Genetic diversity and variation in North American orchardgrass (Dactylis glomerata L.) cultivars and breeding lines
Abstract
Orchardgrass (Dactylis glomerata L.) is a high quality forage grass naturalized to temperate climates. Used extensively in hay and grazing agriculture, hundreds of orchardgrass cultivars have been released over the past 50 years. However, progress in yield and other agronomic characteristics in orchardgrass cultivars has occurred slowly and often inconsistently. One cause of the slow progress could be a lack of genetic diversity among orchardgrass cultivars, or an over-abundance of diversity within cultivars. With an emphasis on North American cultivars, this study assessed the genetic diversity within and among 52 orchardgrass cultivars, breeding lines and accessions. Genetic similarity within cultivars ranged from 52 to 71%, similar to values from wild-land, unselected accessions. Populations from Wisconsin and Missouri breeding efforts that resulted from two cycles of genotypic recurrent selection were included as checks. Neither group of selection populations exhibited more within-population similarity compared to the wild-land accessions (ecotypes) and cultivars. Genetic differentiation was detected only for the selection populations and several cultivars and breeding lines that had a tendency to originate from eastern Asian germplasm and have late flowering times. These results indicated an abundance of genetic variation within the orchardgrass cultivars, but a paucity of genetic differentiation among cultivars.
Introduction
Orchardgrass, or cocksfoot (Dactylis glomerata L.), is a high quality forage grass that is commonly used in hay and grazing systems (van Santen and Sleper 1996). Naturalized to most continents (Stewart and Ellison 2010), hundreds of orchardgrass cultivars have been developed from European, Oceania and Asian countries (Organisation for Economic Co-operation and Development [OECD] 2013). Orchardgrass is also naturalized across North America, with approximately 50 cultivars released therein by the year 2000 (Casler et al. 2000). Available breeding histories of orchardgrass cultivars suggest that germplasm has been primarily drawn from D. glomerata ssp. glomerata, with periodic uses of other D. glomerata subspecies either advertently or inadvertently.
Several studies have examined orchardgrass populations with an objective to understand the genetic relationships among specific breeding materials. Molecular markers were used to compare orchardgrass populations or cultivars across Europe and Asia (Lumaret 1988; Koelliker et al. 1999; Tuna et al. 2004; Zeng et al. 2008; Litrico et al. 2009; Xie et al. 2010; Bushman et al. 2011; Last et al. 2013, 2014); where each study found the majority of variation apportioned within cultivars or populations, and that entries did not always group as predicted by geographic provenance or ploidy level. However, the relationships among most North American cultivars and the levels of molecular variation within cultivars have not been characterized. Additionally, a previous report showed that genetic distance between orchardgrass germplasm can be a predictor of combining ability (Robins et al. 2012) suggests the importance of identifying genetically differentiated pools of germplasm. Further analysis of genetic diversity may produce new insights into the distribution of genetic diversity at the cultivar level and identify differentiated pools of germplasm useful for breeding.
As a perennial, self-incompatible, auto-tetraploid grass, orchardgrass improvement for yield and other traits has occurred at a slow or insignificant pace, especially in North America (Casler et al. 2000, 2001; Wilkins and Humphreys 2003). Improvement of traits in forage grasses requires recurrent selection with multiple location and year testing, large field trials and progeny testing; all of which can be prohibitively time-consuming and expensive. Orchardgrass variety development has usually involved phenotypic recurrent selection leading to a 5–10 clone synthetic population. Breeders have been careful to avoid inbreeding effects, often infusing novel germplasm into each new synthetic rather than continuing to select within that population. However, as an auto-tetraploid, genetic variation within orchardgrass is expected to be higher than in diploid grasses (Lumaret 1988; Moody et al. 1993), and infusion of novel genotypes may prevent the accumulation of beneficial linkage blocks (Bingham et al. 1994). Thus, it would be beneficial to know if orchardgrass cultivars contain sufficient genetic diversity to allow direct selection within synthetic populations that would accelerate cultivar improvement.
In this study, we present molecular marker assessments of 52 orchardgrass cultivars, accessions and breeding lines from North America, and several cultivars originating from Japan, Europe and New Zealand. Simple sequence repeat (SSR) markers previously developed (Bushman et al. 2011) were used to estimate genetic similarity and relationships within and among orchardgrass cultivars and breeding lines. Assessments of accessions from several D. glomerata subspecies were also included because they are likely to be involved in the ancestry of orchardgrass cultivars. Our purposes were to estimate if variation within orchardgrass entries is sufficient for plant improvement, and if genetically differentiated groups exist that could be used to develop new and broad-based cultivars.
Materials and methods
Fifty-two orchardgrass cultivars, accessions and breeding lines were included in this study (Table 1). Cultivars were selected from available seed of USA and Canadian breeding companies and universities, Japanese government institutions and European cultivars. Most cultivars were developed as 5–10 clone synthetics preceded by some form of phenotypic selection (Table 1). Accessions were selected to represent the orchardgrass subspecies aschersoniana (Graebn.) Thell. and woronowii (Ovcz.) Stebbins & Zohary as well as one accession of ssp. himalayensis Domin (Bushman et al. 2011). Selection populations from Missouri (MO) and Wisconsin (WI) (Casler et al. 1997), each with two cycles of genotypic recurrent selection without infusion of novel genotypes, were breeding lines specifically distinguished and included to test the effects of recurrent selection on molecular marker-based genetic diversity. Four UTDG breeding lines were included (Table 1) that represent internal breeding efforts to improve drought or freezing tolerance. All cultivars were reported tetraploids, all breeding lines were confirmed as tetraploids using flow cytometry (data not shown), and accession ploidy levels were previously tested with flow cytometry in suspect accessions (Bushman et al. 2011) and only seeds from tetraploid plants of the accessions were used. Breeding histories were available for most entries through personal communications, published histories (Alderson and Sharp 1994), plant registration articles and the digitized PVP database (GRIN; http://www.ars-grin.gov/).
| Entry | Source | Breeding history |
|---|---|---|
| AC Killarney | Agri-Food Canada | 8-Clone synthetic from Kay |
| AC Kootenary | Agri-Food Canada | ~12-Clone synthetic from Kay and four Northern Russian collections |
| Ambassador | Intl. Seeds of Oregon | Synthetic of unknown cultivars and breeding lines |
| Athos | DLF-Trifolium | Synthetic of French and Russian origin |
| Barexcel | Barenbrug | Synthetic of breeding lines from Spain, Portugal and Yugoslavia |
| Benchmark Plus | FFR Cooperative | Mass selection of 66 clones from Benchmark |
| Barspry | Barenbrug | Synthetic of Russian origin |
| Century | Forage First Genetics | 7-Clone synthetic from cultivars and private breeding lines |
| Command | Forage first | 8-Clone synthetic from cultivars and private breeding lines |
| Crown Royale | Grassland Oregon | 15-Clone synthetic from Crown, Condor, Warrior and US collections |
| Dawn | Land O'Lakes | 8-Clone synthetic from Jackson and Caucasus Mtn. region collections |
| Endurance | DLF-Trifolium | Selections from Hallmark |
| Frode | Swedish Seed Assoc. | 4 Generations of mass selections from material collected in central Sweden |
| Harujiman | NARCH, Japan | 7-Clone synthetic from Kay, HD-4, and collections from India and former USSR |
| Harunemidori | NARCH, Japan | 5-Clone synthetic tracing to USSR collections, Latar and Frontier |
| Harvestar | Radix Research | 5-Clone synthetic from Dawn, Arly, Lude and Berber |
| Icon | Forage first | 8-Clone synthetic from commercial and public varieties |
| Intensiv | Barenbrug | 4-Clone synthetic of Romanian ecotypes |
| Olathe | DLF-Trifolium | 11-Clone synthetic from diverse cultivars and breeding lines |
| Justus | U. Missouri | 6-Clone synthetic from material collected in MO pastures |
| Latar | USDA cooperation | Derived from PI 111536 “Westover-Enlow” collection in Turkmenistan |
| Excellate SA | Radix Research | Polycross of Dawn, Haymate, Quantum and a British Columbia collecton |
| Paiute | USDA-FS | Derived from PI109072 collection in Ankara, Turkey |
| Pawnee | FFR Cooperative | 12-Clone synthetic from cultivars and breeding lines |
| Pennlate | Pennsylvania State U. | 4-Clone synthetic from four synthetic lines |
| Persist | U. Tennessee | 6-Clone synthetic from old stands in Tennessee |
| Pizza | DLF-Trifolium | Synthetic of four families, originating from various European countries |
| Prairie | U. Kentucky | Synthetic from old cultivars that performed well in Kentucky |
| Profit | AMPAC Seed | Synthetic tracing back to Justus |
| Quickdraw | Grassland Oregon | Not available |
| Renegade | FFR Cooperative | 8-Clone synthetic of multiple breeding lines |
| Rushmore | Mt View Seeds | 4-Clone synthetic from Arly and southwestern Oregon collections |
| Seco | Forage first | 12-Clone synthetic from multiple cultivars and breeding lines |
| Tekapo | Grasslandz Tech. | Unknown origin obtained from a Portuguese breeding program |
| Toyomidori | NARCH, Japan | 7-Clone synthetic from Nordstern, Masshardy, Okamidori and a USSR collection |
| Warrior | Will. Vall. Seeds | Not available |
| UTDG-466 | USDA-ARS FRRL | Breeding line from old stands at high elevations in Utah |
| UTDG-102 | USDA-ARS FRRL | Breeding line from a Russian collection, PajbjergII, Pozan and Condor |
| UTDG-LOG | USDA-ARS FRRL | Breeding line from Annankovskaja18, Dedinovskaja4, Latar and Russian collections |
| UTDG-101 | USDA-ARS FRRL | Collection from Xinjiang Province, China, selected against seed dormancy |
| PI 538922 | USA NPGS | ssp. woronowii from Azerbaijan |
| PI237610 | USA NPGS | ssp. woronowii from Iran |
| GR 5930 | Germany IPK | ssp. aschersoniana from Sweden |
| PI 372621 | USA NPGS | ssp. aschersoniana from Germany |
| PI 420745 | USA NPGS | ssp. aschersoniana from Australia |
| PI 295271 | USA NPGS | ssp. himalayensis from India |
| MO base | U. Missouri | 6-Clone synthetic from pastures in MO |
| MO cycle-1 | U. Missouri | Recurrent selection from MO-base |
| MO cycle-2 | U. Missouri | Recurrent selection from MO-cycle1 |
| WI base | U. Wisconsin | Composite from 16 cultivars and experimental populations |
| WI cycle-1 | U. Wisconsin | Recurrent selection from WI-base |
| WI cycle-2 | U. Wisconsin | Recurrent selection from WI-cycle1 |
- MO, Missouri; WI, Wisconsin; NARCH, National Agriculture Research Center for Hokkaido, Japan; NPGS, National Plant Germplasm System of the USA.
Plant leaf tissue was collected from young seedlings at the five-tiller stage of growth, lyophilized, and used for DNA extractions. DNA was extracted using Qiagen DNeasy96 kits, following the manufacturer's protocol (Qiagen, Valencia, CA, USA). DNA quantities and qualities were verified with spectrophotometry and agarose gel electrophoresis. Twenty-eight SSR markers were genotyped on eight plants from each entry (Table S1). Homology searches between the orchardgrass SSR marker sequences, and barley (Hordeum vulgare L.) and Brachypodium distachyon L. were conducted using the BLASTn algorithm with a cutoff threshold of e(−)100. Polymerase chain reaction used Jumpstart (Sigma Aldrich, St. Louis, MO, USA) hot-start Taq polymerase following the manufacturer's protocol, except that 1 μmol L−1 R110-5 dCTP (Perkin Elmer, Waltham, MA, USA) was spiked into each reaction and amplification products were resolved on an ABI3730 (Applied Biosystems, Foster City, CA, USA) at the Utah State University Center for Integrated Biosystems (Logan, UT, USA).
Because orchardgrass is an auto-tetraploid, and proper dosage of alleles cannot be determined from banding profiles (Kosman and Leonard 2005), SSR amplification products were scored as binary scores rather than alleles. Similarity within plants of entries was determined using the similarity coefficient (Dice 1945; Lynch 1990), and corrected standard errors obtained as per Leonard et al. (1999). Pairwise tests of differences among MO and WI selection population's similarity values were conducted with 1000 permutations to test the null hypothesis of no difference between mean similarity values within two respective groups compared to mean similarity between the two groups; also according to Leonard et al. (1999). Variance was apportioned within and among entries using analysis of molecular variance (AMOVA), implemented in Genalex Ver. 6.41 (Peakall and Smouse 2006). A total binary genetic distance matrix was computed between all pairs of SSR profiles, and ф-st values between all pairs of varieties was computed using the method described by Peakall and Smouse (2006), using R Ver. 2.14.2 (R Core Team 2013). These values were used to construct a neighbor joining dendrogram in PAUP Ver. 4.0 (Swofford 2002). Bootstrap support for the dendrogram nodes was computed by sampling with replacement the within and between variety distances from the total binary genetic distance matrix representing each pair of varieties, then calculated using the Consense command in PHYLIP (Felsenstein 2009). Resampling was performed 500 times.
A model based Bayesian clustering analysis was conducted to assess population structure, using STRUCTURE Ver. 2.2 (Falush et al. 2007) without advanced assignment of plants into entries. The data were analyzed with the Recessive Alleles option and the Admixture Model with correlated marker frequencies. Probabilities of the dataset for K = 1 through K = 9 groups were tested with three replications for each level of K. The Markov chain Monte Carlo (MCMC) procedure was used with 10 000 burn-in and 100 000 MCMC steps after burn-in to determine the probability of each structure model. The average estimated log probability of the data was plotted against the K-values to observe structural fit of the data. Additionally, the second order rate of change of log probability (ΔK) between two successively tested models was plotted against the corresponding number of groups tested as described by Evanno et al. (2005). As the ΔK procedure requires subtractions of former and latter structures, only groups K = 2 through K = 8 are shown for that graph.
Results and discussion
Twenty-eight SSR markers were drawn from Bushman et al. (2011) and previously mapped to orchardgrass linkage groups by Xie et al. (2012) (Table S1). The markers were homologous to barley genes on the seven barley chromosomes and B. distachyon genes on the five B. distachyon chromosomes (Table S1). Based on B. distachyon homologs, uncharacterized predicted proteins or gene annotations were assigned to 24 of the 28 markers (Table S1). The number of alleles per plant for each of the 28 SSR markers ranged from 1.3 to 6.4 (Table S1), highlighting allele dosage uncertainties for markers with less than four bands per plant and possibly indicating multiple gene copies or pseudogenes present for markers with more than four bands per plant. The total number of bands per marker detected across all plants ranged from 2 to 25 (Table S1), with lower numbers corresponding to loci that were relatively homogeneous (e.g. two bands total) while higher numbers likely corresponded to gene families or loci rich with alleles (e.g. 25 bands total).
Eighty-four percent of the genetic variance was apportioned within orchardgrass entries and 16% was apportioned among entries (P < 0.01), similar to values previously reported for tetraploid orchardgrass (Koelliker et al. 1999; Xie et al. 2010). Average molecular similarity within entries ranged from 52% (PI 237610) to 71% (UTDG-101) (Figure 1). Five accessions of subspecies were included, with PI 237610 being the most diverse (52% similarity) and PI 420745 being one of the least diverse (69% similarity) (Figure 1). The UTDG-101 breeding line, which was subjected to two cycles of genotypic recurrent selection from an original group of collections in China, did not have a significantly higher average similarity value than PI 420745 (P = 0.18). Assuming that seed increases during accession maintenance did not appreciably affect their genetic diversity, orchardgrass subspecies aschersoniana, woronowii and himalayensis present a boundary of average similarity values expected from wild-land, unselected ecotypes. The cultivars, UTDG breeding lines, and MO and WI selection populations were all at or within this range of subspecies' similarity values (Figure 1). Thus it is unlikely that current cultivars, subspecies or breeding lines have similarity values high enough to potentiate inbreeding depression if genotypic recurrent selection were imposed.
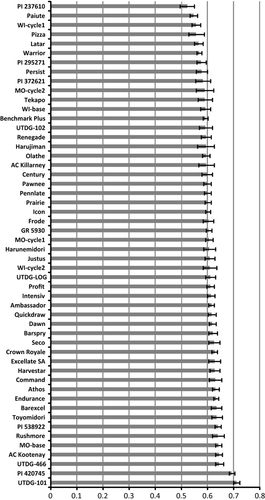
This range of subspecies' average similarity values was similar to that of a previous report for diploid and tetraploid orchardgrass subspecies (Bushman et al. 2011), and was not inconsistent with heterozygosity values reported using several heterologous SSR markers on orchardgrass populations in Litrico et al. (2009). Auto-polyploidy per se can increase heterozygosity (Moody et al. 1993) and exhibit higher recombination rates when compared to diploid populations of the same species (Bever and Felber 1992). Indeed, the average similarity values of these tetraploid orchardgrass entries were greater than those reported for diploid Lolium perenne L., Festuca pratensis Huds., or 11 D. glomerata subspecies (Koelliker et al. 1999; Bushman et al. 2011), and are consistent with the predictions of higher recombination and allelic diversity for auto-polyploids (Bever and Felber 1992). Interestingly, diploid perennial ryegrass was previously reported as abundantly diverse, and stricter genotypic recurrent selection was recommended in that species in order to obtain gains (Wilkins and Humphreys 2003).
Multiple cycles of genotypic recurrent selection within a population would be expected to increase average similarity, as long as no new genotypes were infused during the selection process. For the MO and WI selection populations, that increase was not detected (Table 2). On the contrary, no significant differences were found among the MO populations while the WI cycle-1 selected population was significantly more diverse (less similar) than the WI base population (Table 2). Additionally, both populations were previously reported to show gains without measurable reductions in phenotypic variation (Casler et al. 1997), suggesting that selection improved the target traits without restricting overall genetic diversity within those populations. However, the similarity trends in the selection cycles may yet be immature, as responses to selection in auto-tetraploids are typically slower than that for diploids (Bever and Felber 1992) and neither of these populations was subjected to strict selection (Casler et al. 1997). Changes in auto-tetraploid similarity trends, and population structure, would likely require more than two cycles of selection to show higher molecular marker similarities. Likewise, orchardgrass breeders may find additional cycles of selection are necessary to obtain uniformity of traits.
| MO-base | MO-cycle1 | MO-cycle2 | Justus | WI-base | WI-cycle1 | WI-cycle2 | |
|---|---|---|---|---|---|---|---|
| MO-base | 0.642 | ||||||
| MO-cycle1 | 0.614ns | 0.607 | |||||
| MO-cycle2 | 0.617ns | 0.606ns | 0.590 | ||||
| Justus | 0.564* | 0.578* | 0.563* | 0.609 | |||
| WI-base | n/t | n/t | n/t | n/t | 0.592 | ||
| WI-cycle1 | n/t | n/t | n/t | n/t | 0.553* | 0.557 | |
| WI-cycle2 | n/t | n/t | n/t | n/t | 0.608ns | 0.570ns | 0.610 |
- nsNot significant; *Significant at P < 0.05. MO, Missouri; n/t, not tested; WI, Wisconsin.
One possible contribution to high genetic diversity within entries could be the selection methods used during most orchardgrass cultivar development. A synthetic breeding population is often developed by intermating selected genotypes from a limited number of diverse maternal backgrounds and a relatively larger pool of pollen donors. The selected genotypes are then polycrossed, and the first synthetic population is created and designated as breeder's seed (Casler et al. 2000, 2001). In some instances, further phenotypic recurrent selection occurred after a release of the progenitor cultivar, but with one or several novel genotypes infused to produce a new synthetic. That introduction and recombination of unrelated germplasm, followed by selection, likely resets the process of developing beneficial linkage disequilibrium blocks (Bever and Felber 1992). The data presented in this study indicate that there is ample room for genotypic recurrent selection within populations without the infusion of novel genotypes. Orchardgrass appears to have been bred for the retention of maximum variation (Bingham 1980), and that goal was realized.
Another objective of this study was to identify genetically differentiated groups of orchardgrass cultivars that could allow plant breeders to diversify their cultivar portfolio or maximize heterosis. However, using cluster analysis of genetic distances to construct a neighbor joining dendrogram, statistical support for only three nodes was detected: the MO selection populations, the WI-base and WI cycle-2 node, and the UTDG-101 and PI 295271 node (Figure 2). Aside from these three nodes, all other groupings were unsupported with bootstrap analysis. The entries UTDG-101 and PI 295271 did show a tendency to group with Harujiman, AC Kootenay, and AC Killarney (Group III, Figure 2); as well as the Toyomidori, PI 538922, UTDG-LOG, Latar and UTDG-466 (Group II, Figure 2). Additionally, the cultivars Benchmark Plus, Seco, Command, Century, and Pawnee tended to group as products of the same breeding program (Table 1 and Figure 2). The major subspecies aschersoniana and woronowii were distributed across the dendrogram rather than grouping as subspecies.
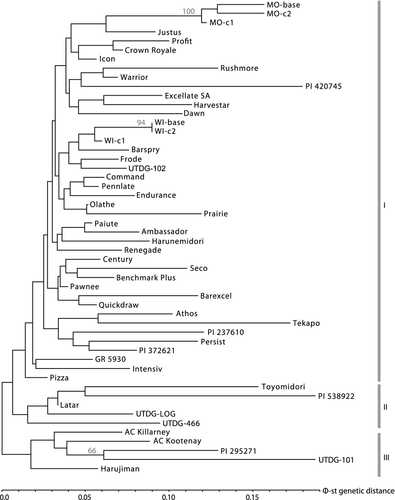
As most dendrogram groupings were tentative and unsupported, Bayesian clustering was used to further test for genetic structure among the entries. The second order rate of change supported a three-structure model (K = 3), above which the log likelihood values improved only marginally (Figure 3a,b). Under the K = 3 model, 10 entries were represented by a majority of co-ancestry in one structure (black, Figure 3d) and persisted from the K = 2 test (black, Figure 3c) throughout K = 3 (black, Figure 3d) and higher K-value tests (data not shown). The 10 entries comprised the same two tentative groups of entries detected in the dendrogram, namely: UTDG-101, PI 295271, AC Killarney, AC Kootenay, Harujiman, Toyomidori, PI 538922, Latar, UTDG-LOG and UTDG-466. The other two structures under the K = 3 model exhibited several “pure-type” representative entries with co-ancestries predominantly related to a single structure. The MO selection populations, and their relatives Justus and Profit represented one of the structures (medium grey, Figure 3d) while cultivars Tekapo and Barexcel, which may contain partial Mediterranean germplasm in their breeding history, represented the third structure (light grey, Figure 3d). Aside from these few entries with pure-type co-ancestries in a single structure, the majority of entries showed admixture or split co-ancestries (Figure 3d). Even the MO selection populations and Tekapo and Barexcel did not persist with pure-type co-ancestry at higher structure tests (data not shown).
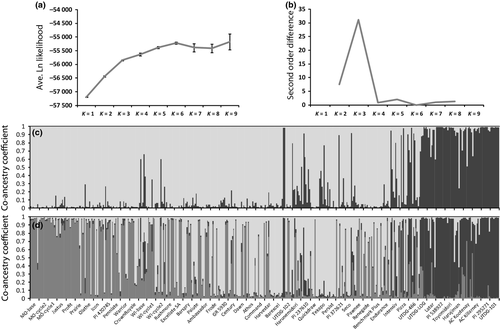
These data present orchardgrass cultivars as broad based (Figure 1) with minimal genetic structure (Figure 3). The two exceptions were the MO selection populations with bootstrap support in the dendrogram and Structure support in the K = 3 model, and the 10 entries detected as a trend in the dendrogram yet supported as a structured population at the K = 3 model. Of those latter 10 entries, most were partially derived from late flowering germplasm from central or eastern Asia. Subspecies himalayensis (PI 295271) was collected from India and is the latest flowering subspecies of orchardgrass (Stebbins and Zohary 1959). PI 538922 is late flowering (Bushman et al. 2012) and was collected in central Asia; Toyomidori and Harujiman are medium-to-late flowering and contain ancestry from eastern Asia; UTDG-101 is a late flowering breeding line collected from the Xinjiang Province of China; and several of the 10 entries included the cultivar Kay in their ancestry, which itself is late flowering and originated from the former USSR (Table 1). Although late flowering entries also existed in the other two structures at the K = 3 model (e.g. Athos and Barspry), whether or not those entries drew any ancestry from central or eastern Asia is unclear from available breeding histories. If orchardgrass breeders are interested in incorporating late flowering into germplasm of interest, the use of these 10 materials may provide differentiated genotypes when compared to the other cultivars examined in this study.
Previous genetic diversity studies of orchardgrass suggested the need to collect and maintain subspecies as unique gene pools of variation (Stebbins and Zohary 1959; Stewart and Ellison 2010). In the present study tetraploid populations of subspecies aschersoniana and woronowii were included, which are considered the ancestors of nearly all ssp. glomerata cultivars (Lumaret 1988). Neither of these subspecies grouped as genetically differentiated from other orchardgrass cultivars, further confirming that tetraploid populations of these subspecies are already present in the orchardgrass cultivar gene pool. Other more unique pools of orchardgrass subspecies' germplasm were not included in the present study, such as diploid populations and winter active Mediterranean types (Stewart and Ellison 2010; Bushman et al. 2011). Those unique pools have generally been excluded from North American orchardgrass breeding due to an inability to persist in target environments (Bushman et al. 2012). Thus, aside from the sole ssp. himalayensis accession that grouped with eastern Asian entries, this study represented a sampling of ssp. glomerata. Given this sampling of ssp. glomerata germplasm, these data showed a paucity of differentiated germplasm sources despite an abundance of genetic variation.




