQoL’ and ‘mobility’.
Time: Defined a minimum of 1 year and a maximum of as long as possible for all outcome measures, except ‘pain, tenderness, swelling, need for medication (analgesics)’, which is a minimum of 7 days and a maximum of 3 months, and OHRQoL, which is minimum of 6 months and maximum of as long as possible.
Study type: Human clinical trials studies (randomized control trials, comparative clinical trials [CCTs]—nonrandomized, longitudinal observational studies [retrospective and prospective comparative cohort and case–control studies]). The number of patients need to be at least 20 (10 in each arm) at the end of the study.
WG4: The regenerative treatment of apical periodontitis
Outcomes: Main outcome(s): The most critical outcome is ‘tooth survival’, Other critical outcomes are ‘pain, tenderness, swelling, need for medication (analgesics, antibiotics)’, ‘radiographic evidence of reduction of apical lesion size (loose criteria)’, ‘radiographic evidence of normal periodontal ligament space (strict criteria)’ and ‘radiographic evidence of increased root thickness and length’. Additional outcome(s): Important outcomes are as follows: ‘tooth function (fracture, restoration longevity)’, ‘need for further intervention’, ‘adverse effects (including exacerbation, restoration integrity, allergy, discolouration)’, ‘OHRQoL’, ‘presence of sinus tract’ and ‘response to sensibility testing’.
Time: Defined as a minimum of 1 year and a maximum of as long as possible for all outcome measures, except ‘pain, tenderness, swelling, need for medication (analgesics)’, which is a minimum of 7 days and a maximum of 3 months, and OHRQoL, which is a minimum of 6 months and a maximum of as long as possible.
Study type: Human experimental studies (randomized control trials, comparative clinical trials [CCTs]– nonrandomized). Our search will be supplemented by longitudinal observational studies (retrospective and prospective comparative cohort and case–control studies) to ensure that all relevant clinical information that is often not tested in experimental studies is captured.
The number of patients is to be at least 20 (10 in each arm) at the end of the study.
Search strategy
All SRs utilized a comprehensive search strategy of a minimum of three different databases (mandatory from PubMed, Embase, Google Scholar, Scopus and Cochrane Library) dependent on database availability in reviewers' institutions. The electronic search period was from inception to current date and a grey literature search was mandatory. Furthermore, a hand search of (i) reference lists of included papers and previously published reviews and (ii) the last 20 years of International Endodontic Journal, Journal of Endodontics. In case of the interdisciplinary systematic reviews, the Journal of Clinical Periodontology, the Journal of Dental Research, the Journal of Dentistry and Clinical Oral Investigations were also included. The reviewers were given a designated time period to complete the review process, according to the CPG timetable (Table 5).
| Time point | Activity/Action |
|---|---|
| October 2020 | Decision by the Executive Board of the ESE to proceed with S3-level guideline development process for diagnosis and treatment of pulpal and apical disease |
| November to December 2020 | ESE assigns guideline lead and retains services of independent methodologist and co-lead from European Federation of Periodontology (EFP). Guideline lead divides topic into 4 WGs and nominates 2 senior members of the endodontic profession to act as WG chairs. Outline of timetable, topics to be covered and potential reviewers are made by guideline leads |
| December 2020 | WG leads and guideline leads form guideline steering group (GSG). First online meeting of GSG. Process, review topics finalized and potential reviewers discussed |
| January 2021 | Systematic review topics and reviewers agreed, ratified by ESE board and subsequently invited. Methodological online session provided to give overview of the process and agree standardized tools for assessing risk of bias and quality of evidence |
| 29 January 2021 | Online plenary session focussing on methodological aspects to give overview of the process and agree standardized tools for assessing risk of bias and quality of evidence |
| January to March 2021 | PICOTS prepared and submitted to GSG. Gaps assessed. GSG and reviewers completed conflict of interest (CoI) forms |
| April 2021 | Online GSG meeting |
| April to September 2021 | Relevant outcomes for each WG listed and ranked by consensus by GSG and SRs in a systematic online process. Time, study type and study size confirmed. After GSG assessment, one further SR added. PROSPERO protocol completed, checked by guideline leads and submitted by ESE guideline lead. SRs started |
| December 2021 to January 2022 | Representative stakeholder list compiled by guideline leads and invitations sent. Four patient representatives identified |
| January to April 2022 | Deadline for SRs. Process of internal peer review by GSG. Revision. Submission to International Endodontic Journal. Invitations resent to stakeholders who were unresponsive |
| April 2022 | Online GSG meeting |
| May to June 2022 | Online WG meetings to discuss potential conflicts, systematic reviews discussed and GRADE/recommendations introduced. SRs published online early in the International Endodontic Journal |
| June to November 2022 | Preparation of background text and provisional recommendations |
| November to December 2022 | Online WG meetings to assess and discuss progress |
| 29 January to 1 February 2023 | Face-to-face guideline summit in Lisbon, Portugal |
| February to June 2023 | Formal stakeholder consultation, finalization of guideline method report and background text |
| March 2023 | Online plenary meeting to finalize recommendations |
| August 2023 | Submission of guideline document to the International Endodontic Journal. Approval by ESE board |
| September to November 2023 | Publication of guideline and contributory systematic reviews in special edition of the International Endodontic Journal |
| Winter 2023 | Process of adaptation and adoption by National Societies |
The Language was restricted to studies published in English and excluded unpublished work. No more languages were considered as the review authors are not universally fluent in other languages, and the time for the preparation of this systematic review for a consensus workshop is limited. The search strategy will be performed as described by two independent reviewers with disagreement and doubts resolved by discussion with a third reviewer. Duplicates identified in the searches of the various databases were removed. Relevant and appropriate studies selected in the systematic review will be performed based on a three-step process: 1. Identification; 2. Screening; 3. Eligibility. The search was rerun before conducting the final analyses, and newly discovered eligible texts were included.
Quality assessment of included studies
Critical appraisal of the included studies was performed depending on the type of study (Table 6).
| Study design | Risk of bias tools |
|---|---|
| Randomized control trials |
RoB2 https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials |
| Controlled clinical trials (nonrandomized) |
ROBINS-I |
| Comparative cohort, case–control |
Newcastle Ottawa Scale for observational studies http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp |
| Diagnostic accuracy studies |
QUADAS-2 https://www.bristol.ac.uk/population-health-sciences/projects/quadas/quadas-2/ |
Risk of publication bias in cross-studies: Possible publication bias was assessed using Funnel plots and Egger's linear regression method for the primary outcome, if appropriate. Furthermore, if applicable, we will perform sensitivity analyses during meta-analysis.
Data synthesis
All data were analysed qualitatively and quantitatively and a narrative synthesis of the included studies will be performed. If the included studies were homogeneous in nature, a quantitative meta-analysis was considered. The forest plot will be calculated considering 95% of CI and p-values. Meta-regression and tests of sensitivity were also conducted to examine the effectiveness of each investigated parameter that contributes to the heterogeneity. The software used to perform a potential meta-analysis was determined by the review team based on previous experience and availability in respective review centres.
Evidence to recommendations: Structured consensus
The structured consensus development conference was held during the inaugural ESE S3-level treatment of pulpal and apical disease held in Lisbon, Portugal, on 29 January to 1 February 2023. Using the 14 SRs as background information, evidence-based recommendations were formally debated by the guideline panel (Table 2) using the format of a structured consensus development conference. This consisted of small group discussions and open plenary discussions, where the proposed recommendations were presented, voted upon and adopted by consensus (Murphy et al., 1998). Delegates declaring potential conflicts of interest abstained from voting and abstentions were recorded. Prior to the in-person meeting, up to 10 online meetings were organized (two at the plenary level and eight at the working group level) in May, June and November 2022, in order to advance the process of guideline development to a suitable stage prior to the face-to-face consensus meeting.
In the small group phase at the guidelines summit, delegates convened in four WGs directed by the two WG chairs belonging to the ESE GSG, addressing the following four subtopics: WG1—The treatment of pulpitis; WG2—The nonsurgical treatment of apical periodontitis; WG3—The surgical treatment of apical periodontitis; and WG4—The regenerative treatment of apical periodontitis. WG4 covered the treatment of immature and mature apex teeth and was reported in the guideline manuscript alongside WG2.
Plenary meeting 1 (One online session, January 2021)
Introduction to timetable and guideline methodology, including presentation from the methodologist (I.K.) and guideline leads (H.D. and M.K.).
Working group meetings 1 (Four online sessions, May and June 2022)
- Peer review of declarations of interest was discussed and managed (3.5.2)
- Summary of evidence from each systematic review by WG chairs and reviewers
- Consider relevance of PICOTS to practice and specific outcomes
- Introduce GRADE assessment (3.4.1) and recommendations (3.4.2)
- Discussion on the structure and standardization of the background text
Working group meetings 2 (Four online sessions, November and December 2022)
- GRADE assessment example for construction of recommendation prior to face-to-face plenary meeting
- Background text discussion
- Invitation to comment on draft recommendations and completed background text
- Collection and merging of amendments by group chairs
Plenary session 2 (In-person meeting, January/February 2023)
- Presentation of WG results including background text and draft recommendations to the guideline panel (Table 2) in a plenary session
- Invitation to suggest problems and reasonable amendments of the group by the independent guideline methodologist (I.K.)
- Preliminary vote and assessment of strength of consensus
- Recording of abstentions due to potential conflict of interest
- Moderated debate where no consensus was reached
- Further task delineation to individual working groups
Working group session 3 (In-person meeting, January/ February 2023)
- Discussion of tasks and formulation of guidelines led by WG leads
- Formulation of reasonable amendments to take back to the plenary
- Preliminary voting on recommendations and text in preparation for the plenary session.
Plenary session 3 (One online meeting, March 2023)
- Presentation pending expert-based general recommendations and overview flowcharts
- Suggestions received and discussed
- Strength of consensus assessed
- Voting
- Debate in cases of lack of consensus and alternative recommendations formulated
- Final vote with abstentions noted
- Local implementation discussed
Definitions and determining strength of evidence
For all evidenced-based recommendations and statements contained in parts 5 and 6, this guideline clearly highlights: (1) the ‘quality of evidence’ available to support each specific outcome, an evaluation that reflects the degree of certainty or uncertainty of the evidence as well as the robustness of the results; (2) the ‘grade of the recommendation’, reflecting the criteria considered to make the judgement; the strength of consensus and the percentage number of abstentions were due to potential conflicts of interest.
Quality of evidence
The quality of evidence was evaluated for every outcome in each systemic review and designated as being of ‘high’, ‘moderate’, ‘low’ or ‘very low’ quality according to GRADE (Balshem et al., 2011; Guyatt et al., 2008).
Strength of recommendations
- relevance of substantial nature of outcomes and quality of evidence for each outcome
- consistency of study results
- direct applicability of the evidence to the target population/PICOTS specifics
- precision of effect estimates using confidence intervals
- magnitude of the effects
- balance of benefit and harm
- ethical, legal and economic considerations
- patient preference
| Grade of recommendation | Syntax |
|---|---|
| Strong | We recommend to (⇑⇑) |
| We recommend not to (⇓⇓) | |
| Weak | We suggest to (⇑) |
| We suggest not to (⇓) | |
| Open | We do not know/may be considered (⇔) |
Strength of consensus
The consensus determination process followed the recommendations of the German Association of the Scientific Medical Societies (AWMF) & Standing Guidelines Commission (http://www.awmf.org/leitlinien/awmf-regelwerk.html). Where consensus could not be reached, different points of view were documented in the guideline text and the issue was voted on again after amendment (Table 8). Participants with an agreed conflict of interest, who were not permitted to vote, were excluded from the consensus calculations.
| Level of consensus | % Agreement |
|---|---|
| Strong consensus | Agreement of >95% of participants with voting rights |
| Consensus | Agreement of 75%–95% of participants with voting rights |
| Majority agreement | Agreement of >50%–75% of participants with voting rights |
| No majority agreement | Agreement of ≤50% participants with voting rights |
| Justified dissent | To be reported in the ESE S3 Guideline report |
- Note: Participants excluded from voting due to CoI are not included in the reported percentages.
Editorial independence
Funding of the guideline
The development of this guideline and its subsequent publication was financed entirely by internal funds of the European Society of Endodontology (ESE), without any support from industry, other organizations or stakeholders.
Declaration of interests and potential conflicts
All members of the guideline panel (Table 2) declared secondary interests potentially relevant to the guideline process using the standardized form provided by the International Committee of Medical Journal Editors (ICMJE) (International Committee of Medical Journal Editors, 2012).
Management of potential conflicts of interest (CoIs) was discussed in the online working group meetings as well as the plenary sessions, following the principles provided by the Guidelines International Network (Schünemann et al., 2015). According to these principles, panel members with relevant, potential CoIs abstained from voting on guideline statements and recommendations during the consensus process. Those percentage abstentions are recorded in each recommendation table in Section ‘Clinical Recommendations—Overall Strategy for the Management of Patients with Pulpal and Apical Disease: Evidence-Based Recommendations’. All CoIs are described in Appendix S1.
Peer review
All 14 SRs underwent several stages of peer review as previously described. The submitted draft documents were first evaluated by members of the GSG and the methodological consultant using the following appraisal tools: (i) the AMSTAR 2 checklist to check the methodological quality (Shea et al., 2017), and (ii) a bespoke checklist to verify that all PICOTS questions were addressed as described. Detailed feedback was then provided via the WG chairs to the SR authors. This process was completed up to three times until the GSG was content that the SR was ready for submission. Thereafter, all 14 systematic reviews entered into regular editorial peer review process in the International Endodontic Journal.
The recommendation section of the guideline text was drafted by the chairs of the working groups, in close cooperation with the senior reviewers, methodological consultant and guideline leads, and circulated amongst the members of the guideline group prior to the workshop. The methodological quality was formally assessed by an external consultant using the AGREE framework. The guideline was subsequently peer reviewed for its publication in the International Endodontic Journal following the standard evaluation process of the journal.
Dissemination and implementation
For this S3-level guideline, a multi-stage communication plan will be established and implemented by the ESE, supported specifically by the ESE Executive Board, Communications Committee and Benefits of Endodontics Committee. This will include: (1) the publication of the guidelines as an Open Access publication in the International Endodontic Journal alongside all 14 SRs. (2) A programme of Adoption and Adaptation (Schunemann et al., 2017) by 37 ESE national member societies including generation of educational material. (3) Dissemination of the findings in designated symposia sessions at the ESE biennial conference (Helsinki 2023 and 2025). (4) Dissemination of simple ‘bite-sized’ outputs from the guidelines through the ESE and member societies. (5) Dissemination of simplified versions (including lay terms and flowcharts) of the guidelines for the benefit of stakeholders and patients.
Validity and update process
The guideline is valid for 5 years until 2028. However, the ESE represented by the members of the GSG will continuously assess current developments in the field. Where there are significant and major changes in circumstances, for example, new comparative evidence, an update of the guideline will be undertaken to potentially amend the recommendations. It is planned to update the current guideline regularly and dynamically on demand and consistent with the format of a living guideline.
PULPAL AND APICAL DISEASE DIAGNOSIS AND TREATMENT SEQUENCE
Endodontic diagnosis
There have been recent calls to replace the current diagnostic terminology used in endodontics, particularly in relation to the nomenclature used to describe pulpal disease (AAE, 2021; ESE, 2019; Galicia & Peters, 2021; Rechenberg & Zehnder, 2020; Wolters et al., 2017); however, a new classification has not been agreed or adopted. In terms of pulpitis, the use of the terms reversible and irreversible pulpitis is common in the endodontic literature (AAE, 2013), so in order to reflect this without endorsing potentially outdated terminology, the terms spontaneous and nonspontaneous pain were used in WG1. In terms of apical periodontitis, the generally accepted term apical periodontitis was used throughout with minimal subdivision although it is recognized that acute and chronic forms of AP are relevant clinically.
Diagnostic pathways in relation to management
- Evaluation of the level of pulpal damage
- Prognosis and restorability of teeth
Establishment of tooth prognosis preoperatively or sometimes intraoperatively after investigation is critical prior to embarking on expensive and often time-consuming treatment. Individual tooth prognosis is frequently complicated by the need to assess the possibility of a lack of sufficient coronal tooth substance, a periodontally compromised tooth and also consider what function the tooth will serve in the future, for example, as an abutment for a fixed or a removable restoration. It is important that for each endodontic treatment, the outcome of the intervention is evaluated against recognized benchmarks (Duncan, Nagendrababu, et al., 2021a, 2021b).
Differential diagnosis
After the completion of the history, examination, clinical tests and analysis of radiographic images, a differential diagnosis should be established. This will likely include other dental conditions including marginal periodontitis, occlusal issues, cracked teeth as well as nonodontogenic conditions. It is accepted and should be made clear to the patient that in the case of pulpitis and apical periodontitis, it may not be possible to reach a definitive diagnosis preoperatively and a decision on the status of the tissues may have to be changed intraoperatively as further information comes to light after, for example, removal of carious dentine, pulp exposure or during surgery.
Treatment sequence
The treatment plan for managing pulpitis or apical periodontitis should start with a working diagnosis and outline the steps of treatment required to manage the disease. This should include the evidence supporting the decision choices as well as alternative or further options if the treatment fails. It should be evident that certain features, such as root maturity, medical history, age, physical/ cognitive well-being and dependency status as well as patient choice may influence the decision-making process and subsequent treatment sequence. It is essential that the patient is fully aware of the diagnosis, including the cause of the disease, risk factors and balanced treatment alternatives (with expected risks and benefits) including the option of no treatment. The option of no treatment must, however, be carefully conveyed so that the patient is aware of the risks of not treating the disease. This discussion should be followed by agreement on a personalized care plan. The patient should also be informed that the plan might need to be modified during the course of treatment, depending upon intraoperative findings, technical challenges and evolving patient preferences.
Specific treatment pathways according to the stage of root development: Immature apex
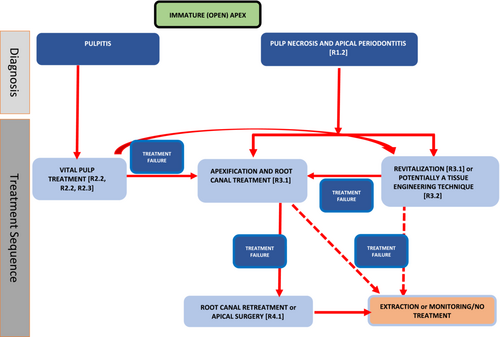
Common to all treatment pathways is the need to establish a working diagnosis through meticulous history, examination and special tests (see Section ‘Diagnostic pathways in relation to management’). If the carious lesion or restoration is not close to the pulp tissue clinically or radiographically, the carious tissue can be nonselectively removed and a well-placed sealing restoration applied. If the caries is deep, defined as ‘caries reaching the inner quarter of dentine, but with a zone of hard or firm dentine between the caries and the pulp, which is radiographically detectable when located on an interproximal or occlusal surface; there is a risk of pulp exposure during operative treatment’ (ESE, 2019), or extremely deep defined as ‘caries penetrating the entire thickness of the dentine, radiographically detectable when located on an inter-proximal or occlusal surface; pulp exposure is unavoidable during operative treatment’ (ESE, 2019), measures to avoid pulp exposure through stepwise excavation or selective caries removal can be employed. In cases of deep/extremely deep caries, pulp capping/pulpotomy procedures can be carried out if the pulp is exposed or there is spontaneous pain. Generally, with an open apex (Cvek, 1992; Stage I- IV), it is preferable to preserve the pulp in order to promote continued root development. If efforts to maintain the pulp are not successful, other techniques including apexification (with root canal treatment) or revitalization can be considered. Apical surgery remains a possibility for management of immature teeth with necrotic pulps, but only after root canal treatment has been carried out.
Specific treatment pathways according to the stage of root development: Mature apex
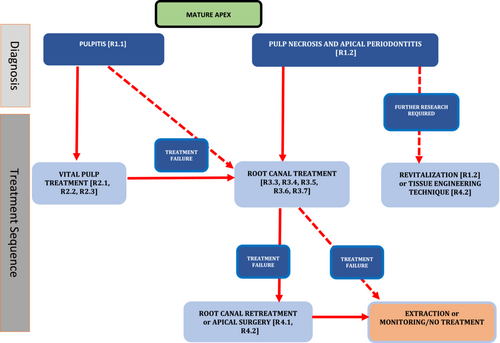
As with immature roots, if the caries or restoration is not close to the pulp tissue clinically or radiographically, the carious tissue can be removed and a well-placed sealing restoration applied. If the caries is deep, measures to avoid pulp exposure through stepwise excavation or selective caries removal should be employed if the symptoms do not indicate spontaneous pain (and potentially severe pulp damage). With extremely deep caries or when there is spontaneous pain, a pulpotomy procedure or root canal treatment may be carried out. If efforts to maintain the pulp are not successful, root canal treatment can be carried out. Apical surgery remains a possibility for management of mature teeth with necrotic pulps, potentially after failure of root canal retreatment, although a decision should be made on the need to retreat on an individual case-by-case basis. In selected circumstances, intentional replantation can also be considered as an alternative to traditional apical surgical, but again only after failure of root canal treatment.
CLINICAL RECOMMENDATIONS: OVERALL STRATEGY FOR THE MANAGEMENT OF PATIENTS WITH PULPAL AND APICAL DISEASE: EXPERT EVIDENCE-BASED RECOMMENDATIONS
The salient features of symptomatic pulpitis are pain and sensitivity related to a tooth, symptomatic (acute) apical periodontitis is associated with pain and swelling whilst asymptomatic (chronic) disease presents primarily with radiographic evidence of an apical radiolucency. These features arise as a result of a bacterial challenge to the pulp prior to tissue breakdown, infection in the root canal system and subsequent inflammation of the periapical tissues. These various disease forms can severely impact the patient's quality of life and put the offending tooth at risk of being lost if appropriate remedial treatment is not carried out. The competencies required for appropriate diagnosis and management of these diseases may be complex, whilst the evidence base supporting the different choices is frequently limited. In fundamental areas of uncertainty, the experts and stakeholders participating in the ESE S3-level consensus summit agreed on a series of expert-based recommendations that provide critical guidance for the management of endodontic disease in order to assist understanding of the general strategic principles for therapeutic management of patients with a compromised tooth.
Can pulpitis be successfully managed and the tooth preserved?
| Additional questions raised by the WG |
|---|
| Expert consensus-based recommendation 1 |
| For the management of restorable teeth with pulpitis, we recommend either vital pulp treatment or root canal treatment, appropriate restoration to function and supportive postoperative care rather than extraction |
| Supporting literature Expert opinion and ESE position statements (ESE, 2006, 2019) and S3 systematic reviews (Jakovljevic et al., 2022; Rossi-Fedele & Ng, 2022; Tomson et al., 2022) |
| Quality of evidence Expert-based evidence |
| Grade of recommendation Strong |
| Strength of consensus Strong (0% of the group abstained due to potential CoI) |
Can apical periodontitis be successfully managed and the tooth preserved?
| Additional questions raised by the WG |
|---|
| Expert consensus-based recommendation 2 |
| For the management of restorable teeth with apical periodontitis, we recommend root canal treatment, appropriate restoration and supportive postoperative care, rather than extraction |
| Supporting literature Expert opinion, ESE quality guidelines (2006) and S3 systematic reviews (Bürklein & Arias, 2022; Meire et al., 2022; Pirani & Camilleri, 2022; Rossi-Fedele & Rödig, 2022) |
| Quality of evidence Expert-based evidence |
| Grade of recommendation Strong |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
In the long-term management of pulpitis and apical periodontitis, retention of the natural dentition with adequate treatment, whenever possible, is advantageous as it defers the provision of prosthodontic replacement (fixed or removable) and shortens their required longevity. The option of tooth retention needs to be considered along with the alternatives and should be justified on a case-by-case basis, including the tooth's prognosis, technical challenges, patient preference and cost–benefit considerations.
Is endodontic treatment effective for the emergency management of symptomatic pulpitis or apical periodontitis?
| Additional questions raised by the WG |
|---|
| Expert consensus-based recommendation 3 |
| For the emergency management of symptomatic pulpitis or apical periodontitis in a restorable tooth, we recommend either vital pulp treatment or root canal treatment, rather than extraction or systemic antibiotic prescription |
| Supporting literature Expert opinion and based on contributory data from ESE position statement on antibiotics (Segura-Egea et al., 2018) and WHO document on antibiotic stewardship (2021) |
| Quality of evidence Expert-based evidence |
| Grade of recommendation Strong |
| Strength of consensus Consensus (0% of the group abstained due to CoI) |
Background
For the immediate treatment of symptomatic teeth presenting with pulpitis, symptomatic apical periodontitis or apical abscess, retention of the tooth should be considered as the standard care. Dental treatment either in the form of vital pulp treatment or pulpectomy is necessary to relieve discomfort rather than the prescription of antibiotics. Critically, systemic antibiotic use should only be adjunctive in conjunction with endodontic treatment in selected circumstances (see antibiotic position statement—Segura-Egea et al, 2018), which is in line with the 2021 world health organization (WHO) document on antibiotic stewardship (https://www.who.int/publications/i/item/9789240025530). The indications for adjunctive (not related to prophylaxis) antibiotics are (Segura-Egea et al., 2018); (1) Acute apical abscess in medically compromised patients; (2) Acute apical abscess with systemic involvement (localized fluctuant swellings, elevated body temperature >38°C, malaise, lymphadenopathy and trismus); (3) Progressive infections (rapid onset of severe infection in <24 h, cellulitis or a spreading infection, osteomyelitis) where onward referral to oral surgeons may be necessary; (4) Replantation of avulsed permanent teeth; and (5) Soft tissue trauma requiring treatment (e.g. sutures and debridement). The option of tooth retention needs to be considered along with the alternatives and should be justified on a case-by-case basis, including the tooth's prognosis, technical challenges, patient preference and cost–benefit considerations.
How important is the use of an aseptic technique and optimal surgical field for vital pulp treatment, nonsurgical root canal treatment and revitalization procedures?
| Additional questions raised by the WG |
|---|
| Expert consensus-based recommendation 4 |
| For the nonsurgical management of pulp exposure, pulpitis and apical periodontitis, we recommend the use of a meticulous aseptic technique and optimal surgical field, including the use of dental dam, good light and magnifying devices |
| Supporting literature Expert opinion, ESE quality guidelines, position statements (ESE, 2006, 2019) and based on contributory data that both test and control groups used dental dam and magnification (Bürklein & Arias, 2022; Pirani & Camilleri, 2022; Rossi-Fedele & Rödig, 2022) |
| Quality of evidence Expert-based |
| Grade of recommendation Strong |
| Strength of consensus Consensus (0% of the group abstained due to CoI) |
How important is the use of an aseptic technique and optimal surgical field for surgical endodontic treatment?
| Additional questions raised by the WG |
|---|
| Expert consensus-based recommendation 5 |
| For the surgical management of apical periodontitis, we recommend the use of a meticulous aseptic technique and optimal surgical field, including the use of good light and magnifying devices |
| Supporting literature Expert opinion and based on contributory data groups using magnification had improved outcomes (Setzer & Kratchman, 2022; Setzer et al., 2010, 2012) |
| Quality of evidence Expert based |
| Grade of recommendation Strong |
| Strength of consensus Strong consensus (0% of the group abstained due to CoI) |
Background
As the essential cause of pulpitis and apical periodontitis is microbial in nature (Kakehashi et al., 1965; Nair, 2004), strict adherence to asepsis should be observed including use of sterile burs, limiting waterline contamination and nonsurgical treatment procedures should only be carried out when the tooth is isolated by dental dam to prevent microbial contamination (ESE, 2006). Dental dam will have additional benefits with regards to preventing inhalation and ingestion of instruments and facilitating the use of strong disinfectants. Endodontics is a technically sensitive discipline, which is simplified by ensuring good light and vison when operating.
How important is further postgraduate training for advanced endodontic techniques?
| Additional questions raised by the WG |
|---|
| Expert consensus-based recommendation 6 |
| For advanced nonsurgical techniques including complex retreatment and for surgical management of apical periodontitis, we suggest further postgraduate training |
| Supporting literature Expert opinion and based on current ESE undergraduate curriculum guidelines (De Moor et al., 2013) and ESE postgraduate training programme accreditation guideline (ESE, 2010) |
| Quality of evidence Expert based |
| Grade of recommendation Weak |
| Strength of consensus Strong consensus (0% of the group abstained due to CoI) |
Background
The ESE mission is to promote the subject of endodontology to all dentists, not merely specialists; however, it is recognized that certain endodontic procedures are technically more difficult than others and as a result in order to carry out some procedures (e.g. complex retreatment on multi-rooted teeth or surgical procedures) further supervised training at the postgraduate level may be required. Many of these procedures are not within the remit of current undergraduate training and in order to carry them out predictably further supervised training is essential or the cases should be appropriately referred. It is noted within current guidelines that undergraduate students should gain the assigned level of competence in assessing endodontic treatment complexity with a view to deciding what they can or cannot carry out themselves (De Moor et al., 2013).
How long should the follow-up be after vital pulp treatment or nonsurgical or surgical treatment?
| Additional questions raised by the WG |
|---|
| Expert consensus-based recommendation 7 |
| After vital pulp treatment to manage pulpitis or nonsurgical or surgical treatment of apical periodontitis, we recommend that cases are monitored for a prolonged period with the review period extended if there is uncertainty about healing |
| Supporting literature Expert opinion, ESE quality guideline (ESE, 2006) and position statements (ESE, 2019) |
| Quality of evidence Expert based |
| Grade of recommendation Strong |
| Strength of consensus Strong consensus (0% of the group abstained due to CoI) |
Background
After vital pulp treatment procedures, assuming that the tooth has been adequately restored to function, the ESE previously recommended that continued pulpal health should be carefully monitored by history and clinical examination at 6 months, supplemented by periapical radiograph at 1 year; if symptoms persist or there is uncertainty regarding healing, the tooth should continue to be assessed at regular intervals (ESE, 2019). The benefit of a radiographic analysis in addition to clinical tests at 1-year follow-up is to assess for potential root resorption (Careddu & Duncan, 2021; ESE, 2006) or continued root development. Previously, the ESE (2006) has also recommended that nonsurgical and surgical treatment should be assessed at least after 1 year and subsequently as required to 4 years with the following findings indicating a favourable outcome: absence of pain, swelling and other symptoms, no sinus tract, no loss of function and radiological evidence of a normal periodontal ligament space around the root. A radiolucency which is obviously reducing on sequential radiographs may also indicate a favourable or healing outcome. If case of uncertainty about healing an extended period of review can be considered.
CLINICAL RECOMMENDATIONS: OVERALL STRATEGY FOR THE MANAGEMENT OF PATIENTS WITH PULPAL AND APICAL DISEASE: EVIDENCE-BASED RECOMMENDATIONS
Diagnosis
In order to manage pulpal and apical disease effectively, the ability of current diagnostic methods to accurately diagnose the presence or absence of disease as well as the level of disease is critical. Systematic reviews addressed the effectiveness of the diagnosis of pulpits (Donnermeyer et al., 2022) and apical periodontitis (Hilmi et al., 2023) respectively.
Effectiveness of diagnosing pulpitis (R1.1)
The study population (P) was patients suspected of having pulpitis with no pain, nonspontaneous pain or spontaneous pain. The diagnostic interventions (I) were clinical findings such as symptoms/signs, depth of caries lesion, pulp exposure, bleeding or any other method and evaluation of the presence of inflammatory mediators (biomarkers). The reference comparisons (C) were (i) pulp survival, when teeth with suspicion of pulpitis were treated with any type of vital pulp treatment; (ii) histological evaluation of the pulp tissue after extraction; and (iii) quantification of inflammatory mediators obtained from dentinal fluid or pulp tissue of teeth suspected of pulpitis in comparison of teeth with normal (healthy) pulp tissue; and the outcomes (O) were diagnostic accuracy (sensitivity and specificity) of pre- or intraoperative diagnosis of the level of pulp inflammation.
| PICOTS addressed by a SR | |
|---|---|
| R1.1 | Evidence-based recommendation |
| Grade of recommendation | In patients suspected of having pulpitis with no pain, nonspontaneous pain or spontaneous pain: |
| Weak (⇑) | We suggest cold testing possibly supplemented by electric pulp testing (EPT) to assess pulp vitality |
| Weak (⇑) | We suggest a combination of pain history (presence of pain, history of previous pain and occurrence of spontaneous pain) with clinical conditions (presence of pulp exposure, tenderness to percussion and pain on heat stimuli) to assess pulpal condition |
| Open (⇔) | We do not know whether biomarkers can predict the inflammatory status of the pulp |
| Quality of the evidence | Supporting literature (Donnermeyer et al., 2022) |
| Diagnostic accuracy of pulp vitality: Low ⊕⊕⊝⊝ | Diagnostic accuracy studies included: 12 studies on diagnostic accuracy of pulp vitality (n = 3035 teeth plus 16 controls; age of patients 6–99 years) |
| Diagnostic accuracy of pulpal conditions: Low ⊕⊕⊝⊝ | 10 studies on diagnostic accuracy of pulpal conditions (n = 1827 teeth; age of patients 13–75 years) |
| Diagnostic accuracy of biomarkers: Very low ⊕⊝⊝⊝ | 6 studies on expression of biomarkers (n = 191 teeth; age of patients 11–72) |
| Strength of consensus | Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
With respect to the assessment of diagnostic accuracy of pulp vitality assessment, cold and heat testing, electric pulp tester (EPT), pulse oximeter and percussion tests were investigated. The reference standards used were histology of pulp after tooth extraction (Dummer et al., 1980; Seltzer et al., 1963) or direct visual inspection of pulp tissue (Dastmalchi et al., 2012; Farid et al., 2015; Gopikrishna et al., 2007; Hazard et al., 2021; Jespersen et al., 2014; Kamburoglu & Paksoy, 2005; Petersson et al., 1999; Pigg et al., 2016; Villa-Chávez et al., 2013; Weisleder et al., 2009). With regards to diagnostic accuracy of the pulpal condition, thermal tests, presence and history of pain, spontaneous pain, pain at night (nocturnal), sensibility to percussion, pulp exposure by caries and expression of biomarkers were used as criteria. For assessment of biomarker expression, only studies that confirmed pulpal inflammation using classical histopathological criteria were included. For assessment of pulp condition, histology of the pulp tissue after extraction was used as the reference standard.
Available evidence
Twenty-eight diagnostic accuracy studies, 12 focusing on pulp vitality assessment (Dastmalchi et al., 2012; Dummer et al., 1980; Farid et al., 2015; Gopikrishna et al., 2007; Hazard et al., 2021; Jespersen et al., 2014; Kamburoglu & Paksoy, 2005; Petersson et al., 1999; Pigg et al., 2016; Seltzer et al., 1963; Villa-Chávez et al., 2013; Weisleder et al., 2009), 10 on diagnosis of the pulp condition (Barańska-Gachowska et al., 1969; Barańska-Gachowska & Waszkiewicz-Gołoś, 1969; Cisneros-Cabello & Segura-Egea, 2005; Dummer et al., 1980; Garfunkel et al., 1973; Hasler & Mitchell, 1970; Johnson et al., 1970; Ricucci et al., 2014; Seltzer et al., 1963; Tyldesley & Mumford, 1970) and 6 on expression of biomarkers (Abd-Elmeguid et al., 2013; Di Nardo Di Maio et al., 2004; Giuroiu et al., 2017; Petrini et al., 2012; Rauschenberger et al., 1997; Silva et al., 2009) were included in the review (Donnermeyer et al., 2022). Due to the considerable heterogeneity between the included studies and the fact that only one study provided 95% confidence intervals for all measures of validity (Pigg et al., 2016), and furthermore, due to the prevalence values of disease being highly heterogeneous, pooling predictive values from different studies was not possible. Therefore, calculation of effect sizes and confidence intervals was regarded as inappropriate.
None of the studies looked specifically at teeth without spontaneous pain. The studies examined the accuracy of the diagnostic methods including clinical findings such as symptoms, depth of caries lesion, pulp exposure, bleeding or presence of biomarkers in detecting the level of pulpal inflammation and pulpal condition with respect to if it is possible to maintain pulpal health in teeth with suspected pulpitis and no spontaneous pain. The comparator was a gold standard reference, such as (i) pulp survival, when teeth with suspicion of pulpitis were treated with any type of vital pulp treatment (Careddu & Duncan, 2021; Marques et al., 2015; Matsuo et al., 1996), (ii) histological evaluation of the pulp tissue after extraction and (iii) quantification of inflammatory mediators.
Risk of bias
All included studies regarding diagnostic accuracy of pulp vitality and accuracy of the pulp condition were considered to have a certain degree of bias. According to the QUADAS-2 tool for diagnostic accuracy studies and the Newcastle–Ottawa scale, the study quality was considered to be moderate for seven studies (Cisneros-Cabello & Segura-Egea, 2005; Gopikrishna et al., 2007; Hasler & Mitchell, 1970; Hazard et al., 2021; Pigg et al., 2016; Ricucci et al., 2014; Villa-Chávez et al., 2013) and unsatisfactory for all other studies included. For expression of biomarkers, most studies were considered as satisfactory according to the risk of bias assessment, whilst one study was rated as good (Abd-Elmeguid et al., 2013).
Consistency
Considerable heterogeneity between the included studies was obvious with regard to aspects related to patients (age, gender distribution and history of previous pain), assessors (blinded, two or more independent assessors and level of experience), type and clinical conditions of included teeth (caries, intrapulpal mineralization, condition of the pulp tissue, type and quality of coronal restorations) and prevalence of disease.
Clinical relevance and effect size
Effect sizes were not reported in any of the included studies. The diagnostic odds ratios (DOR) for cold used to determine pulp vitality were in the range 2.19–664.29. Regarding assessment of pulp conditions, DOR values were in the range 5–88 to 11.01 for ‘presence of pain’, 17.73 for ‘previous pain’, 31.41 for ‘spontaneous pain’, 24.62 for ‘pulp exposure’, 11.6 for ‘pain on heat’ and in the range 2.54–14.27 for ‘tenderness to percussion’.
Balance of benefits and harm
No serious adverse effects were reported, but the benefit of accurate diagnosis for provision of correct treatment and avoidance of overtreatment is obvious.
Ethical considerations
Not applicable.
Legal considerations
Not applicable.
Economic considerations
No cost-effectiveness outcomes were reported. Cost associated with use of combination of diagnostic test can be justified by the need to arrive at accurate diagnosis to facilitate provision of conservative less costly vital pulp treatments.
Patient preferences and values
No patients' preference/acceptability were reported for any of the diagnostic accuracy studies.
Applicability
Most tests are technically easy to use. Most of the studies were carried out in hospital or university settings (Efficacy) but few in general dental practice ‘real-world’ environments (Effectiveness). Although pulse oximetry was found to represent a reliable method to assess pulp vitality with an accuracy of 97.5% (Gopikrishna et al., 2007) and the fact that pulp necrosis was correctly identified in 93% to 100% of the teeth (sensitivity) and vital pulps in 95% to 100% of the teeth (specificity) (Dastmalchi et al., 2012; Gopikrishna et al., 2007), eligible commercially available devices for this specific test are currently not available. Therefore, at the moment, application of pulse oximetry would represent an off-label use and require modification of a probe for dental purposes.
Effectiveness of diagnosing apical periodontitis (R1.2)
The study population (P) was human patients and cadavers. The diagnostic interventions (I) were imaging techniques assessing the periapical tissues; the reference comparisons (C) were histology, microscopy or direct in situ visualization of the periapical tissues; and the outcome measure (O) was the diagnostic accuracy. Eligible studies must have a primary objective to evaluate the accuracy of a diagnostic imaging technique to detect signs of apical periodontitis and a reference standard.
| PICOTS addressed by a SR | |
|---|---|
| R1.2 | Evidence-based recommendation |
| Grade of recommendation | In patients suspected of having apical periodontitis |
| Strong (⇑⇑) | *We recommend periapical radiography be routinely used to diagnose apical periodontitis |
| Open (⇔) | **CBCT may be considered as an additional diagnostic measure in cases where there is doubt about the diagnosis. Presence of radiopaque materials in the root canal and periapex may affect the diagnostic accuracy of CBCT |
| Quality of the evidence | Supporting literature (Hilmi et al., 2023) |
| Diagnostic accuracy of periapical radiography: Low ⊕⊕⊝⊝ | Information from 5 studies (1 study providing duplicate information) |
| 2 studies based on roots (n = 53), (n = 86) | |
| 3 studies based on teeth (n = 217), (n = 39) and (n = 96) | |
| Diagnostic accuracy of CBCT: Moderate ⊕⊕⊕⊝ | CBCT |
| 2 studies based on roots (n = 86), (n = 335) | |
| Strength of consensus | *Strong consensus (4.4% of the group abstained due to potential CoI) |
| **Strong consensus (0% of the group abstained due to potential CoI) | |
- Recommendation marked* relates to consensus vote*. Recommendation marked** relates to consensus vote**.
Background
Intervention
Patient symptoms and clinical information alone cannot reliably be used to assess the presence or absence of apical periodontitis, as the disease is chronic in nature, often with minimal pain and discomfort for the patient. It, therefore, becomes important to study to what extent imaging techniques display histopathological changes associated with apical periodontitis in humans. Studies using human cadavers have been used to investigate this since taking adequate biopsies in vivo, including the entire periapical area, would be considered unethical. The present study included only diagnostic accuracy studies including healthy reference teeth as a comparison. The only two radiographic modalities providing such information were studies on periapical radiography and CBCT.
Available evidence
In total, six studies comparing radiographic imaging with the true state of the periapical tissues verified by histology were included (Barthel et al., 2004; Brynolf, 1967; Green et al., 1997; Kanagasingam, Hussaini, et al., 2017; Kanagasingam, Lim, et al., 2017; Kruse et al., 2019). Two of the included studies were performed on the same study sample (Kanagasingam, Hussaini, et al., 2017; Kanagasingam, Lim, et al., 2017).
Periapical radiography: Five studies were identified and included (Barthel et al., 2004; Brynolf, 1967; Green et al., 1997; Kanagasingam, Hussaini, et al., 2017; Kanagasingam, Lim, et al., 2017). Considerable heterogeneity was seen amongst the radiographic protocols, including both conventional and digital radiographs. Furthermore, the exposure settings, object tube distance and beam angulations varied. In two studies, additional radiographs of each study object were taken. In two studies, the study object was teeth; one of these included only single-rooted teeth. In two studies, the study object was root. The two studies performed on the same study sample included only roots that were not root filled. One study included only root filled roots (Barthel et al., 2004), and two studies included both root filled and non-root filled teeth (Brynolf, 1967; Green et al., 1997).
CBCT: Two studies were identified and included. In both studies, the study object was root. Heterogeneity was seen in relation to CBCT unit, radiographic settings and protocols and disease threshold. One study included only non-root filled teeth (Kanagasingam, Lim, et al., 2017), whereas the other study included both root filled and non-root filled roots (Kruse et al., 2019).
Risk of bias
Periapical radiography: Three of the studies were based on teeth, one of these was assessed to have a high risk of bias (n teeth = 39) and one study was assessed to have some concerns regarding risk of bias (n teeth = 217). Two studies were based on roots, and for these studies, some concerns were noted when assessing risk of bias (n roots = 139).
CBCT: Two studies were identified and included (n roots = 421). Both studies were assessed to have some concerns regarding risk of bias.
Consistency
Considerable heterogeneity between the studies was obvious, regarding the study object, radiographic protocol and threshold for disease. However, specificity for both periapical radiographic imaging and CBCT was high. Overall, sensitivity was lower compared with specificity. Sensitivity in relation to periapical radiographic imaging was lower compared with CBCT, except for CBCT on root filled roots. Two studies, based on the same study sample, reported considerably lower sensitivity in relation to periapical radiography compared with the other three studies reporting on that parameter.
Clinical relevance and effect size
Periapical radiography: Specificity, the ability to identify the healthy, was high in all the studies ranging from 0.86 to 1.00. Sensitivity, the ability to identify disease, varied amongst studies from 0.27 to 0.90 (Barthel et al., 2004; Brynolf, 1967; Green et al., 1997; Kanagasingam, Hussaini, et al., 2017; Kanagasingam, Lim, et al., 2017).
- For non-root filled roots, specificity was 0.80 and sensitivity was 0.90.
- For root filled roots, specificity was 0.83 and sensitivity was 0.79.
CBCT: Specificity, the ability to identify the healthy, ranged from 0.69 to 1.00. Sensitivity, the ability to identify the diseased, ranged from 0.63 to 0.89 (Kanagasingam, Lim, et al., 2017; Kruse et al., 2019).
- For non-root filled roots, specificity was 0.90 (95% CI [0.85–0.94]) and sensitivity was 0.95 (95% CI [0.84–0.99]).
- For root filled roots, specificity was 0.69 (95% CI [0.58–0.80]) and sensitivity was 0.63 (95% CI [0.46–0.77]) (Kruse et al., 2019).
The studies used different thresholds, and sensitivity and specificity may differ accordingly. A low level of demineralization may create more uncertainty in the diagnostic accuracy.
Balance of benefits and harm
Additional cost and radiation of CBCT may be justified in selected cases. However, in relation to root filled teeth, there may be a significant risk of overdiagnosis, which could result in overtreatment of the patient if not combined with information from the clinical examination and testing.
Ethical considerations
Additional costs and radiation of CBCT data acquisitions should be considered in light of potential benefits in selected cases.
Applicability
Knowledge of the diagnostic accuracy related to radiographic imaging techniques should be applied when diagnosing apical periodontitis in patients. However, radiographic images in accuracy studies on cadavers have been acquired under optimal conditions, with no patient movement and on selected roots/teeth. It should be recognized that these conditions may not directly apply to the clinical situation, and may affect the certainty of a diagnosis.
Treatment of pulpitis
In order to manage pulpal disease, the most appropriate treatment strategy for a given clinical scenario needs to be evaluated in comparative studies. Systematic reviews addressed the effectiveness of vital pulp treatment in managing pulpitis with no or nonspontaneous pain (Jakovljevic et al., 2022), spontaneous pain (Tomson et al., 2022) as well as the effectiveness of RCTx in managing teeth with vital and necrotic pulps (Rossi-Fedele & Ng, 2022).
Effectiveness of vital pulp treatment in managing nontraumatic pulpitis associated with no or nonspontaneous pain (R2.1)
Research question 1
In patients with nontraumatic pulpitis associated with no or nonspontaneous pain in immature and mature permanent teeth, is direct pulp capping or pulpotomy (partial/full) as effective as selective or stepwise caries removal, in terms of a combination of clinical outcomes (O), with ‘tooth survival’ as the most critical outcome?
| PICO addressed by a SR | |
|---|---|
| R2.1 | Evidence-based recommendation 1 |
| Grade of recommendation | |
| No studies included | In patients with nontraumatic pulpitis associated with no or nonspontaneous pain in immature and mature permanent teeth, we do not know whether direct pulp capping or pulpotomy (partial/full) is as effective as selective or stepwise caries removal regarding the long-term survival of the pulp or the tooth |
| Quality of the evidence | Supporting literature (Jakovljevic et al., 2022) |
| No studies identified or included | |
| Strength of consensus | Strong consensus (0% of the group abstained due to a potential CoI) |
| PICO addressed by SR |
|---|
| Expert consensus-based recommendation 2.1 |
| In patients with nontraumatic pulpitis associated with no or nonspontaneous pain in immature and mature permanent teeth, the use of either selective/stepwise caries removal without pulp exposure or after pulp exposure direct pulp capping or pulpotomy (partial/full) may be considered |
| Supporting literature Expert opinion, position statements (ESE, 2019) and published studies within the endodontic literature (Asgary et al., 2018; Bjørndal et al., 2010, 2017; Careddu & Duncan, 2021; Maltz et al., 2012; Marques et al., 2015) |
| Quality of evidence Expert-based evidence |
| Grade of recommendation Strong |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
| PICO addressed by SR |
|---|
| Expert-based recommendation 2 |
| In patients with nontraumatic pulpitis associated with no or nonspontaneous pain in immature and mature permanent teeth, if direct pulp capping or pulpotomy (partial/full) is performed, we suggest an enhanced protocol (i.e. dental dam, antimicrobial lavage, magnification and use of a hydraulic calcium silicate cement) |
| Supporting literature Expert opinion, position statements (ESE, 2019) and published studies within the endodontic literature (Ballal et al., 2022) |
| Quality of evidence Expert evidence |
| Grade of recommendation Strong |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
In teeth with caries in proximity to the pulp, different treatment options intended to preserve pulp tissue are available to the clinician. A conservative approach aims to avoid pulp exposure by retaining and sealing residual caries in proximity to the pulp and either restoring with immediate placement of a permanent restoration (selective caries removal) or with the placement of a temporary restoration and subsequent re-entry and permanent restoration (stepwise caries removal). Selective one-stage carious-tissue removal or stepwise excavation was recommended for the management of deep carious lesions to avoid pulp exposure when the tooth is asymptomatic or has signs and symptoms indicative of no worse than reversible pulpitis (ESE, 2019). In RCTs after performing selective/stepwise carious removal, 56 to 80% of teeth responded to sensibility tests and had no signs of emerging apical radiolucency after 1 to 3 years (Bjørndal et al., 2010; Maltz et al., 2012).
In contrast, direct pulp capping or pulpotomy (partial/full) after nonselective caries removal to ‘hard’ dentine aims to treat the inflamed/infected pulp tissue and capping with a suitable bioactive material. After direct pulp capping or pulpotomy (partial/full), 9% to 100% of teeth responded to sensibility tests and/or had no signs of emerging apical periodontitis after 1 to 5 years (Asgary et al., 2018; Bjørndal et al., 2017; Careddu & Duncan, 2021; Kundzina et al., 2017; Marques et al., 2015). Furthermore, an enhanced disinfection protocol has been recommended for these procedures (Ballal et al., 2022; ESE, 2019).
Available evidence
No studies with direct comparison were available.
Risk of bias
No studies were identified.
Consistency
N/A.
Clinical relevance and effect size
Maintaining pulp vitality is important for long-term survival of the tooth (Duncan, 2022). Therefore, treatment methods that create better conditions for the long-term preservation of a vital pulp are important and clinically relevant. This PICO highlights an important area for further research.
Balance of benefits and harm
The increased risk for pulp exposure associated with nonselective caries removal can be considered as a potential harm. However, this does not apply if vital pulp treatment following pulp exposure would achieve comparable or better results in terms of on-term survival of the pulp compared with selective excavation. The choice of treatment is hampered by the lack of objective measures of pulp inflammation and there are no available studies comparing these treatments and/or evaluating potential harms and benefits.
Ethical considerations
A direct comparison between selective caries removal and treatment after pulp exposure is challenging but high priority.
Accessibility, affordability and equity issues
Selective caries removal is a simple and affordable treatment that should be available in every setting. For teeth with pulp exposures due to caries, an enhanced protocol has been recommended regarding pulp capping or pulpotomy (partial/full), adding the use of aseptic conditions, magnification, disinfection of the exposed pulp and the use of suitable bioactive capping material. In general, vital pulp treatment is considered less technically demanding than root canal treatment.
Legal considerations
Not applicable.
Economic considerations
No cost-effectiveness analysis has been made based on a study directly comparing treatments. Vital pulp treatment following pulp exposure is anticipated to be more expensive than vital pulp treatment without pulp exposure but cheaper than root canal treatment.
Patient preferences and values
There is no evidence supporting one approach over the other but a preference for a less invasive and more affordable method would be likely.
Applicability
Data are lacking on treatment of older age groups. Furthermore, studies have mainly been performed in university settings.
Research question 2
In patients with nontraumatic pulpitis associated with no or nonspontaneous pain in immature and mature teeth (P), is pulpotomy (partial/full) (I) as effective as direct pulp capping (C), in terms of a combination of clinical outcomes (O), with ‘tooth survival’ as the most critical outcome?
| PICO addressed by a SR | |
|---|---|
| R2.1 | Evidence-based recommendation 2 |
| Grade of recommendation | In patients with nontraumatic pulpitis associated with no or nonspontaneous pain and pulp exposure in mature permanent teeth |
| Open (⇔) | |
| Either direct pulp capping or pulpotomy (partial/full) may be considered | |
| Quality of the evidence | Supporting literature (Jakovljevic et al., 2022) |
| Postoperative pain: Very low ⊕⊝⊝⊝ | 1 RCT (n = 218 patients) |
| Clinical and evidence of emerging radiolucency: Very low ⊕⊝⊝⊝ | 2 RCTs (n = 276 patients) |
| Other outcomes including survival not reported. | |
| Strength of consensus | Consensus (2.1% of the group abstained due to potential CoI) |
Background
Intervention
In teeth with deep caries in proximity to the pulp, the pulp may be exposed after removal of the carious tissue. For direct pulp capping, a biomaterial is directly applied onto the exposed pulp, whilst pulpotomy involves removal of a small portion (partial pulpotomy) or the complete removal of the coronal pulp tissue (full pulpotomy) after exposure, prior to application of the biomaterial and placement of a permanent restoration.
Available evidence
Two RCTs (Asgary et al., 2018; Bjørndal et al., 2010, 2017) with at least 12-month follow-up. One trial has published two reports at different time-points involving same cohorts (Bjørndal et al., 2010, 2017). Bjørndal et al. (2010) at 12 months follow-up reported 32.3% and 25.9% success for partial pulpotomy and direct pulp capping, respectively, with no difference between the groups (success was defined as pulp vitality without apical radiolucency). At 60 months, follow-up success decreases to 9.7% for partial pulpotomy and 3.7% for direct pulp capping (Bjørndal et al., 2017). On the other hand, Asgary et al. (2018) reported 40.8% success for partial pulpotomy, 56.5% for full pulpotomy and 61.6% for direct pulp capping at 12 months follow-up. The overall success rate from the study by Asgary et al. (2018) was a combination of clinical success (absence of signs/symptoms of inflammation/infection) and radiographic success. Postoperative pain was reported by Asgary et al. (2018) and no difference between the groups was noted.
Risk of bias
Low risk of bias for both RCTs (RoB 2). One study reported industry support, and one was supported by university funding.
Consistency
No meta-analysis could be performed due to the low number of studies and their heterogeneity in terms of methodology and reported success rates. Both included studies reported no difference between groups, although the effect size varied considerably between the two studies.
Clinical relevance and effect size
It is considered clinically relevant which treatment approach creates better conditions for the long-term preservation of a healthy pulp. The reported proportion of successful treatments varied greatly between the two included studies at 1 year; 62% (Asgary et al., 2018) and 26% (Bjørndal et al., 2010) for direct pulp capping. At 60 months, the proportion of successful treatments was less than 6% (Bjørndal et al., 2017). This variation brings some uncertainty but could be attributed to the fact that the comparison of partial pulpotomy and direct pulp capping was nested within a trial with another primary comparison in the Bjørndal study (Bjørndal et al., 2010).
Balance of benefits and harm
No data in included studies about harm directly related to procedures and no serious adverse effects were reported.
Ethical considerations
No obvious ethical issues.
Accessibility, affordability and equity issues
Direct pulp capping is considered a technically less demanding procedure compared with pulpotomy. However, for both procedures, an enhanced protocol including the use of aseptic conditions, magnification, disinfection of the exposed pulp and the use of suitable bioactive capping material has been recommended.
Legal considerations
No obvious ones.
Economic considerations
No cost-effectiveness analysis has been made based on a study directly comparing treatments. Initial costs would most likely be comparable for both treatment options, however, it is not possible to foresee whether there might be a higher cost for nonsuccessful cases of full pulpotomies as root canal treatment may be difficult to perform due to the formation of hard tissue at the canal orifices.
Patient preferences and values
There is no evidence supporting one approach over the other but a preference for a less invasive method would be more likely.
Applicability
There is a shortage of data for the treatment of older patient groups. Clinicians should be aware that the depth of the carious lesion and how the excavation is performed may affect the outcome of the treatments. Furthermore, studies have mainly been performed in university settings, which reduces the effectiveness and generalizability of the results.
Research question 3
In patients with nontraumatic pulpitis associated with no or nonspontaneous pain in mature permanent teeth (P), is pulpotomy (partial/full) (I) as effective as a pulpectomy (C), in terms of a combination of patient and clinical reported outcomes (O), with ‘tooth survival’ as the most critical outcome?
| PICO addressed by a SR | |
|---|---|
| R2.1 | Evidence-based recommendation 3 |
| Grade of recommendation | In patients with nontraumatic pulpitis associated with no or nonspontaneous pain and pulp exposure in mature permanent teeth |
| Open (⇔) | Either full pulpotomy or pulpectomy may be considered |
| We do not know whether partial pulpotomy is as effective as pulpectomy | |
| Quality of the evidence | Supporting literature (Jakovljevic et al., 2022) |
| Postoperative pain: Very low ⊕⊝⊝⊝ | 1 RCT (n = 54 patients) |
| Clinical and evidence of emerging radiolucency: Very low ⊕⊝⊝⊝ | 1 RCT (n = 54 patients) |
| Other outcomes including survival not reported. | |
| No studies comparing partial pulpotomy to pulpectomy were identified | |
| Strength of consensus | Consensus (2.1% of the group abstained due to potential CoI) |
Background
Intervention
In teeth with caries in proximity to the pulp, the pulp may be exposed during operative treatment. Pulpotomy involves removal of a small portion (partial pulpotomy) or the complete removal of the coronal pulp tissue (full pulpotomy). Pulpectomy is a treatment with total removal of the pulp from the root canal system followed by root canal treatment, prior to placement of a permanent restoration. In cases of pulpitis associated with no or nonspontaneous pain when the pulp is cariously exposed, clinicians often prefer to carry out pulpectomy in assumption that the pulp is contaminated with bacteria, however, vital pulp treatment offers a less invasive treatment option.
Available evidence
One RCT (Galani et al., 2017) included 54 patients with deep caries randomly allocated to full pulpotomy or pulpectomy. Success was defined as combined clinical success (lack of pain, swelling and sinus tract and presence of intact restoration) and radiographic success (radiographs displayed PAI 1 at end of follow-up). Overall success rate was 81.5% in the pulpotomy group (full pulpotomy) and 77.8% in the RCTx group (pulpectomy) at 18-month follow-up, with no significant difference between groups (p > .05). No studies are currently available comparing partial pulpotomy with pulpectomy.
Risk of bias
RCT (Galani et al., 2017) with low risk of bias (RoB 2) but condition of the pulp is not precisely described. No statement of funding.
Consistency
Only one study was included.
Clinical relevance and effect size
It is considered clinically highly relevant which treatment approach creates better conditions for the long-term survival of the tooth. No reported difference between groups; proportion of successful treatments was 82% for full pulpotomy and 78% for pulpectomy at 18 months.
Balance of benefits and harm
No serious adverse effects were reported. Vital pulp treatment is generally considered quicker, less technically complex and less invasive than pulpectomy.
Ethical considerations
None.
Economic considerations
Pulpotomy (partial or full) is less costly and quicker to perform compared with pulpectomy. There are no data on cost-effectiveness of pulpotomy versus root canal treatment.
Patient preferences and values
No data are reported, but a preference for a less invasive method would be more likely.
Applicability
Evidence provided by only one study conducted in a well-controlled research environment; therefore, generalizability to general dental practice settings is unclear. Data are lacking on treatment of older-age groups.
Effectiveness of pulpotomy compared with root canal treatment in managing nontraumatic pulpitis associated with spontaneous pain (R2.2)
Does pulpotomy (partial or full) (I) result in better patient and clinical reported outcomes (O) compared with root canal treatment (C) in permanent teeth with pulpitis characterized by spontaneous pain (P) evaluated at various time intervals (T)?
| PICO addressed by a SR | |
|---|---|
| R2.2 | Evidence-based recommendation |
| Grade of recommendation | For patients diagnosed with nontraumatic pulpitis associated with spontaneous pain in permanent teeth |
| Weak (⇑) | We suggest treatment with either root canal treatment or full pulpotomy |
| Quality of the evidence | Supporting literature (Tomson et al., 2022) |
| Postoperative pain: Low: ⊕⊕⊝⊝ | 2 RCTs with low risk of bias (n = 769 patients) |
| Radiographic healing 1 year after treatment: Low ⊕⊕⊝⊝ | 1 RCT with a high risk of bias (n = 407 [at start] patients) |
| Survival and other outcomes not reported | |
| Strength of consensus | Consensus (21.2% of the group abstained due to a potential COI) |
Background
Intervention
In cases of pulpitis associated with spontaneous pain, root canal treatment is considered by many clinicians to be the only choice of treatment to retain the tooth. Even though areas of bacterially infected or already necrotic tissue can be detected histologically beneath the carious lesion in the coronal pulp, this process does not affect the entire pulp tissue. Recent clinical studies have shown that high success rates may be achieved after partial or full pulpotomy. Partial pulpotomy is defined as removal of a small portion of coronal pulp tissue after exposure, followed by application of a biomaterial directly onto the remaining pulp tissue prior to placement of a permanent restoration. However, full pulpotomy is defined as complete removal of the coronal pulp and application of a biomaterial directly onto the pulp tissue at the level of the root canal orifice (s), prior to placement of the permanent restoration (ESE, 2019).
The studies included in the present SR performed one-visit root canal treatment with similar techniques. Instrumentation was performed with manual K-files, the working length was determined radiographically, whilst obturation was completed using cold lateral condensation (Asgary & Eghbal, 2010; Asgary et al., 2013, 2014, 2015). Eghbal et al. (2020) used rotary NiTi to prepare the canals, electronic apex locators/ radiographs to determine working length and obturation was carried out by cold lateral condensation.
In both studies, pulpotomy was performed with diamond burs in a high-speed handpiece. Haemorrhage control was achieved with saline in the Asgary et al.'s cohorts and with chlorhexidine (0.2%) and NaOCl (5.25%) in Eghbal et al.'s study. Calcium silicate cements were used in both studies, calcium-enriched mixture (CEM) was used as a pulp covering material in the Asgary et al's studies but Eghbal et al.'s study also included an MTA group. Permanent cavity filling was performed with dental amalgam 7 days after the pulpotomy in Asgary et al.'s cohort and a sandwich technique (glass–ionomer + light cured resin-bonded composite) was used in Eghbal et al.'s trial (Eghbal et al., 2020).
Available evidence
The systematic review (Tomson et al., 2022) included two RCTs (Eghbal et al., 2020; Asgary & Eghbal, 2010; Asgary et al., 2013, 2014, 2015). It is worth noting that one trial has published four reports at different time-points involving the same patient cohorts (Asgary et al., 2013, 2014, 2015; Asgary & Eghbal, 2010).
Postoperative pain meta-analysis revealed no difference in postoperative pain (day 7) between RCTx and pulpotomy (OR = 0.99, 95% CI 0.63–1.55, I2 = 0%). Clinical success was high at year 1, 98% for both interventions, however, decreased over time to 78.1% (pulpotomy) and 75.3% (RCTx) at 5 years.
Risk of bias
Two RCTs with low risk of bias were available to study the ‘pain’ outcome (Asgary & Eghbal, 2010; Eghbal et al., 2020), whereas an RCT with high risk of bias (Asgary et al., 2013, 2014, 2015) was available to study ‘clinical and radiographic outcome’.
Consistency
Regarding postoperative pain outcome, meta-analysis was performed using two studies. The forest plot showed that I2 statistic was 0%, which suggests that no heterogeneity was observed for the effect of two interventions (pulpotomy and root canal treatment). Regarding ‘clinical and radiographic’ outcomes, meta-analysis was not conducted because the studies have data from the same patient cohort published at different time-periods.
Clinical relevance and effect size
Considered as clinically relevant. No difference in postoperative pain (day 7) between RCTx and pulpotomy (OR = 0.99, 95% CI 0.63–1.55, I2 = 0%). Clinical success was high at year 1, 98% for both interventions, however, decreased over time to 8.1% (pulpotomy) and 75.3% (RCTx) at 5 years.
Balance of benefits and harm
Vital pulp treatment including pulpotomy is generally quicker, less technically complex and less invasive than root canal treatment. It also reduces the risk of unwanted effects such as fracture, or residual periapical inflammation (ESE, 2019). There is a perception that root canal sclerosis could occur after pulpotomy, however, its prevalence is unknown and the degree to which it could prevent subsequent treatment to treat pulp necrosis or apical periodontitis cannot be predicted until longer-term clinical trials are conducted (Duncan et al., 2022).
Ethical considerations
None.
Accessibility, affordability and equity issues
Pulpotomy would be considered easier to perform than root canal treatment and therefore it would be anticipated it would be more widely available than root canal treatment which requires more time, greater expertise and more instruments to perform.
Legal considerations
None.
Economic considerations
Root canal treatment has additional costs compared to pulpotomy, which may not appear to be justified by the added benefits (Asgary et al., 2014).
Patient preferences and values
No data are reported. From the patient's perspective, pain control is an important issue. However, since both interventions (pulpotomy and root canal treatment) are equally effective in reducing postoperative pain, pain control does not seem to be a meaningful, determining factor in the choice of the final treatment approach.
Applicability
All included studies have been published by the same research group, involving patients from one country. Therefore, the generalizability of the results needs to be supported in future high-quality RCTs from other geographical regions. Both studies used calcium silicate cements for the biomaterial following pulpotomy, however, CEM was used in the Asgary et al. study cohort and this material is not available commercially currently in Europe.
Effectiveness of root canal treatment for vital pulps compared with necrotic pulps in the presence or absence of signs of periradicular pathosis (R2.3)
Does root canal treatment of permanent teeth (P) with vital pulps (I) results in better patient- and clinician-reported outcomes (O), compared with teeth with pulp necrosis (nonvital) with or without radiographic signs of periradicular pathosis (C)?
| PICO addressed by a SR | |
|---|---|
| R2.3 | Evidence-based recommendation |
| Grade of recommendation | We suggest root canal treatment to be performed on teeth with nonvital pulps as soon as the diagnosis is confirmed |
| Weak (⇑) | 28 cohort studies were included |
| Quality of the evidence | Supporting literature (Rossi-Fedele & Ng, 2022) |
| Tooth survival: Moderate ⊕⊕⊕⊝ | 5 studies |
| Postoperative pain: Moderate to high ⊕⊕⊕⊝ | 7 studies |
| Radiographic healing 1 year after treatment: Moderate to high ⊕⊕⊕⊝ | 16 studies |
| Other outcomes not reported | |
| Strength of consensus | Strong consensus (23% of the group abstained due to a potential CoI) |
Background
Intervention
Root canal treatment is a ‘non-surgical’ approach used to treat two distinct endodontic disease entities: (1) ‘extirpation’ of vital, but ‘inflamed’ or ‘unsavable’ pulps, where the goal is to maintain existing periapical health and thus prevent periapical disease; this category also includes elective root canal treatment for prosthodontic reasons; or (2) the nonvital or dying, infected pulp, associated with radiographic signs of apical periodontitis. The goal of treatment for apical periodontitis is to restore the periradicular tissues to health. Additionally, the overall goal of root canal treatment is to ensure the survival and functionality of the teeth.
Available evidence
All 28 included studies were classified as cohort studies. Four of the five included studies, which reported on the outcome of tooth survival, showed pulp status is not a significant predictor (RR 1; 95% 1.00; n = 2 172 186 teeth). Presence of periapical radiolucency following RCTx in teeth with necrotic pulp was higher than teeth with vital pulp (RR 1.09; 95% CI 1.05, 1.13; n = 17 studies). In seven studies, no difference in postoperative pain in necrotic versus vital pulp was demonstrated. The quality of evidence for the outcome ‘tooth survival’ was considered to be moderate (one study of high RoB, one study of moderate RoB and one study of low RoB). The quality of evidence for the outcomes, ‘pain’ and ‘periapical health’, was considered to be moderate to high. The GRADE was dominated by the RoB and was not affected by heterogeneity; indirectness of evidence; imprecision; or publication bias.
Considerable heterogeneity amongst the included studies was obvious with regard to the periapical status of teeth with necrotic pulps, and criteria for determining ‘periapical health’ outcome. Statistical heterogeneity remained substantial after excluding data on teeth with necrotic pulp in the absence of radiographic signs of periradicular pathosis for analyses.
Risk of bias
Using the Risk of Bias 2.0. tool, one study (outcome ‘tooth survival’) was rated moderate risk of bias and two other studies (outcome ‘pain’ and ‘evidence of apical radiolucency’) were rated moderate-to-high risk of bias.
Consistency
The results from the different included studies are consistent.
Clinical relevance and effect size
Considered as clinically relevant. The CI was narrow in the meta-analysis (1.05, 1.13).
Balance of benefits and harm
Root canal treatment for teeth with vital and necrotic pulps is a predictable procedure when carried out to a high technical standard with no serious adverse effects reported by the reviewed studies. However, sodium hypochlorite accidents, extrusion of calcium hydroxide canal medicament or root filling material into the periapical tissues, maxillary sinus or inferior dental nerve canal have been reported to be associated with serious adverse effects (Alves et al., 2020; Gluskin et al., 2020; Guivarc'h et al., 2017; Yamaguchi et al., 2007).
Ethical considerations
The outcome data comparing root canal treatment on teeth with vital pulps versus necrotic pulps could only be obtained from observational studies because it is not ethically sound to electively devitalize and/or infect healthy teeth to generate randomized trial data.
Legal considerations
Pulp status should be confirmed to avoid overtreatment.
Economic considerations
The costs of root canal treatment on teeth with vital or necrotic pulps are comparable, although protocols may differ according to the pulp status. The setting will also influence costs and other economic considerations.
Patient preferences and values
No data are reported. It can be assumed that patients would rather not have root canal treatments in the absence of disease.
Applicability
In the majority of the included studies, the treatments were carried out in hospital or institution settings by undergraduate or postgraduate students under supervision and only some involved the primary care setting. Inference of the findings of the present systematic review can therefore not be necessarily drawn for the general dental practice setting, and in domiciliary setting which limits external validity. Furthermore, some earlier component studies used clinical techniques or materials not necessarily representative of contemporary practice.
Nonsurgical treatment of apical periodontitis
As described previously (Section ‘Treatment sequence’), the management of teeth with immature apices differs particularly in relation to the treatment of apical periodontitis as conventional root canal preparation and root canal filling may not be possible. For this reason, this section is divided into the management of apical periodontitis in immature teeth (Sections ‘Effectiveness of treatment of pulp necrosis with or without apical periodontitis in immature permanent teeth’ and ‘Effectiveness of endodontic tissue engineering in treatment of pulp necrosis with or without apical periodontitis in immature permanent teeth’) and mature teeth (Sections ‘Effectiveness of root canal instrumentation for the treatment of apical periodontitis in teeth with mature apices’, ‘Effectiveness of root canal irrigation and dressing for the treatment of apical periodontitis’, ‘Effectiveness of root canal filling materials and techniques for the treatment of apical periodontitis’ and ‘Effectiveness of adjunct therapy for treatment of apical periodontitis’). For the purposes of this guideline process, an immature root was defined according to Cvek's classification (1992) with stages I–IV considered immature (i.e., stage I [< half of root length], stage II [half], stage III [two-thirds of root length] and stage IV [nearly completed root length with wide open foramen]). Stage V is considered a mature completed root formation, with closed apex.
Effectiveness of treatment of pulp necrosis with or without apical periodontitis in immature permanent teeth (R3.1)
In patients with permanent immature teeth and pulp necrosis with or without signs of apical periodontitis (P), what is the effectiveness of revitalization (I) in comparison with calcium hydroxide apexification, apical plug and root canal treatment (C) in terms of tooth survival, pain, tenderness, swelling, need for medication (analgesics, antibiotics), radiographic evidence of reduction in apical lesion size, radiographic evidence of normal periodontal ligament space, radiographic evidence of increased root thickness and length (not for mature teeth), tooth function (fracture, restoration longevity), need for further intervention, adverse effects (including exacerbation, restoration integrity, allergy, discolouration), oral health-related quality of life (OHRQoL) and presence of sinus tract and response to sensibility testing (O)?
| PICO addressed by a SR | |
|---|---|
| R3.1 | Evidence-based recommendation |
| Grade of recommendation | In patients with immature permanent teeth with pulp necrosis with or without apical periodontitis |
| Open (⇔) | |
| The apical plug technique or revitalization procedures may be considered | |
| Quality of the evidence | Supporting literature (Meschi et al., 2022) |
| Survival: Low ⊕⊕⊝⊝ | 1 year after treatment: 1 RCT—Lin et al., 2017 (n = 103); and 1 NRCT—Silujjai & Linsuwanont, 2017 (n = 43) |
| Radiographic and clinical success: Low ⊕⊕⊝⊝ | 1 year after treatment: 1 RCT—Lin et al., 2017 (n = 103); and 1 NRCT—Silujjai & Linsuwanont, 2017 (n = 43) |
| Other outcomes not reported | |
| Strength of consensus | Consensus (0% of the group abstained due to a potential CoI) |
Background
Intervention
Immature permanent teeth with pulp necrosis with or without apical periodontitis pose particular technical challenges to the practitioner, as root morphology does not allow for conventional root canal treatment. Different treatment options are applied, which may be chosen, taking the stage of root development into account (Cvek, 1992). These include calcium hydroxide apexification, the apical plug technique and revitalization. Apexification refers to promoting the formation of a mineralized tissue barrier in teeth with an open apex and is considered suitable for Cvek stages II to IV. Calcium hydroxide has been used traditionally, where its repeated application led to the formation of an apical barrier which allowed for the subsequent root canal filling with Gutta–percha (Cvek, 1992; Kahler et al., 2014). After the introduction of hydraulic calcium silicate cements in endodontics, the apical plug technique was introduced as an alternative treatment (AAE, 2020). Revitalization as another treatment option was established more recently with benefits, particularly for Cvek stage I. The aim of the procedure is to create an environment to enable continued root formation. This may be achieved by triggering blood from the periapical area to clot in the root canal which can initiate the re-population of the pulp space with cells and subsequent formation of vital tissue (Wigler et al., 2013). Alternatively, mesenchymal stem cells could be transplanted inside the root canal (cell-based concept). The generally desired outcomes for revitalization are healing of periapical lesions, further root development and regaining of tooth sensitivity (AAE, 2018; Galler et al., 2016).
Available evidence
Two studies (Lin et al., 2017; Silujjai & Linsuwanont, 2017) addressed the PICO question, evaluating revitalization versus mineral trioxide aggregate (MTA) apical plug technique or calcium hydroxide apexification. Not all outcomes were addressed in the included studies. The most critical outcome was ‘survival’ and a combination of clinical and radiographical critical outcomes (absence of pain, tenderness, swelling, ‘radiographic evidence of reduction in apical lesion size’ and ‘radiographic evidence of increased root thickness and length’) was defined as ‘success’ in the current review. The survival and success rates seem to be high (76.5%–100%) during the first year after treatment and independent of the tooth and treatment type.
- Lin et al. (2017): root lengthening and thickening were significantly different and in favour of the revitalization group. All cases presented apical lesion size reduction.
- Silujjai and Linsuwanont (2017): root thickening was significantly different and in favour of the revitalization group, but not root lengthening. The apical lesion size reduction occurred in 80.77% of the apical plug group and 76.47% of the revitalization group.
The additional outcome of sensitivity testing was not assessed in studies on immature permanent teeth.
Risk of bias
Survival after 12 months: Lin et al., 2017, and Silujjai & Linsuwanont, 2017, are both highly biased.
Success after 12 months: Lin et al., 2017, and Silujjai & Linsuwanont, 2017, are both highly biased.
Consistency
The studies differed in terms of study design, evaluation period, subject characteristics, treatment protocol and assessment method.
Clinical relevance and effect size
Effect size: <400 events = few events and hence not enough power to obtain a reliable level of certainty.
Clinical relevance: Due to limited evidence, revitalization may be considered a treatment option for apical periodontitis in immature permanent teeth.
Balance of benefits and harm
Revitalization may be a last resort for retention of very immature teeth (stages of root formation CVEK 1–2). This procedure offers a potential for root maturation, but still preserves options for future treatment due to retrievability; in cases of failure, all options to retreat still remain. Compared to calcium hydroxide apexification, there is a reduced number of visits. The apical plug technique similarly offers a reduced number of visits compared to calcium hydroxide apexification. The most frequently reported adverse event after revitalization was tooth discolouration due to the use of bismuth oxide-containing materials or other reasons (e.g blood, antibiotics).
Ethical considerations
For immature permanent teeth, revitalization is an established but not well-documented procedure.
Applicability
All clinical trials were conducted in well-controlled research settings and included specifically selected populations with no systemic diseases.
Effectiveness of endodontic tissue engineering in treatment of pulp necrosis with or without apical periodontitis in immature permanent teeth (R3.2)
In patients with permanent immature teeth and pulp necrosis with or without signs of apical periodontitis (P), what is the effectiveness of approaches based on the introduction of scaffolds or biomaterials (natural or synthetic, allogenic or xenogenic, cell-based or cell-free, etc.) into the root canal to facilitate tissue formation (I) in comparison with calcium hydroxide apexification, apical plug and root canal treatment (C) in terms of tooth survival, pain, tenderness, swelling, need for medication (analgesics and antibiotics), radiographic evidence of reduction of apical lesion size, normal periodontal ligament space and increased root thickness and length, tooth function (fracture and restoration longevity), need for further intervention, adverse effects (including exacerbation, restoration integrity, allergy and discolouration), oral health-related quality of life (OHRQoL), presence of sinus tract and response to sensibility testing (O)?
| PICO addressed by a SR | |
|---|---|
| R3.2 | Evidence-based recommendation |
| Grade of recommendation | In patients with immature permanent teeth with pulp necrosis with or without apical periodontitis |
| Open (⇔) | |
| We do not know whether endodontic tissue engineering represents a valid treatment option. Further research is necessary to address this lack of evidence | |
| Quality of the evidence | Supporting literature (Widbiller et al., 2022) |
| Survival and radiographic evidence of healing: Moderate ⊕⊕⊕⊝ | 1 year after treatment: 1 RCT (n = 36) (Xuan et al., 2018) |
| Other outcomes not reported | |
| Strength of consensus | Strong consensus (0% of the group abstained due to a potential CoI) |
Background
Intervention
With the objective of achieving biological regeneration of the dental pulp and providing a predictable and reproducible clinical outcome, researchers have made great efforts in recent years to develop tissue engineering strategies for application in the root canal. In this context, endodontic tissue engineering (ETE) can be subdivided into basic cell-based (CB-ETE) and primarily cell-free procedures (CF-ETE) (Widbiller & Schmalz, 2021; Dohan Ehrenfest et al., 2014; Lin et al., 2021). In the CB-ETE, the cells used must be expanded ex vivo and introduced into the root canal by transplantation into prefabricated scaffolds with added growth factors. In contrast, CF-ETE uses endogenous sources of stem or progenitor cells and bypasses ex vivo cell manipulation. Here, primarily cell-free scaffold materials are introduced into the root canal together with signalling molecules, where they are supposed to attract cells of the periapical tissue. A special application CF-ETE are autologous platelet products such as platelet-rich fibrin (PRF), platelet-rich growth factor (PRGF) or platelet-rich plasma (PRP), which can also be introduced in an orthograde direction into the root canal (Dohan Ehrenfest et al., 2014). Here, a fibrin matrix encapsulates blood components and platelets as a source of signalling molecules and, according to the concept of CF-ETE, provides the opportunity for cells to populate the root canal and form tissue.
Available evidence
One RCT (Xuan et al., 2018) addressed the PICO question, evaluating only approaches of cell-based endodontic tissue engineering. Survival after 12 months was reported. Furthermore, the study reported on pulp sensitivity and blood perfusion, however, it is not in a technically comparable form. Parameters of root maturation were addressed; radiographic evidence of periapical healing was also shown.
Risk of bias
Survival after 12 months: Xuan et al. (2018) with moderate concerns in terms of RoB.
Consistency
Not applicable due to only one study.
Clinical relevance and effect size
Effect size: <400 events = few events and hence not enough power to obtain a reliable level of certainty.
Clinical relevance: In patients with immature permanent teeth with pulp necrosis with or without apical periodontitis, CB-ETE may potentially be a valid treatment option.
Balance of benefits and harm
The study did not report on potential harm or adverse effects.
Ethical considerations
The possible advantages of endodontic tissue engineering with controllable risk together with the option to use the alternative therapy (calcium hydroxide apexification) in case of failure justify the treatment indication from an ethical perspective.
Applicability
The clinical trial (Xuan et al., 2018) was conducted in a well-controlled research setting and included specifically selected populations and age groups. In the underlying literature, only CB-ETE approaches were undertaken, limiting applicability to specialized facilities with appropriate equipment, expertise and authorization to perform cell transplantation. Therefore, generalizability of the results to general dental practice is not possible at this time.
Effectiveness of root canal instrumentation for the treatment of apical periodontitis In teeth with mature apices. (R3.3)
Research question 1
In patients with apical periodontitis (P), what is the effectiveness of root canal instrumentation performed with contemporary techniques (I) in comparison with a ‘traditional’ (conventional stainless-steel instruments) technique (C) in terms of clinical and patient-related outcomes (O)?
| PICO addressed by a SR | |
|---|---|
| R3.3 | Evidence-based recommendation 1 |
| Grade of recommendation | In patients with apical periodontitis in permanent teeth |
| Weak (⇑) | |
| We suggest root canal preparation should be performed using contemporary engine-driven techniques with nickel–titanium (NiTi) root canal instruments | |
| Quality of the evidence | Supporting literature (Bürklein & Arias, 2022) |
| Survival, postoperative pain: Moderate ⊕⊕⊕⊝ | Survival: 1 cohort study (n = 289 patients) |
| Postoperative pain: 2 RCTs (n = 223 patients) | |
| Quality of life: Very low ⊕⊝⊝⊝ | Quality of life: 1 RCT (n = 87 patients) |
| Radiographic healing: Low ⊕⊕⊝⊝ | Radiographic healing 1 year after treatment: |
| 1 RCT (n = 87 patients) and 2 cohort studies (n = 245 patients) | |
| Strength of consensus | Consensus (18% of the group abstained due to potential CoI) |
Research question 2
In patients with apical periodontitis (P), what is the effectiveness of root canal instrumentation performed with contemporary engine-driven NiTi instruments (I) compared with other types of contemporary engine-driven NiTi instruments (with different design and/or technology) (C) in terms of clinical and patient-related outcomes (O)?
| PICO addressed by a SR | |
|---|---|
| R3.3 | Evidence-based recommendation 2 |
| Grade of recommendation | In patients with apical periodontitis in permanent teeth |
| Open (⇔) | |
| Any tested type of engine-driven NiTi instruments may be considered for root canal preparation | |
| Quality of the evidence | Supporting literature (Bürklein & Arias, 2022) |
| Postoperative pain: Low: ⊕⊕⊝⊝ | Postoperative pain: 3 RCTs (n = 272 patients) |
| Radiographic healing 1 year after treatment: Low ⊕⊕⊝⊝ | Radiographic healing 1 year after treatment: 1 RCT (n = 47 patients) |
| Survival and further outcomes not reported | |
| Strength of consensus | Strong consensus (21% of the group abstained due to a potential CoI) |
Background
Intervention
Treatment of patients diagnosed with apical periodontitis includes root canal preparation and chemo-mechanical debridement, which is accomplished with various types of instruments. Observable end-points of treatment are survival of teeth and postoperative pain (patient centred) and observable bony infill of apical radiolucent areas. Outcomes were compared between contemporary NiTi rotary instruments and classic techniques based on conventional stainless-steel hand instruments. Secondarily, differences in efficacy amongst various contemporary techniques were assessed.
Available evidence
The nine selected studies (Cheung & Liu, 2009; de Figueiredo, Lima, Lima, et al., 2020; Diniz-de-Figueiredo et al., 2020; Eyuboglu & Özcan, 2019; Fleming et al., 2010; Kandemir Demirci et al., 2021; Neves et al., 2020; Spili et al., 2005; Topçuoğlu & Topçuoğlu, 2017) included 1599 patients of which 1219 qualified for the systematic review. Three studies were retrospective studies (Cheung & Liu, 2009; Fleming et al., 2010; Spili et al., 2005) with overall good quality, whilst six of the studies were RCTs (de Figueiredo, Lima, Oliveira, et al., 2020; Diniz-de-Figueiredo et al., 2020; Eyuboglu & Özcan, 2019; Kandemir Demirci et al., 2021; Neves et al., 2020; Topçuoğlu & Topçuoğlu, 2017). Relative to the selected outcomes, five studies solely addressed the patient-centred outcomes survival (Fleming et al., 2010), quality of life (Diniz-de-Figueiredo et al., 2020) and postoperative pain (Eyuboglu & Özcan, 2019; Kandemir Demirci et al., 2021; Neves et al., 2020; Topçuoğlu & Topçuoğlu, 2017), three studies addressed periapical healing (Cheung & Liu, 2009; Spili et al., 2005), whilst one study included both outcomes (de Figueiredo, Lima, Lima, et al., 2020). A meta-analysis limited to three studies that addressed PICOT 1 and radiographic outcomes were performed (Cheung & Liu, 2009; de Figueiredo, Lima, Lima, et al., 2020; Spili et al., 2005).
The majority of studies (6/9) (Cheung & Liu, 2009; Diniz-de-Figueiredo et al., 2020; Fleming et al., 2010; Neves et al., 2020; Spili et al., 2005; de Figueiredo, Lima, Lima, et al., 2020) compared various forms of contemporary treatment in a variety of clinical scenarios with classic procedures defined as manual preparation with stainless-steel files. Two further studies (Eyuboglu & Özcan, 2019; Kandemir Demirci et al., 2021) provided outcome data comparing different forms of contemporary root canal preparation, such as rotary versus reciprocation or different types of contemporary instruments, whilst one study addressed both clinical questions.
Risk of bias
The retrospective studies (Cheung & Liu, 2009; Fleming et al., 2010; Spili et al., 2005) were judged as overall of good quality, whilst each of the three RCTs had a high risk of bias (de Figueiredo, Lima, Lima, et al., 2020; Diniz-de-Figueiredo et al., 2020; Eyuboglu & Özcan, 2019) or some concerns were noted (Kandemir Demirci et al., 2021; Neves et al., 2020; Topçuoğlu & Topçuoğlu, 2017). There was unclear publication bias in 3/6 reports for the RCTs (de Figueiredo, Lima, Lima, et al., 2020; Diniz-de-Figueiredo et al., 2020; Topçuoğlu & Topçuoğlu, 2017).
Consistency
Evidence was fragmented overall and only few data points were available for certain outcomes and comparisons, therefore the overall quality of evidence was considered moderate or weak. Patient-reported or -centred outcomes were inconsistently reported, however, adverse events, when reported, were rare. Study heterogeneity was high.
Clinical relevance and effect size
Survival: Current evidence suggested a benefit for contemporary over traditional root canal preparation techniques in terms of survival without post-treatment intervention (odds ratio = 0.13 [95% CI = 0.04–0.47]) from a retrospective study that included 289 patients (Fleming et al., 2010).
Oral health-related quality of life: Currently, there is weak evidence analysing OHIP-14 scores for a more favourable outcome in patients who received a root canal treatment using contemporary instrumentation with NiTi instruments (46 patients) compared with the classic group using stainless-steel hand instruments (42 patients) after 6 months. At 12-month follow-up, there were no differences between the contemporary and classic techniques, which included 42 and 45 patients respectively. Also, root canal filling techniques differed amongst groups (Diniz-de-Figueiredo et al., 2020).
Pain: Current evidence suggests that any type of mechanized root canal instrumentation with NiTi instruments may reduce postoperative pain after retreatment. No difference in pain score was seen amongst studies 1-week post-treatment, except for one study with high risk of bias (Topçuoğlu & Topçuoğlu, 2017).
Radiographic healing: Current evidence suggests a benefit for contemporary over traditional root canal preparation techniques. The results of the meta-analysis based on three studies (Cheung & Liu, 2009; de Figueiredo, Lima, Lima, et al., 2020; Spili et al., 2005) with 332 evaluable participants showed that contemporary instrumentation improved radiographic healing (p = .005) compared with traditional root canal preparation with stainless-steel instruments (odds ratio = 2.07 [95% CI = 1.25–3.44]) with no evidence of statistical heterogeneity (I2 = 0%).
Current evidence is weak when suggesting that the use of reciprocating instrumentation in root canal retreatment may be associated with a higher intensity of postoperative pain. This statement is based on an RCT that showed a significantly higher intensity of postoperative pain in retreatment (p = .001) performed with reciprocating instrumentation (33 patients) up to day 7 when compared with different rotary instruments (66 patients) (Eyuboglu & Özcan, 2019). Other RCTs reported no significant differences between multifile rotary versus reciprocating single file for primary endodontic treatment (103 patients) (Kandemir Demirci et al., 2021) or retreatment (70 patients) (Topçuoğlu & Topçuoğlu, 2017). The overall effect size of the instrument type selected appears small.
Balance of benefits and harm
The majority of the studies did not report on potential harm/adverse effects.
Ethical considerations
Additional cost of nickel–titanium root canal instruments may be justified; reprocessing issues should be considered.
Applicability
The majority of studies were conducted in well-controlled research environments and included specifically selected populations, that is, those with no systemic diseases. Moreover, different operator competence levels were evident. However, the majority of the studies were performed by endodontic specialists. This appears to reduce applicability or external validity to different provider competence levels. The evidence presented illustrates ‘efficacy’ rather than ‘effectiveness’; therefore, generalizability to general dental practice settings is unclear.
Effectiveness of root canal irrigation and dressing for the treatment of apical periodontitis (R3.4)
Research question 1
In patients with asymptomatic AP in permanent teeth (P), what is the effectiveness of instrumentation and irrigation performed with any root canal irrigant(s) and sequence (I) in comparison with instrumentation and irrigation with NaOCl and EDTA (C) in terms of clinical and patient-related outcomes (O).
| PICO addressed by a SR | |
|---|---|
| R3.4 | Evidence-based recommendation 1 |
| Grade of recommendation | In patients with asymptomatic apical periodontitis in permanent teeth for irrigation |
| Open (⇔) | |
| NaOCl (1%–5.25%) followed by EDTA, and NaOCl (1%–5.25%) may be considered | |
| Quality of the evidence | Supporting literature (Rossi-Fedele & Rödig, 2022) |
| Postoperative pain: Very low ⊕⊝⊝⊝ | 2 RCTs (n = 212 patients) |
| Radiographic healing: 1 year after treatment: Very low ⊕⊝⊝⊝ | Radiographic healing 1 year after treatment: 1 RCT (n = 86 patients) |
| Survival and other outcomes not reported | |
| Strength of consensus | Consensus (15% of the group abstained due to a potential COI) |
Background
Intervention
In association with chemo-mechanical root canal preparation with instruments, different types and/or concentrations of irrigant solutions have been suggested to improve outcomes for root canal treatment of teeth with an infected root canal system and apical periodontitis.
Available evidence
Two RCTs addressed the PICOTS question. One study involving 126 patients evaluated different types of irrigants: 5.25% sodium hypochlorite (NaOCl) and a mixture of 2% chlorhexidine gel and normal saline in association with 17% EDTA (Almeida et al., 2012). The other study, involving 86 patients evaluated the effect of different concentrations of NaOCl (1% and 5%) in association with 17% EDTA (Verma et al., 2019). Radiographic healing was reported in one study (Verma et al., 2019), whilst pain after 7 days was reported in two RCTs (Almeida et al., 2012; Verma et al., 2019). Meta-analysis was not performed.
Risk of bias
One RCT had low risk of bias, and one had some concerns.
Consistency
Study reporting was consistent with respect to pain. Only one study reported on radiographic healing.
Clinical relevance and effect size
Radiographic healing: One RCT assessed periapical healing at 1 year after root canal treatment using the Periapical Index. An overall healing rate of 76.7% (66/86) was reported. In the high-concentration (5% NaOCl) group, 81.4% healed, and in the low-concentration group, 72.1% healed, but the difference was not statistically significant (p > .05) (Verma et al., 2019).
Pain: Two RCTs assessed pain after 7 days, and in both studies, no patients reported severe pain at any postoperative time interval (Almeida et al., 2012; Verma et al., 2019). After 7 days, no patients (Verma et al., 2019), or only a few patients (Almeida et al., 2012), experienced even mild pain, with no significant difference between the groups. In one study, 20%–24% of patients required analgesics; however, there was no significant difference between groups (Verma et al., 2019). There is currently insufficient evidence to recommend any type and concentration of irrigant over another for the treatment of apical periodontitis or management of postoperative pain.
Survival and other outcomes were not reported in any included study.
Balance of benefits and harm
None of the included studies reported on potential harm/adverse effects.
Ethical considerations
As the use of inert irrigation solutions alone, such as water or saline, may be considered unethical, this could contribute to a relative scarcity of studies on irrigation solutions.
Applicability
The studies were conducted in well-controlled research environments and included specifically selected subpopulations, for example, with no systemic diseases (i.e. ‘no relevant comorbid conditions’ ‘immunocompromised or immunosuppressed’) (Almeida et al., 2012) or ‘diabetes, immunocompromised patients’ (Verma et al., 2019). Moreover, different operator competence levels were evident. This appears to reduce applicability to different provider competence levels. The evidence presented illustrates ‘efficacy’ rather than ‘effectiveness’; therefore, generalizability to general dental practice settings is unclear.
Research question 2
In patients with asymptomatic AP in permanent teeth (P), what is the effectiveness of intracanal dressing with any root canal dressing(s) or calcium hydroxide mixed with other vehicles, dressings or no dressing (I) in comparison with calcium hydroxide (mixed with glycol, glycerine, saline, distilled water or unmixed) (C) in terms of clinical and patient-related outcomes (O).
| PICO addressed by a SR | |
|---|---|
| R3.4 | Evidence-based recommendation 2 |
| Grade of recommendation | In patients with asymptomatic apical periodontitis in permanent teeth |
| Strong (⇑⇑) | |
| Where adequate clinical procedures have been performed, we recommend using a single-visit approach without the use of inter-appointment calcium hydroxide | |
| Quality of the evidence | Supporting literature (Rossi-Fedele & Rödig, 2022) |
| Radiographic healing: Moderate ⊕⊕⊕⊝ | 4 RCTs (n = 437 patients) |
| Radiographic healing 1–5 years after treatment | |
| Survival and other outcomes not reported | |
| Strength of consensus | Consensus (8.8% of the group abstained due to potential COI) |
Background
Intervention
Treatment of patients diagnosed with asymptomatic apical periodontitis may include, in addition to chemo-mechanical canal preparation, the use of various inter-appointment medicaments, with the aim of improving overall debridement and disinfection.
Available evidence
Four studies, all RCTs, reported on use of inter-appointment medication when treating asymptomatic apical periodontitis (Paredes-Vieyra & Jimenez-Enriquez, 2012; Peters & Wesselink, 2002; Waltimo et al., 2005; Weiger et al., 2000). Three studies compared single-visit treatment with multiple visits including inter-appointment medication in the form of calcium hydroxide, powder or mixed with water (Paredes-Vieyra & Jimenez-Enriquez, 2012; Peters & Wesselink, 2002; Weiger et al., 2000). All four studies reported on periapical healing, of which three used strict radiographic criteria and were sufficiently homogenous to be further synthesized via meta-analysis and showed a low statistical heterogeneity.
Risk of bias
One study was assessed to have low risk of bias, and three to have some concerns.
Consistency
Consistency was demonstrated across the studies, even though only one study individually showed a significant effect (Paredes-Vieyra & Jimenez-Enriquez, 2012).
Clinical relevance and effect size
It was demonstrated that there was a benefit for single-visit root canal treatment [without Ca(OH)2] compared with multiple-visit root canal treatment [with Ca(OH)2] in relation to radiographic healing using strict criteria (RR 1.10; 95% CI 1.03–1.19: p = .007).
Balance of benefits and harm
From a patient's point of view, there is a benefit of one visit compared to several visits in relation to discomfort, time and economy. The majority of the studies did not report on potential harm/adverse effects. The use of calcium hydroxide in case of extrusion may be associated with adverse effects based on case reports (Gluskin et al., 2020).
Ethical considerations
No relevant ethical considerations were identified.
Applicability
The majority of studies were conducted in well-controlled research environments and two included specifically selected populations that is those with no systemic diseases (i.e. ‘non-contributory medical history’ [Peters & Wesselink, 2002]; ‘patients in good health’ [Paredes-Vieyra & Jimenez-Enriquez, 2012]). The studies included only teeth with asymptomatic apical periodontitis, and findings therefore do not apply to treatment of acute apical periodontitis. Operator expertise was not disclosed, which reduces the applicability of the data to different provider competence levels. The evidence presented illustrates ‘efficacy’ rather than ‘effectiveness’; therefore, generalizability to general dental practice settings is unclear.
Effectiveness of root canal filling materials and techniques for the treatment of apical periodontitis (R3.5)
Research question 1
In patients with apical periodontitis (P), what is the effectiveness of chemo-mechanical preparation and root canal filling with any type of nonlateral compaction technique (I) in comparison with cold lateral compaction technique using Gutta–percha (C) in terms of clinical and patient-related outcomes (O)?
Research question 2
In patients with apical periodontitis (P), what is the effectiveness of chemo-mechanical preparation and root canal filling with any other type of sealer (I) in comparison with epoxy resin (AH Plus/AH 26) using Gutta–percha (C) in terms of clinical and patient-related outcomes (O)?
| PICOS addressed by a SR | |
|---|---|
| R3.5 | Evidence-based recommendations |
| Grade of recommendation | In patients with apical periodontitis in permanent teeth |
| Open (⇔) | *Root canal filling with Gutta–percha and sealer using any of the included techniques (cold lateral compaction, warm vertical compaction, carrier based or single cone) may be considered |
| Open (⇔) | **Root canal filling with Gutta–percha in combination with any of the included sealers (epoxy resin, ZOE or calcium silicate) may be considered |
| Quality of the evidence | Supporting literature (Pirani & Camilleri, 2022) |
| Survival, postoperative pain and quality of life: Low ⊕⊕⊝⊝ | Root canal filling techniques |
| Survival—1 prospective study (n = 58 teeth) | |
| Postoperative pain—3 RCTs (n = 409 teeth) | |
| Quality of life—1 RCT (n = 87 teeth) | |
| Radiographic healing 1 year after treatment: Low ⊕⊕⊝⊝ | Radiographic healing 1 year after treatment |
| 6 RCTs (n = 385 teeth) | |
| 1 retrospective study (n = 177 teeth) | |
| Postoperative pain: Low ⊕⊕⊝⊝ | Sealer types |
| Postoperative pain | |
| 1 RCT (n = 57 patients) | |
| Radiographic healing 1 year after treatment: Very low ⊕⊝⊝⊝ | Radiographic healing 1 year after treatment |
| 1 retrospective study (n = 177 teeth) | |
| Survival and other outcomes not reported for sealers | |
| Strength of consensus | *Strong consensus (12.5% of the group abstained due to potential COI) |
| **Strong consensus (12.1% of the group abstained due to potential COI) | |
- Recommendation marked* relates to consensus vote*. Recommendation marked** relates to consensus vote**.
Background
Intervention
After chemo-mechanical debridement of the root canal system, treatment of patients diagnosed with apical periodontitis includes root canal filling, accomplished with various materials and clinical techniques. Observable end-points of treatment are retention of teeth in function (survival) and postoperative pain as well as observable bone fill of apical radiolucent areas described based on semi-quantitative or qualitative measures. Outcomes were contrasted between lateral compaction of Gutta–percha with epoxy resin sealer and other clinical techniques. Secondarily, differences in efficiency amongst various sealers were assessed.
Available evidence
Ten studies were included. Nine prospective studies included 709 teeth (Chu et al., 2005; de Figueiredo, Lima, Lima, et al., 2020; de Figueiredo, Lima, Oliveira, et al., 2020; Diniz-de-Figueiredo et al., 2020; Graunaite et al., 2018; Kandemir Demirci & Çalışkan, 2016; Michanowicz et al., 1989; Özer & Aktener, 2009; Wong et al., 2015) and one retrospective study included 177 teeth (Aqrabawi, 2006). Eight studies were RCTs, including in total 651 teeth (de Figueiredo, Lima, Lima, et al., 2020; de Figueiredo, Lima, Oliveira, et al., 2020; Diniz-de-Figueiredo et al., 2020; Graunaite et al., 2018; Kandemir Demirci & Çalışkan, 2016; Michanowicz et al., 1989; Özer & Aktener, 2009; Wong et al., 2015).
Relative to the respective selected outcomes, seven studies addressed various patient-centred outcomes such as survival and postoperative pain, eight addressed a combination of periapical healing and clinical symptoms, whilst one study included both outcomes. The majority of studies (9/10) compared various root canal filling techniques to lateral compaction. Two further data sets provided outcome data comparing different types of sealers used for root canal filling.
Risk of bias
Nine studies were found to be at a high risk of bias, and one study had some concerns (Kandemir Demirci & Çalışkan, 2016). There was unclear publication bias in all reports.
Consistency
Evidence was overall fragmented and only few data points were available for some outcomes and comparisons, therefore the overall quality of evidence was considered moderate or weak. Patient-reported outcomes were inconsistently reported, however, adverse events, when reported, were rare. Study heterogeneity was high.
Clinical relevance and effect size
Survival: Current evidence suggested that tooth survival was similar in teeth treated using lateral compaction and a carrier-based system from a prospective study that included 58 teeth (Chu et al., 2005). Four teeth in the lateral compaction group and three teeth in the carrier-based group—a total of seven teeth—were extracted due to fracture before recall examination but it is not known whether these teeth had preoperative AP. All other studies did not report information on extracted teeth.
Radiographic healing: Current evidence suggested similar outcomes for nonlateral compaction techniques over cold lateral compaction techniques using Gutta–percha. The results of the systematic review based on seven studies with 385 evaluable teeth showed that any type of no lateral compaction technique did not improve radiographic healing compared to cold lateral compaction technique using Gutta–percha.
Current evidence did not suggest any consistent effect of varying sealer types on radiographic healing from a prospective study that included 177 teeth (Aqrabawi, 2006). Six of seven studies (n = 385) showed no significant difference (Chu et al., 2005; de Figueiredo, Lima, Lima, et al., 2020; Diniz-de-Figueiredo et al., 2020; Kandemir Demirci & Çalışkan, 2016; Michanowicz et al., 1989; Özer & Aktener, 2009).
Pain: Current evidence suggested that neither varying root canal filling techniques (from 3 prospective studies with 409 evaluable teeth; Diniz-de-Figueiredo et al., 2020; Kandemir Demirci & Çalışkan, 2016; Wong et al., 2015) nor the sealer type (from 1 RCT with 57 patients; Graunaite et al., 2018) resulted in different incidence of postoperative pain.
Sealers of various types, for example, epoxy resin, ZOE based or calcium silicate based, were tested and the overall effect size of sealer type on investigated outcomes appears to be limited.
Balance of benefits and harm
Nine studies did not report on potential harm/adverse effects. One study showed a higher risk of overfilling in relation to carrier-based technique but did not show any difference in outcome. Time required for root canal filling may be relevant for the patient.
Ethical considerations
Cost of materials should be considered.
Applicability
The majority of studies were conducted in well-controlled research environments and included specifically selected populations, that is, those with no systemic diseases. Moreover, different operator competence levels were evident. This appears to reduce applicability to different provider competence levels. The evidence presented illustrates ‘efficacy’ rather than ‘effectiveness’; therefore, generalizability to general dental practice settings is unclear.
Effectiveness of adjunct therapy for treatment of apical periodontitis (R3.6)
In patients with apical periodontitis in permanent teeth (P), what is the effectiveness of any intracanal procedure going beyond chemo-mechanical preparation with instruments and traditionally delivered irrigants (I) in comparison with chemo-mechanical preparation with instruments and traditionally (syringe-needle based) delivered irrigants (C) in terms of clinical and patient-related outcomes (O)?
| PICO addressed by a SR | |
|---|---|
| R3.6 | Evidence-based recommendation |
| Grade of recommendation | In patients with apical periodontitis in permanent teeth |
| Weak (⇓) | |
| We suggest not to use adjunct therapy in addition to traditionally (syringe-needle-based) delivered irrigants | |
| Quality of the evidence | Supporting literature (Meire et al., 2022) |
| Postoperative pain: Low ⊕⊕⊝⊝ | Postoperative pain: 7 RCTs (n = 636 patients) |
| Radiographic healing 1 year after treatment: Low ⊕⊕⊝⊝ | Radiographic healing 1 year after treatment: 6 RCT (n = 726 patients), 1 cohort (n = 46 patients) |
| Survival and other outcomes not reported | |
| Strength of consensus | Consensus (12.2% of the group abstained due to potential COI) |
Background
Intervention
After chemo-mechanical root canal preparation with instruments and traditionally delivered irrigants, different forms of adjunct therapy have been investigated to optimize root canal cleaning and disinfection. These include irrigant activation methods/devices, light-mediated root canal disinfection (photo-activated disinfection and direct laser irradiation) and ozone therapy. Adjunct therapy has been suggested to improve treatment outcomes after root canal treatment of teeth with an infected root canal system.
Available evidence
Fourteen studies (13 RCTs and 1 retrospective cohort) addressed the PICO question, evaluating different types of adjunct therapy: antimicrobial photodynamic therapy (aPDT) (3 RCTs: [Barciela et al., 2019a; Guimarães et al., 2021; Souza et al., 2021]), diode laser canal irradiation (2 RCTs: [Kaplan et al., 2021; Morsy et al., 2018]), 1 retrospective cohort: (Masilionyte & Gutknecht, 2018), Nd:YAG laser canal irradiation (2 RCTs: [Koba et al., 1999, Verma et al., 2020]), Er:Cr:YSGG laser canal irradiation (one RCT: [Martins et al., 2014]), ozone therapy (2 RCTs: [Kist et al., 2017; Pietrzycka & Pawlicka, 2011]) and ultrasonically activated irrigation (UAI) (4 RCTs: [Liang et al., 2013; Middha et al., 2017; Tang et al., 2015; Verma et al., 2020]).
Radiographic healing was reported in seven studies, but meta-analysis was only possible for two RCTs investigating the use of UAI (Liang et al., 2013; Verma et al., 2020) and two RCTs investigating ozone therapy (Kist et al., 2017; Pietrzycka & Pawlicka, 2011); none of them showed any difference between intervention and control. The other trials (Martins et al., 2014; Masilionyte & Gutknecht, 2018; Tang et al., 2015) did not find any significant difference either.
Pain after 7 days was reported in seven RCTs. Meta-analysis was performed on three studies that used aPDT (Barciela et al., 2019a; Guimarães et al., 2021; Souza et al., 2021), and on two studies using diode laser irradiation (Morsy et al., 2018; Kaplan et al., 2021). None of the meta-analytic estimates showed a difference between intervention and control. Middha et al. (2017) found no difference in pain after 7 days between UAI and control, whilst Tang et al. (2015) reported significantly lower pain levels in the UAI group.
Risk of bias
Radiographic healing: three RCTs had high risk of bias, two had some concerns and only one had low risk of bias.
Pain: four RCTs had high risk of bias, two had some concerns and one had low risk of bias.
Consistency
Studies differed in terms of type of adjunct therapy, practical details of applied adjunct therapy (such as laser wavelength, type of photosensitizer and irradiation/activation time), outcome determination (e.g. clinical vs. radiographic success, strict vs. loose radiographic criteria) and several possible combinations of these. Within the same adjunct therapy, consistency was demonstrated across the studies.
Clinical relevance and effect size
Radiographic healing: The lesion reduction after 12 months in the ultrasonically activated irrigation group was 12% higher than in the control group; this was, however, not statistically significant (RD = −0.12; 95% CI: −0.25–0.02, p = .09). Two studies investigating the use of ozone, also showing no statistically significant difference (RD = −0.04; 95% CI: −0.23–0.16, p = .72). One RCT investigating Er;Cr:YSGG laser showed no significant difference in healing after 12 months. One cohort study (940 nm diode laser) showed no significant difference in healing after longer observation times.
Pain: Meta-analysis on three studies that used aPDT, and on two studies using diode laser irradiation showed no significant difference in the prevalence of pain after 7 days between the control and adjunct therapy—(RD = −0.07; 95% CI: −0.27–0.13, p = .51) and (RD = −0.03; 95% CI: −0.11–0.06, p = .50) respectively. One individual RCT (UAI) showed no significant difference (p = .154). One cohort study (UAI) indicated more pain after 7 days in the control group (p < .05).
There is currently insufficient evidence to recommend any adjunctive therapy for the treatment of apical periodontitis or management of pain occurring after 7 days.
Balance of benefits and harm
None of the studies reported on potential harm/adverse effects.
Ethical considerations
Additional costs associated with adjunctive therapy may not be justified in the absence of benefit for the patient.
Applicability
Most studies were conducted in well-controlled research environments and included specifically selected populations that is those with no systemic diseases. Moreover, different operator competence levels were evident. This appears to reduce applicability to different provider competence levels. The evidence presented illustrates ‘efficacy’ rather than ‘effectiveness’; therefore, generalizability to general dental practice settings is unclear.
Effectiveness of revitalization for the treatment of pulp necrosis with or without apical periodontitis in mature permanent teeth (R3.7)
In patients with permanent mature teeth and pulp necrosis with or without signs of apical periodontitis (P), what is the effectiveness of revitalization (I) in comparison with calcium hydroxide apexification, apical plug and root canal treatment (C) in terms of tooth survival, pain, tenderness, swelling, need for medication (analgesics and antibiotics), radiographic evidence of reduction in apical lesion size, evidence of normal periodontal ligament space, tooth function (fracture and restoration longevity), need for further intervention, adverse effects (including exacerbation, restoration integrity, allergy and discolouration), oral health-related quality of life (OHRQoL), presence of sinus tract and response to sensibility testing (O)?
| PICO addressed by a SR | |
|---|---|
| R3.7 | Evidence-based recommendation |
| Grade of recommendation | In patients with mature permanent teeth with pulp necrosis with or without apical periodontitis |
| Weak (⇓) | We suggest not to use revitalization procedures |
| Quality of the evidence | Supporting literature (Meschi et al., 2022) |
| Survival and success after 1 year: Low ⊕⊕⊝⊝ | Success 1 year after treatment: Arslan et al., 2019 (n = 46) and Jha et al., 2019 (n = 30) |
| Other outcomes not reported | |
| Strength of consensus | Consensus (2.2% of the group abstained due to potential COI) |
Background
Intervention
The gold standard for treating mature permanent teeth with pulp necrosis with or without apical periodontitis is root canal treatment. Nevertheless, revitalization, a treatment established for immature permanent teeth, has been explored also for mature teeth more recently. The treatment may enable good bony healing and a biological root canal filling (Glynis et al., 2021).
Available evidence
Two RCTs (Arslan et al., 2019; Jha et al., 2019) addressed the PICO question, evaluating revitalization versus root canal treatment. Not all outcomes were addressed in all included studies. The most critical outcome was ‘survival’ and a combination of clinical and radiographical critical outcomes (pain, tenderness, swelling and ‘radiographic evidence of reduction in apical lesion’) was defined as ‘success’ in the current review. The survival and success rates reported in these two studies seem to be high (≥80%) during the first year after treatment independent of the tooth and treatment type.
The additional outcome ‘Sensitivity Testing’ was not assessed by Jha et al., 2019 (16), but in Arslan et al., 2019, it was positive in 50% of the revitalized teeth. Brizuela et al., 2020, were also included in this systematic review, assessing revitalization in mature permanent teeth. Nevertheless, this study was excluded from the current recommendation, as it is a Phase I/II study (assessing safety and efficacy) and was not performed to present the superiority of this treatment approach in mature teeth.
Risk of bias
Success after 12 months: Two RCTs, Arslan et al., 2019 (15) and Jha et al., 2019 (16), both highly biased.
Consistency
Studies differed in terms of study design, subject characteristics, treatment protocol, assessment method and study outcome.
Clinical relevance and effect size
- Effect size: <400 events = few events and hence not enough power to obtain a reliable level of certainty.
- Clinical relevance: Due to limited and low-quality evidence, revitalization in mature permanent teeth with pulp necrosis with or without apical periodontitis is not recommended.
Balance of benefits and harm
- Root canal treatment has a solid body of evidence.
- Revitalization: The most frequently reported adverse event was tooth discolouration due to bismuth oxide containing MTA.
Ethical considerations
Revitalization of mature permanent teeth seems to be experimental so far.
Applicability
All clinical trials were conducted in well-controlled research settings and included specifically selected populations with no systemic diseases.
Surgical treatment of apical periodontitis
Nonsurgical root canal treatment and retreatment versus apical surgery in treating apical periodontitis (R4.1)
In patients with apical periodontitis in permanent teeth (P), what is the effectiveness of apical surgery (I), as compared with nonsurgical root canal treatment or retreatment (C), in terms of clinical, radiological and patient-related outcomes (O)?
| PICO addressed by a SR | |
|---|---|
| R4.1 | Evidence-based recommendation |
| Grade of recommendation | The evidence does not suggest a difference in the effectiveness of apical surgery, as compared with nonsurgical root canal treatment or retreatment, in terms of clinical, radiological and patient-related outcomes, for managing permanent teeth with apical periodontitis |
| Open (⇔) | |
| When nonsurgical root canal treatment or retreatment is impractical, apical surgery may be considered for the management of permanent teeth with apical periodontitis | |
| Quality of the evidence | Supporting literature (Bucchi et al., 2022) |
| Tooth survival: Low ⊕⊕⊝⊝ | 1 RCT and 2 NRCTs (n = 229) |
| Need for further intervention: Low ⊕⊕⊝⊝ | 1 RCT, 1 NRCT and 1 retrospective cohort study (n = 357) |
| Radiographic healing 1 year after treatment: Low ⊕⊕⊝⊝ | 1 RCT, 2 NRCTs and 1 retrospective cohort study (n = 408) |
| OHRQoL (presence of symptoms): Low ⊕⊕⊝⊝ | 1 RCT (n = 37) |
| Other outcomes (pain, tenderness, swelling, presence of sinus tract, satisfactory soft tissue, radiological evidence of normal periodontal ligament space healing, adverse effects and tooth mobility) were not reported. | |
| Strength of consensus | Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Apical surgery consists of periapical curettage, root-end resection/apicectomy, root-end preparation and root-end filling for the management of teeth with apical periodontitis following nonsurgical root canal treatment and when nonsurgical root canal retreatment is impractical or offers a poorer prognosis. However, the studies identified did not employ comparable surgical techniques that meet the currently accepted clinical standards.
Available evidence
Five studies (2 RCTs, 2 NRCTs and 1 retrospective cohort) addressed the PICOT question, evaluating root canal treatment or retreatment versus apical surgery. These studies showed large heterogeneity in many aspects, from the study protocol to the materials and techniques used, as well as in the radiographic evaluation. The included studies did not mention clinical aspects such as the initial size of the periapical lesion, the quality of the previous root canal treatment and the coronal restoration, or the training of the operator.
Tooth survival was reported in three studies (1 RCT and 2 NRCTs), but meta-analysis was not possible (Estrela et al., 2014; Prati et al., 2018; Riis et al., 2018).
Radiographic healing was reported in four studies (1 RCT, 2 NRCTs and 1 retrospective cohort study), but meta-analysis was not possible (Danin et al., 1996; Liu et al., 2021; Prati et al., 2018; Riis et al., 2018).
Need for further intervention was reported in three studies (1 RCT, 1 NRCT and 1 retrospective cohort study), but meta-analysis was not possible (Danin et al., 1996; Estrela et al., 2008; Liu et al., 2021).
Presence of symptoms (issues related to OHRQoL) was reported in one study (1 RCT), but meta-analysis was not possible (Danin et al., 1996). Further controlled clinical trials comparing the clinical and patient-related outcomes of apical surgery, nonsurgical root canal treatment or retreatment are still needed.
Risk of bias
Tooth survival: Two studies with high risk of bias (NRCT) and one with moderate risk of bias (RCT).
Radiographic healing: All studies had high risk of bias (1 RCT, 2 NRCTs and 1 retrospective cohort study).
Need for further intervention: Two studies had high risk of bias (1 NRCT and 1 retrospective study) and one had moderate risk of bias (1 RCT).
Presence of symptoms (issues related to OHRQoL): The one study had high risk of bias (1 RCT).
Consistency
The consistency of the studies is uncertain since confidence intervals of effect estimates were not reported.
Clinical relevance and effect size
Due to heterogeneity, no meta-analysis was possible for any outcome.
Tooth survival: 84.04% of teeth in the experimental group versus 88.19% in the control group (No. of patients at follow-up = 229).
Need for further intervention: 7.1% of teeth in the experimental group versus 18.3% in the control group (No. of patients at follow-up = 357).
Radiographic healing (complete or partial) 1 year after treatment: 82.9% of teeth in the experimental group versus 71.7% in the control group (No. of patients at follow-up = 408).
Oral health-related quality of life (presence of symptoms): 5.3% of teeth in the experimental group versus 22.2% in the control group (No. of patients at follow-up = 37).
Balance of benefits and harm
The studies did not report on potential harm/adverse effects, so no conclusion can be drawn.
Ethical considerations
No ethical considerations were detected.
Applicability
All of the studies were conducted in university or academic dental clinical settings. The majority of the studies did not report on the clinicians' experience or the patients' general health status, so this reduces applicability.
Effectiveness of root resection techniques in the treatment of apical periodontitis (R4.2)
In patients with apical periodontitis in permanent teeth (P), what is the effectiveness of root resection techniques (root resection/crown resection/root amputation) (I) compared with nonsurgical root canal retreatment or apical surgery (C), in terms of clinical, radiological and patient-related outcomes (O) one-year post-treatment (T)?
| PICO addressed by a SR | |
|---|---|
| R4.2 | Evidence-based recommendation |
| Grade of recommendation | There is no comparative evidence of the effectiveness of root resection techniques versus nonsurgical root canal retreatment or apical surgery for managing permanent teeth with apical periodontitis |
| Weak (⇓) | |
| We do not suggest root resection techniques as an alternative to nonsurgical root canal retreatment or apical surgery in the management of permanent teeth with apical periodontitis | |
| Quality of the evidence | Supporting literature (Corbella et al., 2022) |
| Tooth survival: Very low ⊕⊝⊝⊝ | 3 retrospective noncomparative studies |
| Other outcomes not reported | |
| Strength of Consensus | Consensus (2.1% of the group abstained due to potential CoI) |
Background
Intervention
Root resection or root amputation techniques are defined as the complete removal of an entire root and of the surrounding adherent soft tissues, leaving the crown intact and supported by other remaining roots. Hemisection is the procedure where the root is resected and removed with the corresponding portion of the crown, as it can happen in maxillary and mandibular molars. The procedure was reported for the management of teeth with periodontal involvement of the furcation, or apical periodontitis involving a single root in a multi-rooted tooth, with the aim of tooth retention, when other treatments were not considered feasible or had failed.
Available evidence
The systematic review on the topic (Corbella et al., 2022) reported that no comparative randomized or nonrandomized trials were available for the addressed comparisons. Sparse data were extracted and considered from three papers on retrospective case series (Alassadi et al., 2020; Derks et al., 2018; El Sayed et al., 2020) that reported clinical outcomes after root resection was performed for endodontic reasons. The validity of the available results, which are just partly focussed on the objective of the review, is substantially limited by the presence of confounding factors; such as the extent of periodontal involvement, heterogeneity of the study protocols and insufficient information about the endodontic status of the samples. Clinical trials comparing the effectiveness of root resection techniques with nonsurgical root canal retreatment or apical surgery are required.
Risk of bias
The assessment of the risk of bias in the studies found that two papers were classified as low quality (Alassadi et al., 2020; Derks et al., 2018), and one study was classified as moderate quality (El Sayed et al., 2020). No funding bias was found in relation to these three studies.
Consistency
The consistency of the studies is uncertain since confidence intervals of effect estimates were not reported.
Clinical relevance and effect size
Tooth survival: The studies included in the review consisted of data from 305 resected teeth, from 254 patients, with a follow-up period of 1–16.8 years. Overall, 151 teeth were extracted during the follow-up period. In these studies, root resection treatment was carried out on 42 teeth exclusively for endodontic reasons.
Balance of benefits and harm
The studies did not report on potential harm/adverse effects. However, whilst it allows for the potential of tooth retention, root resection techniques are surgical procedures that weaken tooth structure, potentially risking tooth/root fracture and prosthodontic failure.
Ethical considerations
No ethical considerations were detected.
Applicability
The applicability of root resection technique should be weighted by considering the periodontal condition of the teeth (periodontal attachment loss and furcation involvement), which was not acknowledged in the studies included in the review by Corbella et al. (2022).
Effectiveness of intentional replantation in managing teeth with apical periodontitis (R4.3)
In patients with apical periodontitis in permanent teeth (P), what is the effectiveness of intentional replantation (I) compared with nonsurgical root canal treatment/retreatment or apical surgery (C) in terms of clinical and patient-related outcomes (O)?
| PICO addressed by a SR | |
|---|---|
| R4.3 | Evidence-based recommendation 1 |
| Grade of recommendation | There is no comparative evidence of the effectiveness of intentional replantation versus nonsurgical root canal treatment/retreatment or apical surgery for managing permanent teeth with apical periodontitis |
| Weak (⇓) | |
| We do not suggest intentional tooth replantation as a routine alternative to nonsurgical root canal treatment/retreatment or apical surgery for managing permanent teeth with apical periodontitis | |
| Quality of the evidence | Supporting literature (Plotino et al., 2022) |
| Empty review | No studies identified |
| Strength of consensus | Consensus (2.2% of the group abstained due to potential CoI) |
| PICO addressed by SR |
|---|
| Expert-based recommendation 1 |
| We do not know whether intentional replantation is as effective, compared with nonsurgical root canal treatment/retreatment or apical surgery, in terms of clinical and patient-related outcomes, in managing permanent teeth with apical periodontitis as there are no longitudinal studies comparing intentional replantation with any other forms of intervention |
| Noncomparative clinical studies reported high overall survival rates in the mid-to-long term, with relatively low complication rates. Therefore, in the absence of other treatment alternatives and rather than extraction, if anatomical conditions permit atraumatic extraction and an extraoral time of less than 15 minutes, then intentional replantation may be considered for the management of permanent teeth with apical periodontitis |
| Supporting literature Expert opinion, position statements (ESE, Krast, et al., 2021; ESE, Mannocci, et al., 2021) and published studies within the endodontic literature (Cho et al., 2016; Choi et al., 2014; Jang et al., 2016; Wu & Chen, 2021) |
| Quality of evidence Expert-based evidence |
| Grade of recommendation Strong |
| Strength of consensus Malassada (0% of the group abstained due to potential CoI) |
Background
Intervention
Intentional replantation entails intentional atraumatic tooth extraction, extra-alveolar evaluation of the root surfaces and endodontic management, followed by re-insertion of the tooth into its original position in the tooth socket. If needed, intentional replantation can also be combined with surgical extrusion, the repositioning of the tooth more coronally than its original position. Intentional replantation is a treatment option for permanent teeth with apical periodontitis that have not responded favourably following nonsurgical root canal treatment/retreatment or apical surgery.
Available evidence
None of the studies fulfilled the selection criteria. Therefore, we do not know the effectiveness of intentional replantation when compared with nonsurgical root canal treatment/retreatment or apical surgery in terms of clinical and patient-related outcomes in managing permanent teeth with apical periodontitis. Clinical trials comparing the effectiveness of intentional replantation with nonsurgical root canal treatment/retreatment or apical surgery are required. Despite being excluded, four noncomparative clinical studies (Cho et al., 2016; Choi et al., 2014; Jang et al., 2016; Wu & Chen, 2021) reported high overall survival rates in the mid-to-long term, with relatively low complication rates.
Risk of bias
None of the studies fulfilled the selection criteria. Hence, the risk of bias analysis was not performed.
Consistency
None of the studies fulfilled the selection criteria. Hence, the consistency cannot be assessed.
Clinical relevance and effect size
No effect size could be determined as no studies were included.
Balance of benefits and harm
There are certain risks associated with intentional replantation, such as tooth fracture during extraction, periodontal breakdown or possible future root resorption (mainly replacement root resorption or ankylosis), that are not objectively controllable. If anatomical conditions permit atraumatic extraction and an extraoral time of less than 15 minutes, then intentional replantation can be considered for the management of apical periodontitis, thereby preserving the tooth.
Ethical considerations
No ethical considerations were detected.
Economic considerations
Intentional replantation can be an alternative to tooth extraction and implant placement, thus potentially reducing the comparative costs for patients.
Patient preferences and values
In the absence of high-quality evidence from comparative clinical trials (CCTs), clinical decision-making should be on a case-by-case basis and in accordance with the clinician's experience and the patient's preference.
Applicability
Clinical studies showed that intentional replantation may be a treatment modality to manage problems of endodontic origin.
AUTHOR CONTRIBUTIONS
H.F.D. (planned guidelines, organized working groups, chaired online and in-person summit and wrote and edited manuscript); L.-L.K., O.A.P., I.E.-K., G.K., M.D.F., B.S.C., K.M.G. and J.J.S.-E. (chaired working groups, attended online and chaired working group sessions at in-person summit, wrote recommendations and edited manuscript); M.K. (planned, organized working groups, co-chaired sessions online and at in-person summit and edited manuscript); ESE workshop participants (engaged in working group activity, wrote recommendations and attended online and in-person summit); I.K.—methodological consultant (offered independent methodological advice and training, co-chaired online sessions and chaired consensus sessions at in-person summit).
ACKNOWLEDGEMENTS
The authors express their gratitude to all reviewers involved in the preparation of the systematic reviews and sincerely thank those organizations that participated in the guideline development process: Association for Dental Education in Europe (ADEE), Council of European Chief Dental Officers (CECDO), Council of European Dentists, European Dental Hygienist Federation (EDHF), European Dental Students Association (EDSA), European Association for Osseointegration, European Association of Dentomaxillofacial Radiology (EADMFR), European College of Gerodontology (ECG), European Federation of Conservative Dentistry, European Federation of Periodontology (EFP), European Organization for Caries Research (ORCA), European Prosthodontic Association, International Association of Dental Traumatology (IADT) and Pan-European Region—International Association for Dental Research (PER-IADR) as well as four patient representatives (Amanda Jackson, Cathy Dillon, Massimo Gruffanti and Thomas Schratzenstaller). Open access funding provided by IReL.
FUNDING INFORMATION
This study was funded by the European Society of Endodontology (ESE).
CONFLICT OF INTEREST STATEMENT
Individual potential conflict of interest forms were completed by all participants and are available on file at the European Society of Endodontology and in the Supporting Information, available online (Appendix S1). Potential conflicts of interest, in the previous 36 months, reported by the chairs of the workshop (in alphabetic order) will be listed here. Bun San Chong (Chair: Working Group) reports—Grants or contracts from any entity: None; Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events: National University of Singapore (External examiner), Universiti Kebangsaan Malaysia (External examiner), Universiti Malaya Malaysia (External examiner), University of Birmingham UK (External examiner), University of Dundee UK (External examiner), University of Otago New Zealand (External examiner), Royal College of Surgeons of Edinburgh Scotland (Examiner), PanEndo Egypt, Quintessence Symposium Berlin (Speaker fees); Participation on a Data Safety Monitoring Board or Advisory Board: None; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: International Endodontic Journal (IEJ—Editorial Board), University of Thessaloniki Greece (External Evaluator Appointments & Promotions), Universiti Kebangsaan Malaysia (External Advisor Promotions and Titles), International Federation of Endodontic Association 2024 (Organizing Committee); American Association of Endodontists, British Dental Association, British Endodontic Society (BES) and Royal College of Surgeons of England and Royal College of Surgeons of Edinburgh (All society membership); Patents, copyrights and retail licences: None. Massimo Del Fabbro (Chair: Working Group) reports—Grants or contracts from any entity: IRCCS Orthopaedic Institute Galeazzi (Grant); Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events: Dental Tech Srl Misinto Italy; Zimmer Biomet/Zimmer Day (Speaker fees); Participation on a Data Safety Monitoring Board or Advisory Board: None; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: Clinical Implant Dentistry and Related Research (Editorial Board); Academy of Non-Transfusional HEmo-Components (ANTHEC) (President) and Italian Academy of Endodontics (AIE) (Honorary member); Patents, copyrights and retail licences: None. Henry F. Duncan (Chair: Steering Group) reports—Grants or contracts from any entity: Fulbright—Health Research Board Ireland (Grant); Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events: American Association of Endodontists (AAE), ESE, BES, Irish Dental Association, Belgian Association of Endodontics and traumatology (BAET) (All speaker fees); Participation on a Data Safety Monitoring Board or Advisory Board: None; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: ESE (President-Elect), IEJ (Editor-in-Chief), Frontiers in Dental Medicine (Associate Editor), Journal of Dental Research (Editorial Board) and Pulp Biology and Regeneration Group IADR (President-Elect); Patents, copyrights and retail licences: None. Ikhlas El-Karim (Chair: Working Group) reports—Grants or contracts from any entity: Public Health Agency, Northern Ireland; National Institute for Health Research, BES, Versthus Arthritis (All grants); Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events: ESE (Speaker fees); Participation on a Data Safety Monitoring Board or Advisory Board: None; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: IEJ (Associate Editor), Frontiers in Dental Medicine (Associate Editor) and Pulp Biology and Regeneration Group IADR (President-Elect); Patents, copyrights and retail licences: None. Kerstin M. Galler (Chair: Working Group) reports—Grants or contracts from any entity: Norwegian Research Council, Septodont Company (All grants); Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events: German Association of Endodontology and Dental Traumatology (DGET), German Association of Conservative Dentistry (DGEZ), ESE meetings (Speakers fees and society expenses); Participation on a Data Safety Monitoring Board or Advisory Board: None; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: IEJ (Editorial board), Journal of Dental Research (Editorial board); ESE (Executive board), DGZ, DGET and IADR (Committees/membership); Patents, copyrights and retail licences: None. Moritz Kebschull (Chair: Steering Group) reports—Grants or contracts from any entity: Bredent, EMS, Genolytic, NIHR UK, Unilever (All Grants); Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events: Camlog, Curaden, Dexcel, EMS, Geistlich, IAI, GSK, Hu-Friedy, NSK, Procter, Unilever (Speaker fees); Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: European Federation of Periodontology (Executive Committee, Workshop Committee), British Society of Periodontology (Council member) and Guideline Office (DG PARO). Patents, copyrights and retail licences: Periodontal diagnostics patent with Genolytic. Lise-Lotte Kirkevang (Chair: Working Group) reports—Grants or contracts from any entity: Danish Dental Association, Dentsply-Sirona, Acteon, Sendoline, VDW, FKG, KerrDental, Dentaire produits, Nordenta and Zeiss (All grants); Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events: Danish Dental Association, ESE (Speaker fees); Participation on a Data Safety Monitoring Board or Advisory Board: None; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: ESE (Executive Board), Danish Dental Association (Postgraduate committee), Danish Endodontic Society (Member), International Association of Dentomaxillofacial Radiology (Member) and Aktuel Nordisk Odontologi and IEJ (Editorial board); Patents, copyrights and retail licences: None. Gabriel Krastl (Chair: Working Group) reports—Grants or contracts from any entity: None; Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events: German Association of Operative Dentistry (DGZ), DGET (Speaker fees); Participation on a Data Safety Monitoring Board or Advisory Board: None; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: IEJ, Dental Traumatology, Journal of Adhesive Dentistry, Endodontie and Das Deutsch Zahnärzteblatt (All editorial board); Patents, copyrights and retail licences: None. Ove A. Peters (Chair: Working Group) reports—Grants or contracts from any entity: Dentsply Sirona, MicroMega, Coltene, ReDent Nova, VDW, Sonendo, Gbr. Martin (All grants); Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events: VDW, Dentsply Sirona, ESE (All speaker fees); Participation on a Data Safety Monitoring Board or Advisory Board: Advisory Board Sonendo (Advisory board); Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: AAE, Australian Endodontic Society; Australian and New Zealand Academy of Endodontists; ESE, IADR; DG Endo/DGET (All member), IEJ (Associate Editor) and Australian Endodontic Journal (Associate Editor); Patents, copyrights and retail licences: Gbr. Martin—Instruments for nonsurgical and surgical endodontics. Juan J. Segura Egea (Chair: Working Group) reports—Grants or contracts from any entity: Programa financiador—Plan Estatal 2017–2020 Generación Conocimiento—Proyectos I + D + I Entidad financiadora: Ministerio de Ciencia e Innovación. Spain (All grants); Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events: ESE, Opinion-leader meeting Dentsply Sirona (Speaker fees); Participation on a Data Safety Monitoring Board or Advisory Board: None; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: IEJ and Journal of Clinical and Experimental Dentistry (Editorial Board), Board of Directors of the Asociación Española de Endodoncia (AEDE) (Board member) and Academia Española de Ciencias Odontológicas (Vice President); Patents, copyrights and retail licences: Machine for testing Endodontic Instruments.
APPENDIX 1
ESE Workshop Participants: Marta Adam, Ana Arias, Carsten Appel, Kathrin Becker, Sema Belli, Cecilia Bourguignon, Christos Boutsioukis, Sebastian Bürklein, Charlotte Carter, Antonis Chaniotis, Stefano Corbella, Valerie Chevalier, Elisabetta Cotti, Till Dammaschke, Roeland De Moor, Paul Dummer, Ken Eaton, Fernando Durán-Sindreu, Vittorio Franco, Helena Fransson, Gianluca Gambarini, Antonio Ginjeira, Aleksandar Jakovljevic, Amanda Jackson, Anastasia Kossioni, Casper Kruse, Maarten Meire, Paulo Melo, Nastaran Meschi, Giampiero Rossi-Fedele, Chiara Pirani, Barry Quinn, Tina Rödig, Eyal Rosen, Edgar Schäfer, Hagay Shemesh, Christian Spleith, Jale Tanalp, Igor Tsesis, Leo Tjäderhane, Phil Tomson, Nicola West, John Whitworth. Methodological Consultant: Ina Kopp. Workshop Organization: European Society of Endodontology (ESE). Scientific societies involved in the guideline development process: Association for Dental Education in Europe (ADEE), European Association for Osseointegration (EAO), European Association of DentoMaxilloFacial Radiology (EADMFR), European College of Gerodontology (ECG), European Federation of Conservative Dentistry, European Federation of Periodontology (EFP), European Organization for Caries Research (ORCA), European Prosthodontic Association (EPA), International Association of Dental Traumatology (IADT), Pan-European Region—International Association for Dental Research (PER-IADR). Other organizations involved in the guideline development process: Council of European Chief Dental Officers (CECDO), Council of European Dentists (CED), European Dental Hygienist Federation (EDHF), European Dental Students Association (EDSA).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.