Impact on colorectal cancer pathology reporting practice of migration from TNM 5 to TNM 8
Abstract
Aims
The introduction of TNM 8 into UK pathology practice in January 2018 considers tumour deposits in colorectal cancer staging for the first time. The impact of this new classification on pathology reporting practices has yet to be evaluated.
Methods and results
A clinical audit was conducted, comparing consecutive colorectal cancer resection specimens reported under TNM 5 classification guidelines in 2017 (n = 177) and TNM 8 guidelines in 2018 (n = 234). Tumour features (venous invasion, perineural invasion, lymph node metastatic disease, tumour deposits) and changes in reporting practices were evaluated among four specialist gastrointestinal pathologists working within a large pathology department. Adoption of TNM 8 practice led to an approximate doubling in the use of ancillary stains (41.0% of TNM 8 versus 22.0% of TNM 5 cases, P < 0.001) to help evaluate tumours. A narrowing of the range between pathologists was observed in reporting cases as having one or more form of regional, extramural, discontinuous tumour (TNM 5 range = 50.0–79.0%, TNM 8 range = 57.8–65.7%), with no change in the overall proportion of cases reported as such (62.7% versus 62.4%, P = 0.95). However, significant interobserver variation in reporting rates for individual parameters remained.
Conclusion
TNM 8 colorectal cancer staging offers potentially greater reproducibility in pathology reporting of regional, extramural, discontinuous disease with similar proportions of patients reported as having one or more of these forms of tumour spread compared with TNM 5. Further guidance in defining individual features is required to reduce interobserver variation in pathology assessments and to help elucidate the clinical significance of each parameter.
Introduction
The anatomical extent of tumour invasion, locally, within regional lymph nodes or distant metastases [tumour–node–metastasis (TNM) stage], remains the most powerful predictor of outcome for patients with colorectal cancer (CRC). Foci of tumour discontinuous from the primary tumour but within pericolic or perirectal fat, now called tumour deposits (TD), have been defined and classified variably within different editions of the Union for International Cancer Control (UICC) TNM classification. This directly impacts the handling and interpretation of CRC cases by pathologists and, in turn, cancer staging and potentially patient care.
The fifth edition of the UICC classification (TNM 5), published in 1997, was the first to address discontinuous foci of CRC and considered any such tumour nodule measuring greater than 3 mm in diameter, without microscopic evidence of residual lymph node tissue, to be classified under the pN category as regional nodal metastatic disease.1 Those nodules less than 3 mm were classified under the pT category, interpreted as discontinuous primary tumour extension.
The sixth edition of UICC TNM (TNM6), published in 2002, discounted size but classified discontinuous tumour nodules on the basis of contour, with smooth rounded nodules considered nodal metastases and nodules with irregular contours considered likely to represent extramural venous invasion (EMVI), the latter classified under the V1 (microscopic venous invasion) or V2 (macroscopic venous invasion) coding systems.2 The seventh edition of UICC TNM (TNM 7), published in 2009, formally introduced the term ‘tumour deposits’, defined as discrete macroscopic or microscopic nodules of cancer in the pericolorectal adipose tissue's lymph drainage area of a primary carcinoma that are discontinuous from the primary and without histological evidence of residual lymph node.3 TDs were considered to represent discontinuous spread, venous invasion or totally replaced lymph nodes. Such nodules with a smooth contour could still be interpreted as nodal metastases, rather than TDs, regardless of size, retaining this TNM6 ruling. Importantly, any nodules interpreted as TDs under TNM 7 were to be recorded under the pN category as pN1c, provided that all identifiable lymph nodes showed no metastatic disease. Given concerns over a lack of evidence base for introducing these changes and over reproducibility, some countries, including the United Kingdom, did not adopt TNM6 or TNM 7 and continued to apply TNM 5 staging for CRC.4-6
The eighth edition of UICC TNM (TNM 8), published in 2017, introduced more clarity to the definition of TD, adding to the TNM 7 definition that TDs should also lack histological evidence of identifiable vascular or neural structures, or residual lymph node tissue.7 Furthermore, if a vessel wall is identifiable on haematoxylin and eosin (H&E), elastic or other stains, under TNM 8 it should be classified as EMVI or extramural lymphatic invasion (EMLI) and, similarly, if neural structures are identifiable, the lesion should be classified as extramural perineural invasion (EMPNI). Evidence from a systematic review and meta-analysis also indicated the now undoubted prognostic significance of TDs, representing a stronger predictor of adverse outcome than either lymph node metastatic disease or EMVI alone, and offering additional prognostic value in combination with these.8 For these reasons, UICC TNM 8 staging was recommended by the Royal College of Pathologists (UK) for implementation from 1 January 2018.9
This more rigorous assessment of discontinuous tumour foci is likely to have brought about changes in pathology practice, with respect to processing and reporting of CRC resections specimens. This is the focus of the current study, which examines consecutive cohorts of such cases, reported pre- and post-TNM 8 introduction within a large UK pathology laboratory. Changes in approaches to ordering ancillary stains to facilitate interpretation of discontinuous tumour foci and the impact on reporting profiles for key morphological features are compared within a group of four specialist gastrointestinal pathologists.
Methods
Study Population
This study represents a clinical audit of pathology reporting of CRC resection specimens processed in the cellular pathology department of a large teaching hospital, the Royal Victoria Hospital, Belfast, Northern Ireland. This department reports on resection specimens from patients undergoing surgery in the Belfast and South Eastern Health and Social Care Trusts, representing approximately 40% of all patients diagnosed with CRC in Northern Ireland (population 1.8 million).10 Specimens reported by four specialist gastrointestinal pathologists working in this department were included in the study. Consecutive cases reported in 2017 and 2018 were chosen for comparison, i.e. the time period immediately before and after implementation of the change in recommended TNM classification from TNM 5 to TNM 8, which took effect in the United Kingdom from 1 January 2018.
Data Collection
Participating pathologists prospectively completed a Microsoft Excel database for TNM 8 cases, recording information on tumour site, venous invasion, lymphatic invasion, perineural invasion, numbers of nodes involved, number of TDs and overall TNM stage. All forms of regional, extramural, discontinuous tumour, specifically nodal metastatic disease, extramural venous, lymphatic or perineural invasion or TDs were included in the study analysis. A medical student and a pathology trainee (O.K./M.M.) retrospectively compiled an equivalent database for 2017 (TNM 5) cases from review of patients' electronic clinical records and the pathology laboratory information management system. They also collected information on any ancillary immunohistochemical and histochemical stains, specifically desmin, S100 and elastic van Gieson (EVG) requested by pathologists to aid interpretation of discontinuous tumour foci, and on neoadjuvant therapies received by the patient. Database completeness and quality were reviewed by one of the consultant pathologists (M.B.L.) participating in the study.
Statistical Analysis
Of the total 465 patients included in the audit, 54 patients who had undergone neoadjuvant therapies (n = 23 in the TNM 5 time-frame and n = 31 in TNM 8 time-frame) were excluded from analysis, as these therapies may impact staging and interpretation of discontinuous tumour foci is particularly difficult in this setting, necessitating specific studies on such cases. This left 177 and 234 CRC resections that were classified under the TNM 5 and TNM 8 systems, respectively, for further analysis. Characteristics of patients and features of tumours were compared between those specimens reported under TNM 5 and TNM 8. χ2 tests were applied for categorical variables, and independent-sample t-tests or Mann–Whitney tests were applied for continuous variables. Due to changes in requirements for recording of some features under TNM 5 and TNM 8 (for example TDs, perineural invasion and lymphatic invasion), direct comparison was not always possible. According to the Royal College of Pathologists (UK) 2018 reporting guidelines for colorectal cancer, lymph node involvement by tumour is also considered a form of EMLI, lymph nodes representing a component of the extramural lymphatic system. Therefore, EMLI was excluded from this analysis as a separate data item. Intra- and interobserver variation in reporting patterns between pathologists was evaluated. Statistical analysis was conducted by study team member H.G.C., independently of the contributing study pathologists, using Intercooled Stata version 14.2 (StataCorp, College Station, TX, USA).
Results
Table 1 outlines the descriptive characteristics of cases included in the study. Patients reported under TNM 5 (n = 177) and TNM 8 (n = 234) did not significantly differ in terms of age, sex or location of their tumour. The majority of cases in both cohorts were staged as pT3 or pT4. The proportion of cases reported by the four pathologists participating in the audit ranged from 17% to 35%, demonstrating that each contributed a reasonable caseload in both TNM 5 and TNM 8 cohorts, with some variation in the proportion of cases reported by individual pathologists upon transfer from TNM 5 to TNM 8 rules.
| Features | TNM5 | TNM8 | P-value |
|---|---|---|---|
| n = 177 (%) | n = 234 (%) | ||
| Sex | |||
| Male | 76 (42.9) | 123 (52.6) | 0.053 |
| Female | 101 (57.1) | 111 (47.4) | |
| Age, years | |||
| Mean (SD) | 70.5 (11.7) | 69.1 (11.2) | 0.22 |
| pT category | |||
| pT1 | 6 (3.4) | 16 (6.8) | 0.34 |
| pT2 | 25 (14.1) | 26 (11.1) | |
| pT3 | 65 (36.7) | 92 (39.3) | |
| pT4 | 81 (47.8) | 100 (42.7) | |
| Location* | |||
| Proximal colon | 96 (54.5) | 111 (48.9) | 0.41 |
| Distal colon | 51 (29.0) | 68 (30.0) | |
| Rectum | 29 (16.5) | 48 (21.1) | |
| Reporting pathologist | |||
| A | 46 (26.0) | 64 (27.4) | 0.02 |
| B | 61 (34.5) | 60 (25.6) | |
| C | 32 (18.1) | 70 (29.9) | |
| D | 38 (21.5) | 40 (17.1) | |
- TNM, Tumour–node–metastasis; SD, Standard deviation.
- * Eight tumours were recorded as unspecified or multifocal location.
Ancillary stains were requested in 39 (22.0%) of TNM 5 and 96 (41.0%) of TNM 8 cases, representing an almost twofold increase in laboratory work-load associated with reporting of these cases (Figure 1A, P < 0.001). EVG was the most common stain requested and increased from 18.6% to 32.9% (P = 0.001), while substantive increases were observed in requests for desmin and S100 immunostains under TNM 8 compared to TNM 5 (from 4.0% to 21.4% for desmin, P < 0.001 and from 1.7% to 7.7% for S100, P = 0.006). Increases in the proportion of cases being interpreted with the aid of ancillary stains was observed among all four pathologists, and was statistically significant for two (Figure 1B). Of note, the interpathologist variation in requesting of stains did not significantly narrow on migration from TNM 5 reporting (range = 13.0–29.5%) to TNM 8 reporting (range = 32.8–47.1%).
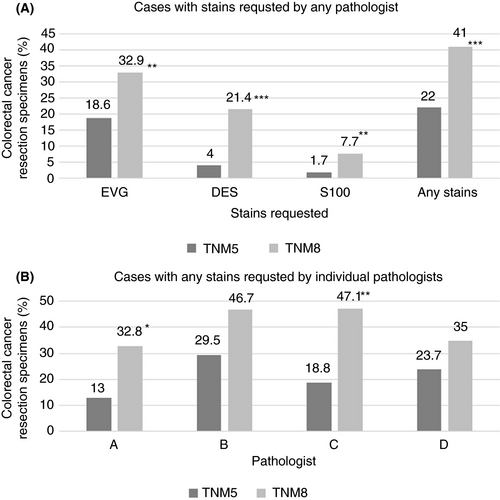
Table 2 displays key pathology features reported for the TNM 5 and TNM 8 cohorts. Overall there was no significant change in the percentage of cases classified as pN1 from TNM 5 to TNM 8 (32.8% versus 35.9%, P = 0.31), despite the introduction of the new pN1c category, which accounted for 5.1% of cases in TNM 8. The median total nodal count per case and mean number of positive nodes in node-positive cases were both lower when comparing TNM 8 with TNM 5, although these differences did not achieve statistical significance. There was no change observed for overall EMVI reporting rate between TNM 5 and TNM 8 (46.9% versus 44.9%, P = 0.89). EMPNI, EMLI and TDs, all new reporting parameters in TNM 8, were reported in 17.1%, 45.7% and 23.9% of cases, respectively. Interestingly, despite these new parameters in TNM 8, there was no change in the proportion of cases that displayed one or more form of regional, extramural, discontinuous tumour collectively between TNM 5 and TNM 8 cohorts (62.7% versus 62.4%, P = 0.95). These changes are also illustrated graphically in Figure 2A.
| Features | TNM5 | TNM8 | P-value |
|---|---|---|---|
| n = 177 (%) | n = 234 (%) | ||
| pN stage* | |||
| pN0 | 85 (48.0) | 118 (50.4) | |
| pN1 | 58 (32.8) | 84 (35.9) | |
| pN2 | 34 (19.2) | 32 (13.7) | 0.31 |
| pN1 or 2 | 92 (52.0) | 116 (49.6) | 0.63 |
| Total nodal count, median (IQR) | 21 (16–27) | 19 (15–25) | 0.09 |
| Nodes-positive count if any positive, mean (SD) | 4.5 (5.2) | 3.4 (3.2) | 0.07 |
| Venous invasion | |||
| No | 85 (48.0) | 118 (50.4) | |
| Intramural | 9 (5.1) | 11 (4.7) | |
| Extramural | 83 (46.9) | 105 (44.9) | 0.89 |
| Lymphatic invasion | |||
| No | – | 121 (51.7) | – |
| Intramural | 6 (2.6) | ||
| Extramural | 107 (45.7) | ||
| Perineural invasion | |||
| No | – | 187 (79.9) | – |
| Intramural | 7 (3.0) | ||
| Extramural | 40 (17.1) | ||
| Tumour deposits | |||
| No | – | 178 (76.1) | – |
| Yes | 56 (23.9) | ||
| Any extramural discontinuous tumour† | |||
| No | 67 (37.9) | 88 (37.6) | 0.95 |
| Yes | 111 (62.7) | 146 (62.4) | |
- TNM, Tumour–node–metastasis; IQR, Interquartile range; SD, Standard deviation.
- * Under TNM8 classification, pN1 staged tumours comprised pN1a (37, 15.8%), pN1b (35, 15%) and pN1c (12, 5.1%), and pN2 staged tumours comprised pN2a (18, 7.7%) and pN2b (14, 6%) of all cases.
- † Any cases with: pN1 or pN2 stage, extramural venous/lymphatic/perineural invasion or tumour deposits.
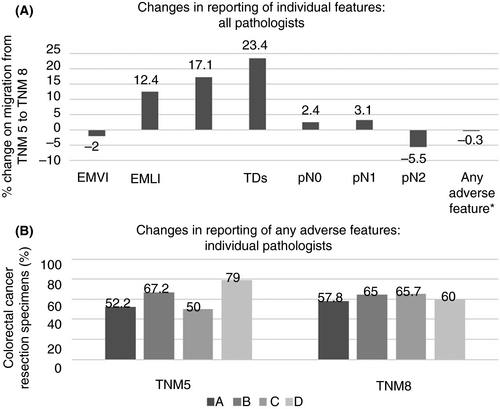
Figure 2B depicts changes among the four study pathologists, between TNM 5 and TNM 8, in reporting cases with any one or more form of regional, extramural, discontinuous tumour described. Of note, despite minimal change in the overall percentage of cohort cases reported with any one or more form of regional, extramural, discontinuous tumour when considering all pathologists collectively, there was considerable variation between individual pathologists. However, the range among individual pathologists in reporting the presence of any one or more of these features narrowed significantly, from 50–79% under TNM 5 to 57.8–65.7% under TNM 8. Of note, the marked increase in reporting regional, extramural, discontinuous tumour by pathologist C was due largely to a 31.2–52.9% increase in reporting EMVI and the marked decrease in reporting regional, extramural, discontinuous tumour by pathologist D was due largely to a 60.5–42.5% decrease in reporting EMVI (Table 3).
| Features | Pathologist A | Pathologist B | Pathologist C | Pathologist D | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TNM5 | TNM8 | P-value | TNM5 | TNM8 | P-value | TNM5 | TNM8 | P-value | TNM5 | TNM8 | P-value | |
| n = 46 (%) | n = 64 (%) | n = 61 (%) | n = 60 (%) | n = 32 (%) | n = 70 (%) | n = 38 (%) | n = 40 (%) | |||||
| pN stage* | ||||||||||||
| pN0 | 27 (58.7) | 32 (50.0) | – | 28 (45.9) | 35 (58.3) | – | 18 (56.2) | 34 (48.6) | – | 12 (31.6) | 18 (45.0) | – |
| pN1 | 12 (26.1) | – | 22 (36.1) | – | 7 (21.9) | – | 17 (44.7) | – | ||||
| pN1a | – | 13 (20.3) | – | 6 (10.0) | – | 12 (17.1) | – | 6 (15.0) | ||||
| pN1b | – | 6 (9.4) | – | 9 (15.0) | – | 11 (15.7) | – | 9 (22.5) | ||||
| pN1c | – | 7 (10.9) | – | 0 (0.0) | – | 1 (1.4) | – | 3 (7.5) | ||||
| pN2 | 7 (15.2) | – | 11 (18.0) | – | 7 (21.9) | – | 9 (23.7) | – | ||||
| pN2a | – | 2 (3.1) | – | 4 (6.7) | – | 9 (12.9) | – | 3 (7.5) | ||||
| pN2b | – | 4 (6.3) | – | 6 (10.0) | – | 3 (4.3) | – | 1 (2.5) | ||||
| Venous invasion | ||||||||||||
| No | 29 (63.0) | 43 (67.2) | 22 (36.1) | 26 (43.3) | 19 (59.4) | 30 (42.9) | 15 (39.5) | 19 (47.5) | ||||
| Intramural | 0 (0.0) | 1 (1.6) | 6 (9.8) | 3 (5.0) | 3 (9.4) | 3 (4.3) | 0 (0.0) | 4 (10.0) | ||||
| Extramural | 17 (37.0) | 20 (31.3) | 0.59 | 33 (54.1) | 31 (51.7) | 0.50 | 10 (31.2) | 37 (52.9) | 0.11 | 23 (60.5) | 17 (42.5) | 0.07 |
| Lymphatic invasion* | ||||||||||||
| No | – | 38 (59.4) | – | – | 30 (50.0) | – | – | 32 (45.7) | – | – | 21 (52.5) | – |
| Intramural | 0 (0.0) | 3 (5.0) | 2 (2.9) | 1 (2.5) | ||||||||
| Extramural | 26 (40.6) | 27 (45.0) | 36 (51.4) | 18 (45.0) | ||||||||
| Perineural invasion* | ||||||||||||
| No | – | 59 (92.2) | – | – | 39 (65.0) | – | – | 13 (18.6) | – | – | 32 (80.0) | – |
| Intramural | 0 (0.0) | 7 (11.7) | 0 (0.0) | 0 (0.0) | ||||||||
| Extramural | 5 (7.8) | 14 (23.3) | 13 (18.6) | 8 (20.0) | ||||||||
| Tumour deposits* | ||||||||||||
| No | – | 43 (67.2) | – | – | 47 (78.3) | – | – | 61 (87.1) | – | – | 27 (67.5) | – |
| Yes | 21 (32.8) | 13 (21.7) | 9 (12.9) | 13 (32.5) | ||||||||
| Any regional, extramural, discontinuous tumour† | ||||||||||||
| No | 22 (47.8) | 27 (42.2) | 0.56 | 20 (32.8) | 21 (35.0) | 16 (50.0) | 24 (34.3) | 8 (21.0) | 16 (40.0) | |||
| Yes | 24 (51.2) | 37 (57.8) | 41 (67.2) | 39 (65.0) | 0.80 | 16 (50.0) | 46 (65.7) | 0.13 | 30 (79.0) | 24 (60.0) | 0.12 | |
- * Direct comparisons not made due to changes under tumour–node–metastasis (TNM)8 classification.
- † Any cases with: pN1 or pN2 stage, extramural venous/lymphatic/perineural invasion or tumour deposits.
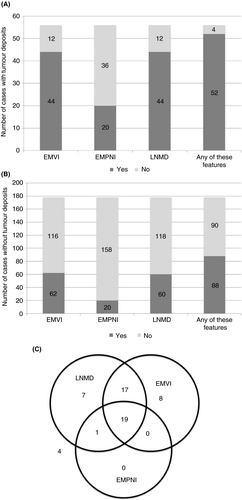
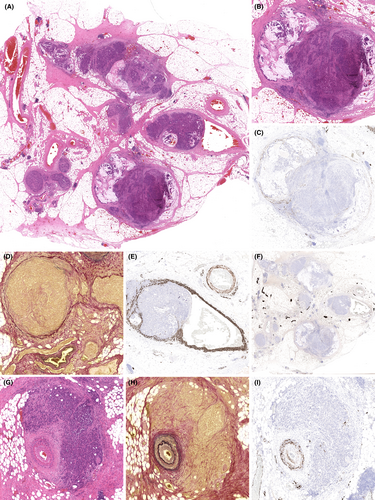
TDs were strongly correlated with the presence of other forms of regional, extramural, discontinuous tumour (Figure 3). Fifty-six (23.9%) TNM 8 cases were reported with TDs, 52 (92.9%) of which had one or more other forms of discontinuous tumour (Figure 3A). Forty-four (78.6%) cases had co-existent EMVI, 20 (35.7%) cases had co-existent EMPNI and 44 (78.6%) cases had co-existent overt lymph node metastatic disease (stage pN1a/b or pN2a/b). Only four (7.1%) pN1c cases had no other form of regional, extramural, discontinuous tumour. In contrast, of the 178 TNM 8 cases reported without TDs, 62 (34.8%) had co-existent EMVI, 20 (11.2%) had co-existent EMPNI and 60 (33.7%) had co-existent overt lymph node metastatic disease (Figure 3B). Ninety (50.6%) of the cases lacking TDs had no form of regional, extramural, discontinuous tumour. The relationships between these features is further depicted by Venn diagram in Figure 3C, for those cases with TDs. Fifty-two (92.9%) of the 56 cases with TDs were also reported as having lymph node metastatic disease or EMVI, and 36 (64.3%) of these cases had both. EMPNI was extremely rare in the absence of other forms of regional, extramural, discontinuous tumour. Nineteen cases (33.9%) demonstrated a ‘full house’ of EMVI, EMPNI, nodal metastatic disease and TDs.
Discussion
In comparison to TNM 5, introduction of TNM 8 staging criteria for CRC has necessitated greater scrutiny and evaluation of any discontinuous foci of tumour in the vicinity of the primary cancer. Specifically, in defining a TD, exclusion of nodal tumour involvement, EMVI, EMLI and EMPNI requires careful consideration, and separate reporting, of each of these potential metastatic pathways. Application of various ancillary histochemical and immunohistochemical stains can help to detect involvement of these pathways, notably histochemistry for elastic tissue and immunohistochemistry for smooth muscle markers (caldesmon or desmin) to detect EMVI and immunohistochemistry for S100 to detect perineural invasion.11-18 Under TNM 8, TD is essentially a classification of exclusion, the pathologist having failed to be convinced that the regional, extramural, discontinuous tumour focus in question has a clear origin in any of these pathways.
We found significantly increased use of these ancillary stains under TNM 8 by all four specialist gastrointestinal pathologists in this study. However, there was significant interobserver variation in stain application by individual pathologists before and after migration to TNM 8. Although not quantified in this study, this change in practice will inevitably impact upon associated laboratory work-loads and costs, and probably also impact upon turnaround times of pathology reports, although the latter is not considered likely to be of clinical significance.
Given the introduction of a new pN1 subcategory of pN1c, applied under TNM 8 when TDs are identified in the absence of overt lymph node metastatic disease, one may have anticipated an increase in the proportion of cancers reported as ‘node-positive’ (pN1/2) or overall clinical stage III. This was not found to be the case in this study. A slight increase in pN1 cases was compensated by a greater reduction in pN2 cases, neither statistically significant. There was also a small, but non-significant, increase in pN0 (stage II) cases. Only 5% of cases were reported as pN1c. The probable explanation for these findings relates to application and interpretation of ancillary stains applied to extramural discontinuous tumour foci. Whereas some such foci will have been interpreted as TDs under TNM 8, other foci previously considered nodal metastases under TNM 5 (applying the ‘3 mm rule’) may have been interpreted under TNM 8 as EMVI or EMPNI, with greater use of ancillary stains. Further indirect evidence for this comes from the decrease in mean number of involved lymph nodes in node positive cases, from 4.5 to 3.4 per case, together with a reduction in median total nodal counts, from 21 to 19 per case, although neither change achieved statistical significance. Similar to our findings, Jin et al. reported a 4% reduction in node-positive cases and a slight reduction in lymph node yields, from 22.2 to 21.2 nodes per case, on restaging a large series of CRC cases applying TNM 5 and TNM 7.19
Although TDs were detected overall in almost 25% of cases, the vast majority of such cases also had overt nodal disease, EMVI and/or EMPNI. These associations are unsurprising, as TDs must represent an unidentified pathway and the presence of other forms of regional, extramural, discontinuous tumour may represent a clue to the aetiology of TDs in specific cases. Strong associations in particular with EMVI and lymph node metastatic disease suggest that these represent the most likely metastatic pathways involved in the pathogenesis of TDs, much less so EMPNI. This strong association between TDs and other features limits the impact of the new TD classification on TNM 8 staging. However, as it is recognised that the presence of TDs offers additional adverse prognostic value in the setting of node-positive CRC, it is now recommended that details of TDs are reported separately to TNM stage.8, 9
Inevitably, introduction of the new TD category will have led to some foci, interpreted as EMVI under TNM 5, being classified as TD under TNM 8, applying the new criteria in this assessment. Furthermore, the subtle but important change to the definition of TD between TNM 7 and TNM 8 now stipulates that any discontinuous tumour focus which has an origin in EMVI, EMLI or EMPNI is classified as such, and not as a TD. In contrast, the TNM 7 definition somewhat confusingly accepted that some TDs may be vascular or perineural in origin.3 The TNM 8 definition of TD is therefore more stringent, and may have different clinical relevance compared to TDs defined under TNM 7, possibly representing more aggressive disease. TNM 7 was not used in the United Kingdom, and therefore does not form part of this study, but it would be of interest to see in other countries what impact, if any, this definitional change has had on TD reporting and associated clinical outcomes.
The increased use of ancillary stains between TNM 5 and TNM 8 evident in this study was associated with a narrowing of the range of reporting of discontinuous tumour foci by the study pathologists. More widespread application of adjunctive stains has probably led to more judicious evaluation of discontinuous TDs and, specifically, classification as EMVI or TD. This represents a surrogate marker of improved reproducibility of interpretation, although formal reproducibility studies are required in this regard.
However, there is still significant variation in reporting these key parameters under TNM 8. One potential explanation for this is a lack of guidance on robust morphological criteria for classifying regional, extramural, discontinuous tumour foci and including interpretation of such ancillary stains. For example, one approach is that any identifiable vein wall remnant, evident on H&E, elastic or desmin stains, is sufficient to classify a tumour focus as EMVI rather than a TD, but what is sufficient staining in this regard (Figures 4 and 5)? Is proximity to a ‘widowed’ arteriole in itself sufficient to designate a discontinuous tumour focus as EMVI rather than TD? Similarly, what H&E or S100 staining patterns in the vicinity of tumour are considered sufficient to represent perineural invasion rather than TD? These interpretations are likely to be subject to interobserver variation. Previous studies have examined morphological features applied to distinguish lymph node metastatic deposits from TDs.20 Similar studies are required focusing on ancillary stain interpretation. Such guidance is not provided currently by UICC TNM to facilitate interpretation of new TNM 8 criteria, but would be welcomed and may improve all-important reproducibility of pathology assessment. In the meantime, regular intradepartmental discussion of difficult cases can help to calibrate thresholds for such classifications within pathologist teams. The current study was conducted in the routine clinical environment, reflecting real-life pathology reporting without formal discussion of all cases among the participating pathologists. Post-study discussion identified variation in interpretation of ancillary stains as an important contributory factor to differing interpretations of extramural, discontinuous tumour.
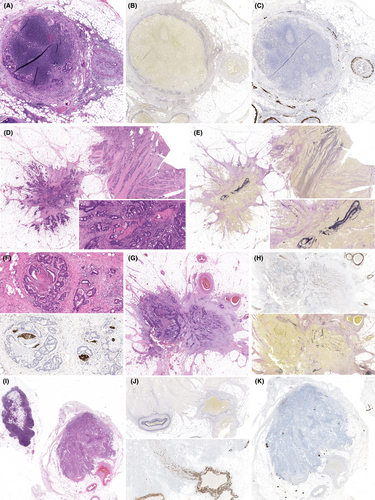
This study has several limitations. Unlike comparable studies, we did not restage cases using different versions of TNM classifications to allow a direct comparison between classifications. Our ‘real-world’ approach instead aimed to reduce bias by adopting consecutive cases from daily, routine reporting practice. Nevertheless, study pathologists were aware their TNM 8 cases were to be included in a study and this may have influenced practice with respect to ordering ancillary stains, for example. This represents an important potential study bias to highlight, although the increase in ordering ancillary tests is entirely plausible, given the new wording of TNM 8. There is also the potential for reporting practice to have evolved in 2017, leading up to TNM 8 implementation, and inclusion of more historical cases may have yielded greater differences in practice. The study included only specialist gastrointestinal pathologists, probably familiar with subtleties of changes in TNM staging, and the findings may therefore not be generalisable to the wider pathology reporting community. As the study included a population-representative cohort of cases, however, the overall findings can be readily generalised to the wider patient population. It is acknowledged that overall case numbers are relatively small, and larger studies are required over a longer time-frame to further explore our findings.
Under TNM 8, the greater scrutiny of regional, extramural, discontinuous tumour foci to elucidate the metastatic pathway or pathways involved is welcomed. This should lead to more complete and biologically meaningful pathology reports, accepting that these will require greater pathology time and resource to produce. The inclusion of a TD category, now proved to be of undoubted prognostic significance, is a pragmatic solution when the metastatic pathway leading to that deposit cannot be identified. TNM 8 colorectal cancer staging offers potentially greater reproducibility in identification of cases having some form of regional, extramural, discontinuous tumour. Further guidance in defining individual features and morphological interpretation is required to further reduce interobserver variation in pathology assessments and to help elucidate the clinical significance of involvement of each of these alternative metastatic pathways.
Conflicts of interest
The authors declare no conflicts of interest.
Author contributions
MBL devised the study. OK and MM collated data. MBL, CC, PK and GM provided raw data and input to study design. HGC led the statistical analysis and provided input to study design. MBL drafted the manuscript and generated the figures. All authors approved the final version.