Janzen's Hypothesis Revisited for Soil Microorganisms: Bacteria Align More Strongly With Its Postulates Than Fungi
Funding: This research was supported by the Second Tibetan Plateau Scientific Expedition and Research (STEP) programme (2024QZKK0200), Yunnan Applied Basic Research Project (202401CF070057), National Natural Science Foundation of China (32201315), Introducing talents start-up fund of Kunming Institute of Botany, Chinese Academy of Sciences, Yunnan Caiyun Postdoctoral Programme (to Yazhou Zhang). Yunnan Revitalisation Talent Support Programme ‘Young Talent’ Project (XDYC-QNRC-2024-561).
Yazhou Zhang and J. Aaron Hogan contributed equally to this study.
ABSTRACT
Aim
Mountains support disproportionately high biodiversity relative to the land area they cover. Yet, how montane biodiversity stratification varies with elevation, and whether patterns are similar among mountains, is a contentious issue. In the 1960s, Janzen proposed that the reduced climatic variability in tropical mountains compared with their temperate counterparts constrains species' thermal niches and dispersal abilities, ultimately leading to greater compositional differentiation across equivalent elevation gradients in tropical regions. This pattern has been confirmed for plants and animals but remains largely unexplored for microorganisms.
Location
Global.
Time Period
2008–2023.
Major Taxa Studied
Fungi and bacteria.
Methods
Here, we synthesised global soil microbiome distributions from 268 elevational transects across 17 mountains to test Janzen's hypothesis for microorganisms.
Results
Bacterial communities primarily respond to latitude, temperature variability and elevation, whereas fungal communities are slightly shaped by temperature variability and latitude. The Bray–Curtis distance decay rate of tropical bacteria and fungi is higher than that of temperate regions. As latitude and temperature variability increase, bacterial endemism significantly decreases, while the trend for fungi is weaker. Bacterial community assembly is primarily governed by environmental selection and dispersal limitation, whereas fungi are mainly influenced by dispersal limitation and drift. Notably, the impact of dispersal limitation on bacteria diminishes with increasing latitude.
Main Conclusions
We confirm that bacterial communities align more closely with Janzen's hypothesis than fungal communities, showing steeper distance decay, higher endemism and greater dispersal limitation at low latitudes. The application of classical ecological theories to microorganisms should carefully consider the specific characteristics of different regions and taxa. Our study provides a basis for understanding the mechanisms that maintain microbial diversity along elevational and climatic gradients.
1 Introduction
Mountains are biodiversity hotspots (Rahbek, Borregaard, Antonelli, et al. 2019; Rahbek, Borregaard, Colwell, et al. 2019) because of their strong environmental gradients over short geographic distances (Körner 2007; Rahbek 2005), making investigations along mountain gradients an area of intense interest since at least von Humboldt's early explorations (Grytnes and McCain 2013; Körner 2007; Lomolino 2001; Nogués-Bravo et al. 2008; Wulf 2015). Patterns of biodiversity and species compositional turnover along mountain elevation gradients are largely driven by physiological responses to large changes in climate over short spatial distances (Ghalambor et al. 2006). However, the magnitude of change in climate with elevation depends on climate at the base of the mountain (Stevens 1992). In the tropics, where the base of a mountain is quite warm, climate variation across an elevational gradient can be much more extreme than in temperate areas where the base is cooler. Thus, Janzen (1967) proposed the iconic hypothesis for ‘why mountain passes are higher in the tropics’.
The logic is that because tropical mountains exhibit more uniform climates (with less temperature variability) at a given elevation, as well as less temperature overlap among elevations than temperate mountains, tropical species will tend to have narrower climatic niches than temperate species, resulting in greater elevational stratification of communities and reduced dispersal along elevational gradients (Janzen 1967). This leads to greater compositional differences and higher species richness over similar elevation ranges in tropical mountains compared to temperate mountains (Ghalambor et al. 2006; Smith 2018). This hypothesis exemplifies a fundamental niche–dispersal framework, where a physiological barrier (thermal niche processes) is the primary mechanism that generates dispersal limitation (manifested as effectively ‘higher’ mountain passes) (Polato et al. 2018; Zhang, Hogan, Crowther, et al. 2024). Empirical support for this hypothesis has accumulated for the distribution (McCain 2009) and compositional turnover (Huey 1978) of plants and animals, as well as patterns of temperature tolerances (Shah et al. 2017; Mammola et al. 2019), genetic differentiation within populations (Eo et al. 2008) and dispersal rates (Polato et al. 2018).
Microbes also respond strongly to many biogeographic gradients (Ma et al. 2022) including elevation (Wang et al. 2022). However, these patterns can be highly variable among studies from different mountains (Xu et al. 2023). Furthermore, microbes are often thought to respond differently to biogeographic gradients than plants or animals, because of the conceptual paradigm that ‘Everything is everywhere’ (de Wit and Bouvier 2006). Some studies support that tropical montane microbial communities and distributional range sizes are more affected by climatic variability and show higher endemism (Feng et al. 2023; Wang and Soininen 2017; Zhang, Hogan, Crowther, et al. 2024), whereas others have found no clear patterns of microbial beta diversity and dispersal limitation with latitude (Zhang et al. 2020). Compared with plants and animals, the biogeographical patterns of microorganisms involve more complex ecological contexts, creating uncertainty in the application of Janzen's hypothesis to microorganisms.
Firstly, along large-scale elevational gradients, tropical microorganisms exhibit higher turnover/distance–decay rate, endemism and dispersal limitation than those in temperate regions, thus fitting Janzen's hypothesis; however, at smaller elevational gradients, there is no difference in the above processes across different climate zones, indicating a scale dependency (Zhang, Hogan, Crowther, et al. 2024). Moreover, Janzen's hypothesis, which is based on comparisons across large-scale climate zones, may overlook other significant factors in microbial biogeography. For example, climate dependency: Mountain microorganisms exhibit independent biogeographical patterns and drivers across different climate zones (Xu et al. 2023); taxonomic dependency: Single-celled bacteria and filamentous fungi differ significantly in dispersal capabilities, with fungi also experiencing strong dispersal limitation in temperate regions (Powell et al. 2015; Zhang, Hogan, Crowther, et al. 2024; Zhang, Hogan, Ye, et al. 2024); biotic interactions: The intensity of plant–microbe interactions varies across climate zones, strongly shaping microbial patterns, particularly in low-latitude mountains (Zhang, Worthy, Xu, et al. 2024); and microenvironments: Tropical mountains do not necessarily possess richer microclimate or soil property heterogeneity compared to other mountains (Ma et al. 2022). Due to the variability of questions and analyses, as well as the absence of a unified framework for examining these questions across temperate and tropical mountains, it remains uncertain whether microbes follow the same general constraints as other organisms.
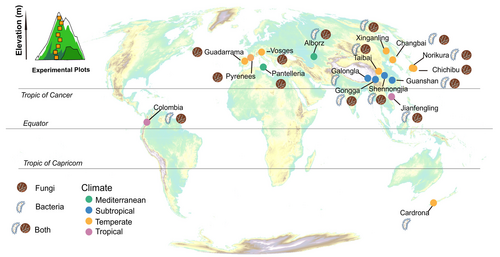
In this study, we analysed a comprehensive data set of soil microbiome data from 268 elevational transects across 17 mountains worldwide, representing a distinct climatic gradient (Figures 1 and S1). Our aim was to test whether the diversity of soil microorganisms along montane gradients aligns with the predictions of Janzen's (1967) hypothesis. Building upon recent methodological advances in testing Janzen's hypothesis (Shimabukuro et al. 2023; Zhang, Hogan, Crowther, et al. 2024; Liu et al. 2025), we developed an integrated analytical framework that bridges observed patterns with mechanistic understanding. Our approach began with constrained ordination (dbRDA) and variation partitioning to quantify the relative contributions of energy–water availability, temperature variability and spatial factors to community dissimilarities, with a particular emphasis on evaluating whether core Janzen's explanatory variables (temperature variability, latitude and elevation) serve as the primary drivers. We then conducted independent analyses examining the relationship between community dissimilarity and elevational distance, specifically testing for differences in distance–decay rates across distinct climatic zones. Further investigation focused on latitudinal patterns of microbial endemism and its associations with temperature variability, latitude and elevation. Finally, we employed community assembly models to assess the relative importance of selection versus dispersal processes in shaping montane microbial communities, while simultaneously quantifying how dispersal limitation varies along latitudinal gradients. Consistent with Janzen's hypothesis and its extensions, our predictions were as follows: (1) Microbial community composition would be jointly shaped by climatic and spatial factors, with latitude, temperature variability and elevation serving as the primary drivers; (2) distance–decay rates would be steeper in tropical than temperate regions; (3) microbial endemism would decrease with increasing latitude, temperature variability and elevation; and (4) community assembly would be predominantly governed by selection processes accompanied by strong dispersal limitation, with the strength of dispersal limitation diminishing at higher latitudes.
2 Materials and Methods
2.1 Data Collection and Processing
2.1.1 Soil Microbiomes
We collected soil microbiome data from studies published between 2008 and 2023, which sampled microbial composition along montane elevational gradients (Wang et al. 2022; Supporting Information 1 and 2). These microbiome data were classified based on several criteria, including ecosystem (terrestrial and aquatic), sampled habitat (e.g., soil, plant tissue, rhizosphere), domain (bacteria, fungi, archaea, and diatoms) and sequencing target groups (e.g., all taxa, photosynthetic bacteria, mycorrhizal fungi; Wang et al. 2022). We selected a subset of the data that targeted entire bacterial and fungal communities from terrestrial soil ecosystems to identify broad ecological patterns that might be obscured by including studies that only investigated certain groups (e.g., pathogenic or mycorrhizal taxa) (Li, Tedersoo, Crowther, Dumbrell, et al. 2023; Li, Tedersoo, Crowther, Wang, et al. 2023). We excluded plant-associated microbial data due to their close interlinkage with plant communities that could diminish environmental effects (Sasse et al. 2018; Zhang, Worthy, Xu, et al. 2024). Community-level data were chosen for the final analyses. Data from each mountain in our analysis spanned an elevational range of more than 700 m to ensure sufficient spatial variability within mountains. We only selected studies that included raw sequence data and spatial sampling metadata so that we could explicitly match microbial communities to spatially derived environmental covariates (such as elevation). In all, our analyses include 1584 sequenced fungal and bacterial communities (from the same number of plots) from 268 elevational transects on 17 mountains worldwide (Supporting Information 1). To account for the interference caused by different experimental designs, we collected confounding factors (sampling depth and year) to account for their relative contributions in the subsequent models. This study primarily encompasses three categories of predictor variables: climatic factors, spatial factors (elevation, latitude, and longitude) and confounding factors.
2.1.2 Climatic Data
We extracted bioclimatic variables for each mountain from WorldClim2 (https://www.worldclim.org/) (Fick and Hijmans 2017). These variables included those associated with energy and water (mean annual temperature [bio1] and cumulative annual precipitation [bio12]) and temperature variability (mean diurnal temperature range [bio2], temperature seasonality [bio4] and annual temperature range [bio7]) (Zhang et al. 2022, Figure S1).
2.1.3 Bioinformatic Analyses
From each plot, we processed sequences, clustering, taxonomic assignment and quality control using QIIME2 ver. 2.0 (Bolyen et al. 2019). First, we processed raw sequence data with the Demux plugin and then used the Cutadapt plugin (Martin 2011) to trim primers from read sequences. We separated bacterial and fungal sequences for quality filtering, denoising, merging and chimera removal. We identified operational taxonomic units (OTUs) through an open-reference OTU assignment approach, which clustered sequences against the Greengenes database (DeSantis et al. 2006) at a 97% similarity threshold. We constructed a maximum-likelihood phylogenetic tree using FastTree2 (Price et al. 2010). We plotted the rarefaction curves for fungi and bacteria, which indicated uneven sequencing depth (Figure S2). Therefore, we filtered out low-read samples (excluding bacteria with < 16,794 reads and fungi with < 2484 reads) for subsequent analyses. For consistency in downstream analyses, we normalised all samples to the same read number using the lowest read count among all samples. We conducted parallel analyses to compare the consistency between the raw data and the filtered data. The results showed that both exhibited highly significant correlations in OTU abundance (bacteria: r = 0.831, p < 0.001; fungi: r = 0.91, p < 0.001) and Bray–Curtis dissimilarity (bacteria: r = 0.994, p < 0.001; fungi: r = 0.999, p < 0.001) (Figure S3).
2.2 Statistical Analyses
2.2.1 Community Composition Differences and Drivers
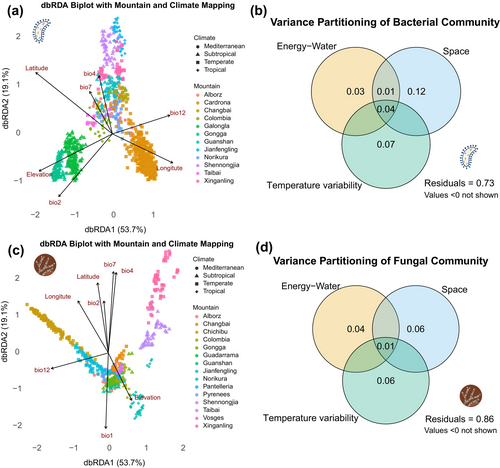
In the context of Janzen's (1967) hypothesis, we first explored whether climate variability, latitude and elevation determined differences in microbial community composition and quantified their relative contribution. Predictor variables were categorised into three groups: energy and water (bio1 and bio12), temperature variability (bio2, bio4 and bio7) and spatial factors (latitude, longitude and elevation). We performed distance-based redundancy analysis (dbRDA) using Bray–Curtis dissimilarities via the ‘dbrda’ function in the vegan R package (Oksanen et al. 2015), followed by a stepwise variable selection procedure (‘ordiR2step’ function) with bidirectional elimination based on AIC criteria (permutations = 999) (Table S2). The final model retained only significant explanatory variables, explaining 25% (bacteria)/14% (fungi) of the variance compared to 27% (bacteria)/14% (fungi) in the full model, while maintaining model parsimony. We used the constrained ordinations to do variation partitioning (‘varpart’ function), which quantifies the unique and shared contributions of the three groups of explanatory variables to community compositional differences (Shimabukuro et al. 2023).
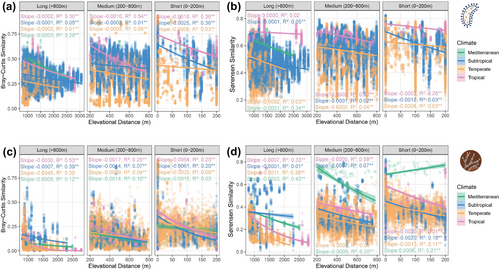
To test whether microbial community similarity decays with increasing elevational distance and whether this pattern differs between climatic zones, we quantified pairwise compositional dissimilarities (Sørensen and Bray–Curtis indices) within and among climatic zones, then analysed their relationships with elevational distance using distance–decay models. The Sørensen index uses presence/absence data and is highly sensitive to rare species, whereas the Bray–Curtis index uses abundance data, which are based on read number, weighting changes in more common species more heavily (Legendre and Legendre 2012). Based on Janzen's hypothesis, tropical mountains should harbour more rare/endemic species; thus, the aforementioned two methods may exhibit distinct patterns, each with unique implications for mechanisms of community assembly. We used distance–decay relationships rather than raw composition dissimilarity because the elevational distances from each study were not standardised, and calculating the slope of the distance–decay relationship is an appropriate way to account for spatial sampling bias in beta-diversity analyses (Anderson et al. 2011). We fitted negative exponential, power law and linear models for distance–decay relationships, ultimately selecting the negative exponential model as the best-fitting model based on AIC values (Tables S3 and S4). To compare the distance decay relationships within the same elevation ranges, we divided elevation distances into three categories: short (0–200 m), medium (200–800 m) and long (> 800 m) (based on Zhang, Hogan, Crowther, et al. 2024). We then compared the distance decay relationships within each of these ranges. We calculated the decay rate by using the slope from generalised linear models (GLMs) with a quasi-binomial family and a log-link function, fitting community similarities against elevation distance. Consequently, the slopes represent the rate of a negative exponential relationship between community similarity and distance (Graco-Roza et al. 2022). To assess the significance of exponential models, we employed a pseudo-R2 metric based on the Kullback–Leibler divergence (Cameron and Windmeijer 1997). This statistic was selected because it tests the null hypothesis of independence between similarity and distance and quantifies the proportion of model deviance explained by the fitted relative to the null model, providing a robust measure of explained variation (Martínez-Santalla et al. 2022).
2.2.2 Endemism
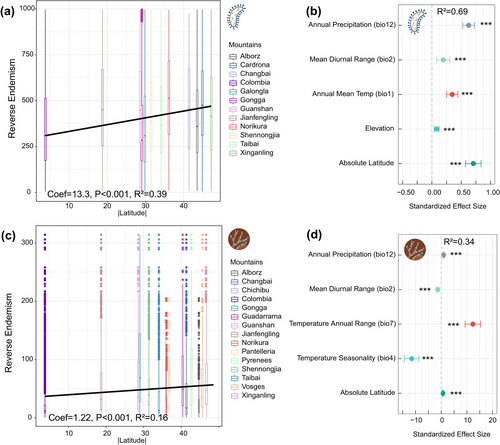
According to Janzen's hypothesis, limited dispersal ability in tropical mountains relative to temperate mountains leads to greater species endemism (Zhang, Hogan, Crowther, et al. 2024). To synthesise studies across mountains with variable elevational intervals between sampling plots, we quantified the number of plots occupied by each OTU in all samples to estimate its occupancy and provide insights into patterns of endemism across mountains: Lower occupancy reflects higher endemism and vice versa (Zhang, Hogan, Crowther, et al. 2024). The relationship between occupancy and endemism is referenced in the calculation of weighted endemism, which is the inverse of the number of distribution points (plots in this case) of a species within the study area (Linder 2001). We subsequently explored the relationship between endemism and explanatory variables. To investigate the relationship between endemism and latitude, we first employed linear models to reveal general latitudinal trends. We further fitted the relationships between endemism and explanatory variables using mixed-effects models, the latter incorporating year and depth as random effects to account for their influence on the model. For multivariate mixed-effects models, we selected the best-supported model based on AIC values (Table S5) using ‘dredge’ function in the MuMIn package (Bartoń 2019). Additionally, we incorporated a spatial distance covariance matrix in the aforementioned models to account for the effects of spatial autocorrelation. We fitted the mixed effect models using the nlme package (Pinheiro et al. 2022).
2.2.3 Community Assembly Associations
3 Results
We first explored the drivers behind the differences in microbial community composition, quantifying the relative importance of the factors related to Janzen's hypothesis (temperature variability, elevation and latitude). The dbRDA revealed that latitude (R2adj = 0.100, F = 115.41, p < 0.001) and elevation (R2adj = 0.151, F = 62.44, p < 0.001) were the strongest initial explanatory variables of bacterial community variation (Figure 2a, Table S2). However, temperature variability significantly improved model fit: Mean diurnal temperature range (bio2) added substantial explanatory power (ΔR2adj = 0.026, F = 32.84, p < 0.001); annual temperature range (bio7) further increased variance explained (ΔR2adj = 0.028, F = 37.45, p < 0.001) (Table S2). Variation partitioning also revealed that spatial factors (R2 = 0.12) play a dominant role in shaping bacterial composition differences, followed by temperature variability (R2 = 0.07) (Figure 2b). Fungal communities were most strongly influenced by precipitation (bio12, R2adj = 0.023, F = 13.86, p < 0.001), followed by latitude (ΔR2adj = 0.020, F = 12.48, p < 0.001) and temperature seasonality (bio4, ΔR2adj = 0.016, F = 10.51, p < 0.001). Elevation contributed weakly but significantly (p < 0.001) (Figure 2c, Table S2). Variation partitioning revealed that spatial factors (R2 = 0.06) and temperature variability (R2 = 0.06) had equal explanatory power for fungal communities (Figure 2d). In general, bacterial communities were mostly structured by spatial gradients and temperature variability, suggesting strong dispersal processes tied to niche filtering. Fungal assembly, however, was driven by water availability, temperature variability and latitude and showed weak elevation-dependent patterns and some influence by confounding factors (Figure S4).
We subsequently investigated the patterns in distance–decay relationships of microbial communities across climatic zones. Overall, the distance–decay relationships of bacteria and fungi in tropical mountains showed greater variation explained (R2) across different elevation ranges (short, medium and long) than Mediterranean, temperate or subtropical mountains, as evidenced by both Bray–Curtis and Sørensen dissimilarities (Figure 3a–d). Based on the results from Bray–Curtis dissimilarities, bacterial communities in tropical regions exhibited a greater distance–decay slope than temperate regions, which held for short and medium elevation distances (Figure 3a). Similarly, fungi displayed a comparable pattern within short and medium elevation spans (Figure 3c). However, no such patterns were observed in the analysis based on the Sørensen dissimilarities (Figure 3b,d). The results of beta diversity partially aligned with the predictions of Janzen's hypothesis, particularly with the Bray–Curtis distance–decay rate of bacteria and fungi.
We explored how patterns of endemism vary with latitude and their drivers. We found that endemism significantly decreases with increasing latitude for bacteria, exhibiting a stronger relationship (reverse endemism: slope = 13.3, R2 = 0.39, p < 0.001) than for fungi (reverse endemism: slope = 1.22, R2 = 0.16, p < 0.001) (Figure 4a,c). The best-supported model revealed significant negative relationships (p < 0.001) between bacterial endemism and the following explanatory variables, ranked by standardised effect size: latitude, bio12 (annual precipitation), bio1 (mean annual temperature), bio2 (diurnal temperature range) and elevation (Figure 4b). In contrast, fungal endemism showed significant positive relationships to bio4 (temperature seasonality) and bio2 (p < 0.001), but significant negative relationships to bio7 (annual temperature range), bio12 and latitude (p < 0.001) (Figure 4d). This indicates that endemism patterns were more pronounced in low-latitude (i.e., low temperature variability) mountains than in high-latitude (i.e., high temperature variability) ones, as predicted by Janzen's hypothesis—particularly for bacteria. While fungal endemism was also influenced by temperature variability, its latitudinal pattern was weaker than that of bacteria.
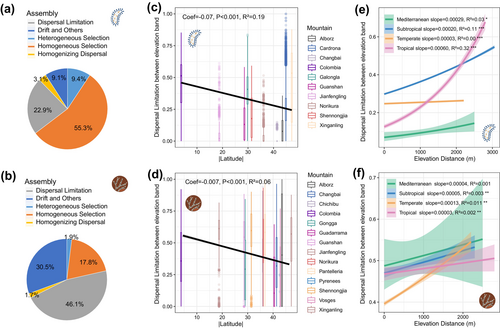
We further examined the relative importance of environmental selection and dispersal limitation, as well as the latitudinal patterns of dispersal limitation. For bacterial community assembly, we found that homogeneous selection processes were the most important (55.3% importance), followed by dispersal limitation (22.9% importance) (Figure 5a). For fungi, dispersal limitation was the dominant process (46.1% importance), with a substantial contribution from drift and other processes (30.5% importance) (Figure 5b). We observed that the importance of dispersal limitation exhibited a clear latitudinal pattern, decreasing with increasing latitude. This pattern was more pronounced for bacteria (R2 = 0.19, p < 0.001) than for fungi (R2 = 0.06, p < 0.001) (Figure 5c,d). For bacteria, the slope of increasing dispersal limitation with elevation distance was steepest in tropical mountains, followed by subtropical, Mediterranean and temperate mountains (Figure 5e). In contrast, for fungi, the slope of dispersal limitation strength was steepest in temperate mountains (Figure 5f). In summary, bacterial community assembly processes were dominated by environmental selection and influenced by a latitudinal gradient pattern of dispersal limitation. In contrast, fungal community assembly process were mostly driven by dispersal limitation, showing a weaker latitudinal pattern. Thus, bacteria aligned better with the niche-dispersal coupling framework of Janzen's hypothesis, reflecting ‘a higher mountain pass’ in tropical mountains as a physiological barrier to dispersal.
4 Discussion
Synthesising a global sample of elevational transects of montane bacterial and fungal communities, we found that bacteria align more closely with the predictions of the Janzen's hypothesis than do fungi. This is reflected in the temperature variability-driven community compositions, distance–decay rates, patterns of endemism, and selection × dispersal processes. First, we found that temperature variability was a key factor driving microbial community compositional differences. The climate variability hypothesis posits that seasonal climate variability increases with latitude, selecting for species with more dispersal capability and greater thermal tolerances (Gaston and Chown 1999; Stevens 1989). Janzen's hypothesis is an extension of the climate variability hypothesis applied to elevational gradients, emphasising that tropical mountains have lower climate variability compared to temperate mountains (Martin and McKay 2004). It states that species differ in their dispersal and thermal tolerance capabilities along elevational gradients in different climatic zones, encapsulated in the notion that ‘tropical mountain passes are higher’. Indeed, previous studies have supported this notion, verifying the important role of climate variability in shaping patterns of microbial diversity (Feng et al. 2023; Wang and Soininen 2017). Furthermore, bacterial communities were primarily shaped by spatial gradients (latitude and elevation) and temperature variability, reflecting strong dispersal limitation linked to niche filtering. In contrast, while fungal assembly was influenced by temperature variability and latitude, it exhibited weak elevation-dependent patterns and was more affected by confounding factors (e.g., sampling year and depth). Therefore, we have clarified the important roles of temperature variability, latitude and elevation—key elements of the Janzen's hypothesis (Archibald et al. 2013; Mammola et al. 2019; Montano-Centellas et al. 2021)—in shaping microbial communities (especially for bacteria), providing a background for further hypothesis testing.
Species in tropical regions tend to exhibit narrower temperature tolerances, resulting in effective ‘physiological’ barriers to dispersal along elevational gradients in tropical mountains (Ghalambor et al. 2006; Smith 2018). The differences in dispersal distances and thermal tolerance across elevational gradients also suggest that tropical mountains should exhibit greater beta diversity compared to temperate ones (Huey 1978; Zhang, Hogan, Crowther, et al. 2024). We found that the Bray–Curtis distance decay rate of tropical bacteria was greater than that of temperate bacteria, while fungi exhibited a similar pattern. Dominant tropical microbes exhibit strong dissimilarities (high Bray–Curtis distance decay), which aligns with Janzen's prediction of a narrower range in physiological temperature tolerance. However, Sørensen dissimilarity, which focuses on presence–absence rather than abundances (as in Bray–Curtis similarity), did not show this pattern. Specifically, in tropical mountains, common species are more uniformly distributed (resulting in lower Sørensen dissimilarity), but there are larger abundance differences among rare species (leading to higher Bray–Curtis dissimilarity), whereas the opposite is true for temperate mountains. Rarer species in tropical mountains were also consistent with Janzen's hypothesis, where lower dispersal ability and more climatic niches resulted in higher endemism (Graham et al. 2014; McCain 2009; Zhang, Hogan, Crowther, et al. 2024). Like Janzen's hypothesis, Rapoport's rule suggests that species should have larger distributional ranges, and thus lower endemism, at higher latitudes or elevations because of greater climatic variability (Rapoport 1982; Stevens 1992). Our results further reveal that the endemism of montane microorganisms decreases with greater latitude and that temperature variability greatly influences this pattern.
In addition to examining biogeographical patterns, we also explicitly focused on the selection × dispersal assembly mechanism, which is the coupled ecological processes underlying Janzen's mountain passes hypothesis (Polato et al. 2018; Zhang, Hogan, Crowther, et al. 2024). This work illustrates how Janzen's framework, originally for macroorganisms, bifurcates for microbes: Bacteria align with its niche-based predictions (i.e., selection-dominated physiological constraints) and dispersal-based consequences (‘higher’ mountain passes as dispersal barriers), while fungi follow a neutral-like model (i.e., dispersal/drift-dominated). Our findings also confirm that bacterial dispersal limitation weakens with latitude, showing the steepest elevational distance–decay in tropical mountains, which aligns with the main prediction of Janzen's hypothesis (Ghalambor et al. 2006).
However, for microbial communities, tropical mountain elevational gradients are not necessarily ‘higher’, nor are temperate mountain ones always ‘lower’ (Currie 2017; Mammola et al. 2019). Indeed, not all tropical montane regions have greater climatic variability than all temperate mountains. Mountain temperature gradients are greatly influenced by slope aspect and vegetation cover, which can alter local environmental factors and change predictions of the elevational stratification of biodiversity (Ghalambor et al. 2006; Smith 2018). For instance, daily temperature fluctuations in tropical high-elevation areas can resemble seasonal changes in temperate regions, and environmental changes at the base of many tropical mountains can be more dramatic than at mountain summits (Smith 2018). In addition, dispersal limitation may be a common mechanism in the assembly of mountain microbial communities, even in temperate mountains, especially for fungi (Zhang, Hogan, Ye, et al. 2024), resulting in an indistinct latitudinal gradient pattern. These differences between bacteria and fungi in responsiveness likely stem from body size and life-history differences. For example, bacteria, which typically grow as single cells, are smaller and, therefore, have greater dispersal capabilities compared to filamentous fungi (Powell et al. 2015; Jiao and Lu 2020). Fungal communities in some models partially align with Janzen's hypothesis, likely reflecting the influence of multiple complex mechanisms. Due to the influence of confounding factors on fungi, whether they conform to Janzen's hypothesis still requires more confirmations through future standardised global studies. Finally, although climate significantly influences soil microbial diversity patterns, they are also affected by soil properties (e.g., soil moisture, texture and nutrient availability) and plant–microbe interactions (Wang et al. 2022; Zhang, Worthy, Xu, et al. 2024). Therefore, ecological hypotheses developed for plants and animals may not perfectly apply to microbes, necessitating more comprehensive exploration in microbial biogeography in the future (Bodelier 2011; Guerra et al. 2020).
While our study provides insights into mountain microbial diversity maintenance mechanisms, several limitations must be acknowledged. First, our analyses integrate datasets with heterogeneous sampling designs, differing spatial coverage, sequencing depth and environmental measurements, which may introduce bias into our cross-regional comparisons. Second, the uneven distribution of sampling sites, particularly the underrepresentation of tropical mountains, constrains our ability to fully validate latitudinal gradients in microbial dispersal limitation. These methodological inconsistencies mirror broader challenges in testing Janzen's hypothesis, where divergent sampling schemes complicate assessments of climatic barriers (Ma et al. 2022). Future studies should adopt standardised protocols across complete latitudinal transects (tropical to temperate) following Janzen's original comparative framework (Janzen 1967). Additionally, three key frontiers warrant attention: (1) Multitaxa integration to test synchronous/asynchronous responses across trophic levels (plants, animals, microbes; Xu et al. 2023); (2) functional biogeography to assess whether selection and dispersal act on traits (e.g., metabolic constraints; Bodelier 2011) rather than taxonomy; and (3) experimental validation through transplant studies (Midolo and Wellstein 2020) and climate manipulations (Dainese et al. 2024). Addressing these gaps will clarify boundary conditions—such as exceptions in host-dependent fungi (Powell et al. 2015) or nontropical endemism hotspots (Currie 2017)—while enhancing predictions for biodiversity under global change.
In summary, we generally confirm that across a global sample of mountains and their climates, bacteria align more closely with the predictions of Janzen's hypothesis than fungi. Montane bacterial communities are shaped by the coupled effects of environmental selection and dispersal limitation, which jointly determine their biogeographic patterns. According to previous predictions, if climate change drives temperatures beyond bacterial thermal tolerances and affected species cannot disperse effectively to facilitate migration, bacterial diversity could decline, especially in the tropics (Polato et al. 2018; Zhang, Hogan, Crowther, et al. 2024). For fungi, which exhibit extensive dispersal limitation at the global scale, climate change may pose severe threats to fungal diversity across all latitudes, from the tropics to polar regions. The results of this study provide empirical and mechanistic insights into the maintenance of mountain microbial diversity and biogeographic patterns and highlight the need to focus on microorganisms in vulnerable mountain regions under the effects of global change.
Author Contributions
Hang Sun, Yazhou Zhang and J. Aaron Hogan designed the study. Yazhou Zhang, Minshu Song and Shijia Xu compiled and curated the data. Yazhou Zhang and Minshu Song analysed the data. Hang Sun, Thomas W. Crowther, Yazhou Zhang and J. Aaron Hogan wrote the first draft of the manuscript. All authors contributed to the several revisions of the manuscript.
Acknowledgements
This research was supported by the Second Tibetan Plateau Scientific Expedition and Research (STEP) programme (2024QZKK0200), Yunnan Applied Basic Research Project (202401CF070057), National Natural Science Foundation of China (32201315), Introducing talents start-up fund of Kunming Institute of Botany, Chinese Academy of Sciences, Yunnan Caiyun Postdoctoral Programme (to Yazhou Zhang). Yunnan Revitalisation Talent Support Programme ‘Young Talent’ Project (XDYC-QNRC-2024-561). We thank Professor Jonathan Chase for his valuable advice in organising the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data were obtained directly from online databases or previously published studies cited in the Methods and Supporting Information. Raw data used to perform analyses are available in the Science Data Bank (https://doi.org/10.57760/sciencedb.2).




