Causes of variable reproductive performance by Southern Ground-hornbill Bucorvus leadbeateri and implications for management
Abstract
Range occupancy of the cooperatively breeding Southern Ground-Hornbill Bucorvus leadbeateri in South Africa has decreased by 65% in the last three generations and the effective management of the remaining populations is hampered by a lack of fundamental understanding of the factors determining reproductive performance. We examined the influence of social and environmental factors on the species' reproductive success in South Africa using data gathered from 23 groups over eight breeding seasons. Some groups had access to artificial nest-sites, others did not. High rainfall (> 500 mm) over the breeding season led to a decrease in reproductive success, with groups being most successful in years when rain in the vicinity of the nest ranged from 300 to 500 mm. Groups breeding in natural nests were successful only when the proportion of open woodland surrounding the nest-site was high. Those that bred in artificial nests, where overall breeding success was more than twice as high as those in natural nests, were less dependent on the availability of open woodland. Large groups (more than three birds) bred more successfully than groups comprising only two to three individuals. Group size, helper effects and rainfall cannot be managed to increase the productivity of Ground-Hornbills but the fact that the availability of artificial nest-sites and the amount of open woodland around the nest-site both contribute positively to breeding performance identifies practical and simple management options for increasing the reproductive output of Southern Ground-Hornbill populations.
Cooperative breeding is an uncommon and specialized reproductive system that occurs in an estimated 9% of bird species (Cockburn 2006). The most common form of cooperative breeding involves a single pair of birds breeding on an all-purpose territory within which they retain offspring from previous broods to assist in the current breeding effort (Magrath & Yezerinac 1997, Nelson-Flower et al. 2011). Several studies have investigated the effects that environmental and social factors have on reproductive success in cooperatively breeding birds (Supporting Information Table S1). Most have focused on group size and helper effects to explain all aspects of breeding performance. However, there is relatively little understanding of how different factors (e.g. environmental, social and phenotypic) interact and possibly influence intra-specific variation in breeding performance among cooperatively breeding species.
Southern Ground-Hornbill Bucorvus leadbeateri is the largest (2.2–6.2 kg), cooperatively breeding bird in the world (Kemp 1995). It occurs at low densities, in cohesive groups of 2–11 individuals, occupying year-round territories (in South Africa) at a group density of approximately one group per 100 km2 (Kemp et al. 1989). Non-breeding group members are subordinate helpers, which are generally retained offspring of the dominant pair (Kemp 1995). They nest in natural cavities in large trees and cavities on rock faces, laying a clutch of one to two (very rarely three) eggs (Kruger National Park average 1.85 ± 0.20 se, n = 34; Kemp 1990) and fledge a maximum of one chick per season (Kemp & Begg 1996). Because the birds are so large, nest-sites are considered to be a limiting resource due to the scarcity of large nesting cavities in the landscape (Kemp et al. 1989). It has been suggested that the shortage of nesting cavities reflects the impacts of increasing numbers of African Elephants Loxodonta africana across the southern African portion of the Ground-Hornbill's range (Owen-Smith et al. 2006, Shannon et al. 2008). However, in the Kruger National Park, 80% of the Southern Ground-Hornbill nest-sites were in only five tree species, of which only the Marula Tree Sclerocarya birrea is regularly seriously damaged (through pollarding and toppling) by Elephants (K. Morrison & A. C. Kemp unpubl. data), accounting for much of the high mortality rate observed in Marulas (Helm et al. 2011). Although the number of large trees (and hence the potential number of nest cavities) may not yet have been significantly impacted by increasing Elephant numbers, the future recruitment of large trees may well be influenced by the rapidly increasing Elephant population because they remove medium-sized trees from the landscape and negatively impact sapling recruitment (Helm et al. 2011). The availability of suitable nesting sites may be a critical determinant of territory quality and may influence reproductive success (Kemp & Begg 1996, Henley & Henley 2005).
Southern Ground-Hornbills are listed as globally Vulnerable (BirdLife International 2011). However, recent analyses of the South African population conclude that a 65% reduction in range, and presumably in numbers, has occurred over the past three generations (100 years – A.C. Kemp & R. Webster unpubl. data), suggesting that their regional conservation status may be even less favourable. At the same time, however, their very long generation time requires that many years of comprehensive data are needed to analyse their reproductive traits and breeding performance before informed conservation-management plans can be formulated and implemented for such long-lived species.
Fewer than 1500 Ground-Hornbills (equating to c. 400–450 breeding pairs) remain in South Africa, most of which are in protected areas (Kemp 1995). Community expansion, bush encroachment and agricultural practices such as forestry have reduced the natural habitat available to the birds, and changing distribution patterns show a direct correlation with habitat loss (A.C. Kemp & R. Webster unpubl. data). If it can be determined why certain groups are more successful than others, it may be possible to introduce an adaptive conservation-management plan for the species.
In this study, we use multivariate analyses to assess both the social (group size) and the environmental factors (rain, and nest-site and habitat availability) that may explain variation in reproductive performance between groups of Ground-Hornbills. Specifically, we assessed which groups were the most successful, whether landscape features and/or rainfall influenced breeding success, whether there was an influence of group size, whether artificial nestboxes influenced breeding success and whether the results of these analyses could contribute towards an adaptive conservation-management plan for the species.
Methods
Study area
The study was initiated in 2001 at the Associated Private Nature Reserves (APNR) in the Limpopo and Mpumalanga Provinces of South Africa (24°02′–24°33′S; 31°02′–31°29′E). The APNR is located on the western boundary of the Kruger National Park and encompasses an area of 180 000 ha, making it among the largest private nature reserves in the world (Greyling et al. 2004). The area is characterized by a highly seasonal, sub-tropical climate of hot, humid summers and warm, dry winters. There is a pronounced gradient of increasing rainfall along a northwest/southeast axis, with the mean annual rainfall at different locations ranging from 375 to 625 mm. Summer rains fall between October and March and account for approximately 90% of the annual rainfall. Ground-Hornbills breed during the wet season, with the first eggs being laid in October and the last chicks fledging in March/April (G. Wilson and P.A.R. Hockey 1991, pers. obs.). Dominant vegetation types within the APNR include lowland savanna, open tree savanna, mixed and open woodland, low thicket, and shrubveld. The dominant tree species are the Red Bushwillow Combretum apiculatum, Acacia spp. (Acacia nigrescens, Acacia tortilis), Marula and Mopane Colophospermum mopane.
Data collection
Breeding performance of 23 groups of Southern Ground-Hornbills in the APNR was monitored over eight breeding seasons from 2001 to 2008, providing 184 group-years of data. All groups and nest-sites were documented for all years and group identity was determined using photographs, group demography data and colour-ringed individuals. At the start of the breeding season (September/October) all nests were checked on a weekly basis until a breeding attempt was discovered, after which the active nest was checked every 4–5 days until hatching occurred. The chicks were monitored up to fledging. When no breeding was recorded for a group, efforts were made to locate any alternative natural nest-site possibly used by the group (although this was difficult and often unsuccessful) and, subsequently, any evidence of a chick having been raised during the season (at an undiscovered nest).
The first artificial nestboxes were constructed and erected before the breeding season in 2002. A total of 28 were erected between 2002 and 2008: these were sited randomly within territories, not least because precise territory boundaries were unknown. The boxes were placed in trees large enough to prevent toppling by Elephants. The type (natural or artificial) and location of the nest for each breeding season were recorded for each group. When groups bred successfully but the nest-site was not found, the successful breeding attempt was recorded as being in a natural, but unknown, nest (because all nestboxes within the territory had been checked). This occurred in four of the 184 group-years.
Group size was recorded as the number of individuals present in each group at the start of each breeding season. In years when sightings of a particular group were few and the breeding site was unknown (a total of 12 group-years), group size could not be determined accurately and was omitted from analyses. Age/sex ratios were determined at 843 of the 924 encounters with groups during the study period.
The average monthly rainfall data for the years spanning the study were collated from 16 sites spread throughout the APNR. Each group was then associated with the rainfall station within or closest to its territory using ArcView GIS 3.3 and analysed using total rainfall over the whole 6-month breeding season (October–March), with an assumption that rainfall influences food availability (Kemp & Kemp 1991). The overall mean (± sd) distance of rainfall gauges from the nests was 3.2 ± 1.8 km (n = 16), with a range of 0.4–7.2 km.
A detailed digitized vegetation map of the APNR, based on a combination of aerial photography and ground truthing, was used to estimate the proportion of different vegetation types within a 3-km radius of each nest using ArcView GIS 3.3. This map was based on a combination of vegetation composition and structure with detailed information on 24 different vegetation classes and dominant species.
The distances that Ground-Hornbills move during the breeding season to forage and provision the dominant female and chick at the nest are unknown. However, radiotracking of birds outside the breeding season showed that they can travel large distances (8–10 km) in a single day. In the absence of empirical evidence about the extent of breeding-season movement, we analysed vegetation characteristics within 3 km of each nest, based on the assumption that nests were not positioned on territory boundaries. The proportions of the different vegetation types within this radius were used to calculate vegetation diversity associated with each nest using the Shannon–Wiener Diversity Index. Ground-Hornbills often forage and nest in open areas, where prey are most easily detected (Kemp et al. 1989, Kemp & Begg 1996). Using the same vegetation map, the proportion of open woodland and other open habitat such as airstrips within a 3-km radius of each nest was calculated. Our definition of ‘open woodland’ was based on the woody component of habitat structure, i.e. extent of closure of the canopy, rather than floristics.
The nature of the terrain and habitat made it impossible to quantify rates of food acquisition. We included grass biomass as a potential explanatory variable based on the assumption that it was a reasonable surrogate for food availability. It is well established that above-ground grass biomass and insect richness are positively correlated and that grass biomass is dependent on rainfall (Schowalter 2006, Covas et al. 2008, Blaum et al. 2011). The grass fuel load was estimated annually in March/April at 103 sites across the APNR using a disc pasture meter (Trollope & Potgieter 1986, Zambatis et al. 2006). The grass biomass values of the five closest sites to each nest were used to calculate the average grass biomass for each Ground-Hornbill group in each year.
Statistical analyses
Generalized linear mixed models (GLMMs) were used to investigate the factors affecting overall breeding success. Because Ground-Hornbills can only fledge a maximum of one chick per breeding season, annual productivity is a binary outcome; they are either successful or they fail at breeding in that year. Two dependent variables were analysed: breeding success (where 0 = failed and 1 = success) and breeding attempts (where 0 = no attempt and 1 = attempt) in GLMMs using binomial distributions and logit link functions in R (version 2.10.0, R Development Core Team 2009) and following the model selection methodology of Burnham and Anderson (2002). A model was selected over another model when ∆AIC > 2 (Burnham & Anderson 2002). Mixed models are necessary to analyse such data correctly, because random as well as fixed effects can both be included (Bennington & Thayne 1994, Johnson & Omland 2004). To control for the fact that turnover of the dominant pair in Ground-Hornbills is infrequent, we included group identity as a random factor in all analyses of breeding success. The random term was retained in all models.
Akaike's information criterion (AIC) was used to select the most parsimonious model (Akaike 1973, Burnham & Anderson 2002, Brouwer et al. 2006). The potential explanatory variables fitted to the model included: (1) availability and type of nest-sites (i.e. whether an artificial nest was or was not available to the group); (2) environmental factors (rainfall over the breeding season, grass biomass, vegetation diversity, proportion of open woodland); and (3) group size. Because the inclusion of strongly associated variables in a GLMM analysis can lead to false estimates of significance, the association between all potential explanatory variables was verified prior to analysis using linear regression methods and comparing correlation coefficients between variables. There was no relationship between the environmental and social factors incorporated in the analysis and therefore all terms were included simultaneously. However, within the category of environmental factors, rainfall and grass biomass were highly correlated (r178 = 0.73), as were vegetation diversity and the proportion of open woodland (r37 = 0.70). These terms are all ecologically meaningful and therefore the terms that contributed the most to the models' power and had the lowest AIC values when interchanged in the full model were retained. The proportion of open woodland and rainfall over the breeding season generated the lowest AIC values and were retained; vegetation diversity and grass biomass, the latter assumed to be a surrogate for prey abundance, were removed. All possible two-way interactions of the reduced variable set were tested, but only those that resulted in the lowest change in AIC were considered further.
Results
Reproductive success
There were major differences in reproductive success among the Ground-Hornbill groups. Some groups bred and fledged a chick almost every year, whereas others either did not attempt to breed or did not rear a single chick over the 8-year period. Over the study period, 67 breeding attempts were made by 17 of the 23 groups (6% of the 184 group-years). Of these, 51 (76%) were successful, equating to 26% success over the 184 group-years, with seven of the groups (30%) collectively contributing 60% to the total reproductive output (Table 1). These seven groups all bred in artificial nests. Only five of the 23 groups bred in natural cavities: of the latter, three groups did not fledge a single chick over the 8 years and the other two groups fledged a total of six chicks. Overall, therefore, pairs with artificial nests each produced an average of 0.33 chicks per year: the equivalent figure for pairs in natural nests was 0.15 chicks per year.
| Group | Breeding attempts | Chicks reared | Group | Breeding attempts | Chicks reared |
|---|---|---|---|---|---|
| 1 | 6 | 6 | 13 | 3 | 2 |
| 2 | 7 | 5 | 14 | 2 | 2 |
| 3 | 5 | 4 | 15 | 2 | 2 |
| 4 | 5 | 4 | 16 | 1 | 0 |
| 5 | 5 | 4 | 17 | 1 | 0 |
| 6 | 4 | 4 | 18 | 0 | 0 |
| 7 | 4 | 4 | 19 | 0 | 0 |
| 8 | 6 | 3 | 20 | 0 | 0 |
| 9 | 5 | 3 | 21 | 0 | 0 |
| 10 | 4 | 3 | 22 | 0 | 0 |
| 11 | 3 | 3 | 23 | 0 | 0 |
| 12 | 4 | 2 |
The best-supported model explaining variation in reproductive success retained rainfall over the breeding season, group size and the interaction of artificial nests with the amount of open woodland within 3 km of the nest (Model 1 – Table 2). Both social (group size) and environmental factors (rainfall, proportion of open woodland, nest type) thus contributed to explaining variation in breeding success.
| Model | K | AIC | ΔAIC | Deviance |
|---|---|---|---|---|
| (a) | ||||
| Nest type * open woodland + group size + rain | 7 | 165.6 | 0 | 151.6 |
| Nest type * open woodland + group size | 6 | 170.7 | 5.1 | 158.7 |
| Nest type + group size + rain + open woodland | 6 | 173.4 | 7.8 | 161.4 |
| Nest type * rain + group size + open woodland | 7 | 175.3 | 9.7 | 161.3 |
| Explanatory terms (best model) | Effect | se |
|---|---|---|
| (b) | ||
| Intercept | –7.312 | 1.926 |
| Group size | 0.971 | 0.202 |
| Rain | –0.005 | 0.002 |
| Nest type * Open woodland | ||
| Artificial nest | 1.222 | 4.558 |
| Natural nest | 13.642 | 4.558 |
- K: total number of parameters (explanatory terms + random term + residual variance); AIC, Akaike's Information Criterion; ΔAIC, difference between the AIC value for a given model and the model with the lowest AIC; Deviance: −2 log-likelihood.
- Interactions are indicated by an asterisk.
The best-supported model explaining the occurrence of breeding attempts among groups, rather than their breeding success, contained the same terms as the minimum adequate model for explaining reproductive success. The differences between the best and second-best models (ΔAIC) in both analyses were similar. Thus, the same environmental and social parameters that influenced reproductive success determined whether a group attempts to breed, implying that the factors causing breeding attempts to fail are the same factors that cause some groups not to attempt breeding in some years.
Effects of environmental factors
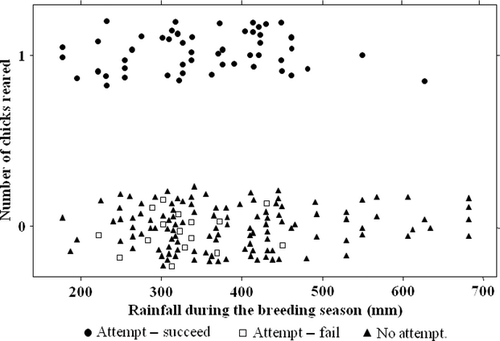
Breeding success was highest in years when rainfall near the nest over the 6 months of the breeding season ranged from 300 to 500 mm, decreasing rapidly when rainfall exceeded 500 mm (Fig. 1). Only two breeding attempts were made (by two different groups) when rainfall exceeded 500 mm (Fig. 1).
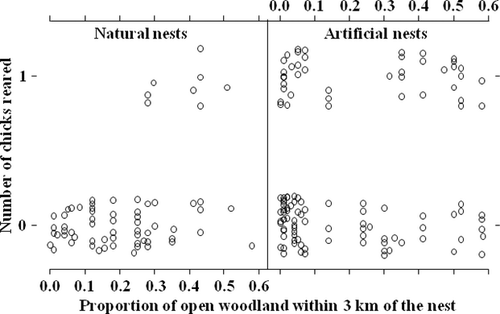
The inclusion of a significant interaction term linking nest type with the proportion of open woodland within 3 km of the nest-site (Table 2) indicated that groups that had artificial nests available to them as well as a relatively large area of open woodland close to the nest were the most successful breeders. In support of this, groups that bred in natural cavities were most successful when they also had access to extensive areas of open woodland (Table 2, Fig. 2). When the proportion of open woodland near the nest was low, groups using artificial nests achieved higher reproductive success than those in natural nests. The combination of an artificial nest and large open areas therefore contributed positively towards reproductive success (Fig. 2).
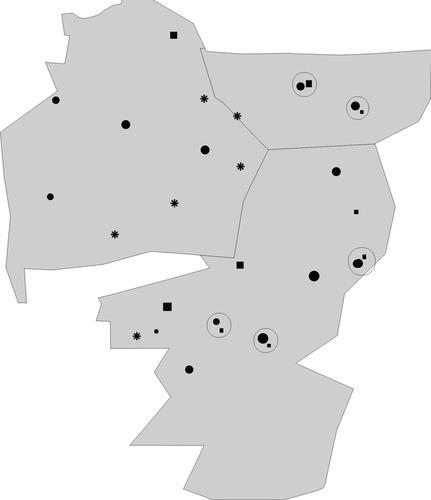
There was no apparent clustering of groups according to breeding success across the study site (Fig. 3): the most successful and least successful groups were scattered across the APNR. The results are therefore unlikely to have been due to some unmeasured factor operating at a larger scale. What is clear from Figure 3, however, is that of those groups that switched nest-site during the study from a natural to an artificial nest, all except one improved their performance as a result. Only one group switched nest-site in the opposite direction, moving from a natural nest to an artificial one, and then back to a natural nest.
Effects of group size
Mean (± sd) group size over the study period was 3.4 ± 1.2 birds, the largest group comprising seven individuals. This falls within the range of group sizes in the Kruger National Park (Kemp et al. 1989), the Eastern Cape (Vernon 1986) and KwaZulu-Natal (Knight 1990). Group size and reproductive success were positively correlated and exclusion of group size from models markedly elevated their AIC values.
Discussion
In recent decades, there has been escalating concern about decreases in the range and numbers of Southern Ground-Hornbills in South Africa (Kemp 1980, 1987, 2000, Kemp & Begg 1996). Statistical models identified three strong positive correlates of reproductive performance by Southern Ground-Hornbills: rainfall over the breeding season, the interaction of nest type with the amount of open woodland available in the nest vicinity and group size.
Reproductive success was determined primarily by environmental factors, although there was no suggestion of spatial autocorrelation in breeding success across the site (Fig. 3). The amount of rainfall over the breeding season significantly influenced the reproductive performance of Ground-Hornbills. In high-rainfall years, reproductive success declined greatly (Fig. 1). Breeding success was highest when rainfall was in the range 300–500 mm, when up to 12 groups (52%) were successful in one breeding season. Reproductive success decreased when breeding-season rainfall fell below 300 mm; in total, only 13 attempts were successful in low-rainfall years. Rainfall is one of the main determinants of food availability (Cockburn et al. 2008, Covas et al. 2008) and, in Ground-Hornbills, precipitates egg-laying (Kemp & Kemp 1991). These factors could explain why breeding performance was highest in years of constant, steady rainfall. High rainfall may have restricted other aspects of the Ground-Hornbills' reproduction, for example by influencing foraging ability. Several of the natural nests are open to the sky, and flooding of eggs and small chicks has been recorded (Kemp & Begg 1996), although it did not occur in our study. However, dense ground cover associated with wet years is likely to lower foraging performance by making walking more difficult, making prey less easy to detect and elevating the risk from ambush predators. The position of the nest could therefore play an important role in facilitating successful breeding, especially if nests are in open areas. The only two successful breeding attempts in high-rainfall years were both in artificial nests sited in very open areas.
Understanding the effects of helpers on reproductive success can be complicated because it is often difficult to separate the true effects of helpers from effects of territory or parental quality (Legge 2000a,b, Cockburn et al. 2008, Covas et al. 2008). Among birds, the beneficial effects of helpers are usually ascribed to the extra food that they bring to the nest (Legge 2000a,b, Canestrari et al. 2008, Covas et al. 2008). High-quality parents and those occupying high-quality territories may be the most successful breeders, with large group size therefore being a consequence rather than a cause of this success (Cockburn 1998, Magrath 2001, Cockburn et al. 2008). This conundrum is unlikely to have confounded the conclusions of this study because group identity was accounted for in all models, thereby accounting for differences in parental quality. Larger groups (more than three individuals) were the most successful, suggesting that these groups may be more efficient at provisioning the dominant female and her chick (i.e. it is a genuine social effect of group size). Larger groups are more likely to contain a larger number of older and therefore experienced helpers.
Since the artificial nestboxes (one per territory) were first placed in the APNR in 2002, they have facilitated successful breeding, even though the sole criterion for their positioning was availability of a tree too large to be toppled by Elephants (and not the presence of open habitats in the immediate vicinity). The seven most successful groups that contributed 60% of the reproductive output all bred in artificial nests. In 2001, before the erection of artificial nests, only three of these same seven groups even attempted to breed (in natural nests), with only one group being successful. Since 2002, all three groups that attempted to breed in 2001 have moved from natural nests to artificial nests, where they now breed successfully. This supports the conclusion that the artificial nests are favoured by the Ground-Hornbills and result in improved breeding success.
Ground-Hornbills may prefer artificial nests to natural nests because (1) they are better protected from rain and direct sunshine, reducing the risks of nests becoming waterlogged or overheating, (2) their positioning and construction make them less accessible to arboreal predators (one natural nest, when not occupied by Ground-Hornbills, is occasionally used as a roost site by a Leopard Panthera pardus, and another by a large African Rock Python Python sebae) and/or (3) they are more spacious for the developing chick. Kemp and Begg (1996) found that attractiveness of natural nests was correlated with features of the nest (nest height and cavity depth) and suggested that the structural arrangement of the entrance or gradient and texture of the inner walls as well as the topography of the nest floor might all influence breeding success. In this study, artificial nests were on average larger than natural nests in terms of overall height (mean ± sd: 610.4 ± 156.1 cm, n = 14) vs. 362.5 ± 114.4 cm, n = 4) but had smaller entry hole widths (26.3 ± 3.2 cm, n = 13 vs. 28.7 ± 8.5 cm, n = 3), and offered shallower cavities (43.5 ± 10.3 cm, n = 13 vs. 57.0 ± 18.0 cm, n = 4). Some of the natural nests lacked side entrances and were accessed from the top of the cavity: these nests thus lacked a roof.
The interaction of nest type with the proportion of open woodland in the nest vicinity also influenced reproductive performance. Groups nesting in natural cavities were most successful when there were extensive areas of open woodland nearby. The amount of open woodland also had a positive influence on groups breeding in artificial nests, but the effect was not as strong. This suggests that having an artificial nest is the more important of the two variables but if groups are nesting in natural cavities, then the amount of nearby open woodland is important for successful reproduction. The most successful combination involves both access to an artificial nest and extensive open habitat surrounding the nest-site. The reason for the positive effect of open habitat is probably food-related. Ground-Hornbill prey items such as snakes, lizards, small mammals and insects may be easier to find and catch in open areas than in dense, shrubby habitats where the mobility of the birds and visibility of prey would both be constrained by habitat architecture. Proximity of prime foraging habitat to the nest would therefore reduce travel costs during the breeding season when the birds are effectively central place foragers. In the adjacent Kruger National Park, most nests are located in isolated, large or dead trees in open areas with bare ground, or with grass of short to medium height (Kemp & Begg 1996). In combination, therefore, these results strongly suggest that the siting of artificial nests within territories could be optimized by placing them close to or within areas of open woodland.
In conservation terms, managing social factors such as group size and helper effects are not practical options, but manipulating environmental factors such as nestboxes and the amount of open woodland are potential management considerations. The fact that the availability and position of artificial nest-sites and the amount of open woodland surrounding the nest-site both contribute positively to breeding performance identifies options for increasing the reproductive output of Ground-Hornbill populations in South Africa and elsewhere. Areas of open woodland need to be identified within territories and nestboxes deployed within or close to these areas. Alternatively, if sites (Elephant-proof trees) for the deployment of nestboxes are limited, then bush clearance (a management tool used for other reasons in the APNR) becomes a potential means of manipulating habitat quality. Interestingly, and subsequent to this study, a group that had failed to rear a single chick from 2001 to 2008 bred successfully in a natural nest that we failed to locate. Unknown to us, the landowner had undertaken considerable bush clearance in the winter preceding that breeding season.
The groups in our study that used artificial nests (sited without bias towards open habitats) achieved more than twice the reproductive output of groups using natural nests, a strong indication that this is a highly effective management tool. If artificial nests were also sited in open areas, we predict that, based on our model of the correlates of reproductive success, this performance would increase, especially in wet years. Provision of such nest-sites should therefore be considered as a priority for short- to medium-term conservation of Southern Ground-Hornbills.
Acknowledgments
This study was made possible through funding provided by the DST/NRF Centre of Excellence at the Percy FitzPatrick Institute of African Ornithology, Dow Southern Africa (Pty) Ltd and the Abax Foundation. This work was additionally supported through bursaries to G.W. from the South African National Research Foundation and the University of Cape Town. Thanks to Doug Schaefer, Quentin Hagens, Sieglinde Rode, Kate Meares and other field workers for data collection and collation; to the wardens and managers in the APNR who provided the rainfall and vegetation data; and to Mike Peel from the Agricultural Research Council who kindly provided the grass biomass data. Thanks to Rowan Martin, Rita Covas and Colin Attwood for assistance with analyses. Field work for the vegetation map was done by Andrew Purchase and the final map was produced by Noel and Gretel Van Rooyen (2005), under the auspices of the University of Pretoria.




