Exploratory in vitro evaluation of thrombin generation of eptacog beta (recombinant human fviia) and emicizumab in congenital haemophilia A plasma
Abstract
Introduction/Aim
Eptacog beta is a recombinant activated human factor VII approved to treat and control bleeding in haemophilia A and B patients with inhibitors. Emicizumab is a factor VIIIa mimetic antibody approved for prophylactic treatment of haemophilia A with and without inhibitors (HAI and HA, respectively). Inhibitor patients treated with emicizumab should expect breakthrough bleeding that requires bypassing agent treatment to restore haemostasis. The aim of this study is to quantify the in vitro thrombin generation induced by the addition of eptacog beta to HAI and HA plasma containing emicizumab.
Methods
Thrombin generation assays were performed using HAI and HA plasma. Thrombin generation parameters were examined using a fixed effects model with inhibitor titre, eptacog beta concentration and emicizumab concentration as main effects, and eptacog beta concentration with inhibitor and emicizumab concentration with inhibitor as interaction effects.
Results
A significant increase in peak thrombin, ETP and velocity was observed when combinations of eptacog beta (0, 1, 2 or 5 µg/ml) and emicizumab (0, 50 or 100 µg/ml) were evaluated in HA and HAI plasma; the effect remained below that observed in Normal Plasma (NP). A small shortening of lag time below that of NP was observed.
Conclusions
Eptacog beta and emicizumab induced thrombin generation in haemophilia A plasma (with and without inhibitors) with the thrombin generation parameters remaining below those of normal plasma. These data provide in vitro proof of concept supporting the concept of use of eptacog beta for the treatment and control of breakthrough bleeding in patients on emicizumab prophylaxis.
1 INTRODUCTION
Eptacog beta (Sevenfact®, LFB SA) is a new recombinant human factor VIIa (rFVIIa) developed as a bypassing agent (BPA) for the treatment and control of bleeding in persons with haemophilia A and B and inhibitors.1, 2 Based on the results of a phase 1 and phase 3 clinical trial, eptacog beta was approved for use in adults and adolescents by the FDA in April 2020.1-4
The effects of eptacog beta on the coagulation system have been characterized using in vitro, in vivo, ex vivo and clinical studies. Chevreux et al5 reported the in vitro thrombin generating capacity of eptacog beta using plasma from patients with haemophilia A (HA) and haemophilia A with inhibitors (HAI) and Grandoni et al subsequently investigated the mechanism of action of eptacog beta. These mechanistic studies showed that eptacog beta has greater affinity for the endothelial protein C receptor (EPCR) than eptacog alfa (NovoSeven® RT, Novo Nordisk A/S)6; EPCR is a receptor that is known to affect localized haemostatic response through regulation of the activated protein C anticoagulant pathway.7
Emicizumab (Hemlibra®, Hoffman-La Roche) is a bispecific antibody that can replace factor VIIIa (FVIIIa) in the coagulation cascade; it functions as a FVIIIa mimetic that binds factor IXa (FIXa) and factor X (FX), resulting in the activation of FX.8, 9 Emicizumab has been approved for use in multiple countries for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in children and adults with congenital haemophilia A with and without inhibitors.10, 11 During clinical trials, thrombotic microangiopathy (TMA) and thrombosis were observed in subjects who administered activated prothrombin complex concentrate (aPCC) to treat breakthrough bleeding events; such thrombotic events were not observed when rFVIIa alone was used to treat a breakthrough bleed.12 In vitro studies performed by Hartmann et al suggested a plausible explanation for these observations: the combination of aPCC and emicizumab resulted in synergistic thrombin generation several times greater than that observed in normal reference plasma; whereas, the combination of rFVIIa and emicizumab resulted in thrombin generation that remained below normal.13
The objective of this in vitro study is to characterize the extent of eptacog beta-induced TG in HA and HAI plasma containing clinically relevant levels of emicizumab and to compare it to that found in NP. This in vitro study therefore provides a foundation for an improved understanding of eptacog beta-induced TG in the presence of emicizumab.
2 MATERIALS AND METHODS
Twelve study design points (13, including NP) were evaluated for each plasma lot tested (4 HAI plasma lots and 10 HA plasma lots) with each design point (i.e. eptacog beta/emicizumab combination) for each plasma lot providing 4 experimental results (2 replicates per design point each run in 2 separate assays) yielding a total of 728 data records for analysis. Lag time (min), endogenous thrombin potential (ETP, nM-min), peak height (nM) and the velocity index (nM/min) were derived from the TG curves. TG summary values for each design point represent the mean of all the results from all the HAI or HA plasma lots tested for that design point. NP material was used in every assay as a control and the NP summary values represent the mean from all the duplicate results in each of 28 independent assays.
TG profiles were generated using combinations of eptacog beta (0, 1, 2 and 5 µg/ml) and emicizumab (0, 50 and 100 µg/ml) concentrations. The levels of eptacog beta in this study represent clinical peak plasma levels in a recent phase 1b study2 (1.870 µg/ml for the 225 µg/kg dose and .717 µg/ml for the 75 µg/kg dose approximating the 2 and 1 µg/ml concentrations). The three eptacog beta levels in this study (1, 2 and 5 µg/ml) also encompass the clinical Cmax levels of .566 and 2.4406 µg/ml reported in a phase 3 trial of eptacog beta for the 75 µg/kg and 225 µg/kg doses.3
Similarly, the 50 µg/ml concentration of emicizumab used in this study represents the steady-state plasma level observed in HAVEN 1 (1.5 mg/kg maintenance dose); the 100 µg/ml concentration of emicizumab used in this study encompasses the mean steady-state Cmax plasma level (67 µg/ml) observed with the 6 mg/kg maintenance dose.11, 12, 14
The TG assays were performed using commercial clotting assay reagents. Platelet-poor plasma (PPP) low reagent (1 pM tissue factor plus 4 µM phospholipid mixture) and MP reagent (4 µM phospholipid mixture) ‘simulating’ platelet components, Owren–Koller buffer, FluCa kit and thrombin calibrator were all from Stago (Asnières, France). PPP reagent was reconstituted using 1 ml water for injection (WFI). MP reagent was also reconstituted using WFI (.5 ml vs. 1 ml recommended by Stago). The mixture for induction comprised an equal volume of prepared PPP and MP solutions giving a final concentration of .5 pM TF and 4 µM of phospholipid (PL). Human normal plasma (NP) material was obtained from Siemens Healthcare.
Human serum albumin, water for injection and eptacog beta were obtained from LFB SA. Emicizumab was obtained from Roche.
Plasma (HA and HAI) was obtained from Cryopep; all plasma samples contained <1% FVIII activity (determined by the supplier). Inhibitor titres of HAI plasma samples were 50, 54, 92 and 108 Bethesda unit (BU)/ml. TG assays were performed using a Fluoroskan Ascent fluorometer (ThermoLabsystems).
The TG assay operating procedure is based on that reported by Hemker et al15 Briefly, the sample (80 µl) or normal plasma (80 µl) was dispensed into a 96-well plate containing 20 µl tissue factor (.5 pM) and phospholipids (4 µM). Each TG measurement was calibrated against the fluorescence curve obtained in the same plasma with a fixed amount of thrombin α2-macroglobulin complex to correct for inner-filter effects and substrate consumption.15 Samples were incubated for 10 min (37°C). Starting reagent FluCa Kit (20 µl) containing the fluorogenic substrate and CaCl2 was dispensed and TG was followed by measurement of fluorescence intensity at 390 nm (excitation) and 460 nm (emission). Thrombinoscope® software was used to calculate thrombin activity over time.
TG parameters were summarized and graphically depicted according to combination of eptacog beta and emicizumab concentrations, and presence or absence of inhibitor. TG parameters were examined using a linear model with presence of inhibitor, eptacog beta concentration and emicizumab concentration as main effect variables. The statistical analysis was performed using SAS® Version 9.4.
3 RESULTS
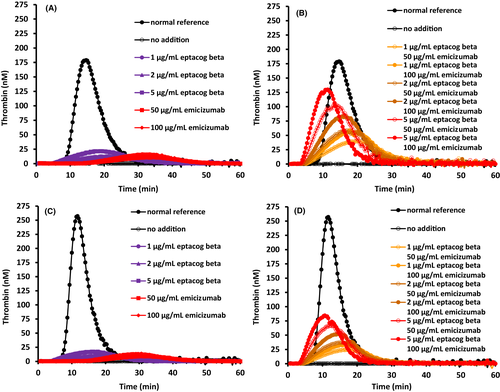
TG assay results on combinations of eptacog beta (0, 1, 2 and 5 µg/ml) and emicizumab (0, 50 and 100 µg/ml) spiked into 10 different lots of HA plasma and 4 different lots of HAI plasma were determined using NP as an assay control. Each combination spiked into each HA/HAI plasma lot was measured in 2 separate assays with 2 results generated per assay. The results of those 728 data points are presented (Figure 1, Table 1). Inherent variability of the TG assay was determined by compilation of TG parameter data from the 56 different results for the NP samples. As expected, the average peak thrombin, ETP and velocity index were reduced and lag time was increased in HA and HAI plasma compared to that found in NP. Addition of emicizumab or eptacog beta individually induced a dose-dependent increase in peak thrombin, ETP, and velocity index in HA or HAI plasma (Table 1, Figure 2).
| Plasma Type | emicizumab (µg/ml) | eptacog beta (µg/ml) | Peak thrombin (nM) | Lag time (min) | ETP (nM min) | Velocity index (nM/min) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HA | HAI | HA | HAI | HA | HAI | HA | HAI | |||
| Testing Combinations | 0 | 0 | 2.2 ± 2.6 | 2.9 ± 1.1 | 9.0 ± 9.7 | 22 ± 7.4 | 39 ± 61.0 | 11 ± 19 | 0.2 ± 0.1 | 0.14 ± 0.05 |
| 50 | 0 | 11 ± 4.3 | 16 ± 15 | 16 ± 5.5 | 14 ± 3.3 | 244 ± 97 | 272 ± 195 | 0.7 ± 0.3 | 1.4 ± 1.6 | |
| 100 | 0 | 18 ± 6.6 | 20 ± 18 | 15 ± 4.5 | 14 ± 2.7 | 371 ± 148 | 311 ± 195 | 1.1 ± 0.5 | 1.9 ± 2.2 | |
| 0 | 1 | 12 ± 4.9 | 11 ± 2.1 | 6.8 ± 2.5 | 5.5 ± 0.56 | 272 ± 123 | 208 ± 36 | 0.9 ± 0.4 | 0.82 ± 0.18 | |
| 0 | 2 | 15 ± 5.0 | 14 ± 2.6 | 6.2 ± 2.2 | 5.1 ± 0.53 | 324 ± 120 | 244 ± 42 | 1.1 ± 0.4 | 1.1 ± 0.24 | |
| 0 | 5 | 24 ± 6.4 | 21 ± 3.8 | 6.0 ± 1.8 | 5.0 ± 0.47 | 477 ± 173 | 349 ± 62 | 2.0 ± 0.5 | 1.9 ± 0.40 | |
| 50 | 1 | 40 ± 9.1 | 29 ± 9.0 | 6.6 ± 1.9 | 5.7 ± 0.35 | 762 ± 246 | 456 ± 98 | 3.3 ± 0.6 | 2.6 ± 0.98 | |
| 50 | 2 | 60 ± 11 | 41 ± 7.8 | 6.0 ± 1.4 | 5.4 ± 0.42 | 993 ± 259 | 595 ± 85 | 5.8 ± 1.0 | 4.1 ± 1.0 | |
| 50 | 5 | 99 ± 18 | 67 ± 4.5 | 5.0 ± 1.0 | 4.6 ± 0. 34 | 1217 ± 223 | 840 ± 83 | 12 ± 1.9 | 8.4 ± 0.79 | |
| 100 | 1 | 56 ± 12 | 40 ± 13 | 6.7 ± 1.6 | 5.8 ± 0.46 | 967 ± 275 | 582 ± 125 | 5.0 ± 0.8 | 4.1 ± 1.8 | |
| 100 | 2 | 84 ± 16 | 56 ± 8.3 | 6.0 ± 1.2 | 5.3 ± 0.26 | 1167 ± 242 | 756 ± 81 | 8.7 ± 1.3 | 6.3 ± 1.3 | |
| 100 | 5 | 128 ± 22 | 85 ± 5.0 | 4.5 ± 0.8 | 4.2 ± 0.38 | 1257 ± 221 | 914 ± 98 | 20 ± 2.9 | 13 ± 1.6 | |
| Normal Plasma | 0 | 0 | 229 ± 34 | 233 ± 33 | 9.1 ± 0.8 | 8.8 ± 0.8 | 1670 ± 139 | 1707 ± 142 | 56 ± 15 | 58 ± 15 |
Note
- Normal Plasma (Human Normal Plasma) was run as a control sample each time an assay was performed.
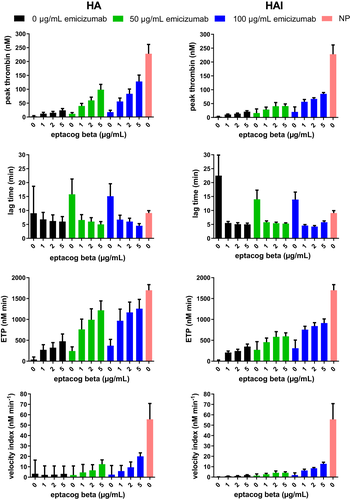
Relative to HA/HAI plasma samples lacking eptacog beta or emicizimab, a significant increase in peak thrombin, ETP and velocity was observed (Figures 2 and 3) when eptacog beta (1, 2 or 5 µg/ml) and emicizumab (50 or 100 µg/ml) were combined in plasma; however, the effect was not super-physiological. None of the data obtained using a combination of the 2 non-zero concentrations of emicizumab and the 3 non-zero concentrations of eptacog beta exceeded values obtained from NP (Figure 2); a small shortening of lag time below that of NP was observed.
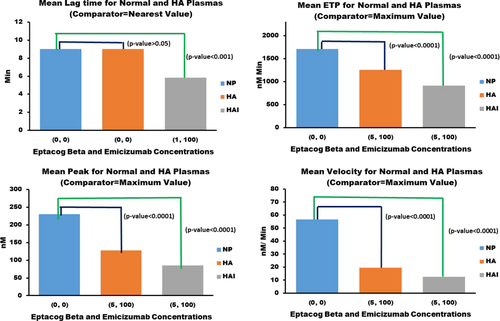
Figure 3 shows the statistical comparison of means of TG assay parameters from NP, HA and HAI plasma. Mean ETP, peak thrombin and velocity index measurements were obtained for each eptacog beta and emicizumab concentration combination in HA and HAI plasma, and the largest mean value for each parameter was then compared to the corresponding mean TG parameter from NP measurements. The largest mean value for a TG parameter (HA and HAI evaluated separately) will have the smallest difference compared to the corresponding mean for NP. If the difference is statistically significant then that indicates that none of the different concentration combinations (eptacog beta and emicizumab) induces ETP, peak thrombin and velocity index levels that are close to the corresponding TG parameter levels for NP. Mean lag times were also obtained for each eptacog beta and emicizumab concentration combination in HA and HAI plasma, and the mean lag time with the smallest difference with NP mean lag time (HA and HAI evaluated separately) was compared to the NP mean lag time. If the difference is not statistically significant then that indicates that at least one concentration combination (eptacog beta and emicizumab) induces lag time level close to NP lag time level.
The statistical comparison of means of TG assay parameters from NP, HA and HAI plasma showed that HA and HAI TG assay parameters (except lag time) were significantly lower than NP TG assay parameters.
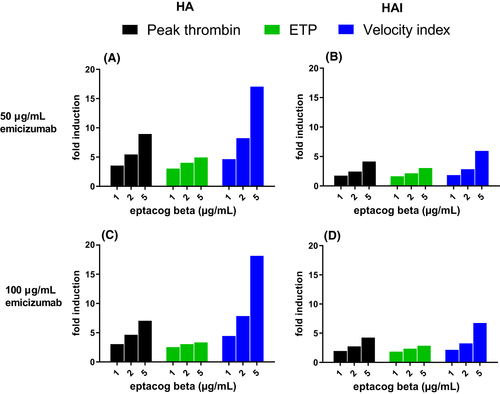
The TG effect of eptacog beta and emicizumab in HAI plasma was significant for all TG parameters examined. The presence of inhibitors (compared to HA and NP) reduced peak thrombin, ETP and velocity in all spiking combinations (Figure 4).
An exploratory linear fixed effects modelling analysis to examine the impact of the presence of inhibitor, eptacog beta concentration and emicizumab concentration (main effects), as well as eptacog beta concentration with inhibitor and emicizumab concentration with inhibitor (interaction effects) on the TG parameters of lag time, ETP, peak and velocity index was performed. Model fitting results showed that the fitted models adequately described the variation in the TG parameters. Table 2 provides full model and partial R2 values for the fitted linear fixed effects models for various TG parameters. Partial R2 value provides a measure of the predictive impact of a factor (main or interaction effect) while discounting the potential impact of the other factors in determining the level of a TG parameter.
| Factors | Parameters | |||
|---|---|---|---|---|
| Lag time | ETP | Peak | Velocity | |
| Inhibitor | 52 | 78 | 74 | 45 |
| Eptacog Beta (EB) | 18 | 85 | 91 | 89 |
| Emicizumab (EM) | 52 | 85 | 91 | 89 |
| Inhibitor*EB | 4 | 63 | 57 | 26 |
| Inhibitor*EM | 51 | 63 | 61 | 34 |
| Full Model | 52 | 86 | 91 | 90 |
Notes
- Partial R2 value for a factor (main or interaction) is calculated as (R2[full]-R2[reduced])*100/(1-R2[reduced]). Full model includes all factors in the model and reduced model includes only the factor in the model.
The R2 values confirm the expected effects of the presence of inhibitors, addition of eptacog beta and addition of emicizumab, with emicizumab demonstrating a greater effect on lag time than on other measured parameters. Also, the presence of an inhibitor showed a predictive effect on all TG parameters. Furthermore, in HAI plasma, the effect of eptacog beta and/or emicizumab was less than that seen in HA plasma though still significant (p < .05).
4 DISCUSSION
Emicizumab is a novel prophylactic agent that significantly reduces the frequency of bleeding events in haemophilia A patients with or without inhibitors.14 Although emicizumab improves haemostasis, it does not normalize it; when breakthrough bleeding occurs in inhibitor patients the use of a bypassing agent is often required to achieve haemostasis.11, 12, 16 Confirming this lack of a fully restored haemostatic system, haemostatic efficacy is reported to be variable in minor surgical procedures with the use of emicizumab prophylaxis alone; this has been compared to the haemostatic outcomes observed in mild haemophilia patients where baseline FVIII levels range from 5% to 40%.16
In HAVEN 1, breakthrough bleeding occurred in 72% of subjects on emicizumab prophylaxis; these were treated with either aPCC, rFVIIa or both agents.12 Five of the subjects who received aPCC (cumulative dose >200 U/kg) experienced thrombotic events, whereas none of the 34 patients who received rFVIIa (85 µg/kg) alone experienced thrombosis. This observation of TMA and serious thrombotic events in patients on emicizumab prophylaxis who administer aPCC raises serious clinical concerns regarding the concomitant use of these procoagulants.16, 17
To investigate this unexpected clinical safety observation, Hartmann et al. reported in vitro TG data that examined the combination of sequence identical analog (SIA)-emicizumab and either rFVIIa or aPCC in HAI plasma.13 In that study, SIA-emicizumab in combination with either aPCC or one of its constituent components (FX or FIX) resulted in a synergistic increase in TG; specifically, the combination of emicizumab and aPCC caused a 4.2-fold increase in peak thrombin above that observed in normal plasma, an outcome that may explain the reported thrombotic complications. That study also demonstrated that all combinations of SIA-emicizumab and rFVIIa (eptacog alfa) resulted in TG that remained below the range observed in normal plasma.
In this study, we examined the TG potential of combinations of different concentrations of eptacog beta and emicizumab, including clinically relevant concentrations.2, 3, 14 As observed with SIA-emicizumab and eptacog alfa,13 the combination of emicizumab and eptacog beta resulted in increased TG; importantly, this TG did not exceed that observed in normal plasma. Specifically, TG lag time, ETP, peak TG and TG velocity obtained from combinations of both emicizumab and eptacog beta did not exceed measured normal plasma values. Interestingly, lag time was shorter than seen in normal plasma, an intriguing observation that requires confirmation in patient care settings to ascertain clinical relevance. The similarity of our results to those observed with eptacog alfa and SIA-emicizumab provide in vitro support for the use of eptacog beta in cases of breakthrough bleeding in patients utilizing emicizumab prophylaxis.
As eptacog alfa is recommended to be used at lower doses (70–90 µg/kg) to treat breakthrough bleeding in patients using emicizumab prophylaxis,16, 18 it might be anticipated that the lower of the 2 approved eptacog beta initial dose regimens (75 µg/kg) might be more commonly used in this patient group.18 The highest concentrations of eptacog beta and emicizumab used were greater than reported clinical levels: the highest concentration of eptacog beta (5 µg/ml) was 8-fold higher than eptacog beta peak plasma levels observed in a phase 3 study (75 µg/kg dose) and 2-fold higher than the 225 µg/kg dose.3 The highest concentration of emicizumab was 100 µg/ml; this is 2-fold higher than steady-state plasma levels reported in HAVEN 1 and 50% higher than the 6 mg/kg steady-state plasma level reported in HAVEN 4. Even so, TG observed with these exaggerated concentrations remained below that observed in normal plasma, supporting our hypothesis that eptacog beta may have a thrombotic safety profile similar to that of eptacog alfa, and might in turn avoid the thrombotic complications observed with the combination of aPCC and emicizumab in this patient population.
In general, we also observed a reduction in TG parameter magnitude in HAI plasma compared to HA plasma. Interestingly, this effect was only seen when emicizumab was present; little difference was seen in TG between HA and HAI plasma when eptacog beta alone was present. It has been reported that TG parameters are reduced in haemophilia plasma containing inhibitors and that this effect is enhanced at low TF concentrations.19, 20
There are several limitations to this study: firstly, a limited number of unique HAI plasma samples (4) were evaluated; however, those samples all contained high titre inhibitors of a magnitude likely to be found in clinical settings. Secondly, we did not use platelet-rich plasma; instead, we used specific reagents to provide a source of phospholipids and tissue factor necessary to support maximum thrombin generation, as is common in in vitro assays measuring coagulation parameters. Furthermore, although our primary results were similar to those observed by Hartmann et al who used eptacog alfa and SIA-emicizumab, our inability to source both eptacog alfa and SIA-emicizumab prevents a direct comparison. Finally, differences in assay conditions may impact the results and their clinical relevance.
The lack of greater than normal TG, TMA and thrombotic events with emicizumab and rFVIIa may relate to the natural self-regulation of rFVIIa. Specifically, rFVIIa has a short plasma half-life of 2–3 h; exerts a maximal procoagulant effect only at the site of injury in the presence of tissue factor and activated platelets; and has multiple natural plasma clearance mechanisms, including antithrombin and tissue factor pathway inhibitor (TFPI).21-23 As a single haemostatic agent and in the absence of thrombotic risk factors, rFVIIa has a very low incidence of thrombotic events in persons with congenital haemophilia A or B with inhibitors24, 25; this favourable thrombotic safety profile appears to hold true when used in combination with emicizumab.
Based on these in vitro data, their similarity to published eptacog alfa data and the proven clinical efficacy of eptacog beta, we plan to initiate a phase 4 clinical trial to investigate the clinical safety of eptacog beta in patients using emicizumab prophylaxis. This study will be coordinated by the American Thrombosis and Hemostasis Network (ATHN-16).
5 CONCLUSIONS
The treatment of breakthrough bleeds in patients with haemophilia A with inhibitors utilizing emicizumab prophylaxis often requires the use of a BPA; rFVIIa is the preferred option, as aPCC use has a heightened risk of TMA.16 Until now, this safety observation has generally limited inhibitor patients to a single BPA for at-home use (eptacog alfa). The results of this in vitro spiking study with emicizumab and eptacog beta demonstrate that TG remains below that observed in normal plasma at clinically relevant concentrations of both agents combined. This observation, and its similarity to results previously reported with eptacog alfa and SIA-emicizumab, suggests that eptacog beta could also be used for the treatment and control of bleeding episodes in patients using emicizumab prophylaxis.
The recent FDA approval of eptacog beta and the recommendation from the Medical and Scientific Advisory Council (MASAC) of the National Hemophilia Foundation supporting the use of eptacog beta for the treatment of breakthrough bleeds in patients using emicizumab prophylaxis presents clinicians with a new human rFVIIa product to add to their armamentarium to support this patient group.18
ACKNOWLEDGEMENTS
We wish to thank Ian S. Mitchell, PhD, Thomas Wilkinson, PhD, and Sonia Nasr, PhD, of GLOVAL LLC, for their assistance during the editing of this article. ISM, TW and SN are affiliated with HEMA Biologics, LLC, per consulting agreement; HEMA Biologics had no involvement or editorial control over this review, which was independently written and submitted by the authors. We wish to also thank Pascal Zeau and Lea Rio of LFB Biotechnologies for their input on some of the statistical analysis performed and Ahmad Al-Sabbagh, MD of LFB USA, Inc., for his assistance during the editing of this article. This research was funded by LFB.
DISCLOSURES
J. G. was an employee of LFB USA at the time the work was done. D. B. and S.E. are employees of LFB USA. V. D. was an employee of LFB Biotechnologies at the time of the work and is now an employee of LFB Biomédicaments. J-L P. is an employee of LFB Biotechnologies.
AUTHOR CONTRIBUTIONS
J. G. designed the study and wrote the manuscript. J-L. P. designed the study and co-wrote the manuscript. S. E. co-wrote the manuscript. D. B. performed statistical analysis. V. D. performed the experiments.
Open Research
DATA AVAILABILITY STATEMENT
Data available from the authors upon reasonable request.




