Separate Determination of Strontium and Barium Mass Fractions in Calcite and Dolomite in Carbonate Rocks by a Multi-step Sequential Leaching Procedure
Abstract
Trace element partitioning in carbonate minerals has shown potential in palaeoenvironment reconstruction, while one of its preconditions of application – the respective extraction of trace element mass fractions in diverse mineral phases in carbonate rocks – leaves room for methodological improvement. We tested a ten-step sequential leaching method to selectively dissolve and extract calcite and dolomite based on their different reactivities to dilute acetic acid, with a focus on strontium and barium release. A group of mineral samples and a group of rock samples were selected for the experiment. The constant release of Sr from both calcite and dolomite mineral samples indicates its uniform distribution in the two carbonate minerals, which is consistent with prior conclusions that Sr is incorporated in the carbonate lattice, substituting for Ca. For carbonate rocks, the Sr and Ba contents present linear correlations with the ratio of calcite and dolomite, as well as Ca/(Mg+Ca) ratio dissolved in each leaching step, suggesting that the determination of their mass fractions in the two minerals can be achieved by regression. Although the Ba abundance in mineral samples is similar to that of Sr, its pattern of release from rock sample powder shows significant non-carbonate contributions. Based on the results of the sequential leaching, we design a multi-step procedure for the separate extraction of Sr and Ba in calcite and dolomite, and a case study is conducted to demonstrate its applicability, the results of which show a significantly lower Ba content in the carbonate minerals compared with bulk Ba concentrations, emphasising the importance of being cautious when using element abundances in carbonate rocks to reconstruct geochemical properties of the palaeoenvironment.
Marine carbonate sediments hold the potential to provide unique information about the chemical composition of palaeo-seawater, and may allow reconstruction of ocean and environmental conditions through time. This is based on the assumption that seawater characteristics are effectively preserved in marine carbonates, which is mainly challenged by effects such as elemental partitioning and isotopic fractionation, post-depositional alteration, and source variability (terrestrial input or deep-sea hydrothermal vents) (Rimstidt et al. 1998, Webb and Kamber 2000, Swart 2008, Eickmann et al. 2009, Hood et al. 2018). To achieve this goal, a variety of geochemical proxies have been developed which serve to reconstruct primary signals. Eliminating the interference of diagenetic alteration has been approached with diverse chemical indicators, for instance concentrations of manganese and strontium, and isotopic proxies including oxygen (δ18O) and carbon (δ13C) isotopes (Brand and Veizer 1980, Allan and Matthews 1982, Banner 1995, Swart 2015). Additionally, high field-strength elements, such as Hf and Ti, and rare earth element characteristics (Y/Ho) that directly respond to terrestrial chemical weathering are accepted to indicate the existence of terrestrial debris as impurities in marine carbonates (Webb and Kamber 2000, Zhao and Zheng 2014, Li W et al. 2019).
Studying high-resolution records of trace elements and impurities in carbonate skeletons, also known as sclerochronology, promises accurate reconstruction of past environments (Prendergast et al. 2017 and references therein). However, the period dominated by biological carbonate regulation is comparatively short, whereas inorganic and authigenic carbonate precipitation was the predominant mechanism over the long history of the Earth (Schrag et al. 2013, Wang et al. 2023). Deciphering the early Earth's geochemical features, especially in Precambrian environments, relies on the latter in the strata to a large extent.
Strontium and barium as palaeoproxies
The alkaline earth elements Sr and Ba have been widely used to reconstruct palaeoenvironments and to constrain their biogeochemical cycles. Strontium isotope stratigraphy has been established from marine carbonate records based on the 87Sr/86Sr ratio, which generally reflects the balance between the hydrothermal release of Sr with lower 87Sr/86Sr ratio and the input of weathered continental crust containing higher 87Rb (and thus higher 87Sr/86Sr) to the oceans (Peterman et al. 1970, Depaolo and Ingram 1985, Elderfield 1986, Derry et al. 1992, Shields and Veizer 2002, McArthur et al. 2012). More recently, a new tool – δ88/86Sr in carbonates – has been combined with 87Sr/86Sr information to account for skeletal carbonate production on the marine strontium budget (Ohno et al. 2008, Krabbenhöft et al. 2010, Pearce et al. 2015) and the role of authigenic carbonate precipitation as a sink for marine carbon, especially in the Earth's early history (Wang et al. 2023).
With respect to barium, the related interests in biogeochemistry concentrate foremost on its nutrient-like distribution in the water column (Chow and Goldberg 1960). Plankton uptake of Ba results in its depletion in surface seawater and the biologically associated Ba is partially released and precipitated as barite at depth through the remineralisation of sinking organic matter by microbial activity (Dehairs et al. 1980, Stroobants et al. 1991). The generated barite appears to redissolve in unsaturated deep water so the accumulation rate of barite is directly controlled by the strength of organic carbon burial and corresponding marine productivity at a certain dissolution rate of barite. Based on this, the accumulation rate of biogenic barite in sediments may serve to estimate palaeoproductivity (Dymond et al. 1992, Francois et al. 1995, Paytan et al. 1996), though it can be difficult to determine precisely due to the limited constraints on sedimentation rate and burial environment (McManus et al. 1998, Paytan and Griffith 2007). More recently, emerging Ba isotope measurement shows potential for understanding these elusive barite-related factors (Horner et al. 2015). While barite accounts for most of the barium in marine sediments, a certain proportion of Ba is associated with carbonates (Dymond et al. 1992). Barium bound in carbonate rocks is thought to provide information on Earth's early marine environments (Cui et al. 2021). Ba/Ca ratios captured by diverse biogenic calcareous skeletons such as corals, foraminifera and some shells are allegedly controlled by aqueous Ba/Ca, which provides various information including riverine barium input fluctuation, nutrients, seawater salinity and climate events as high-resolution records (Lea and Boyle 1991, Torres et al. 2001, McCulloch et al. 2003, Gillikin et al. 2006, Serrato Marks et al. 2017).
Strontium and barium in carbonates
Extraction of trace elements from single carbonate mineral
As natural carbonate sedimentary rocks typically consist of different carbonate minerals (calcite, aragonite, dolomite, etc.), separating these mineral phases and measuring their element mass fractions separately is essential to account for different trace element partitioning due to the different distribution coefficients of these minerals. Previous studies have used stepwise leaching procedures with dilute acetic acid to minimise the potential involvement from non-carbonate phases during carbonate leaching (Bailey et al. 2000, Li et al. 2011, Zhang et al. 2015, Chen and Zhou 2023). In these procedures, the sample is sequentially leached through several steps and the volume of acid solution used in each step is calculated to dissolve only a fixed fraction (1/10 of the initial amount, etc.) of carbonates in the sample. According to Ca and Mg concentrations yielded from each leaching step, calcite in the sample preferentially dissolves first while dolomite begins to dissolve after the depletion of calcite, which can be attributed to the lower reactivity of dolomite to weak acid (Zhang et al. 2015, Chen and Zhou 2023). This allows the separation of calcite and dolomite. Even though the sequential release of some trace elements during this process was discussed, the question of whether this approach allows the separate measurement of trace elements in calcite and dolomite remains unclear due to the different objectives of these studies.
Here, we examine a ten-step sequential leaching procedure and evaluate its applicability and limitations to extract mineral phase-specific Sr and Ba from calcite and dolomite. Based on the test experiment, we propose a corresponding protocol to determine of Sr and Ba mass fractions in carbonate rock samples, which consist of both mineral phases.
Experimental design
Samples
The experiments were conducted with six different samples that were subdivided into two groups (Table 1). Group 1 includes dolomite (D) and calcite (C) crystals, and a 50/50 mixture of these (DC). Group 2 includes dolostone (J) and limestone (S), and their 50/50 mixture (JS). These two groups of samples were used for comparison between minerals and rocks to observe any difference in the release of targeted elements during the sequential dissolution of carbonates. All samples were powdered and passed through a 200-mesh sieve before the acid digestion.
| Sample | Group | Type | Description |
|---|---|---|---|
| D | 1 | Dolomite | A transparent dolomite crystal collected from Azcarate Quarry, Eugui, Esteribar, Navarre, Spain |
| C | 1 | Calcite | A colourless optical calcite crystal from China |
| DC | 1 | Mixture | A 50%/50% mixture of Sample C and sample D (to simulate an ideal carbonate rock that contains 50% calcite and 50% dolomite) |
| J | 2 | Dolostone | A Permian dolostone reference material (JDo-1) purchased from the Geological Survey of Japan, collected from Kuzuu-machi, Tochigi, Japan |
| S | 2 | Limestone | An argillaceous limestone prepared by the National Institute of Standards and Technology as a reference material (NIST SRM 1d) |
| JS | 2 | Mixture | A 50%/50% mixture of Sample S and sample J (to simulate a nature carbonate rock that contains around 50% calcite and 50% dolomite but some other mineral phases) |
Leaching procedure
The ten-step sequential leaching procedure is illustrated in Figure 1. For each sample, powder test portions of 2000 mg were weighed into a 50 ml polypropylene centrifuge tube. In addition to six tubes with samples, an empty tube was set as procedural blank to evaluate potential contamination.
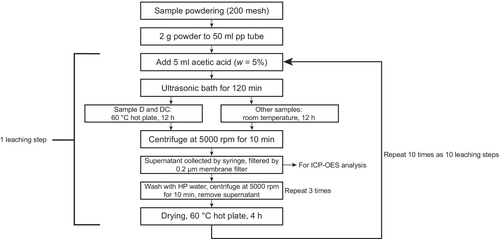
Acetic acid (5 ml, w = 5%) was added to each centrifuge tube for a single leaching step to dissolve approximately 200 mg of carbonate material, which correspond to 10% of the test portion mass. All seven tubes were then treated in an ultrasonic bath for 2 h to accelerate reactions. Finally, all the samples were kept at room temperature for 12 h to ensure the completion of the reaction – except samples D and DC, which were placed on a hot plate for 12 h. Ultrasonic bath treatment for 2 h is sufficient for complete reaction, whereas without it may require up to 24 h (Chen and Zhou 2023, Wei et al. 2021, Zhang et al. 2015). During the ultrasonic processing, we observed that most samples reacted intensely (formation of gas bubbles) which was not the case for samples D (and DC after five leaching steps). We attribute this to the fact that sample D is a nearly pure dolomite whereas the other samples including sample J dolostone have a fraction of calcite that may promote the reactivity dissolution and destruction of the surrounding carbonate lattice structure. Therefore, we decided to place samples D (and DC since step 5) on a hot plate at 60 °C for 12 h instead of at room temperature to ensure full reaction (no more bubbles generated), while the others were kept in mild conditions (room temperature) to avoid over-destruction of the remaining lattice structure, which is important for the subsequent leaching.
After the reaction, the tubes were centrifuged at 5000 rpm for 10 min. The supernatant was collected and then filtered through a 0.2 μm PTFE membrane filter (ADVANTEC_13JP020AN) to remove any suspended particles. The remaining carbonate samples in the tubes were washed with high purity water and centrifuged three times to ensure that remaining solvents from the acid digestion were removed. Finally, they were dried on a hot plate at 60 °C for 4 h. This digestion-washing process was considered as one leaching step. Afterwards, it was repeated nine times, starting from the addition of acetic acid.
Chemical analysis of the leachate
Aliquots of respective supernatants were used for determination of Sr and Ba, as well as other elements to indicate the dissolution of non-carbonate phases including Al, Fe, Mn, Na and K. Measurements were carried out with a SPS5510 CCD ICP-OES (Hitachi High-Tech Analytical Science) at the Department of Earth and Planetary Sciences, Institute of Science Tokyo. The remaining aliquots were diluted in 5% acetic acid solution for measurement of Ca and Mg. A multi-element standard solution (AccuStandard Inc., Prod. No. MES-08-1) was used for method calibration. The repeatability (RSD of intensity from three repeated measurements) for the main target elements (Mg, Ca, Sr, Ba) and other elements was better than 3% and 7.5% respectively. The results (element concentrations in the supernatants) can be found in online supporting information Table S1.
Results of the test experiment
Calcium and magnesium
Figure 2a, b shows two different estimations of dissolved carbonate mass from both groups of samples during the sequential leaching: (1) the mass loss by weighing the sample tubes before and after each leaching step; (2) calculating dissolved carbonate by the concentrations of Ca and Mg in the leachates assuming that almost all of the two elements are released from carbonate. For both groups, the actual dissolved mass fluctuates within a small range compared with the designed value of 200 mg (theoretically 208.3 mg calcite (CaCO3) or 191.9 mg dolomite (CaMg(CO3)2) as expected. There is small difference between the two approximations, which can be attributed to the additional mass loss during the operation such as filtering and washing, as well as the contributions of non-carbonate dissolution to the Ca and Mg concentrations. It can be noticed that there is a rapid drop in the last step for samples C and S owing to the high proportion of calcite, which consumes more mass than dolomite when reacting with the same amount of acid solution.
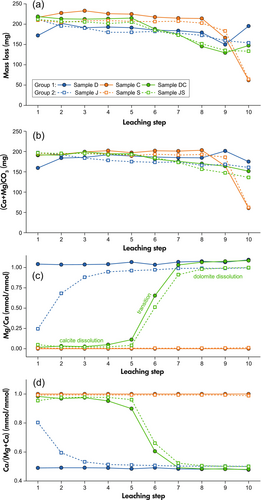
Molar ratios of Mg and Ca in the leachate from both groups of samples during the sequential leaching process are shown in Figure 2c. For Group 1 mineral samples, the dolomite sample D shows a Mg/Ca ratio close to 1 (mean value of 1.06) among all leaching steps, confirming the predominant dissolution of dolomite, whereas the calcite sample C has a constant Mg/Ca = 0, indicating its purity. The Mg/Ca ratio in the mixture DC remained at a low level of about 0.03 in the first four steps. It rose and approached the dolomite ratio in the next two steps, and then approached a constant value, which was equal to that of sample D from steps 8 to 10.
With respect to Group 2 carbonate rock samples, the Ma/Ca patterns of limestone sample S and the mixture JS were the same as samples C and DC respectively. Sample S presented a stable Mg/Ca ratio of 0.01. The Mg/Ca ratio of JS was lower than 0.05 in the first five steps, with a rise in steps 6–7, approximating the value of sample J after that. The main difference between Group 1 and 2 focuses on dolomite and dolostone. The Mg/Ca ratio of dolostone sample J showed a rapidly increasing trend in the first four steps, then gradually stabilised at 1. This suggests the existence of a certain amount of calcite in the natural dolostone, which was responsible for lowering the ratio through its rapid dissolution in the first steps.
The results of major elements Ca and Mg are essentially in agreement with previous studies (Chen and Zhou 2023, Zhang et al. 2015). Low Mg/Ca ratios in both mixtures and even dolostone at first indicates that dilute acetic acid preferentially dissolves calcite. With the depletion of calcite, Mg/Ca rapidly increases, indicating the increasing proportion of dolomite in dissolved carbonates. Based on this, the dissolution of carbonates during the ten-step sequential leaching can be divided into three stages: (1) calcite dissolution, (2) transition and (3) dolomite dissolution.
To show the relative contribution of calcite and for the convenience of subsequent calculations of trace element mass fractions, the Mg/Ca ratio was converted to the molar ratio of Ca over Mg+Ca (Figure 2d). It should be noted that for Group 1, the Ca/(Mg+Ca) in the calcite-dominated dissolution stage of mixed sample DC is slightly lower than the ratios in calcite sample C, then become equal to the ratios in dolomite sample D in the dolomite predominant dissolution stage. A similar pattern was observed in Group 2. This means a minor fraction of dolomite may dissolve in addition to the major contribution of calcite during stage 1 calcite dissolution. In contrast, only dolomite dissolves during the dolomite dissolution stage.
Strontium and barium
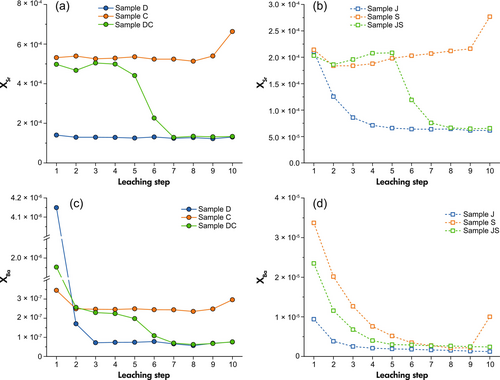
In Group 1, sample D dolomite shows a Sr molar fraction that remains constant throughout steps 1 to 10, while XSr of sample C calcite remains stable but increases in the last step. XSr of mixed sample CD approaches XSr,C from steps 1 to 5, then rapidly decreases in steps 6 and 7 and becomes equal to XSr,D in the last three steps. The patterns of barium of these three samples show similar characteristics to strontium whereas the difference is, all the three samples have significantly high XBa in the first leaching especially for sample D as high Ba release also occurs in step 2.
In Group 2, XSr of sample J decreases from steps 1 to 5 and becomes constant after that. Sample S shows a higher XSr value in the first step than step 2, which then gradually increases from steps 2 to 9 and significantly rises in the last step. XSr of sample JS indicates the same trend of Sr release as sample S during the first five steps, then rapidly decreases in the next two steps and approximates XSr,J. The Ba patterns of Group 2 are different from Group 1 as all the three samples in this show a gradual decrease in Ba concentration. XBa,J is close to the mean of sample J and S.
The release of Sr during the sequential leaching process for both, Group 1 and 2 samples, and Ba for Group 1 (excluding steps 1 and 2) match the variations in Ca/(Mg+Ca) ratios in dissolved carbonates shown in Figure 2. To reveal the relationship between Sr (Ba) pattern and dissolved carbonate phases, XSr and XBa are plotted against Ca/(Mg+Ca) in Figure 3. According to Figure 3a–c, for both, the mixed mineral DC and rock sample JS, as well as dolostone J, XSr is clearly linearly correlated with Ca/(Mg+Ca). XBa vs. Ca/(Mg+Ca) (Figure 3d–f) also shows such linear relationships, except sample JS.
Other elements (Al, Fe, Mn, K and Na)
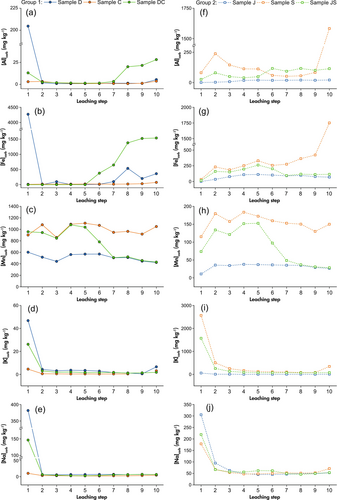
Regarding Group 1, Mass fractions of Al and Fe in sample C remain low from steps 1 to 9 and rises slightly in the last step (Figure 5a, b). These two elements in sample D present strikingly high values (205 and 4270 mg kg-1 respectively) in step 1. The source of this signal is most likely contribution of micro scale black particles that were observed in the dolomite sample. The Al stays constantly low until the last step, while the Fe is also stable throughout steps 2 to 6 but rises after that. Sample DC also shows a slight elevation (13 mg kg-1) in Al in the first step, which is not observed for Fe, and both elements maintain low throughout leaching steps 2 to 6 (Al) and 2 to 5 (Fe). However, a significant increase to values of around 28 mg kg-1 (Al) and 1500 mg kg-1 (Fe) occurs in the following steps. Considering the fraction of sample D in DC, the highest mass fraction in the first step for sample D is likely to be the increased value in steps 6–10 in sample DC, which means a delayed release. That is, black particles may be a kind of Al and Fe bearing mineral that dissolves before dolomite but after calcite. Nevertheless, it does not significantly influence our main targets so it is not further discussed here. Although the Mn mass fractions for group 1 fluctuate to a certain extent, the plot (Figure 5c) still shows similar characteristics to the release patterns of Sr and Ba. For K and Na (Figure 5d, e), a notably high value appears at step 1 for all samples in Group 1. The mass fractions become stable from step 2 to 9 and may rise again in the last step.
For Group 2, the Al mass fraction forms a peak at step 2 for sample S (237 mg kg-1) and JS (81 mg kg-1) (Figure 5f). The Al released from sample S significantly rises in the last step of leaching. For sample J, the mass fraction of Al gradually increases from approximately 1 mg kg-1 to 20 mg kg-1 during steps 1–4 and becomes stable after that. There is a shift at step 5 and 6 for sample JS, which presents a flat curve in the following steps. In terms of Fe (Figure 5g), it shows a gradual increasing trend in steps 1–9 and a rapid rising in the last step for sample S. The Fe in sample J is generally stable, increasing slightly from steps 1 to 4 and decreasing slowly after step 5. For sample JS, its Fe pattern is close to sample S in the first five steps and approximates sample J after that. Similar to group 1, the Mn contents for group 2 show a pattern correlated to major elements but have some fluctuations (Figure 5h). The K and Na (Figure 5i, j) present significantly high values at the beginning, slightly decreasing in steps 2 to 4, stabilising throughout steps 4–9 and tend to rise again in the end.
Applicability of the ten-step leaching
According to the results of sequential leaching experiment, its applicability to determine Sr and Ba mass fractions in calcite and dolomite is discussed in this section, with a protocol proposed in the end.
Non-carbonate contribution to Sr and Ba signals
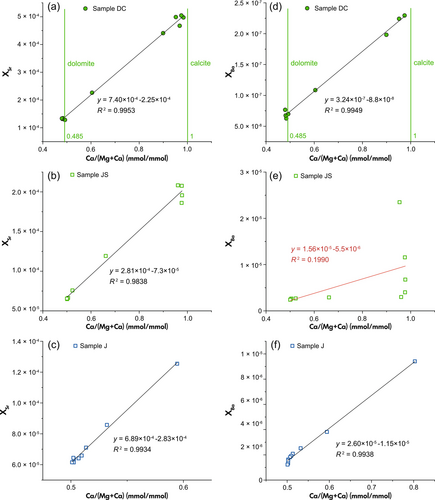
As shown in Figure 3a, Group 1 mineral samples present almost constant Sr concentrations through all leaching steps. The high value in the first step of leaching for Group 2 rock samples can be attributed to the contribution of soluble and exchangeable Sr ions in natural carbonate rocks that can be preferentially washed out. This would also correspond with the elevated K and Na mass fractions that are observed in the first step. Owing to the existence of this fraction, pre-leaching treatment before dissolving carbonates has been applied to eliminate its influence (Halverson et al. 2007, Cox et al. 2016, Zhou et al. 2020). However, a fraction of carbonate will also be removed by chemical pre-treatment. This may cause adverse impact on the results of ten-step leaching, as part of elemental signals from calcite will be removed in this process. Thus, pre-leaching is not adopted in our experiment and instead, steps affected by the dissolution of non-carbonate phases are excluded from the data that are used for the XSr (and XBa for the same reason) vs. Ca/(Mg+Ca) correlation in Figure 4.
The increasing input of Al into the leachate typically indicates successive dissolution of aluminosilicates with the gradual digestion of carbonates (Chen and Zhou 2023, Li F et al. 2019, Tostevin et al. 2016). This explains the notable rise of Sr concentration in the last step in samples C and S as depletion of residual carbonate phase may promote the dissolution of clay minerals in excess acetic acid. It should be noted that a small peak of Al released from sample S appears in step 2 with decreasing Al mass fraction after that (see section Other elements (Al, Fe, Mn, K and Na)). The peak occurs at the early calcite dissolution stage so it is unlikely to be aluminosilicate that is not as reactive as carbonate to acetic acid. A possible interpretation could be the dissolution of a secondary carbonate phase enriched in such as alumohydrocalcite, which may exist in argillaceous limestones such as sample S. The unstable secondary carbonate can be more reactive than calcite, explaining the appearance of the Al peak at the beginning. That is, the gradual increasing Al release during steps 2–6 from slight dissolution of clay minerals may be covered by the dissolution of additional Al-bearing phase, while the trend of Sr may not. The rising Sr concentration from steps 2 to 9 indicates the increasing contribution of Sr from aluminosilicates, consistent with the results of previous studies (Bailey et al. 2000, Chen and Zhou 2023).
Compared with Sr, the results of Ba show different patterns, especially for group 2, which appears to critically suffer from non-carbonate influence. Both groups of samples have strikingly high Ba concentrations at the beginning of leaching, while in Group 2 samples S and JS show gradually decreasing trends from step 2 to steps 6 or 7. The difference between the results of these two elements can be attributed to their different physicochemical properties. The larger radius of Ba ion compared with Sr inhibits its incorporation into the Ca-carbonate lattice, thus resulting in a lower distribution coefficient (Tesoriero and Pankow 1996). That is, lower concentration of Ba than Sr in carbonate phase is more sensitive to non-carbonate influences. By contrast, the lower hydration energy limits the mobility of Ba and it is more likely to be adsorbed by non-carbonate phases including aluminosilicate minerals (Wang et al. 2021). Thus, Ba carried by siliciclastics from terrigenous inputs accounts for a notable portion of Ba content in many marine sediments (Schroeder et al. 1997, Rutten and de Lange 2002). As sample S is argillaceous, the large amount of exchangeable Ba from non-carbonate phases cannot be fully washed out in the first step, but gradually released in the following steps, resulting in a gradual lowering curve of Ba concentration for samples S and JS. Meanwhile, the slight decrease of Ba in the last several steps yielded from sample J is accompanied by the same trend of Fe with a clear linear correlation (see online supporting information Figure S1). This indicates the contribution of Ba from Fe-oxides rather than silicates as the release of Al remains stable in this interval while Fe-oxide is another important Ba-containing phase in natural sediments in addition to silicates (Gonneea and Paytan 2006, Schroeder et al. 1997). Sequential extraction of Ba in different phases of carbonates demonstrates the important role of non-carbonate fractions, which can be even higher than carbonate-bound Ba (Lin et al. 2020). Due to the significant influence of non-carbonate phases in sample S (NIST SRM 1d), we recommend using other reference materials for the future studies.
Carbonate-phase dependence of Sr and Ba released in the sequential leaching process
As presented in Figure 3a, the constant concentrations of Sr released from sample C (calcite) and D (dolomite) during the sequential leaching process provide evidence for the uniform distribution of Sr in these two carbonate minerals, while the carbonate associated Sr is unlikely to exist in the form of strontianite (SrCO3) owing to its different reactivity from calcite and dolomite. Pingitore et al. (1992) studied the incorporation mode of Sr co-precipitated with calcite, demonstrating that Sr ions have similar a local structural environment to Ca ions in both natural and synthetic calcites but different from that in strontianite. This means Sr occupies the carbonate lattice sites in substitution for and through the same principle as Ca. By contrast, the presence of Sr in the supernatant extracted from limestone sample S (Figure 3b) rises slowly as the sequential leaching progresses owing to the contribution of non-carbonate phases.
In samples DC and JS, the coupling between Sr molar fraction and Ca/(Mg+Ca) ratio that is equivalent to the relative ratio of calcite and dolomite dissolved (Figure 4a, b), indicates the carbonate-phase dependence of Sr yielded from carbonate dissolution, further supporting the uniform incorporation of Sr to these minerals by ionic substitution for Ca. As these two samples are artificial mixtures that may have potential difference in physical and chemical behaviours from natural carbonate introduces, this explanation remains unknown. However, the same linear correlation is observed in sample J (Figure 4c), which is a natural rock sample, improving the reliability of the interpretation.
With respect to Ba, the results of Group 1 mineral samples are similar to Sr (Figure 3c). That is, Ba exists uniformly in Ca-carbonate minerals in the form of occupying lattice sites. However, it becomes difficult to distinguish carbonate associated Ba from the results of Group 2 (Figure 3d) due to the significant non-carbonate contribution. Ba extracted from sample S gradually reduces throughout almost the entire sequential leaching process. Sample JS shows a decreasing trend of XBa throughout the first five steps, which then becomes stable after the transition from calcite to dolomite-dominated dissolution. Only the XBa of sample J (Figure 4f) shows similar carbonate-phase dependence as XSr. This means the gradual lowering trend in XBa leaching pattern of sample J in Figure 3d is from the reducing contribution of dissolved calcite rather than non-carbonate Ba, which is different from samples S and JS. Besides, its XBa in the Ca/(Mg+Ca) = 0.5 concentrates at the bottom left. This refers to the Ba signals of steps 6–10 that may be caused by the slight contribution from Fe-oxides among the last five steps as discussed in Non-carbonate contribution to Sr and Ba signals.
It is also noteworthy that the result patterns of Mn for both two groups of samples are similar to those of Sr. That is, the presence of Mn2+ in carbonate minerals is in the same form as Sr and Ba ions, which incorporate to the structural sites of carbonate lattice.
Two methods for calculating Sr and Ba mass fractions in calcite and dolomite
The patterns of Sr and Ba that were obtained from the sequential leaching process (Figure 3a, c) can be directly used to determine their mass fractions in both, calcite and dolomite, by calculating the mean concentration of the steps that Sr or Ba releases stably, which is referred to as ‘stable release zone’ here. The results of Group 1 obtained by this method are shown in Table 2.
| Sample | Mineral | Mean of Steps | Molar fraction XSr | 1s | Mass concentration [Sr] (μg g-1) | 1s (μg g-1) |
|---|---|---|---|---|---|---|
| C | Calcite | 1–9 | 5.29 × 10-4 | 8 × 10-6 | 463 | 7 |
| D | Dolomite | 2–10 | 1.28 × 10-4 | 3 × 10-6 | 121 | 3 |
| DC | Calcite | 1–4 | 4.92 × 10-4 | 1.7 × 10-5 | 431 | 15 |
| DC | Dolomite | 7–10 | 1.32 × 10-4 | 3 × 10-6 | 126 | 3 |
| Sample | Mineral | Mean of Steps | Molar fraction XBa | 1s | Mass concentration [Ba] (μg g-1) | 1s (μg g-1) |
| C | Calcite | 2–9 | 2.45 × 10-7 | 4 × 10-9 | 0.337 | 0.006 |
| D | Dolomite | 3–10 | 7.06 × 10-8 | 6.7 × 10-9 | 0.105 | 0.010 |
| DC | Calcite | 3–4 | 2.27 × 10-7 | 4 × 10-9 | 0.311 | 0.005 |
| DC | Dolomite | 7–10 | 6.91 × 10-8 | 5.8 × 10-9 | 0.103 | 0.009 |
- 1s refers to the standard deviation of steps counted to calculate mean.
For samples C and D, the constant release of Sr among almost all leaching steps was used to calculate the mass fraction in calcite and dolomite, respectively. For sample DC, the mass fraction in calcite can be obtained by the mean of steps 1 to 4, whereas the mass fraction in dolomite is the mean of steps 7 to 10. The mass fraction of Ba is determined in the same way but, to prevent non-carbonate influence, step 1 is excluded for sample C and steps 1 and 2 are excluded for sample D.
As a mixture of sample C and D, the mass fractions of Sr in calcite and dolomite in sample DC should agree with those in samples C and D, respectively. However, it can be found that there is a deviation between [Sr]C and [Sr]cal (Sr mass fraction in calcite in sample DC), also [Ba]C and [Ba]cal. This can be attributed to the minor contribution of dolomite during calcite dissolution in steps 1 to 4 (see section entitled Calcium and magnesium). On the other hand, [Sr]dol and [Ba]dol effectively approximate [Sr]D and [Ba]D that only dolomite dissolves after the depletion of calcite. Nevertheless, this pattern-based method promises for the separate determination of Sr in the two carbonate minerals, though the mass fraction in calcite should be evaluated with caution.
While sample DC is an ideal case, things may become more complicated in natural samples. For instance, as shown in Figure 3b, [Sr]dol in sample JS can be calculated according to the stable release of Sr from steps 8 to 10. In contrast, Sr mass fraction varies during the first five steps due to non-carbonate influence. That is, it can be difficult to identify the ‘stable release zone’ to estimate the mass fraction in some cases. Moreover, a calcareous (high calcite/dolomite ratio) or dolomitic (low calcite/dolomite ratio) carbonate rock, such as sample J, will not present a clear calcite or dolomite dissolution stage with areas of constant Sr or Ba mass fractions in its pattern, for which the method becomes inapplicable.
Compared with the results of first method, it is likely to provide a better approximation of Sr and Ba abundances in calcite, by eliminating the contribution of dolomite effectively. More importantly, this linear regression method is applicable for calcareous (high calcite/dolomite ratio) and dolomitic (low calcite/dolomite ratio) carbonates. The mass fraction of Sr and Ba in dolostone sample J cannot be observed from its dissolution pattern due to the low calcite content, whereas the rapidly dropping trends of Sr and Ba linearly match the variation in Ca/(Mg+Ca) ratio (Figure 4c and f). Thus, its range of application can be wider than the first method. It should be noted that the actual ratio of Mg/Ca in the calcite is unknown in this case, therefore we assume it to be close to 0. Similarly, the small dolomite fraction in the highly calcareous limestone is approximated with Mg/Ca ≈ 1, which is a potential source of uncertainty.
Although these two methods show similar effectiveness on Ba as Sr for Group 1 mineral samples and dolostone J, the presence of non-carbonate contributions such as that in limestone S has narrowed the range of application, requiring the selection of appropriate samples. The applicability of both two methods will be further evaluated through a case study (see Application of the ten-step leaching to unknown sample).
Proposed procedure for determination of Sr and Ba mass fractions in calcite and dolomite separately
- Select and powder appropriate carbonate rocks as samples; avoid using argillaceous carbonates for non-carbonate influence. Optionally, bulk dissolution of carbonate powder is recommended for generally understanding the samples.
- Conduct the ten-step sequential leaching process on the samples shown in Figure 1, with details described in Leaching procedure.
- Measure the concentrations of major elements (Ca, Mg), target elements (Sr, Ba) and other elements (Al, Fe, Mn, Na, K, etc.) in the leachates.
- Convert leachate concentrations to contents in carbonate; calculate and plot referring to Figure 2 – Figure 5.
- Determine the Sr and Ba mass fractions in calcite and dolomite separately using two approaches (see Two methods for calculating Sr and Ba mass fractions in calcite and dolomite). Mean of stable release zone and linear regression.
| Step | Name | Description |
|---|---|---|
| I | Sample selection and preparation | Select an appropriate carbonate rock as sample. *Highly argillaceous carbonate (large amounts of aluminosilicate) should be ruled out to avoid non-carbonate influence. Powder the selected samples (200 mesh). (Optional) Bulk dissolution of sample powder is recommended to determine bulk element mass fractions before sequential leaching; use Ca and Mg mass fraction to calculate calcite and dolomite contents. |
| II | Ten-step sequential leaching | Ten-step sequential leaching of sample powder. |
| III | Chemical analysis | Measure mass fractions of major (Ca, Mg), target (Sr, Ba) and other signal elements (Al, Fe, Mn, K, Na, etc.) in sample leachate. |
| IV | Data processing | Convert leachate concentrations to contents in carbonate. Calculate and plot XSr (XBa), Mg/Ca, Ca/(Mg+Ca) and [Me]carb against leaching step respectively, as well as XSr (XBa) versus Ca/(Mg+Ca). |
| V | Concentration determination | Determine the mass fractions of Sr and Ba in calcite and dolomite based on IV by two methods: (1) Mean of stable release zone: the mean mass fraction of steps with stable release of Sr and Ba that couple with calcite dissolution and dolomite dissolution are considered to be the mass fraction in these two mineral phases, respectively. (2) Linear regression: obtain the function XSr (XBa) = k × Ca/(Mg+Ca) + b based on the plot of XSr (XBa) against Ca/(Mg+Ca). Substitute the Ca/(Mg+Ca) ratio of calcite and dolomite to the function respectively to calculate the Sr and Ba mass fractions in these two minerals. Comprehensively utilise signal elements to address non-carbonate influence. |
Although ten-step sequential leaching traces the release of elements from calcite and dolomite clearly, it is a time-consuming and laborious process that will be difficult for application on a range of samples. In this circumstance, a possible way is to reduce the number of leaching steps. That is, to dissolve most of calcite and dolomite in 1 step respectively and focus on the transition interval from calcite to dolomite dissolution particularly.
To achieve this, the prediction of the transition starting point becomes necessary. For sample DC and JS, that consist of approximately 50% calcite and 50% dolomite, the transition of both began at the fifth leaching. According to this, an estimation is: For a carbonate rock sample containing n% calcite and the volume of acid added is to dissolve 10% of the sample, the transition from calcite to dolomite tends to start at leaching step n/10. However, it is not sufficient enough to summarise only from these two cases, thus the estimation will be further discussed in the next section.
Application of the ten-step leaching to unknown sample
To further evaluate the effectiveness of the procedure proposed in the above section, a case study is conducted on sample YC28, which is a black limestone collected from late Ediacaran Dengying Formation in the Three Gorges area, Yichang, Hubei, China (Kikumoto et al. 2014, Tahata et al. 2013). As described in the first step of the procedure, the sample was powdered first. Before the sequential leaching, 100 mg of powder were weighed and dissolved sufficiently in 5 ml 5% acetic acid solution for an additional bulk dissolution. The mass of calcite and dolomite inside YC28 can be roughly calculated based on the concentration of dissolved Ca and Mg in the solution and the volume of solution. According to the result of this bulk dissolution, YC28 contains about 77% calcite, 13% dolomite and 10% insoluble residue. After that, the ten-step sequential leaching process was conducted. The results of major elements, Sr, Ba, and other signal elements are processed following (IV) to (VI) of the procedure and plotted in Figure 6.

The result of Ca/(Mg+Ca) ratio in Figure 6a indicates the dissolution of calcite from steps 1 to 8, indicated by Ca/(Mg+Ca) ≈ 0.99. A decrease of this ratio to a value of 0.5 in steps 9 to 10 suggests a transition from calcite to dolomite. Due to the low proportion of dolomite, its dissolution only presents in the transition stage.
The transition starts at Step 8 for the sample with around 77% calcite, supporting the previous estimation that the transition occurs at n/10 step for carbonate consisting n% calcite. This provides the possibility to reduce the number of leaching steps by dissolving (n − 10)% of the sample in one step and then conduct 10% leaching from the transition point. Nevertheless, the complete ten-step leaching is still recommended for limited number of samples for more sufficient understanding of targeted elements.
The results of bulk acid dissolution of YC28, [Sr]cal and [Sr]dol are respectively 85% and 38% of the bulk Sr content (approximately 3748 mg kg-1 according to the bulk dissolution). There might be no significant difference between the bulk Sr mass fraction and Sr in calcite while using them as the result of a geochemical analysis, or at least the two values are still comparable. However, with respect to Ba, [Ba]cal and [Ba]dol are only 12% and 9% of the bulk Ba (8.57 mg kg-1) respectively. That is, the difference between using bulk Ba and Ba in calcite to represent ‘Ba content in carbonate’ can be strikingly large. This means that many studies may have overestimated the Ba content recorded in carbonate phases, which could in turn affect conclusions regarding the geochemical properties of ancient seawater and decoding palaeoenvironment changes. Under this circumstance, it is necessary to be more cautious when choosing acid digestion methods to obtain the contents of Ba, and even other elements in carbonate rocks. Though there are already published approaches that focus on Ba contents in different phases in carbonate rocks (e.g., Lin et al. 2020), our method can further provide mineral-based Ba contents that can be used to improve the accuracy of reconstructing ancient seawater chemistry by eliminating the influence of change in carbonate rock composition. Meanwhile, as described above (Extraction of trace elements from single carbonate mineral), the determination of element mass fractions in carbonate minerals can be one of the foundations for reconstructing element concentrations in seawater, which is still a difficult question, by distribution coefficients, showing the possibility to solve this question.
Conclusions
The ten-step sequential leaching process described achieves the separate determination of strontium mass fractions in calcite and dolomite from carbonate rocks. This is based on the preferential dissolution of calcite over dolomite by dilute acetic acid and the structural incorporation of Sr ions into the carbonate lattice, substituting for Ca.
Despite similar results to Sr are observed in carbonate minerals, significant impact of Ba from non-carbonate phases in natural carbonate rocks causes difficulty to the extraction of carbonate-bound Ba. Barium is more sensitive to detrital influence due to its significantly lower abundance than Sr in carbonate phases, which may be attributed to the differences in physical and chemical properties between the two elements.
Based on the results of sequential leaching experiment, we find two different methods, mean of stable release zone and linear regression, can be used to calculate the Sr and Ba mass fraction in single carbonate minerals. Owing to the significance of non-carbonate influence for argillaceous carbonates, we recommend to apply the proposed methods on those relatively clean samples with low aluminosilicate contents. Correspondingly, a practical procedure is proposed for the future research on Sr and Ba in carbonate rocks and a case study on natural carbonate rock is used to test its applicability. The significantly lower Ba content in the carbonate mineral phases compared with the bulk Ba reminds us to be more cautious when dealing with element mass fractions in carbonate rocks. Though there are still limitations, our proposed methods allow the determination of mineral-based Sr and Ba contents, which provides the possibility for improved palaeoenvironment reconstruction.
Acknowledgements
We thank Shio Watanabe for providing calcite sample. This study was supported by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research JP-21H04513 and JP-22H05151. There are no conflicts of interest to declare.
Scientific editing by Thomas C. Meisel.
Open Research
Data availability statement
The experimental data (element concentrations in the supernatants) are provided in the online supporting information.




