Cytomegalovirus serostatus among people with HIV, characterizing the prevalence, risk factors, and association with immune recovery
Abstract
Introduction
Cytomegalovirus (CMV) infection is common among people with HIV (PWH), and may be associated with negative outcomes. We aimed to identify the seroprevalence of CMV between 01 January 1998 and 01 June 2022 among PWH accessing care at the Southern Alberta Clinic (SAC) and the associated risk factors. We also aimed to assess the impact of CMV seropositivity on CD4+ T-cells and CD4+/CD8+ ratio recovery among PWH who maintain HIV viral suppression.
Methods
Poisson regression models with robust variance estimated crude and adjusted prevalence ratios and 95% confidence intervals to identify risk factors for CMV seronegativity. Among PWH maintaining viral suppression, trends in the median CD4+ T-cell count and CD4+/CD8+ ratio were visualized, and continuous time-to-event Cox proportional hazard models estimated hazards ratios (aHR) for CD4+ cell count recovery to ≥500 cells/mm3 and CD4+/CD8+ ratio of >1 at 10 years by CMV serostatus.
Results
Among 3249 PWH, 2954 (91%) were CMV seropositive. CMV seronegativity was associated with younger ages, male sex, non-Hispanic white race and an education of ≥12 years. While CMV seronegativity did not affect CD4+ T-cell recovery following HIV viral suppression (aHR 1.15 [0.89–1.48]), it was associated with a greater likelihood of CD4+/CD8+ ratio normalization (aHR 2.38 [1.85–3.07]) at 10 years of follow-up.
Conclusions
CMV is a common coinfection among PWH. We found that CMV positivity among PWH maintaining HIV viral suppression, while not associated with CD4+ T-cell recovery, was associated with a reduced CD4+/CD8+ ratio recovery. This suggests an association with chronic CMV infection-mediated immune activation and inflammation among PWH.
INTRODUCTION
Prior to the introduction of modern antiretroviral therapies (ART), cytomegalovirus (CMV) infection was responsible for significant morbidity and mortality in people with HIV (PWH) with severe immunosuppression [1-3]. However, ART has led to robust and durable immune recovery, dramatically decreasing CMV-mediated disease [4]. More recently, however, reports have characterized chronic CMV-mediated immune activation in immunocompetent populations and a potential effect of immunosenescence and chronic low-grade inflammation [5-12]. These studies have also suggested CMV seropositivity may be a factor contributing to non-communicable diseases (NCDs) such as frailty, cancer risk, cardiovascular disease, and cognitive impairment [5-7, 9, 13-20]. In PWH, these NCDs frequently occur at younger ages than in people without HIV (PWoH), and chronic low-grade inflammation is believed to be contributory [21]. The underlying pathophysiology of this difference in NCDs and inflammation among PWH remains unknown; however, we hypothesize CMV may play a role [11, 13].
HIV leads to a depletion of CD4+ T-cells and an expansion of CD8+ T cells, resulting in the reduction in the CD4+/CD8+ T-cell ratio [22, 23]. Despite ART suppressing HIV replication, some PWH may experience slow or minimal CD4+ recovery and CD4+/CD8+ ratio normalization. This T-cell dysregulation has been attributed to persistent subclinical immune activation and inflammation, potentially from low-level residual HIV replication, microbial translocation, or other chronic infections, such as CMV [22]. CD4+ and CD8+ T-cells are regulated through different mechanisms, and therefore, dysregulation may predict prognoses independently of each other [24]. In PWoH, the CD4+/CD8+ ratio declines with age and is correlated with the proportion of senescent CD8+ T-lymphocytes [11, 13]. The long-term effect of CMV seropositivity on CD4+ T-cell recovery and normalization of the CD4+/CD8+ ratio following HIV viral suppression among PWH is unknown [24-26].
We aimed to provide estimates of CMV seroprevalence over time and identify associated clinical and demographic factors in a longitudinal and population-based cohort of PWH in southern Alberta, Canada. We then evaluated the association of CMV coinfection on time to recovery of CD4+ T-cells and normalization of the CD4+/CD8+ ratio among PWH who have attained and maintained HIV viral suppression.
METHODS
Study population
We conducted an observational study utilizing a geographically defined population of PWH ≥18 years attending the Southern Alberta Clinic (SAC). SAC has a comprehensive, longitudinal database that collects demographic, clinical, and laboratory data on cohort participants [27]. Individual-level selection criteria for the study population included PWH who were actively enrolled in care during 01 January 1998–01 June 2022 (study period), and had at least one CMV serologic test. The research protocol (REB22-1691) was approved by the University of Calgary Conjoint Health Research Ethics Board.
CMV seroprevalence
CMV serostatus (IgG) is routinely determined at SAC through intake bloodwork and serostatus is known among 87% of the cohort. All CMV serology tests are processed by a central laboratory (Alberta Provincial Laboratory). Between 1998 and 2020 the Behring CMV EIA kits were used which were reported with the following cutoffs: positive >0.2, indeterminate 0.1 to 0.2, negative <0.1. In October 2020, this centralized laboratory transitioned to using the Abbott Alinity CMV IgG assay for all serology testing with the following cutoffs: negative <6.0 AU/mL and positive ≥6.0 AU/mL. Notably, the sensitivity of this assay varies by population with the manufacturer reporting a >92% sensitivity in transplant populations and 97% in pregnant women with specificity reported at 99% [28, 29]. Repeat serology is done in CMV seronegative PWH to detect seroconversions.
CD4+ and CD8+ T-lymphocyte measurement
Immunofluorescence analysis by flow cytometry was used for CD4+ and CD8+ T-lymphocyte measurement. Flow cytometry identifies cellular populations by characterizing their physical and chemical properties as they flow by optical and/or electronic sensors [30]. Monoclonal antibodies are targeted towards specific antigens on the cell surface that are conjugated to fluorochromes to count specific cells. A centralized regional reference laboratory conducts flow cytometry to enumerate CD4+ and CD8+ cells for all patients accessing care at SAC [31].
Covariates
Sex was defined as biological sex assigned at birth. Data on self-identified gender was not available. Race/ethnicity was self-reported and categorized as non-Hispanic White, Indigenous (First Nations/Métis/Inuit), non-Hispanic Black, and Other (Hispanic, East-Asian, West-Asian, Indo-Asian, other). HIV acquisition risks were hierarchically categorized as people who inject drugs (PWID), gay/bisexual/men who have sex with men (gbMSM), heterosexual, or other (blood transfusion or perinatal transmission). Age at initial known HIV diagnosis as well as at initial CMV serology performed locally was included. Birth region and number of household contacts were self-reported at initial intake into the cohort. Number of household members was defined as living with others or living alone, and was included as CMV is transmitted through body fluids and therefore living with others may increase the risk of exposure [32]. Coinfections and comorbidities were determined from the electronic medical record (EMR) as ever reported at the time of study entry, including hypertension, renal disease, diabetes mellitus, cardiovascular disease, and hepatitis C coinfection. Other covariates obtained from the medical record included the highest recorded education level (as a metric of socioeconomic status [SES]), history of incarceration, and CD4+ nadir. CD4+ cell counts and CD4+/CD8+ ratios were assessed as time-varying outcome variables.
Statistical analysis for prevalence estimates and risk factor analysis
We aimed to evaluate factors associated with CMV seronegativity among PWH. We estimated the annual prevalence between 01 January 1998 and 01 June 2022 of CMV seronegativity. Study entry was defined as the latest of the following: (1) Entry into the SAC clinical cohort, (2) initial CMV testing, or (3) 01 January 1998. Poisson regression models with robust variance (approximating log binomial models) estimated crude and adjusted prevalence ratios (aPR) and 95% confidence intervals (CI) comparing risk factors for CMV seronegativity measured at study entry. Risk factors of interest included calendar year and age at CMV measurement, sex, race/ethnicity, HIV acquisition risk, region of birth, prior history of incarceration, highest education level, and household contacts measured at study entry. Due to small numbers, descriptive statistics were utilized to characterize PWH who seroconverted from CMV seronegative to CMV seropositive during the study period.
Immune recovery by CMV serostatus
We conducted a sub-analysis by CMV serostatus of time following initiation of ART and achieving maintained HIV viral suppression to (a) CD4+ T-cell recovery to cells/mm3 (model 1) (b) CD4+/CD8+ ratio recovery to >1 (model 2). Participants must have had CMV serologic testing and initiated on ART prior to study entry. Study entry was defined the first viral load test result of <200 copies/mL following 01 January 1998. Participants were excluded from this analysis if they had a CD4+ cell count of ≥500 cells/mm3 (model 1) or CD4+/CD8+ ratio >1 (model 2) at study entry. Study exit was defined as the first date the patient reached the study outcome (CD4+ cell count of ≥500 cells/mm3 [model 1] or CD4+/CD8+ ratio >1 [model 2]), had a viral load measurement of >200 copies/mL (as a proxy for ART adherence), the date the patient was lost to follow up (defined as their last CD4+ or HIV viral load measurement prior to a gap of >12 months), date of death, or 01 June 2022, whichever came first. Only participants with at least two CD4+ observations and more than 1 day of follow-up were included.
Trends in the median CD4+ T-cell count and CD4+/CD8+ ratio were visualized from time of first attaining HIV viral suppression to individual study exit (defined above). Continuous time-to-event Cox proportional hazard models estimated crude and adjusted hazards ratios (aHR) with 95% confidence intervals for CD4+ cell count recovery to ≥500 cells/mm3 (model 1) or CD4+/CD8+ ratio of >1 (model 2) at 10 years by CMV seropositivity. Models were adjusted for calendar year, age, sex, race/ethnicity, HIV acquisition risk, history of HCV, hypertension, renal disease, diabetes, cardiovascular disease, CD4+ count at baseline and nadir CD4+ count. A 10-year cumulative incidence of CD4+ cell count recovery to ≥500 cells/mm3 (model 1) as well as CD4+/CD8+ ratio of >1 (model 2) by CMV serostatus was estimated using Kaplan–Meier methods. p-value <0.05 with two-tailed tests were considered statistically significant. All analyses were performed using STATA version 18.0 (College Station, TX).
RESULTS
Study population for prevalence estimates and risk factor analysis
Among 3249 PWH meeting inclusion criteria, we observed 4088 CMV serologic tests with 15% of the population having two or more tests during the study period. The majority (86%) of initial CMV serologic tests were done within 6 months of intake into SAC. On initial serology, 2954 (91%) of PWH were CMV seropositive (Table 1). Of the 295 PWH who were CMV seronegative, 45 (15%) seroconverted following the initial serology from negative to positive during observation in the cohort at a median of 3.5 years (IQR 2.1–6.8 years). Of the remaining 250 PWH who were CMV seronegative, 149 (60%) had one or more repeat CMV serologic testing over a median of 8.1 years (IQR 3.9–14.9 years). Among both males and females and across all age ranges, CMV seropositivity was greater than 80% (Figure 1).
| Total | CMV positive | CMV negative | |
|---|---|---|---|
| N = 3249 | N = 2954 | N = 295 | |
| Year of initial CMV testing | |||
| <2007 | 924 (28%) | 794 (27%) | 130 (44%) |
| 2007–2014 | 1450 (45%) | 1338 (45%) | 112 (38%) |
| >2014 | 875 (27%) | 822 (28%) | 53 (18%) |
| Age at HIV diagnosis (years) | 33 (27–41) | 33 (27–41) | 33 (26–42) |
| <30 | 1183 (36%) | 1073 (36%) | 110 (37%) |
| 30–40 | 1121 (35%) | 1027 (35%) | 94 (32%) |
| 40–50 | 608 (19%) | 556 (19%) | 52 (18%) |
| >50 | 295 (9%) | 262 (9%) | 33 (11%) |
| Missing | 42 (1%) | 36 (1%) | 6 (2%) |
| Age at CMV test (years) | 37 (31–45) | 37 (31–45) | 38 (30–44) |
| <30 | 739 (23%) | 666 (23%) | 73 (25%) |
| 30–40 | 1176 (36%) | 1073 (36%) | 103 (35%) |
| 40–50 | 850 (26%) | 776 (26%) | 74 (25%) |
| >50 | 466 (14%) | 424 (14%) | 42 (14%) |
| Missing | 18 (1%) | 15 (1%) | 3 (1%) |
| Sex | |||
| Male | 2358 (73%) | 2126 (72%) | 233 (79%) |
| Female | 891 (27%) | 828 (28%) | 62 (21%) |
| Race/ethnicity | |||
| Non-Hispanic White | 1527 (47%) | 1300 (44%) | 227 (77%) |
| Non-Hispanic Black | 797 (25%) | 787 (27%) | 10 (3%) |
| Indigenous | 356 (11%) | 322 (11%) | 34 (12%) |
| Other/unknown | 569 (18%) | 545 (18%) | 24 (8%) |
| HIV acquisition risk | |||
| gbMSM | 1274 (39%) | 1211 (41%) | 63 (21%) |
| Heterosexual | 1365 (42%) | 1243 (42%) | 122 (41%) |
| PWID | 456 (14%) | 368 (13%) | 88 (30%) |
| Other | 154 (5%) | 132 (5%) | 22 (8%) |
| Birth region | |||
| Africa/Asia | 917 (28%) | 903 (31%) | 14 (5%) |
| Central/South America | 176 (5%) | 168 (6%) | 8 (3%) |
| North America | 1886 (58%) | 1639 (56%) | 247 (84%) |
| Europe | 87 (3%) | 73 (3%) | 14 (5%) |
| Other/unknown | 183 (6%) | 171 (6%) | 12 (4%) |
| Correctional service | 284 (9%) | 221 (8%) | 63 (21%) |
| Highest education level | |||
| <12 years | 644 (19%) | 578 (20%) | 66 (22%) |
| 12 years | 639 (20%) | 572 (19%) | 67 (23%) |
| >12 years | 1403 (43%) | 1289 (44%) | 114 (39%) |
| Other/unknown | 566 (16%) | 515 (17%) | 48 (16%) |
| Household contacts | |||
| Living alone | 558 (17%) | 513 (17%) | 45 (15%) |
| Living with others | 1795 (55%) | 1655 (56%) | 140 (48%) |
| Other/unknown | 896 (28%) | 786 (27%) | 110 (37%) |
| Comorbidities | |||
| HCV coinfection | 292 (9%) | 236 (8%) | 56 (19%) |
| Hypertension | 222 (7%) | 198 (7%) | 24 (8%) |
| Renal disease | 107 (3%) | 96 (3%) | 11 (4%) |
| Diabetes mellitus | 137 (4%) | 125 (4%) | 12 (4%) |
| Cardiovascular disease | 59 (2%) | 50 (2%) | 9 (3%) |
| T-cell measurements | |||
| Median CD4+ nadir (cells/mm3) | 242 (113–382) | 245 (116–384) | 220 (83–363) |
| Median CD4+ count at baseline (cells/mm3) | 381 (237–544) | 380 (238–542) | 389 (230–557) |
| Median CD4/CD8 ratio nadir | 0.3 (0.15–0.54) | 0.3 (0.15–0.54) | 0.3 (0.13–0.56) |
| Median CD4/CD8 ratio at baseline | 0.48 (0.27–0.81) | 0.47 (0.27–0.78) | 0.67 (0.40–0.99) |
- Abbreviations: gbMSM, gay/bisexual/men who have sex with men; HCV, hepatitis C virus; PWID, people who use injection drugs.
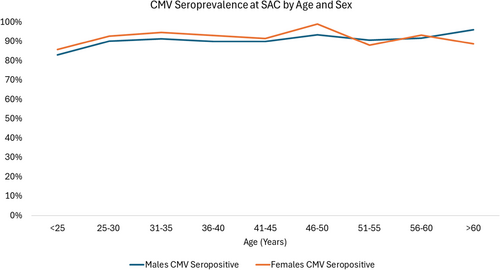
Factors associated with CMV IgG negativity
As CMV exposure is cumulative over time, the likelihood of seronegativity decreases with age, and PWH who were >50 years were 37% less likely (aPR 0.63 [0.44–0.91]) to be CMV seronegative compared with PWH <30 years (Table 2). Compared with non-Hispanic White PWH, non-Hispanic Black (aPR 0.06 [0.02–0.19]) and Indigenous PWH (aPR 0.41 [0.30–0.58]) were less likely to be CMV seronegative. PWH reporting gbMSM HIV acquisition risks had the lowest likelihood of CMV seronegativity, with PWH reporting heterosexual sexual activity, injection drug use, or other risks having a higher likelihood of being CMV seronegative. PWH with lower educational attainment (<12 years vs. 12 years) were less likely (aPR 0.72 [0.53–0.98]) to be CMV seronegative. Having exposure to correctional facilities was associated with CMV seronegativity (aPR 1.42 [1.07–1.89]). CMV serostatus did not differ among PWH who reported living with others compared with those who lived alone.
| cPR (95% CI) | aPR (95% CI) | |
|---|---|---|
| Year of initial CMV testing | ||
| <2007 | Ref | Ref |
| ≥2007 | 0.50 (0.41–0.63) | 0.69 (0.55–0.85) |
| Age at CMV measurement | ||
| <30 | Ref | Ref |
| 30–40 | 0.89 (0.67–1.18) | 0.86 (0.66–1.13) |
| 40–50 | 0.88 (0.65–1.20) | 0.72 (0.54–0.97) |
| >50 | 0.91 (0.64–1.31) | 0.63 (0.44–0.91) |
| Sex | ||
| Male | Ref | Ref |
| Female | 0.71 (0.54–0.94) | 0.67 (0.50–0.89) |
| Ethnicity | ||
| Non-Hispanic White | Ref | Ref |
| Non-Hispanic Black | 0.08 (0.05–0.16) | 0.06 (0.02–0.19) |
| Indigenous | 0.64 (0.46–0.90) | 0.41 (0.30–0.58) |
| Other/unknown | 0.24 (0.14–0.39) | 0.32 (0.16–0.62) |
| HIV risk acquisition | ||
| gbMSM | Ref | Ref |
| Heterosexual | 1.81 (1.35–2.43) | 5.13 (3.72–7.06) |
| PWID | 3.90 (2.88–5.30) | 4.01 (2.85–6.65) |
| Other | 2.89 (1.83–4.56) | 5.63 (3.57–8.89) |
| Birth region | ||
| North America | Ref | Ref |
| Africa/Asia | 0.12 (0.07–0.20) | 0.48 (0.19–1.17) |
| Central/South America | 0.35 (0.17–0.69) | 0.59 (0.21–1.64) |
| Europe | 1.23 (0.75–2.01) | 1.16 (0.71–1.89) |
| Other/unknown | 0.50 (0.29–0.88) | 0.92 (0.46–1.83) |
| Correctional service | 2.84 (2.21–3.64) | 1.42 (1.07–1.89) |
| Highest education level | ||
| <12 years | 0.98 (0.71–1.35) | 0.72 (0.53–0.98) |
| 12 years | Ref | Ref |
| >12 years | 0.77 (0.58–1.03) | 1.02 (0.78–1.34) |
| Other/unknown | 0.81 (0.57–1.16) | 0.96 (0.68–1.36) |
| Household contacts | ||
| Living alone | Ref | Ref |
| Living with others | 1.03 (0.75–1.43) | 0.91 (0.68–1.22) |
| Other/unknown | 1.57 (1.24–1.99) | 1.15 (0.68–1.36) |
- Note: Bold signals statistically significant p-values (<0.05).
- Abbreviations: aPR, adjusted prevalence ratio; CI, confidence interval; cPR, crude prevalence ratio; gbMSM, gay/bisexual/men who have sex with men; PWID, people who use injection drugs.
Incident CMV following study entry
Following engagement with HIV care, 45 CMV seronegative PWH seroconverted. In this population of incident CMV, 93% (N = 42) were male, 84% (N = 38) were non-Hispanic White, and 84% (N = 38) were born in North America. On average, the age at repeat CMV testing that documented seroconversion was 36 years. The majority identified as gbMSM (60%, N = 27), and 13% (N = 6) identified as PWID. Three of these 45 cases of seroconversion resulted in invasive CMV disease, two of which were CMV systemic illness and one CMV retinitis.
Influence of CMV serostatus on immune recovery following HIV viral suppression
With ongoing HIV viral suppression mediated by ART, both the CD4+ T-cell count and the CD4+/CD8+ ratio increase. The median time between HIV viral load measurements did not differ between CMV seropositive (118 days [IQR 85–154 days]) and CMV seronegative (119 days [IQR 89–154 days]) PWH included in this study. Following two years of HIV viral suppression, the median CD4+ count was consistently higher among CMV seronegative compared with seropositive PWH; however, this did not reach a statistically significant difference (Figure 2a). The lowest reported CD4+/CD8+ ratio did not differ by CMV serostatus; however, at the time of HIV viral suppression and consistently following this, the median CD4+/CD8+ ratio was significantly higher among CMV seronegative compared with seropositive PWH (Figure 2b).
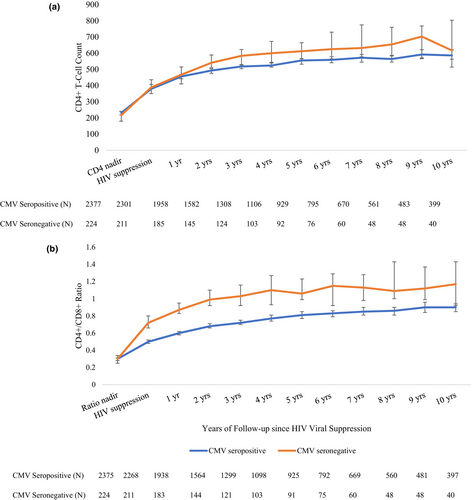
Model 1: CD4+ T-cell trends following HIV viral suppression
Among 2538 PWH who met inclusion criteria for the immune recovery analysis, 787 (31%) had initial CD4+ counts of cells/mm3 and were, therefore, excluded from Model 1. Within 1751 individuals contributing 3953 years of follow-up, we evaluated time to CD4+ T-cell recovery of 500 cells/mm3. Individuals were censored as they reached the study outcome (58%), experienced a detectable viral load (21%), 2% died, and the remainder were lost to follow-up, reached the 10-year follow-up duration or the study end date of 01 June 2022. The median follow-up time was similar among CMV seropositive (1.45 years [IQR 0.55–3.90 years]) and CMV seronegative (1.35 years [IQR 0.55–3.22 years]) PWH. The cumulative probability of achieving a CD4+ count of 500 cells/mm3 within 10 years of observation was 84% among CMV seropositive and 85% among CMV seronegative PWH. We identified no difference by CMV serostatus in the likelihood of reaching a CD4+ count of 500 cells/mm3 (aHR 1.15 [0.89–1.48]) (Table 3, Figure 3a).
| N | % | cHR | 95% CI | aHR | 95% CI | |
|---|---|---|---|---|---|---|
| Model 1: CD4+ recovery to 500 cells/mm3 | ||||||
| CMV seropositive | 1616 | 92.3 | Ref | Ref | ||
| CMV seronegative | 135 | 7.7 | 1.05 | 0.83–1.33 | 1.15 | 0.89, 1.48 |
| Model 2: CD4+/CD8+ ratio recovery to 1 | ||||||
| CMV seropositive | 2002 | 92.3 | Ref | Ref | ||
| CMV seronegative | 167 | 7.7 | 2.20 | 1.74, 2.76 | 2.38 | 1.85, 3.07 |
- Note: Continuous time-to-event Cox proportional hazard models estimated crude and adjusted hazards ratios (aHR) and 95% confidence intervals (CIs) for CD4+ cell count recovery to ≥500 cells/mm3 (yes vs. no, model 1) or CD4+/CD8+ ratio of >1 (yes vs. no, model 2) at 10 years by CMV seropositivity. Models were adjusted for calendar year, age, sex, race/ethnicity, HIV acquisition risk, history of HCV, renal disease, diabetes, cardiovascular disease, hypertension, CD4+ count at baseline and nadir CD4+ count. Bold signals statistically significant p-values (<0.05).
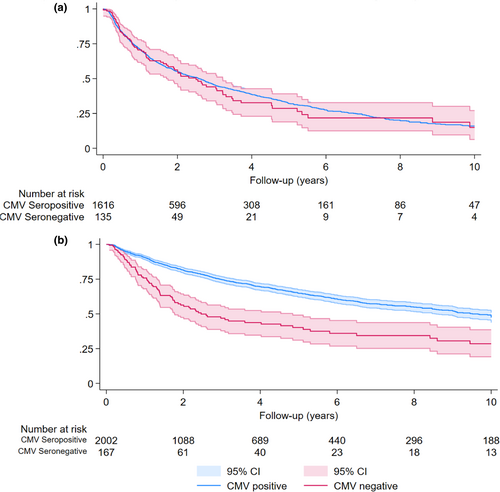
Model 2: CD4+/CD8+ ratio trends following HIV viral suppression
We found that 369 (15%) of the study population had initial CD4+/CD8+ ratios >1 and were excluded from Model 2. Within 2169 individuals contributing 7453 years of follow-up, we evaluated time to CD4+/CD8+ ratio recovery of ≥1. Individuals were censored as they reached the study outcome (32%), or experienced a detectable viral load (29%), 2% died, and the remainder were lost to follow-up, reached the 10-year follow-up duration or the study end date of 01 June 2022. The median follow-up time was longer among CMV seropositive (2.38 years [IQR 0.94–5.38 years]) compared with seronegative (1.65 years [IQR 0.65–3.80 years]) PWH. The 10-year cumulative incidence of CD4+/CD8+ ratio recovery to ≥1 was 53% among CMV seropositive and 72% among CMV seronegative PWH. Compared with CMV seropositive PWH, CMV seronegative were more likely to reach a CD4+/CD8+ ratio of >1 (aHR 2.38 [1.85–3.07]) at 10 years of follow-up (Table 3, Figure 3b).
DISCUSSION
CMV coinfection is common among PWH enrolled in SAC, with 91% of the population seropositive, which is consistently higher than estimates among PWoH [33]. CMV seronegativity did not impact CD4+ T-cell recovery; however, it was associated with normalization of the CD4+/CD8+ ratio, indicating CMV seropositivity may contribute to immune activation and inflammation. A recent study utilized a modelling approach to estimate the CMV seroprevalence in the general population of the United States and Canada, identifying that by the age of 40 years approximately 63% of females and 52% of males are CMV seropositive in the United States, and by age 84 years this increased to 95% among females and 82% among males [33]. We identified in PWH a CMV seroprevalence of >80% among all assessed age groups, which is consistent with other published literature [25, 26, 34].
CMV seropositivity is increasing over time within our cohort; this is likely driven by many factors, including an increasing median age of our study population as well as an increasing proportion of PWH entering the cohort from regions of higher CMV endemicity than North America. There is geographic variability in CMV seropositivity, with higher rates in South America, Africa, Asia, and eastern Europe compared with western Europe and North America [35, 36]. Migrants from higher CMV endemic regions tend to be of younger age compared with PWH from North America, which may, in part, explain why age was not associated with CMV serostatus in unadjusted models; however, following adjustment, increasing age was associated with reduced CMV seronegativity.
In keeping with general population data, we identified a higher CMV seroprevalence among females (vs. males) [33]. In both Canada and the United States, prior studies have identified the lowest CMV seroprevalence among non-Hispanic White populations [33, 37, 38]. The sex and racial/ethnic differences in CMV serostatus are believed to be related to breast-feeding and childcare practices, sexual behaviors, networks, and household contacts [37]. As a marker of SES, an inverse association between a higher level of education and CMV seroprevalence has been identified and is also reflected in our findings [33]. We identified that PWH with prior exposure to correctional services were more likely to be CMV seronegative, as CMV is transmitted through personal contact via body fluids [33, 37, 38]; it is possible that CMV exposure is reduced with the limited personal interaction within correctional facilities. However, due to the small number of individuals with exposure to correctional services, further studies are needed to explore this association.
There is increasing evidence among both PWoH and PWH that CMV seropositivity has a role in persistent immune activation and an association with inflammation-mediated morbidity and mortality [1, 7, 12, 39]. While CMV seronegativity was not associated with CD4+ T-cell recovery following HIV viral suppression, it was associated with a greater likelihood of normalization of the CD4+/CD8+ ratio to ≥1. CD4+ T-cell decline with HIV infection is a predictive marker of immune deficiency and risk of experiencing opportunistic infections, whereas CD8+ T-cells are associated with immune activation and inflammation and are predictive markers of NDCs such as non-AIDS defining cancer, cardiovascular disease, and frailty. A higher CD4+/CD8+ ratio is reflective of both improved immunity and reduction in chronic inflammation. These observations support the growing evidence that CMV seropositivity may be a clinically relevant driver of persistent immune activation and inflammation in treated HIV that is associated with an increased risk of NCDs [7].
A recent randomized controlled trial evaluated the effect of using letermovir, an antiviral agent against CMV among PWH who were HIV virologically suppressed, and identified an increased CD4+ count recovery and increased CD4+/CD8+ ratio among those who received letermovir. This study also identified a reduction in inflammatory markers that are associated with cardiovascular and cancer risk and improved physical function among PWH who received letermovir [40]. Prior studies have evaluated the one-year and three-year association of CMV seropositivity on CD4+ T-cells and CD4+/CD8+ ratio among PWH, identifying an inverse correlation between CMV seropositivity and immune recovery [25, 26]. A cross-sectional study identified that a CD4+/CD8+ of <0.5 was twice as likely to be seen among PWH with CMV seropositivity, whereas a ratio of >1.5 was 3 times as likely to be identified among CMV seronegative PWH [34]. Some suggest this may be explained by socioeconomic disparities associated with both a higher CMV seroprevalence and worse adherence to ART resulting in the identified T-cell dysregulation [26]. However, by only including PWH who had achieved and maintained HIV viral suppression, we were able to evaluate T-cell trends independent of detectable HIV viral replication (a proxy for ART adherence).
There are several limitations to this work. This is a single-centre study and includes only PWH who are engaged in HIV care, which may limit the generalizability of our findings. This study was conducted in Canada, a high-income country; CMV seropositivity is likely higher in lower-income countries, and therefore, our findings may not be generalizable to those settings. Only individuals who had initiated ART and achieved HIV viral suppression were included in the immune recovery analyses; therefore, our findings are not generalizable to individuals not on ART or with active HIV viral replication. As HIV viral replication has known impacts on immune recovery, we chose to include having a detectable HIV viral load as a censoring event. Due to this, the number of individuals included in later years of follow-up had decreased; however, the longitudinal nature of this work to assess the long-term impacts CMV seropositivity has on immune recovery is considered a strength of this study.
CMV seroprevalence is linked to many factors including breastfeeding, contact with children, occupation type, and socioeconomic status, which we did not have data on, and may result in residual confounding. Not all CMV seronegative PWH had repeat CMV serology; therefore, seroconversion may have been underestimated. Estimates also suggest that the seroconversion window to the development of IgG antibodies to CMV is 2–3 weeks; therefore, if PWH were in the seroconversion window at the time of testing, they may have been falsely categorized as CMV seronegative. Full data were not available to assess CD8+ cells directly; however, as we did have data on both CD4+ and CD4+/CD8+ ratios, we used these measures to make inferences about CD8+ cell counts. Due to data limitations, we assessed CMV seropositivity; however, future studies are needed to evaluate CMV viremia on immune recovery among PWH.
CONCLUSIONS
We identified high seroprevalence of CMV among PWH which was associated with a reduced CD4+/CD8+ ratio normalization, suggesting CMV seropositivity is associated with immune activation and inflammation among PWH who maintain HIV viral suppression on ART. CMV co-infection may, in part, contribute to the multifactorial pathogenesis resulting in heightened chronic inflammation and high rates of NCDs experienced by this population.
AUTHOR CONTRIBUTIONS
All authors contributed to the content and writing of this manuscript. RL, MJG, and MDP conceptualized the study. RL, KF, BB, and HBK collected data. RL analysed the data with input and support from SBC. All authors reviewed the analyses and results. JQ and RL drafted the manuscript. All authors reviewed, edited, and approved the final version.
FUNDING INFORMATION
This study was funded by VPR Catalyst grant, University of Calgary, Calgary, Alberta, Canada.
CONFLICT OF INTEREST STATEMENT
A portion of this work was presented as a poster at CROI 2025 in San Francisco USA. MJG declares he serves as an ad hoc member of HIV national Advisory boards to Merck, ViiV, and Gilead. All other authors report no conflicts.
ETHICS STATEMENT
The research protocol (REB22-1691) was approved by the University of Calgary Conjoint Health Research Ethics Board.
Open Research
DATA AVAILABILITY STATEMENT
Due to confidentiality and privacy restrictions, datasets are not available.




