Intraindividual variability of CD30 expression in mycosis fungoides –implications for diagnostic evaluation and therapy
Abstract
Aims
To evaluate the frequency, subcellular localization, and variability of CD30 expression in mycosis fungoides.
Methods
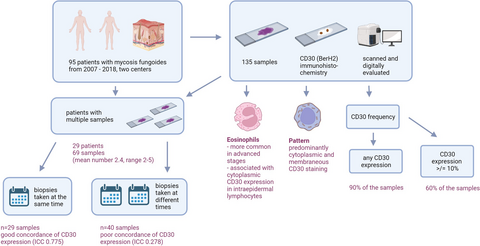
This retrospective multicenter study investigated 135 biopsy specimens of 95 patients with mycosis fungoides for CD30 expression and CD30 staining pattern in relation to histomorphologic criteria, eosinophils, and tumour stage. We used two different CD30 cutoffs: (i) any CD30 positivity (important for in-label treatment with an anti-CD30 antibody-based drug), and (ii) ≥10% (most commonly used cutoff in therapy studies). The histologic slides were digitally evaluated with a slide viewer. None of the patients were treated with an anti-CD30 antibody-based drug.
Results
We found any CD30 expression in 90% of the samples (tumour cells and tumour microenvironment). More than 60% of the samples had CD30 expression ≥10%. CD30 staining was predominantly cytoplasmic and membranous, a Golgi pattern was only found in three samples. In patients with multiple biopsies (69 samples), a highly variable CD30 expression was found, especially in biopsies taken at different timepoints. CD30 and eosinophils were more common in advanced disease stage and cytoplasmic CD30 staining in intraepidermal lymphocytes was significantly associated with the presence of eosinophils.
Conclusions
90% of mycosis fungoides biopsies showed CD30 expression and are principally eligible for anti-CD30-antibody treatment. Because of the high intraindividual variability, investigation of multiple tissue samples for CD30 expression improves the assessment of this therapeutic target. Analysis of only a single skin biopsy might lead to misinterpretation of CD30 and bears the risk of inadequate and potentially unfavourable therapeutic decisions.
Graphical Abstract
Introduction
Mycosis fungoides (MF) is the most common form of cutaneous T-cell lymphoma (CTCL).1 An effective systemic therapy is crucial, especially in cases with disease progression. In the last years, targeted therapies came into focus and a reliable and standardized evaluation of biomarkers is gaining importance. Biomarkers, such as CD30, are heterogeneously expressed in MF (reviewed in Kampa & Mitteldorf)2 and the interpretation and comparability of previous data on CD30 expression in MF is limited.2-4
Brentuximab vedotin (BV) is an anti-CD30 antibody drug conjugate, which was approved for treatment of CD30-positive CTCL after at least one previous systemic therapy. BV is especially used in patients with generalised cutaneous disease or extracutaneous spread, but it is also approved for early stages of MF. A minimal number of CD30-positve cells for the use of BV was not defined, i.e. also patients with very low CD30 expression can be treated in-label with BV.
CD30 belongs to the tumour necrosis factor receptor superfamily. It induces trimerization of TRAF (TNF-receptor associated factors)-binding proteins (TRAF1, 2, and 5). This leads to signal transduction via MAPK/ERK1/ERK2 (mitogen-activated protein kinase/extracellular signal-regulated kinase pathway 1, 2) and gene transcription mediated by NF-κB (nuclear factor kappa-light-chain-enhancer of activated B-cells).5 CD30 is expressed in different types of hematologic malignancies, e.g. (primary cutaneous) anaplastic large T-cell lymphoma and has different biological effects, which are also related to the CD30-expressing cell type or the type of neoplasia.5 The expression and function of CD30 is also related to the cytokine milieu and the composition and function of the tumour microenvironment (TME).6 A link between eosinophils and pathogenesis of MF was suggested, because TME in MF displays a TH2 polarization, which can be promoted by eosinophils.
Material and methods
Study design and patients
This multicenter retrospective study included 135 tissue samples from 95 patients with MF diagnosed from 2007 to 2018 in the Department for Dermatology, Venerology and Allergology, University Medical Center Göttingen and in Kempf & Pfaltz Histologische Diagnostik Zürich. All MF tissue samples of this time-period were collected retrospectively from the authors` case reports. Only samples with a confirmed diagnosis by clinical-pathological correlation were included.
The following clinical parameters were collected in an anonymized form: age, gender, location of the biopsy, date of the biopsy and the tumour stage (TNM classification).
The study was approved by the local Ethics Committees (1/9/18: Ethics Committee of the University Medical Center Göttingen, 2014–0493: Cantonal Ethics Committee Zurich).
Histology and immunohistochemistry
The tissue samples were fixed in 10% buffered formaldehyde, embedded in paraffin, and stained with haematoxylin and eosin (H&E). Immunohistochemical staining for CD30 (BerH2; Medac, Germany, dilution 1:50) was performed according to the manufacturer's protocol.
All samples were scanned with a 3DHISTECH slide scanner and digitally evaluated with the 3DHISTECH slide viewer. The slides were evaluated by one person (F.K.).
Histomorphological criteria
The samples were categorized in patch, plaque, and tumour. Epidermotropism, e.g. the infiltration of the epidermis by atypical lymphocytes (i.e. with cerebriform nucleus and larger than “normal” lymphocytes) was assessed. Basal epidermotropism (predominant distribution of the atypical lymphocytes in the basal third of the epidermis), the presence of lining up (line-up of at least four atypical lymphocytes along the dermoepidermal junction zone), pagetoid spread (scattered distribution of atypical lymphocytes in the epidermis), and the presence of Pautrier's micro-abscesses (clusters of at least three atypical lymphocytes in the epidermis) were evaluated. Moreover, the presence of eosinophils was documented.
Evaluation of the CD30 expression
CD30 expression was assessed separately for the (i) epidermis, (ii) dermis, and the (iii) entire biopsy, because a previous study indicated that the functional and prognostic impact of CD30 expression was related to its epidermal or dermal location.7
(i) Epidermis: CD30-positive cells in relation to CD3-positive cells per five HPF. (ii) Dermis: in analogy to evaluation of PD-L1 expression (see PD-L1 IHC 28–8 pharmDx Interpretation Manual), the slide was divided into quadrants and CD30 expression was given in percentage. In contrast to PD-L1 expression, cytoplasmic and Golgi stains were considered too. (iii) The total CD30 expression was calculated, taking the proportion of epidermis and dermis into account.
CD30-negative cases were defined as those without any CD30-postive cell. To identify such cases is important, as they are not eligible for in-label treatment with BV. Moreover, all cases were assigned to different expression levels of CD30 according to the following cutoff values: ≥10% in the entire infiltrate (most commonly used cutoff in therapeutic studies), as well as cutoffs for epidermal (14%) and dermal (4.7%) lymphocytes as mentioned by Edinger et al.7 Membrane-related, cytoplasmic, and Golgi staining patterns were also assessed.
We evaluated the variability of CD30 expression in patients with multiple biopsies by using the interclass correlation coefficient (ICC). The ICC reflects an agreement of expression levels between two samples. An ICC >0.70 represents a good, 0.40–0.70 a moderate, and <0.40 a poor agreement.8
Statistics
The program IBM SPSS Statistics v. 25 (Armonk, NY, USA) was used for the statistical analysis. A confidence level of 95% corresponding to an α-level (α = 0.05), was set for the statistical evaluations.
Binary logistic regression was used to compare the variability of CD30 prevalence between samples taken at different timepoints and samples taken at the same timepoint.
The correlation of the CD30 expression values within (i) the group of patients with biopsies at the same time, and (ii) the group of patients with biopsies performed at different times was calculated by the ICC. ICC estimates were made based on mean (k = 2), absolute agreement, and 2-way mixed effect models.
To evaluate the significance of the membrane staining in the dermis and epidermis, the McNemar test was used for two dependent samples in the case of dichotomous data.
The single-sample t-test was used to calculate the frequency of exclusive and additional membrane staining in the epidermis and dermis.
The question of the association of eosinophils on CD30 expression was analysed using mixed ANOVA.
The Wilcoxon test was used to check whether epidermal and/or dermal membrane staining was associated with eosinophils in the samples.
All other questions were examined using the Mann–Whitney U test.
Results
Patients' and clinical characteristics
One hundred thirty-five tissue samples from 95 patients were investigated; 41% (39/95) of the patients were female, the median age was 64 years (28–92); 82 (86%) had classical and 13 (14%) had folliculotropic MF. None of the patients received treatment with the CD30-directed antibody drug conjugate brentuximab vedotin (BV) before or at the time of biopsy.
In all, 67 (50%) of the biopsies were taken from the trunk, 33 (24%) from the legs, 10 (9%) from the arms, and 13 (10%) from the head or neck. No information on the collection site was available from nine tissue samples (7%).
The disease of 30 patients were in stage Ia, 39 in Ib, 0 in IIa, 18 in IIb, 2 in III, and 0 in stage IV. In six patients no stage was documented.
Histomorphologic criteria
Overall, 59% of the biopsies were taken from a patch, 32% from a plaque, and 9% from a tumour. Large-cell transformation (LCT) was found in eight of 133 samples (6%). Epidermotropism could be evaluated in 133 biopsies. The results are shown in Table S1.
CD30 expression
CD30 positivity rates
CD30 expression was observed in 122 of 135 samples (90%). A cutoff of ≥10% was exceeded by 81 samples (60%). The mean number of CD30-positive cells was higher in tumour than in patch/plaque stage (P = 0.006), but no differences were found in tissue samples with or without LCT (P = 0.130). Interestingly, 13% of the patches and 17% of the tumours were CD30-negative, while only 2% of the plaques showed no CD30 expression. A stage related (TNM and histologic stage) overview of CD30 expression is given in Table 1.
| CD30+ samples (%) | CD30− samples (%) | |
|---|---|---|
| Histological stage | ||
| Patch (n = 80) | 70 (88%) | 10 (12%) |
| Plaque (n = 43) | 42 (98%) | 1 (2%) |
| Tumour (n = 12) | 10 (83%) | 2 (17%) |
| TNM stage* | ||
| Ia (n = 30) | 25 (83%) | 5 (17%) |
| Ib (n = 39) | 34 (87%) | 5 (13%) |
| IIb (n = 18) | 15 (83%) | 3 (17%) |
| III (n = 2) | 2 (100%) | 0 (0%) |
- * None of the patients were in stage IIa or stage IV.
Table S2 shows the distribution of the samples using the cutoff values mentioned by Edinger et al. (4.7% dermal and 14% epidermal) in relation to the TNM stage.
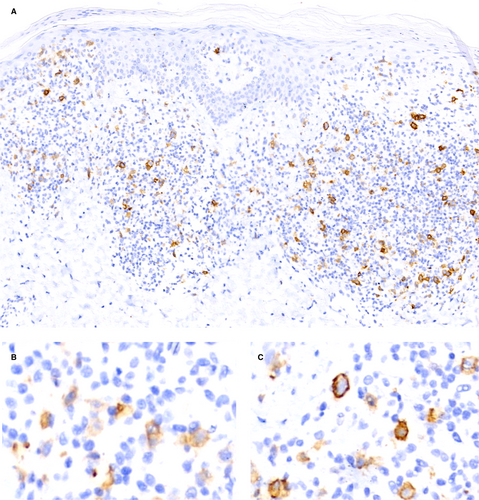
CD30 expression pattern
The predominating expression pattern was cytoplasmic in epidermal (96%) and dermal (81%) lymphocytes (Figure 1A–C). Membrane staining was often combined with cytoplasmic staining (P < 0.001), but was more common in dermal (75%) than in epidermal lymphocytes (41%) (P < 0.0001; χ2 = 36). A Golgi stain was only found in three samples (one patch, one plaque, one tumour) and only in single cells (<1%).
CD30 prevalence (epidermal versus dermal lymphocytes)
In general, CD30 expression was predominantly located in dermal lymphocytes (56% of the samples). In all, 15% of patch and 16% of plaque stages had a predominating CD30 epidermal expression.
CD30 in patients with multiple biopsies
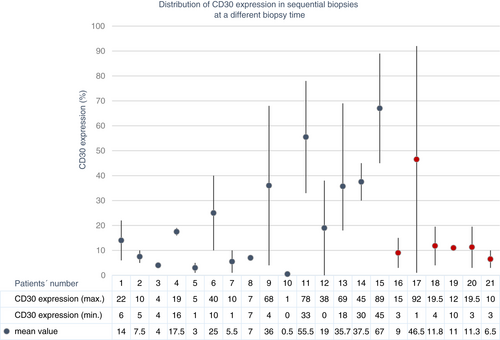
Twenty-nine patients had at least one additional biopsy taken (mean number of biopsies: 2.4, range: 2–5, total number of biopsies: 69). Seven patients had only samples taken at the same time; 15 patients had only samples taken at different times; and six patients had samples taken at the same and at different times. One patient had only CD30-negative samples.
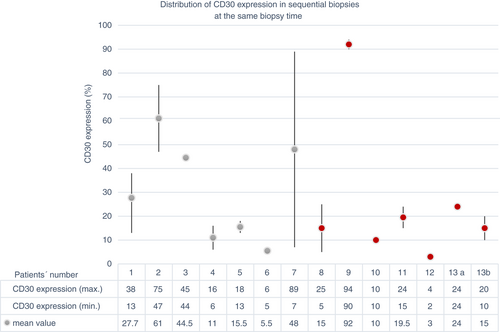
The ICC showed a good concordance of CD30 expression (ICC = 0.775, Figures 2, 3) in biopsies taken at the same time. In contrast, the concordance was poor in biopsies taken at different times (ICC = 0.278, Figure 4, 5). Moreover, the variability of the CD30 prevalence (predominating epidermal versus dermal CD30 expression) was significantly higher in biopsies taken at different rather than at the same time (P = 0.01).
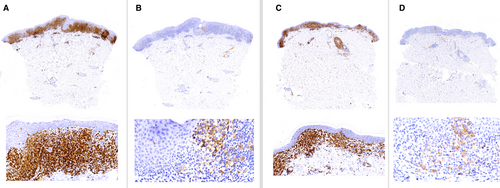
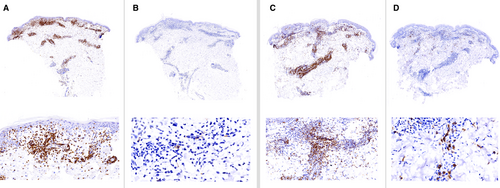
Eosinophils
In 42 of 133 biopsies (31%), eosinophils could be observed in the TME. There was no correlation between the presence of eosinophils and CD30 expression. However, patients with eosinophils showed cytoplasmic more often than membrane staining in epidermal (P < 0.001), but not in dermal lymphocytes (P = 0.225). Eosinophils were significantly more common in tumour (58%) than patch (23%) stage (P = 0.014; Z = −2468; U = 314).
In patients with multiple biopsies, eosinophils were found in only in 17 of 69 (25%) samples. Three patients had eosinophils in all samples, 10 patients had eosinophils only in some samples, and 16 patients had no eosinophils in any of their samples.
Discussion
Although CD30 expression in MF was investigated in several studies, the results cannot be compared due to a missing or heterogeneous definition of CD30 positivity, the investigation of different tumour stages, or inclusion of different lymphoma entities.2 Only eight of 36 studies that investigated CD30 expression in MF and/or Sézary syndrome mentioned cutoffs, which, however, varied from 1%, 5%, and 10–50%.9-15 These heterogeneous cutoff levels are also related to different goals of the studies. In general, in diagnostic settings the thresholds are higher than in therapy studies, which often used a cutoff of 10%. In our study only 10% of the samples were completely negative for CD30. This is similar to the results from Raghavan et al. (CD30 negativity 11%) in 60 MF biopsy samples, but restricting that the proportion of MF with LCT was higher.10
Concerning the prognostic meaning of CD30 expression, the results are heterogenous. While in classical MF (without LCT), three studies found no prognostic relevance14, 16, 17; three others reported an unfavourable impact of CD30 expression.7, 13, 18 Edinger et al. investigated 47 patients and showed that dermal CD30 expression >4.7% was associated with reduced survival, higher stage at diagnosis, and higher maximum stage.7 In contrast, epidermal CD30 expression >14% showed a trend for longer survival.7 When applying these cutoffs to our collective, we could not see a significant stage relation.
Most studies in MF with LCT indicated that CD30 expression was associated with a better prognosis (for details see Kampa & Mitteldorf2) and some authors mentioned CD30 expression as a clue for LCT. Although our study included only eight samples with LCT, the mean CD30 values did not differ from samples without LCT, and the mean CD30 expression was higher in tumour stage, in general, than in LCT.
Knowledge about the probability of CD30 expression is important if the anti-CD30 antibody drug conjugate BV is considered as a therapeutic option. CD30 detection is required for in-label-treatment of CTCL with BV, but no definite cutoff value has been defined. Regarding the relationship between CD30 expression levels and therapeutic response to BV, published data are conflicting. Some authors observed comparatively low response rates in MF, with an expression of <5% of the tumour cells.19 In contrast, others found a therapeutic response regardless to CD30 expression.20, 21 A recent subanalysis of the ALCANZA trial compared baseline CD30 expression (<10% versus ≥10%) in patients with multiple biopsies.22 They found that BV has also a high clinical activity in low CD30 baseline.
Also in other CL entities, examination of immunohistochemical CD30 expression is not a good predictor for clinical response to BV.23, 24 Responses in cases with no or low CD30 expression have been attributed to heterogeneous CD30 expression, insufficient detection by immunohistochemistry, or cytotoxic effects due to the release of monomethyl auristatin E (MMAE).24, 25 Moreover, reactive lymphocytes and macrophages can express CD30. The release of MMAE is not restricted to a binding on CD30-bearing tumour cells and the released drug does not only attack tumour cells but will also influence the TME.
Bartlett et al. found that diffuse large B-cell lymphomas showed a 31% ORR (overall response rate) and a 12% CR (complete response) to BV. All samples were CD30-negative by conventional immunohistochemistry, but with computer-assigned digital image analyses low levels of CD30 were detectable in 11 of 16 responders.26 There was no response to BV in mediastinal B-cell lymphomas with high CD30 expression.27 These results indicate that response rates do not only depend on the CD30-positivity rate, but may also reflect different functions of CD30 in tumours and may be related to the composition of the TME.
CD30 expression is not static and varies in different biopsies in one patient.22, 28, 29 In our collective, this variation was not only related to the stage of the disease but depended on the time of biopsy. We found that the prevalence of CD30 localisation and the agreement of CD30 expression was higher in samples, which had been taken at the same than at different times. Rahbar et al. investigated only samples taken at the same time and showed a higher variability when the biopsies were taken from the same than from different lesions.29 Although the mean level of agreement was better in intra- than in interlesional comparisons, it was not excellent. Again, these finding may be due to heterogenous biological, histological, and clinical characteristics.29 Differences in the composition of the TME (e.g. eosinophils) and a variable cytokine profile (e.g. Il-4) might be additional reasons. All of our patients with multiple biopsies received at least intermittent topical steroids or UV-light-therapy, and some of them were also treated systemically (interferon-alpha, methotrexate, bexarotene, and/or gemcitabin). All of these drugs will influence the tumour cells directly and also the TME. This can also result in differences in CD30 expression, but none of the patients received a specific treatment with the anti-CD30-antibody BV.
In one-third of our samples, we found admixed eosinophils in the TME. Eosinophils can release interleukin (IL)-4, which promotes TH2 polarization and triggers CD30 expression on T-cells via T-cell receptor and IL-4 receptor costimulation.30 MF is a disease with TH2 polarization and a recent study has shown that STAT6 (signal transducer and activator of transcription 6) signalling is important in both pathogenesis and progression of MF.31, 32 The STAT6 pathway activates, not only IL-4 and IL-13 signalling on T-cells, but also regulates gene expression of tumour-associated macrophages, which can also express CD30.31 Moreover, in tumour stage, but not in early-stage MF, IL-4 mRNA was identified.33 In tumour stage, the infiltrate consists predominantly of TH2 tumour cells. In contrast, in early-stage MF, the neoplastic TH2 cells present only a minority of the infiltrate, and reactive TH1 cells release interferon-gamma, which downregulates neoplastic T cells proliferation and reduces IL-4 liberation, and therefore also CD30 expression.33 Our data confirmed that CD30 expression and eosinophils are more common in advanced rather than in early stage MF. We suspect that this is directly related to activation of STAT6 and liberation of TH2 cytokines (e.g. IL-4). Surprisingly, we found no general coexistence of CD30 expression and eosinophils, but cytoplasmic CD30 expression in epidermal lymphocytes was significantly more common in biopsies with eosinophils. The reason for this finding is unclear.
Commonly used monoclonal antibodies directed against CD30 (e.g. BerH-2) primarily stain the cell membrane and sometimes also weakly the cytoplasm.5 Moreover, reactivity of the Golgi apparatus is well known and in many lymphoproliferative disorders (e.g. Hodgkin's lymphoma, anaplastic large T-cell lymphoma) in addition to membrane staining. It has been suggested, that cytoplastic staining reflects a precursor protein.5 We postulate that different CD30 staining patterns (cytoplasmic, cell membrane, Golgi) result from different regulative mechanisms and variable functions of CD30. In contrast to (primary cutaneous) anaplastic large cell lymphoma and Hodgkin's lymphoma, in our cohort a staining of the Golgi apparatus was only found in very few samples and only in the minority of CD30+ cells (less than 1%). If a Golgi staining pattern of CD30 is a helpful additional parameter to differentiate MF from CD30-lymphoproliferative disorders and reactive conditions has to be explored.
In sum, CD30 expression is detectable in 90% of MF samples and 60% of the samples showed a CD30 expression of at least 10%. The CD30 staining pattern was predominantly membranous and cytoplasmic; a Golgi stain was extremely rare in MF.
In patients with multiple biopsies, CD30 expression vared significantly more, if the biopsies were taken at different timepoints than at the same time. CD30 expression and eosinophils were more common in tumour than in patch stage and cytoplastic CD30 staining in intraepidermal lymphocytes was correlated with the presence of eosinophils.
Our results show that most MF patients are potentially eligible for anti-CD30-antibody treatment, but multiple biopies improve the assessment and interpretation of CD30 frequency. Especially in lesions which were initially negative for CD30, evaluation of multiple biopsies can avoid that a potentially effective therapy is unnecessarily withheld to a patient.
Acknowledgement
We thank Dipl.-Psych. Frederike Stucke for her support with the statistical analysis.
Author Contributions
CM: Substantial conception and design, acquisition of data, analysis, and interpretation of data, drafting the article, revising it critically for important intellectual content, and final approval of the version to be published. FK: Acquisition of data, analysis and interpretation of data, revising it critically for important intellectual content, and final approval of the version to be published. PS: Contributions to conception and design, interpretation of data, revising it critically for important intellectual content and final approval of the version to be published. MS: Contributions to conception and design, interpretation of data, revising it critically for important intellectual content, and final approval of the version to be published. WK: Substantial contributions to conception and design, acquisition of data, interpretation of data, revising it critically for important intellectual content, and final approval of the version to be published.
Funding
None.
Disclosure
No study sponsoring.
Conflict of Interest
Advisory and/or lecture honoraria and/or travel support and/or support of meetings, involvement in studies. CM: Kyowa Kirin, Takeda, Recordati rare diseases, 4SC, Stemline. FK: nothing to declare. PS: nothing to declare. MS: nothing to declare. WK: Takeda, Switzerland, Stemline.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
IRB: approved the study (1/9/18 and 2014–0493).