Intronic variation at the CHD1-Z gene in Black-tailed Godwits Limosa limosa limosa: correlations with fitness components revisited
Abstract
Recently, Schroeder et al. (2010, Ibis 152: 368–377) suggested that intronic variation in the CHD1-Z gene of Black-tailed Godwits breeding in southwest Friesland, The Netherlands, correlated with fitness components. Here we re-examine this surprising result using an expanded dataset (2088 birds sampled from 2004 to 2010 vs. 284 birds from 2004 to 2007). We find that the presence of the Z* allele (9% of the birds) is not associated with breeding habitat type, egg size, adult survival, adult body mass or adult body condition. The results presented here, when used in synergy with the previously reported results by Schroeder et al., suggest that there might be a tendency towards female adults with the Z* allele laying earlier clutches than adult females without the Z* allele. The occurrence of the Z* allele was also associated with a higher chick body mass and return rate. Chicks with the Z* allele that had hatched early in the breeding season were heavier at birth than chicks without the Z* allele and chicks with the Z* allele that had hatched late. Collectively, the results suggest that variation in the CHD1-Z gene may indeed have arisen as a byproduct of selection acting on females during the egg fase and on chicks during the rearing stages of the reproductive cycle.
In bird species in which the sexes are morphologically difficult to distinguish, molecular intronic markers (non-coding) have been used to assign sex (Griffiths et al. 1996, 1998, Ellrich et al. 2010, Saino et al. 2010). The chromo-helicase-DNA binding (CHD1) gene, present on both the Z- (CHD1-Z) and W-chromosomes (CHD1-W) in birds, is widely used for this purpose. Using the polymerase chain reaction (PCR), the CHD1-Z and CHD1-W loci are co-amplified and the difference in length between the resulting alleles is used to distinguish male and female genotypes. Males typically have two allele fragments of the same length (ZZ), whereas females typically have allele fragments of different size (ZW). An increasing number of studies report length variation in the PCR-amplified products of the CHD1-Z locus (Dawson et al. 2001, Montel et al. 2001, Lee et al. 2002, Agate et al. 2004, Jarvi & Farias 2006, Casey et al. 2009, Schroeder et al. 2010), which, as the locus comprises an intron, is expected to be selectively neutral.
Thus, it is surprising that some studies have reported correlations between intraspecific variation in the CHD1-Z gene and fitness components. In Common Moorhens Gallinula chloropus a polymorphism in the CHD1-Z gene was associated with increased mortality in heterozygous male chicks (Lee et al. 2002), and in Ovenbirds Seiurus aurocapilla a polymorphism in the CHD1-Z gene was found to correlate with male body mass (Toms et al. 2012). In Black-tailed Godwits Limosa limosa limosa, normally PCR-amplified CHD1-W and CHD1-Z allele fragments have lengths of 393 and 378 base pairs (bp), respectively, whereas a polymorphism on the CHD1-Z gene results in a third possible allele fragment of 374 bp (Z*) (Schroeder et al. 2010). Therefore, male Black-tailed Godwits have three different possible genotypes (ZZ, ZZ* or Z*Z*) and females have two (ZW, Z*W). Based on a sample of 284 Black-tailed Godwits from Friesland, The Netherlands, the rare Z* allele appeared to be positively associated with several fitness components, but homozygote Z*Z* genotypes were never recovered (Schroeder et al. 2010). Males and females with the Z* allele had less extensive breeding plumage and females bred earlier and had larger eggs than birds without the Z* allele. There was also an association with breeding habitat type, such that in areas managed as meadow bird reserves (extensively managed or herb-rich agricultural land) the Z* allele was found in 14% of the birds, whereas no birds with the Z* allele were found on modern, intensively managed agricultural land (Schroeder et al. 2010).
Schroeder et al. (2010) proposed three explanations for such intriguing functional correlations with supposedly neutral genetic variation. First, the polymorphism could reflect underlying population structure. Secondly, the polymorphism may directly influence phenotypic variation. Schroeder et al. (2010) indicated that this latter explanation is unlikely as the CHD1-Z gene has a role in chromatin structure mediation and organization during transcription and should therefore be conserved (Stokes & Perry 1995, Schroeder et al. 2010). The third possibility was that this polymorphism is linked to other, non-neutral loci on the same chromosome.
Here we revisit the results of Schroeder et al. (2010) with a much larger sample of 2088 individuals collected from 2004 to 2010 in the same study area. We include the 284 individuals from Schroeder et al. (2010) in our analyses. Not only was our sample size larger, but we were also able to examine a greater range of fitness correlates, including chick survival and body mass, in addition to habitat type, timing of breeding, egg size and adult survival.
Methods
In late May–June 2004–2010, blood samples of individual Black-tailed Godwits were collected in southwest Friesland in a study area that from 2007 onward comprised 8340 ha of grasslands (see Groen et al. 2012 and Kentie et al. 2013 for more details of the study area). For the agricultural fields the same classification was used as in Kentie et al. (2013). Herb-rich meadows (20% of the study area), henceforth termed herb-rich agricultural land, contained diverse grass and herb species and had water tables not more than 30 cm below the surface. Grassland monocultures (80% of the study area), henceforth termed intensively managed agricultural land, had lower water tables and consisted predominantly of ryegrass Lolium spp. On finding a nest during incubation, the egg floatation method of Liebezeit et al. (2007) was used to predict hatching date, such that around the day of predicted hatching, adults and chicks could be captured on the nest. Each adult bird was fitted with an individual combination of four colour rings plus a flag on their tibia for individual recognition. Since 2008, hatchlings (chicks still in the nest) were fitted with an engraved leg-flag bearing a three letter/number combination. Subsequently, different biometric measurements (bill length, total head, wing length, tarsus length, tarsus toe length) and body weight were recorded for each individual. In addition to ringing the birds and taking their biometrics, we obtained 30 μL of whole blood from the brachial vein of adults or the leg vein of chicks. Blood was stored in individual 1.5-mL Eppendorf tubes containing 97% alcohol buffer, after which the individual tubes were frozen at −80 °C.
DNA was extracted from 6 to 10 μL of blood using either the ammonium acetate method as described by Richardson et al. (2001) or a Qiagen DNeasy Tissue Kit (Qiagen, Hilden, Germany) with minor modifications as described by Trimbos et al. (2009). DNA quality and quantity were checked twice using a NanoDrop ND-1000 instrument (Thermo Scientific, Wilmington, DE, USA). For optimal PCR-amplification, blood samples were diluted to concentrations below 10 ng/μL. For sexing we made use of fluorescently labelled primers P8 and P2 and the PCR protocol described by Griffiths et al. (1998). The PCR products were separated on an ABI 377 automatic sequencer (data from 2004 to 2007) or an AB3730 DNA analyser (data from 2008 to 2010) using a GeneScan™ 500 ROX™ size standard (Applied Biosystems). Subsequently, fragment lengths were determined using genescan 3.1 or genemapper 4.0, respectively.
A total of 2088 individuals, comprising 618 adults and 1470 chicks, were genotyped for sex and the presence of the Z* allele (Table 1). To be able to compare results between Schroeder et al. (2010) and this study, most of the fitness calculations of Schroeder et al. (2010) were repeated for our more extensive samples that included the individuals used by Schroeder et al. (2010). We excluded plumage traits from the fitness analyses because of the difficulty in scoring all birds (especially hatchlings).
| Male adult | Male chick | Total | Female adult | Female chick | Total | Intensively managed agricultural land | Herb-rich agricultural land | Moved between management types | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Z | 266 | 658 | 924 | 296 | 683 | 979 | 98 | 363 | 74 | 535 |
| Z* | 32 | 92 | 124 | 24 | 37 | 61 | 8 | 34 | 13 | 55 |
| Total | 298 | 750 | 1048 | 320 | 720 | 1040 | 106 | 397 | 87 | 590 |
- The number of individuals with and without the Z* allele are subdivided by sex, age and habitat type, and totals for every subcategory are given.
Should the Z* allele be positively correlated with different fitness variables, or should the homozygote Z*Z* genotype be lethal, the relative frequency of different genotypes present in the populations is expected to deviate from Hardy–Weinberg expectations (Frankham et al. 2009). The sex ratio is expected to be 1 : 1. However, possible correlates between the CHD1-Z* allele variant and fitness components may cause reproductive skew. We therefore assessed whether the sex ratio deviated from expected values. Chi-square tests were performed to test whether the differences between observed and expected values for the number of genotypes, sex ratio, and males and females carrying the Z* allele were significant.
We assessed whether the occurrence of the rare Z* allele was restricted to individuals breeding on meadows managed for meadow birds (herb-rich agricultural land, Schroeder et al. 2010, Groen et al. 2012, Kentie et al. 2013). Hatchlings and larger chicks were excluded from this analysis. Because some adults were captured in multiple years, we used a generalized linear mixed model with breeding habitat as response variable, individual as random effect and genotype as fixed effect. In total, 708 observations of 590 different adults were included in this analysis (Table 1).
To determine whether the Z* allele had an effect on yearly apparent survival of adults, ringed Godwits were recaptured and/or resighted with binoculars or with telescopes (Lourenço et al. 2011). Adults with known genotypes marked in 2004–2009 and resighted from 2005 to 2010 were selected for an analysis of annual apparent survival using a Cormack–Jolly–Seber mark-recapture model in the program mark (White & Burnham 1999). Of the 461 individuals included in this analysis, 42 carried the Z* allele. An individual was reported alive if it was seen at least twice and on different dates within the breeding season March–June. The full model was the model in which the survival probability was dependent on year, presence or absence of the Z* allele, and the interaction between year and Z* allele. The resighting probability was able to vary only among years because we thought it is unlikely that the Z* allele would influence resighting probabilities. The goodness-of-fit of the global model was tested with the program u-care (Choquet et al. 2009) and the model fitted the data well (χ2 = 25.7945, df = 26, P = 0.47). Model selection was based on Akaike's information criterion (AIC) to select the most parsimonious model (Burnham & Anderson 2002).
Black-tailed Godwits vary greatly in size, so in addition to body mass, which is body size-dependent, we used a body size-independent mass termed ‘condition’ (van der Meer & Piersma 1994). Of 603 adults body size measurements (bill, total head, wing, tarsus, tarsus toe length), the body mass, molecular sex and CHD1-Z genotype were known. For ‘condition’ the size parameters were collapsed in a principal components analysis (PCA) to extract a single size parameter, representing structural body size. The first principal component (PC1) explained 80.8% of the variation. The residuals of the linear regression of body mass and PC1 (R2 = 0.70, F1,601 = 1429, P < 0.001) were taken as a size-independent measure of condition.
To test for correlations between presence or absence of the CHD1-Z* allele and body mass, as well as body condition, we used a linear mixed effect model, with sex, the interaction of sex and CHD1-Z genotype, habitat (intensively managed or herb-rich agricultural land) and date of capture as fixed effects. Because some individuals were captured over multiple years, we fitted individual as a random factor. Additionally, year of capture was incorporated as a random effect.
Egg volumes and laying dates (the day that the first egg of a clutch was laid) of nests with at least one of the parents carrying the Z* allele were compared with egg volumes and laying dates of nests in which neither of the parents carried the Z* allele. For this analysis we used only nests for which we sampled both parents. We then sought to determine whether there was an effect of the occurrence of Z* on egg volume in males and females separately. A mixed effects model was used, with breeding pairs or individuals (pairs are monogamous and no divorces were observed) and year fitted as random effects. For the egg volume analysis we used laying date, habitat quality and the interaction of laying date with CHD1-Z genotype as fixed effects. The egg volume analysis for two parents per nest resulted in a dataset that was too small to perform the analysis with year as a random factor, and thus year was included as a fixed factor. For the analysis of laying date, habitat quality, the interaction of CHD1-Z genotype with habitat quality, and the interaction of the CHD1-Z genotype with year were fitted as fixed effects.
From 2008 to 2010, 914 newly hatched chicks were marked with an engraved leg flag, enabling us to assess whether there was a difference in return rates of chicks with and without the Z* allele. We used observations of chicks in subsequent years (in either the breeding area or along the migration route) to increase resightings. Since the quality of the hatching habitat (modern or herb-rich agricultural land) and hatching year might influence chick survival, we included these factors in the analysis. Genotype, year, habitat quality, hatching date, body mass, the interaction of genotype and hatching date, the interaction of genotype and body mass, and the interaction of genotype and habitat quality were included in the analyses as fixed effects. We could not fit year as a random factor as only 2 years were included in the analysis. Because of the low number of resightings and observation years, we used a generalized linear model for binomial data with a logit link instead of conducting a mark-recapture analysis. We also assessed whether the CHD1-Z genotype of the chick itself had an influence on its body mass. For this analysis we used the body mass data from 1470 chicks that were caught between 2004 and 2010. To prevent pseudoreplication of chicks that were born within the same nest, our analysis utilized a mixed model with nest and year as random effects. Genotype, sex, habitat quality and hatching date, the interaction of genotype and hatching date, and the interaction of genotype and sex were included in the analyses as fixed effects. We included hatching date as it has been suggested to be correlated with reproductive success (Kruk et al. 1997, Arnold et al. 2006, Schroeder et al. 2012) and hence may be an important explanatory factor. Different analyses have different sample sizes due to missing data for some variables.
Statistical analyses were conducted in r 2.11.1 (R Development Core Team, 2008) unless stated otherwise. Variables that did not have a normal statistical distribution were log-transformed. The function lm() was used for the general linear model, lme() for linear mixed effect models, lmer() for the generalized linear mixed effect model and glm() for generalized linear models. For the linear mixed models we performed a manual backward selection using the anova() function for models fitted with the ML method, while the estimates were provided after refitting the model with the REML method. PCA was conducted with the prcomp() function, using a correlation matrix. Chi-square tests were conducted using chisq.test().
Results
Of the 2088 individuals sampled, 1048 were male (298 adults and 750 chicks) and 1040 were female (320 adults and 720 chicks), with no deviation from the expected sex ratio of 1 : 1 (χ2 = 0.03, df = 1, P = 0.86; Table 1). Of the reported genotypes, 9% carried the Z* allele (Table 1). This corresponded to a Z* allele frequency of 6% (q = 0.06 and P = 0.94). We expected four of 1040 males to be Z*Z* given the Z* allele frequency in the sampled population. We encountered one chick with the homozygous Z*Z* genotype; its biometrics were comparable to that of other chicks (mass 29 g, bill length 17.6 mm). The individual was genotyped twice to check for error. The distribution of observed genotypes did not differ significantly from the expected distribution of genotypes under Hardy–Weinberg equilibrium (Fisher's exact test, P = 0.78). Eight of 106 Godwits caught on intensive meadows and 34 of 397 Godwits caught on herb-rich meadows carried the Z* allele. Additionally, 13 of 87 birds that were caught on meadows of both management types throughout the study period carried the Z* allele (Table 1). There was no difference between the occurrence of the Z* allele in birds on intensively managed and herb-rich agricultural land (z = 0.82, P = 0.4).
The model in which apparent survival of the adults varied by year and resighting probability was constant received the greatest support (Table 2). There was no indication that the presence of the Z* allele had an adverse effect on yearly apparent survival of adults. The most parsimonious model including Z* was the model with an interaction between Z* and year, which had a ∆AICc of 7.6. Resighting probability was high (P = 0.89, se = 0.01), as was average yearly apparent survival (φ = 0.89, se = 0.004).
| Model | AICc | ∆AICc | AICc weights | Model likelihood | No. of parameters | Deviance |
|---|---|---|---|---|---|---|
| Φ(t) p(.) | 1510.24 | 0.00 | 0.92 | 1.00 | 7 | 168.99 |
| Φ(t) p(t) | 1515.85 | 5.61 | 0.06 | 0.06 | 11 | 166.48 |
| Φ(Z*t) p(.) | 1517.81 | 7.58 | 0.02 | 0.02 | 13 | 164.35 |
| Φ(Z*t) p(t) | 1523.44 | 13.20 | 0.00 | 0.00 | 17 | 161.76 |
| Φ(.) p(.) | 1533.54 | 23.31 | 0.00 | 0.00 | 2 | 202.38 |
| Φ(Z) p(.) | 1534.85 | 24.61 | 0.00 | 0.00 | 3 | 201.68 |
| Φ(.) p(t) | 1537.32 | 27.08 | 0.00 | 0.00 | 7 | 196.07 |
| Φ(Z) p(t) | 1538.63 | 28.40 | 0.00 | 0.00 | 8 | 195.36 |
- Apparent survival (Φ) can be year-dependent (t), dependent on genotype (Z) or dependent on the interaction Z*t, or not vary over years and genotype (.). Resighting probability (p) can be year-dependent (t) or not (.).
In the model of adult body mass (Table 3), only sex remained as a significant term after model selection (t595 = −34.97, P < 0.001), males being 56 g lighter than females. Adults with a Z* allele were not heavier than adults without the Z* allele (t594 = 0.46, P > 0.5). The interaction of sex and genotype was not significant at the 5% level (t593 = −1.77, P = 0.08). This interaction compares females without the Z allele (the intercept) with males with the Z* allele. When the interaction between sex and presence of Z* was retained in the model, females with a Z* allele were not significantly heavier (estimate = 6.02 ± 3.82 g, P = 0.11) than females without a Z* allele, and males with a Z* also did not differ significantly in mass relative to males without a Z* allele; they were 3.31 ± 4.47 g lighter. Sex was the only remaining term in the best model of condition (estimate = −7.95 ± 1.49, t595 = −5.33, P < 0.001), suggesting that females had a higher size-independent mass. Genotype was not maintained in this model (t592 = 0.40, P > 0.5). Leaving capture date (t594 = −1.80, P = 0.07) in the model did not alter the effect of genotype (t593 = 1.00, P > 0.5).
| Estimate | se | t-value | df | P | |
|---|---|---|---|---|---|
| Body mass | |||||
| Intercept | 311.07 | 1.28 | 243.47 | 595 | <0.001 |
| Sexa | −56.10 | 1.60 | −34.97 | 595 | <0.001 |
| Zb | 1.21 | 2.64 | 0.46 | 594 | >0.5 |
| Z*Sex | −9.33 | 5.27 | −1.77 | 593 | 0.08 |
| Habitat qualityc | 2.44 | 2.12 | 1.15 | 591 | 0.25 |
| Capture date | −0.11 | 0.09 | −1.23 | 592 | 0.22 |
| Random effects | |||||
| σ2year | 1.68 | ||||
| σ2individual | 18.37 | ||||
| σ2residual | 6.83 | ||||
| Condition Intercept | 3.60 | 1.12 | 3.21 | 595 | 0.001 |
| Sexa | −7.95 | 1.49 | −5.33 | 595 | <0.001 |
| Zb | 0.98 | 2.45 | 0.40 | 592 | >0.5 |
| Capture date | −0.15 | 0.08 | −1.80 | 594 | 0.07 |
| Z*Sex | −6.03 | 4.91 | −1.23 | 591 | 0.22 |
| Habitat qualityc | −2.29 | 1.93 | −1.19 | 593 | 0.24 |
| Random effects | |||||
| σ2year | 1.20 | ||||
| σ2individual | 17.19 | ||||
| σ2residual | 6.08 | ||||
- Terms retained in the model are shown in bold-italic, and the terms are presented in reverse order of exclusion from the model. Estimates and statistics were derived from the last model before exclusion.
- Reference categories: afemale, bZ allele, cintensively managed agricultural land.
The eggs in nests of which one parent had the Z* allele did not differ in volume from eggs in nests where neither of the parents had the Z* allele (t95 = 1.08, P = 0.3; Table 4). The occurrence of the CHD1-Z* genotype (Table 4) was not correlated with average egg volume in a separate analysis for males and females (male: t353 = 1.79, P = 0.08; female: t353 = −0.48, P > 0.5); for males, eggs differed in volume between years and, for females, eggs differed in volume between years and were smaller later in the season (Table 4).
| Laying date | Egg size | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | se | t-value | df | P | Estimate | se | t-value | df | P | ||
| Both | Both | ||||||||||
| Intercept | 5.07 | 0.20 | 25.30 | 115 | <0.001 | Intercept | 40.90 | 0.31 | 131.98 | 97 | <0.001 |
| Zc | −0.23 | 0.17 | −1.36 | 114 | 0.18 | Yeara | |||||
| Habitat qualityb | 0.17 | 0.26 | 0.65 | 113 | 0.5 | 2005 | −1.09 | 1.05 | 1.03 | 20 | 0.3 |
| Z * habitat quality | −1.26 | 0.90 | −1.40 | 112 | 0.16 | 2006 | 0.83 | 0.69 | 1.19 | 20 | 0.2 |
| 2007 | −0.71 | 0.75 | −0.95 | 20 | 0.4 | ||||||
| 2008 | 0.50 | 0.82 | 0.61 | 20 | >0.5 | ||||||
| 2009 | −1.48 | 0.94 | 1.58 | 20 | 0.13 | ||||||
| 2010 | 0.71 | 0.87 | 0.82 | 20 | 0.4 | ||||||
| Zb | 0.78 | 0.72 | 1.08 | 95 | 0.3 | ||||||
| Sqrt laydate | −0.01 | 0.26 | −0.04 | 19 | >0.5 | ||||||
| Z * sqrt(laydate) | −0.55 | 0.57 | −0.55 | 18 | >0.5 | ||||||
| Habitat qualityb | −0.0 | 0.98 | 0 | 94 | >0.5 | ||||||
| Random effects | Random effects | ||||||||||
| σ2year | 0.48 | σ2intercept | 2.65 | ||||||||
| σ2individual | 0.00 | σ2residuals | 1.61 | ||||||||
| σ2residuals | 0.84 | ||||||||||
| Male | Male | ||||||||||
| Intercept | 5.43 | 0.15 | 35.53 | 354 | <0.001 | Intercept | 41.13 | 0.42 | 98.97 | 354 | <0.001 |
| Habitat qualityb | −0.38 | 0.12 | −3.28 | 8 | 0.01 | Zc | 0.77 | 0.43 | 1.79 | 353 | 0.08 |
| Zc | −0.06 | 0.13 | −0.48 | 353 | >0.5 | Habitat qualityb | 0.41 | 0.39 | 1.04 | 8 | 0.3 |
| Z * habitat quality | 0.28 | 0.33 | 0.86 | 352 | 0.4 | Sqrt laydate | 0.02 | 0.18 | 0.10 | 7 | >0.5 |
| Z * sqrt(laydate) | −0.28 | 0.51 | −0.56 | 6 | >0.5 | ||||||
| Random effects | Random effects | ||||||||||
| σ2year | 0.28 | σ2year | 1.01 | ||||||||
| σ2individual | 0.47 | σ2individual | 1.40 | ||||||||
| σ2residuals | 0.67 | σ2residuals | 2.40 | ||||||||
| Female | Female | ||||||||||
| Intercept | 5.38 | 0.16 | 33.97 | 423 | <0.001 | Intercept | 42.73 | 0.66 | 64.67 | 423 | <0.001 |
| Habitat qualityb | −0.36 | 0.11 | −3.23 | 21 | 0.004 | Sqrt laydate | −0.37 | 0.13 | −2.92 | 21 | 0.01 |
| Zc | −0.28 | 0.15 | −1.93 | 422 | 0.05 | Habitat qualityb | −0.06 | 0.27 | −0.22 | 20 | >0.5 |
| Z * habitat quality | −0.86 | 0.65 | −1.32 | 400 | 0.19 | Zc | −0.74 | 0.53 | 1.40 | 422 | 0.16 |
| Z * sqrt(laydate) | 0.34 | 0.68 | 0.50 | 421 | >0.5 | ||||||
| Random effects | Random effects | ||||||||||
| σ2year | 0.31 | σ2year | 0.14 | ||||||||
| σ2individual | 0.36 | σ2individual | 2.92 | ||||||||
| σ2residuals | 0.80 | σ2residuals | 0.89 | ||||||||
- Terms retained in the model are shown in bold-italic, and the terms are presented in reverse order of exclusion from the model. Estimates and statistics were derived from the last model before exclusion.
- Reference categories: a2004, bintensively managed agricultural land, cZ allele.
Laying dates for nests in which only one parent had the Z* allele were not significantly different from nests in which neither of the parents carried the Z* allele (t114 = −0.23, P = 0.18) (Table 4). When the effect of the Z* allele on males and females was tested separately, habitat quality remained in the best model (Table 4) describing lay date. In one of the sexes, the CHD1-Z* genotype had an almost significant statistical effect on laying date (males: t353 = −0.48, P > 0.5; females: t422 = −1.93, P = 0.05). Males and females started breeding earlier on herb-rich agricultural land than in intensively managed agricultural land (males: t8 = −3.28, P = 0.01; females: t21 = −3.23, P = 0.004).
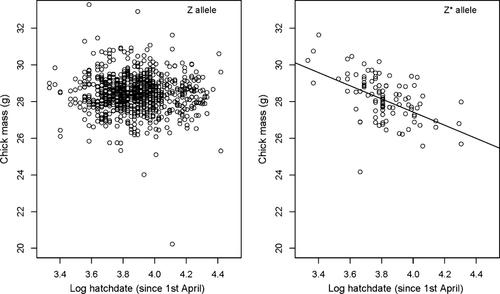
Chicks with a Z* genotype had a higher return rate than chicks with a Z genotype (z = 2.93, P = 0.003). Additionally, chicks that had hatched on intensively managed meadows had a lower return rate (z = −3.07, P = 0.002). Furthermore, the year of hatching influenced return rates of the chicks in consecutive years (2009: z = 1.47, P = 0.14; 2010: z = 2.26, P = 0.02). Hatching date and the interactions of the terms genotype*habitat quality and genotype*hatching date were not retained in the model (Table 5). We had information on the body mass of 1470 chicks from 664 nests, of which 129 had a Z* allele. Chicks carrying the Z* allele were heavier if they hatched early in the season, and lighter if they had hatched late in the breeding season compared with chicks without the Z* allele (Table 5, Fig. 1). At the mean hatching date of 17 May, the weight of the chicks was the same.
| Estimate | se | z-value | P | Estimate | se | t-value | df | P | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Return rates | Chick body mass | |||||||||
| Intercept | −3.28 | 0.37 | −8.88 | <0.001 | Intercept | 28.17 | 1.96 | 14.67 | 803 | <0.001 |
| Za | 1.08 | 0.37 | 2.93 | 0.003 | Za | 13.08 | 4.14 | 3.16 | 803 | 0.002 |
| Habitat qualityd | −1.36 | 0.44 | −3.07 | 0.002 | Z* log(hatching date) | −3.49 | 1.089 | −3.22 | 803 | 0.001 |
| Yearc | log(hatching date) | −0.10 | 0.50 | −0.19 | 803 | >0.5 | ||||
| 2009 | 0.66 | 0.45 | 1.47 | 0.14 | Sexb | −0.14 | 0.09 | −1.51 | 802 | 0.13 |
| 2010 | 0.92 | 0.41 | 2.26 | 0.02 | Sex*Z | 0.01 | 0.38 | 0.02 | 800 | >0.5 |
| Body mass | 0.00 | 0.06 | 0.01 | >0.5 | Habitat qualityd | −0.09 | 0.21 | −0.46 | 801 | >0.5 |
| Z* Body mass | −0.26 | 0.17 | −1.57 | 0.12 | Random effects | |||||
| Z*Habitat quality | 1.30 | 0.96 | 1.36 | 0.18 | σ2year | 0.26 | ||||
| Hatching date | −0.01 | 0.02 | −0.61 | >0.5 | σ2individual | 1.99 | ||||
| Z*Hatching date | 0.03 | 0.04 | 0.86 | 0.4 | σ2residuals | 1.44 | ||||
- Terms left in the model are shown in bold-italic, and the terms are presented in reverse order of exclusion from the model. Estimates and statistics were derived from the last model before exclusion.
- Reference categories: aZ allele, bfemale, c2008, dintensively managed agricultural land.
Discussion
As pointed out by Benedict et al. (2010), links between fitness components and supposedly neutral variation on the sex chromosome are both surprising and interesting. Although there were clear correlations between the occurrence of the Z* allele with apparent chick fitness correlates, on the basis of a greatly enlarged sample size we could not confirm all the correlations between Z allele variants and fitness components in adult Black-tailed Godwits as reported by Schroeder et al. (2010).
Our data indicate that the Z/Z* allele polymorphism does not reflect underlying population structure, a suggestion consistent with a recent study that showed no population structure between Black-tailed Godwits breeding in different parts of The Netherlands (Trimbos et al. 2011). Genotype numbers were not significantly different from the expectations of Hardy–Weinberg equilibrium or a 1 : 1 sex ratio, indicating that the heterozygous genotype probably did not introduce a selective advantage or disadvantage compared with the other genotypes. Only one homozygous Z* individual was found and the biometrics of this chick fell within the population range. On the basis of the rarity of the Z* allele, only four individuals were expected to be homozygous given the size of the dataset used in this study.
The fitness calculations in the adults showed one significant (negative) correlation between occurrence of the Z* allele and laying date. Neither adult males nor females carrying the Z* allele exhibited a higher survival rate, had larger eggs, greater body mass or a higher body condition than birds without the Z* allele. Schroeder et al. (2010) recovered an effect of Z* females breeding earlier than females without the Z* allele (effect size = −4.38 ± 2.15, P = 0.04). However, in their analysis they corrected only for year, whereas we additionally corrected for habitat quality, and recovered only a slight effect of Z* on laying date (almost significant) in contrast to the strong influence of habitat quality. This suggests that the reported correlation in Schroeder et al. (2010) may have been partially confounded by habitat quality, but we cannot dismiss the effect that females carrying the Z* allele may lay their clutch earlier.
In addition, we found apparent fitness correlates between the presence of the Z* allele with chick body mass and with chick return rate. The Z* allele had a positive effect on the body mass of chicks that had hatched early in the breeding season and a negative effect on chicks that hatched later in the breeding season when compared with chicks without the Z* allele. It is often suggested that early hatching is related to a higher chick body mass (Arnold et al. 2006, Schekkerman et al. 2008, 2009, Schroeder et al. 2012), and both early hatch date and high chick body mass often correlate positively with chick survival and should therefore positively influence chick return rates (Arnold et al. 2006, Schekkerman et al. 2008, 2009, Schroeder et al. 2012, Kentie et al. 2013). We did not find a correlation of chick body mass or hatching date with chick return rates. Furthermore, chicks without the Z* allele, the majority of the chicks, did not show a correlation of hatching date with body mass. However, return rates were higher for chicks with the Z* allele. Interestingly, our data indicate that birds breeding on herb-rich agricultural land started breeding earlier than birds breeding on intensively managed meadows. Additionally, return rates of chicks that had hatched on herb-rich agricultural land were higher than return rates of chicks that had hatched on intensively managed agricultural land. This reflects the importance of herb-rich agricultural land for the survival of Black-tailed Godwit chicks, and the decline of herb-rich agricultural land is the factor most responsible for the ongoing annual decline in Black-tailed Godwit breeding populations in northwestern Europe (Teunissen & Soldaat 2005, Schekkerman & Beintema 2007, Schekkerman et al. 2008, Zwarts et al. 2009).
Although not all previous reported associations of the Z* allele with fitness correlates in Black-tailed Godwits presented by Schroeder et al. (2010) could be confirmed here with larger sample sizes, we did find that Z* females laid earlier and laid eggs which, when hatching a chick with the Z* allele, produced chicks that were heavier and had a greater survival probability compared with individuals lacking the Z* allele. Moreover, given the strong correlations between the Z* allele and fitness in chicks, combined with the fact that Z* chicks have at least one Z* parent, the earlier laying date pattern found in adult Z* allele females is likely to be a biological reality. Taken together, the results presented here and by Schroeder et al. (2010) indicate an association of the Z* allele with fitness.
We thank It Fryske Gea, the Friese Fûgelwachten, Staatsbosbeheer, Pedro Lourenço, Niko Groen, Petra de Goeij, João Guilherme, Anneke Rippen, Job ten Horn, Robbie Watt, Valentijn van den Brink, Ysbrand Galama, Sytse-Jan Wouda, Rinkje van der Zee, Martin Bulla, Lucie Schmaltz and the students from the University of Groningen for their help in the field and for the collection of blood samples. Special thanks to all the farmers for providing us with access to their private lands and to the voluntary nest protectors for their most important help in locating the Black-tailed Godwit nests in the field. Klaas Vrieling and Rene Glas of the Leiden Institute of Biology (IBL) were helpful with PCR-amplification and providing laboratory space in Leiden. Bram Verheijen was of great help with DNA extraction and PCR-amplification of the Black-tailed Godwit blood samples. We were financially supported by a start-up grant to T.P. from the University of Groningen, a grant to Julia Schroeder from the Schure-Bijerinck-Popping Foundation, a grant by the Vogelbescherming-Nederland and financial support from the Province of Fryslân and the Weidevogel Kenniskring of the Ministry of LNV. The work done here was conducted in compliance with the current laws of The Netherlands.




