Evolutionary constraints and adaptation shape the size and colour of rain forest fruits and flowers at continental scale
Abstract
Aim
Large-scale patterns in flower and fruit traits provide crucial insights into selection processes and the evolutionary history of plant lineages. To isolate and identify the role of selective pressures, including different plant–animal interactions and the factors driving trait evolution, we investigated the convergence and divergence between flower and fruit traits in shared environments.
Location
Australia to Southeast Asia.
Time period
Eocene (c. 45 Ma) to present.
Major taxa studied
Woody angiosperm rain forest species (2,248 species, 133 families).
Methods
Using a continental-scale data set for all woody angiosperm species in the Australian rain forest (1,816 free-standing and 432 climbing species), we compared the colour and size of fleshy fruits and flowers in relationship to life-form (trees/shrubs and vines), species biogeographical histories and origins (Sunda versus Sahul) and bioregional distributions.
Results
Fleshy fruits in the Australian rain forest are mostly small, with a diversity of colours (<30 mm; 81%), and the flowers are mostly small (<10 mm; 65%) and whitish (c. 80%). Compared with trees and shrubs, climbing species showed a higher proportion of red fleshy fruits and large coloured flowers. Small whitish flowers were dominant across lineages from different biogeographical origins (Sunda and Sahul) and geographical regions, and both small and large fleshy fruits retained a range of disperser-attractant colours.
Main conclusions
Continental-scale size and colour characteristics of flowers and fleshy fruits differed despite sharing environments with similar abiotic selective pressures through time. Plant–animal interactions, including pollination and dispersal, are likely to mediate different evolutionary outcomes for plant traits and reflect both adaptation and evolutionary constraints.
1 INTRODUCTION
As environmental changes occur, the likelihood of populations of species persisting can be enhanced through various combinations of mechanisms, such as dispersal and habitat tracking, phenotypic plasticity and adaptive evolution (Ackerly, 2003). At the core of research endeavours for plants are the organs of reproduction and dispersal (flowers and fruits/seeds) that are inextricably linked to biological fitness. Some aspects of flower and fruit morphology can be measured easily and link directly to both neo-ecological relationships and past selective pressures (Bawa, 1990; Schaefer, Schaefer, & Vorobyev, 2007). Despite the potential for convergence in life-form, morphology and functional traits both within and between families, evolutionary constraints remain fundamental to shaping the limits of species adaptation to shared environments and the persistence of specific attributes (Arnold, 1992).
Floral and fruit traits are subject to strong selective pressures to enhance pollination and dispersal probabilities (Crane, Friis, & Pedersen, 1995; Sauquet et al., 2017; Tiffney, 1984). Floral and fruit traits are likely to experience very different selective pressures, and it has been suggested that greater morphological diversification has occurred in vertebrate and invertebrate animal-pollinated flowers than in animal-dispersed fruits (Schaefer, Schaefer, & Levey, 2004). Whitney (2009) tested this idea by comparing size and colour traits for flowers and fruits from three disparate Northern Hemisphere floras and found more divergence among flowers than fruits.
One explanation for how and why evolutionary pressures on flowers and fruits might differ in relationship to colour signals emerges as a consequence of fundamental differences between the photoreceptors of the key functional groups of animals (pollinators and seed dispersers; Renoult, Valido, Jordano, & Schaefer, 2014; and see Kevan, Giurfa, & Chittka, 1996). Using three floras (two in south America and one in Europe), Renoult et al. (2014) showed that within-animal-group responses to colour signals were found to be more correlated than between-group responses (pollinators versus seed dispersers). In general, the pigments in fruit tissues serve as both signals and rewards (Schaefer et al., 2004), with the carotenoids and anthocyanins that promote fruit colour also providing important dietary requirements for animals (Schaefer, McGraw, & Catoni, 2008). The convergence of signals and rewards suggests that the evolution of visual signal diversity in fruits might have been constrained by the need to produce certain types or amounts of pigments to remain nutritionally attractive to frugivores (Stournaras et al., 2013; Whitney, 2009). The linking of colour as an attractant for dispersers of fleshy fruits (Valenta et al., 2018; Whitney, 2009) to fruit and seed size and dispersal potential (Rossetto, Kooyman, Yap, & Laffan, 2015) forms the basis of the widely cited dispersal syndrome hypothesis (e.g., Van der Pijl, 1982).
Likewise, the size and colour of flowers and the shape of the flower and presentation of sexual parts form the basis of allocations to pollination syndromes (Bawa, 1990). However, in the case of flowers, the structure and shape generally reflect phylogenetic inheritance and family relationships, whereas size and colour are known to be more labile traits with little phylogenetic signal (McEwen & Vamosi, 2010). Bawa, Perry, Bullock, Coville, and Grayum (1985) noted a broad trend for tropical canopy trees to have small, white, whitish, pale green and yellowish open flowers pollinated by generalist insects. In tropical forests, it has been reported that flowers that are pollinated by small bees are often relatively inconspicuous, small, and white, pale or greenish in colour, whereas flowers pollinated by large bees are often colourful (Bawa, 1990). In the Australian tropical and subtropical rain forests, small white and whitish flowers with open form have been described as being pollinated mostly by opportunistic insects, such as micro-beetles, flies and thrips (Irvine & Armstrong, 1990; Osunkoya, 1996; Williams & Adam, 1994).
The biogeographical and climatic history of the Australian rain forests as reflected in the fossil record shows that rain forest vegetation still dominated the Australian continent during the Eocene to Early Oligocene (c. 30 Ma), with increasing aridity since the early Miocene (c. 20 Ma) (Hill, 1994). Within a dominant continental trend of rain forest contraction from the Miocene, palaeobotanical evidence suggests alternation between warm–wet (expansion) and cool–dry (contraction and extinction) cycles during the recent glacial cycles of the Pleistocene (Hopkins, Ash, Graham, Head, & Hewett, 1993; Hopkins, Head, Ash, Hewett, & Graham, 1996; Kershaw, Bretherton, & van der Kaars, 2007). By the early Eocene, the core area of the Sunda plate, which includes the present-day islands of Borneo, Sumatra, Java and Bali, in addition to the Malay Peninsula, became an elevated and emergent extension of continental Asia (Clements & Hall, 2011). The Sahul plate (Australia–New Guinea) broke from Antarctica in the Eocene (c. 40 Ma), and by the Miocene (c. 20 Ma) contact between the Sunda and Sahul plates had commenced, and floristic interchanges (that continue to the present) had begun (Hill, 1994; Kooyman et al., 2014, 2019).
The study of selected reproductive traits and their distributions across continental floras allows us to explore the impact that shared selective filters versus varying adaptive constraints can have on trait evolution. Here, we use a plant trait diversity data set from a clearly defined study system, the Australian rain forest woody flora, that provides full biome representation at the whole-continent scale (Kooyman, Rossetto, Cornwell, & Westoby, 2011; Kooyman, Rossetto, Sauquet, & Laffan, 2013). Such data sets are rare and allow us to explore questions related to trait variability across gradients of latitude (e.g., Rossetto, Kooyman, et al., 2015; Wright et al., 2017), biogeographical history, including the origins (source) of floras, and the outcome of shifts in climate variables (e.g., Yap et al., 2018). Within the context of our study system (Australian rain forests), we have a clear understanding of the biogeography and history of the rain forest flora (Hill, 1994; Kooyman et al., 2019, 2011, 2013, 2014; Rossetto, McPherson, et al., 2015; Yap et al., 2018), species distributions (Kooyman, Rossetto, Allen & Cornwell, 2012), landscape-level dynamics (van der Merwe et al., 2019; Rossetto, Crayn, Ford, Mellick, & Sommerville, 2009), the impact of historical climatic fluctuations (Mellick, Lowe, Allen, Hill, & Rossetto, 2012; Worth et al., 2017) and fruit (and seed) dispersal (Rossetto, Kooyman, et al., 2015). Genetic work in tropical and subtropical Australian rain forest shows strong signals for frugivory-mediated dispersal being a major force in landscape connectivity (van der Merwe, McPherson, Siow, & Rossetto, 2014; Rossetto et al., 2009; Rossetto, Kooyman, Sherwin, & Jones, 2008; Rossetto, Kooyman, et al., 2015).
In light of what is currently understood about the temporal dynamics of Australian rain forests, floristic interchange between Sunda and Sahul, and the influence of both long- and short-term disturbance processes, we evaluate and compare the range and frequency of dominant colour (human visible colour) and size in flowers and fleshy fruits of woody Australian rain forest species using a continental data set of 2,248 woody angiosperm (vine, shrub and tree) species. In any comparison of fruit and flower colours, it must be acknowledged that human visible colour versus high-resolution reflectance data associated with animal (including insect) sight differ significantly (e.g., Stournaras et al., 2013). Likewise, based on available evidence, we largely assume that insects and other animals are the dominant pollinators of rain forest flowers, with wind playing only a minor role (McCulley, Weller, & Sakai, 2002). We posed several questions to frame our study. What continental variation exists in the size and dominant colour traits of flowers and fruits? What is the relationship between size, colours and life-forms (trees versus vines)? Given the shared continental-scale environmental pressures through time, do the size and colour traits of flowers and fruits converge or diverge in relationship to biogeographical history and bioregional distributions? Can we attribute the detected patterns to adaptation (in response to, say, biogeographical history and shifts in pollinators and frugivores) or evolutionary constraints?
2 MATERIALS AND METHODS
2.1 Trait database
We obtained flower colour and size for 2,237 angiosperm species (1,805 free-standing and 432 climbing species) from a trait database including all (2,280) Australian angiosperm rain forest species (with data sources including: CSIRO Flora of Australia; Floyd, 1989; Harden, 1990; Cooper, 2004; Hyland, Whiffin, & Zich, 2010; Harden et al., 2014; Stanley & Ross, 1983; and presented, in part, by Kooyman et al., 2011, 2013). We excluded 43 Ficus species because they have no external flowers. Of the initial number of 2,280 angiosperm species, 1,130 were categorized as having fleshy fruits (seed fully enclosed in flesh; refer to Rossetto, Kooyman, et al., 2015) and 744 as dry (non-fleshy) fruits (33% of the species). We subsequently analysed fleshy fruit colour for 1,125 species (937 free-standing and 188 climbing species) and fleshy fruit size of 1,114 species for which these traits were recorded. Five species were removed from the data set: four had fruits >130 mm in length (and were treated as outliers), and for one species no information for fruit size and colour was available. Given that the colour and size of fleshy fruits is a focus of this study, and previous research has emphasized the potential for different evolutionary outcomes for fruit and flower colour and size (Renoult et al., 2014; Schaefer et al., 2008, 2004; Stournaras et al., 2013; Valenta et al., 2018; Whitney, 2009), we used the reduced data set (n = 1,125 species) for direct comparison of these fruit and flower traits, and we used the full data set (n = 2,237 species) to assign flower colour overall. The total number of species included throughout the fruit and flower database was 2,248 species (belonging to 133 families).
The size and colours (as written descriptions of dominant colour) of flowers and fruits were recorded from literature searches (species descriptions), published floras (CSIRO Flora of Australia online; Cooper, 2004; Floyd, 1989; Harden, 1990; Harden et al., 2014; Hyland et al., 2010; Stanley & Ross, 1983) and field surveys at the species level. Here, we use the dominant colour of fruits and flowers only. Despite identifying >70 different written descriptions of colour variations, the colour range was represented by only 10 dominant colours (e.g., are classified as “white”: white; whitish yellow; cream; cream–greenish; whitish green). The colours presented in this study are human-recognized colours. High-resolution reflectance (colour) data for the Australian rain forest flora was not available, limiting the interpretation of animal responses to colour cues and signal responses that mediate flower visitation, pollination, fruit/seed dispersal, and the non-human visible range of selection process outcomes (Stournaras et al., 2013). We obtained the measured dimensions for the width and length of fruits (continuous data), whereas flower size could be determined only as categorical classes. We divided flower size into three size groups: <10 mm (“small”), >10 mm (“large”) and, to resolve the issue of overlap between the small and large flower size threshold (10 mm) and avoid arbitrary allocations, the “medium” category (10 mm) was added and includes all the flowers that bridged and/or equalled that value (e.g., 8–12, 9–11 and 10 mm). Measurements reflected individual flowers (not inflorescences), and our focus was simply to provide an overview of visual signals (flower size/colour), not to explore pollination mechanisms or morphology.
We assumed that most of the species included in our database were pollinated by animals and that, in most cases, insects had at least some role in pollination regardless of tree species abundances. However, mixed pollination strategies (insect, larger animal and wind) might be more common than currently appreciated in some assemblages with canopy dominants that have gregarious mass flowering events (Williams, 1993; but see McCulley et al., 2002). The role of wind in pollination of other Australian rain forest species is unknown, but it is acknowledged that wind could have a role for species that have synchronous flowering even at lower densities.
2.2 Ancestry and phylogenetic comparisons
Many of the Australian rain forest angiosperm taxa have origins in Sunda (Southeast Asia), and the allocation of species to groups based on origins (Sunda versus Sahul) was central to the exploration of the selected traits (Kooyman et al., 2019; Yap et al., 2018). In that context, the assembly of the Australian flora from the Miocene onwards includes substantial interaction with Sunda (and the Sunda taxa as invaders being dispersed into Sahul). Among the 2,280 angiosperm species, we identified 578 species recognized as of Sunda ancestry and 738 species as of Sahul ancestry (following Yap et al., 2018). All these species were free-standing (trees and shrubs), except for four climbing angiosperms of Sunda ancestry.
To display a simple representation of the distribution of flower and fruit colour across a background phylogeny, we mapped fleshy fruit and flower traits (size categories and dominant colours) of free-standing species, using the Picante package in R, onto a tree (Kembel et al., 2010). We tested for phylogenetic signal on these traits (each size and colour categorical variable for fruits and flowers was entered as a binary variable for each species) using the K statistic (Blomberg, Garland Jr., & Ives, 2003), a Brownian motion-based metric of the strength of phylogenetic signal implemented in the multiPhylosignal function (Picante package). The phylogenetic tree at the species and genus levels was generated using PROTEUS (Sauquet, 2013), as described by Kooyman et al. (2013).
2.3 Regional species pools
We used distributional data from 11 regions (Kooyman et al., 2011; Kooyman, Rossetto, & Laffan, 2012; Figure 1): Western Australia and Northern Territory (WA-NT), Cape York (CY), far north Queensland–wet Tropics (FNQ), Eungella–Proserpine (EP), central Queensland (CQ), south-east Queensland (SEQ), northern New South Wales (NNSW), central New South Wales (CNSW), southern New South Wales (SNSW), Victoria (Vic), and Tasmania (Tas). To test the influence of latitude and tropical versus non-tropical climate (and proximity to Sunda), we also split the regions (and the data for flower and fruit size and colour) into tropical north (WA-NT, CY and FNQ) and sub-tropical to temperate southern (EP, CQ, SEQ, NNSW, CNSW, SNSW, Vic and Tas) based on floristic similarity and regional groupings in non-metric multidimensional scaling ordination (NMDS; Supporting Information Figure S1). The proportion of Sunda and Sahul rain forest species by region across the Australian continent is presented by Yap et al. (2018).

2.4 Statistical analysis
Qualitative differences between life-forms (free-standing versus climbing species), ancestry (Sunda versus Sahul) and biogeographical origins (tropical versus non-tropical), and the relationship between flower size and colour (both categorical variables), were tested statistically using χ2 statistics (χ2 test for equal proportions or Cochran–Mantel–Haenszel test for association when we used categorical data only) implemented in the PROC FREQ in SAS software (SAS 9.4; SAS Institute, Cary, NC, USA). To test the relationship of fruit size (length, quantitative variable) to fruit colour, life-form, ancestry and bioregion, we used a one-way ANOVA in PROC GLM (SAS).
3 RESULTS
3.1 Fruit and flower trait continental-scale patterns
We found 10 dominant colours of fleshy fruits among the 1,125 fleshy-fruited species, with significant differences in the abundance of each fruit colour (Figure 2a; χ2 = 557.6; p < .0001). The most dominant fruit colours were red, black, blue and purple (representing 71% of species), with the average maximum fruit length showing that these were mostly small fruits (Figure 3). Fleshy fruit size was on average <30 mm (81% of species) and differed significantly in relationship to fruit colour (F = 12.86; p < .0001). However, both small and large fruits retained a range of disperser-attractant colours (Figure 3), whereas dry fruits (33% of fruits in total) were predominantly brown in colour (82%).

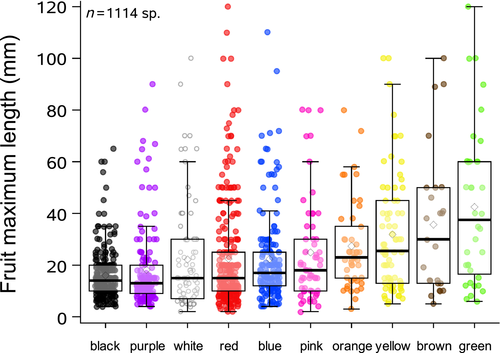
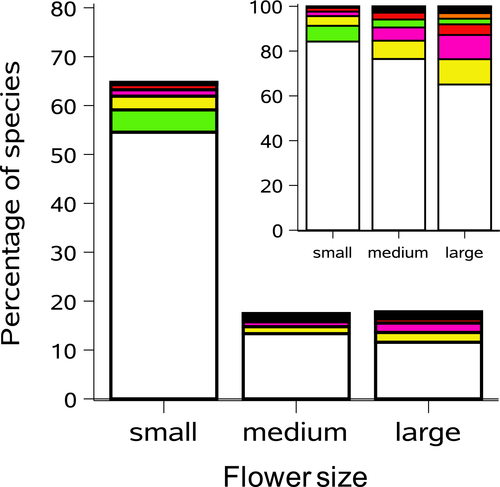
We recorded nine flower dominant colours (Figure 2b). However, a significant majority of species (1,778 species, 79.5%) had white to whitish flowers (all classified as “white” in Figure 2b; χ2 = 10,675.3; p < .0001). A similar trend was found when we analysed flower colours for the subsample of fleshy-fruited species only (n = 1,125 species; Figure 2c). For the full data set, a total of 865 species showed completely pure white flowers, and 61 of 133 families had exclusively white to whitish flowering species. Of the 2,237 species, a significant majority (64.7%) had small flowers (<10 mm; n = 1,448 species), 17.5% had medium flowers (= 10 mm; n = 391 species), and 17.8% had large flowers (>10 mm, n = 398 species) (χ2 = 992.3; p < .0001; Figure 4). Of the total of 133 families, 49 had only small flowers, eight had only medium-sized flowers, and 12 had only large flowers. We found a significant association between flower dominant colour and flower size (F = 180.45; p < .0001; Figure 4). The majority (84.3%) of small flowers were white to whitish (Figure 4, inset). In contrast, medium and large flowers were represented by a higher proportion of coloured flowers (23.5% of medium flowers and 35% of large flowers included all colours other than white; Figure 4, inset).
3.2 Comparing species with contrasting life-forms (free-standing versus climbing)
We found a significant association between fleshy fruit colours and life-form (F = 66.2; p < .0001; Figure 5a). Climbing species had a higher proportion of species (38%) with red fleshy fruits than free-standing species (20%), which had more evenly distributed fruit colours (Figure 5a). However, we found no significant effect of life-form on fleshy fruit size (F = 0.02; p = .88).
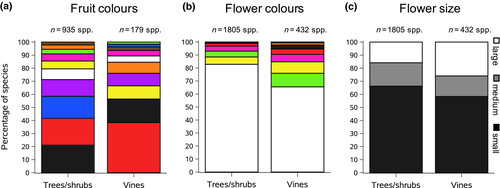
We found a significant association between flower colours and life-form (F = 89.65; p < .0001; Figure 5b). Free-standing tree and shrub species had a higher proportion of white flowers than climbing species (82.8 vs. 65.5%, respectively) and a lower proportion of coloured flowers (17.2 vs. 34.5%, respectively, including all colours other than white) (Figure 5b). Flower size and life-form were also significantly associated (F = 24.21; p < .0001; Figure 5c). Free-standing tree and shrub species had a higher proportion of species with small flowers than climbing species (66.3 vs. 58.3%, respectively) and a significantly lower proportion of species with large flowers (15.8 vs. 25.9%, respectively; Figure 5c).
3.3 Comparing species from Sunda or Sahul ancestry
We found a statistically significant association between fleshy fruit colours and ancestry (F = 66.8425; p < .0001; Figure 6a). Black and red fruits were abundant in species with Sahul or Sunda ancestries (Figure 6a). However, species with Sahul ancestry included a higher proportion of blue fruits than species with Sunda ancestry (23 vs. 9%; Figure 6a). Fleshy fruit size (length) was smaller for species of Sunda ancestry compared with Sahul (21.9 vs. 25.6 mm on average; n = 726 species; F = 7.26; p = .072) as presented by Yap et al. (2018).

We found a significant general association between flower colour and ancestry (F = 26.46; p = .0009; Figure 6b), but overall, white and whitish flowers were dominant among species from both Sunda and Sahul ancestry (>80% of species; Figure 6b). No significant trend was found between flower size and species ancestry (F = 3.8; p = .15).
Phylogenetic comparisons showed no fixed signal from specific families or lineages for any size and colour traits. The strength of phylogenetic signal was low, because K values were <0.26 in all cases (Supporting Information Table S1), suggesting evolutionary lability. Most of the traits had a non-random phylogenetic signal (statistically significant K), except for the flower colours blue, brown and purple–black (Supporting Information Table S1).
3.4 Bioregional comparisons
The results of comparisons between the 11 bioregions for the distribution of flower and fruit colour showed some variability, but red, black, blue and purple fleshy fruits dominated in most regions except Tasmania, while white flowers were dominant in all regions (Figure 1).
The bioregions were also grouped into tropical northern and subtropical to temperate southern to test the broader biogeographical patterns. We found no significant patterns in the distribution of fleshy fruit colours (F = 12.30; p = .20; Figure 6c) among tropical northern and subtropical to temperate southern regions. In both regions, fleshy fruits were generally black or red (42 and 48.5% in tropical northern and subtropical to temperate southern regions, respectively; Figure 6c), and in mainland Australia, red, black, blue and purple fruits dominated, irrespective of the latitudinal gradient (Figure 1). However, the southern regions had significantly smaller fruits (19.6 vs. 23 mm on average; F = 10.85; p = .001).
Likewise, we found no significant patterns in the distribution of flower colours (F = 3.21; p = .92; Figure 6d), with white flowers being the most abundant in each region (78 and 80% for tropical north and subtropical temperate south regions, respectively). For coloured flowers in Tasmania, the key difference was the absence of yellow (Figure 1). Coloured flowers on mainland Australia were predominantly yellow, pink and green, with other colours present but at low percentages (Figures 1 and 2). We found a significant pattern for the distribution of flower size (F = 16.37; p = .0003) among tropical northern and subtropical to temperate southern regions. The relative percentage of small flowers was significantly higher in the south (68 vs. 61%).
4 DISCUSSION
Australian rain forests have many species with colourful fleshy fruits, including those with different life-forms. Fruit colour varies and is dominated by red, black, blue and purple, with large fruits a relatively low percentage overall (19% > 30 mm), more common in the tropics than in subtropical to temperate regions and often restricted to wet forest refugia (Rossetto, Kooyman, et al., 2015). In contrast, flowers show much lower percentage representation in colour (i.e., they are predominantly white or whitish, including different life-forms) and are mostly small. However, climbing species show a higher proportion of red fruits and of larger coloured flowers than trees and shrubs. Overall, white and whitish flowers were dominant among species from both Sunda and Sahul ancestry, whereas fruit colour abundances differed somewhat, with noticeably more blue fruits in Sahul-derived species, whereas Sunda-derived species dispersing into Sahul generally had smaller fruits. The dominance and geographical spread of small white flowers confirmed that the pattern is not simply a signal of conservatism in lineages with ancient Gondwanan origins.
4.1 Colour and size characteristics are mostly homogeneous in flowers but not in fruits
Our study on the woody angiosperm flora of Australian rain forests confirms a broad range of colour and size of fleshy fruits (although relatively few large fruits) and relatively high homogeneity in flowers (small white flowers c. 54%; white/whitish flowers c. 79%; Figures 2-4). We acknowledge that many other floral characteristics are highly heterogeneous across the Australian rain forest flora and have strong phylogenetic signal (The Angiosperm Phylogeny Group et al., 2016).
In relationship to continental patterns of fruit size and colour, our results are largely in agreement with a global study that reported tropical communities as having high diversity in fruit colours (Sinnott-Armstrong et al., 2018). Globally, higher-latitude communities contain a higher percentage of red-fruited species, and the Southern Hemisphere has higher colour diversity overall, similar to the tropics (Sinnott-Armstrong et al., 2018). We found relatively high diversity in rain forest fruit colours, consistent with expectations for the Southern Hemisphere, but in mainland Australia, red, black, and blue fruits dominate irrespective of the latitudinal gradient and despite there being substantially fewer woody angiosperm rain forest species in the subtropical to temperate regions. More generally, the highest concentrations of both specialized and generalist frugivores occur in the tropics (Kissling, Böhning-Gaese, & Jetz, 2009; Kissling, Sekercioglu, & Jetz, 2012).
The exception to the continental trend is Tasmania, where the differences might be attributable to low taxonomic diversity and the very low number of fleshy-fruited species. Fruits in that case are mostly pink, blue, yellow, purple and green, with red and black absent. For flowers in Tasmania, most species are white, and the key differences are that several species are red, whereas none is yellow (Figure 1).
4.2 Disperser syndrome hypothesis
The disperser syndrome hypothesis suggests that fruit traits have evolved in response to different sets of selective pressures by disparate types of seed dispersers. The two major dispersal syndromes reflected in fruit traits in the rain forest are: (a) the bird syndrome, where fruits are brightly coloured and small, because birds have acute colour vision and commonly swallow fruit whole; and (b) the mammal syndrome, where fruits are dull in colour and larger, on average, than bird syndrome fruits, because mammals do not rely as heavily on visual cues for finding fruits (e.g., they often use olfactory cues) and can handle and eat larger fruits piecemeal (Lomáscolo, Speranza, & Kimball, 2008). In general, the fruits in the Australian rain forest are small and colourful, which aligns with the bird dispersal syndrome. Our findings show a greater representation of mammal syndrome dull colours in the Sunda fruits dispersed to Sahul (Australia) (Figure 6), but the Sunda fruits were smaller (rather than larger) overall. For Southeast Asia, Brodie (2017) showed that fruit colour was unrelated to vertebrate diversity or to the representation of birds versus mammals in the frugivore assemblage, but fruit sizes were smaller where frugivore assemblages were depauperate or dominated by small-bodied animals. To some extent, this represents a decoupling of the different components (size and colour) of the mammal dispersal syndrome. Given that the likely dispersers of these species to Australia (Sahul) are birds and flying foxes (Pteropus species), it is not possible (in this case) to allocate these patterns in the data to a particular dispersal syndrome. These findings confirm that plant–seed-disperser interaction networks are complex and diffuse (Howe, 2016; Simmons et al., 2018; Wheelwright & Orians, 1982), making the attribution of different fruit colours to selection by a single dispersal agent or even to particular guilds of dispersers difficult (Herrara, 1992; Jordano, 1995).
4.3 Fruit and flower size and colour in the Australian rain forest
A previous study of multiple vegetation communities carried out in Australia on flower colour reported that species close to the equator had a lower diversity of colours than species at higher latitudes (Dalrymple et al., 2015). Our findings show low levels of flower colour in rain forests across the continent. More generally, our findings are in broad agreement with previous smaller-scale studies focused on different forest communities (tropical to temperate rain forest and drier forests) in New Caledonia (Carpenter, Read, & Jaffre, 2003), India (Selwyn & Parthasarathy, 2006), South Africa (Griffiths & Lawes, 2006), China (Chen & Li, 2008) and New Zealand (McGimpsey & Lord, 2015) that found small white flowers to be common.
The differences between fruit and flower size and colour patterns in the Australian rain forest flora are unlikely to be attributable to chance. Rain forest vegetation has been present on the Australian continent for a very long time, providing ample opportunity for both diversification and adaptation. However, the prevalence of small white flowers in the Australian rain forest flora could also reflect evolutionary stasis and phylogenetic conservatism in ancient Gondwanan lineages (Crisp et al., 2009; Kooyman et al., 2014). For that to be the case, the expectation would be for a concentration of those character traits in specific parts of the phylogeny. An exploration of the distribution of flower size and colour across a supertree of Australian woody rain forest species clearly highlighted a wide and (mostly) even distribution of small white flowers across the phylogeny, suggesting that phylogenetic conservativism alone was unlikely to be responsible for the prevalence of these characters. Alternatively, colour and size homogeneity could reflect a reduction through time in the range of plant–animal interactions (pollination and dispersal) after the loss of some animal species, resulting in the adaptive convergence of some traits across multiple plant lineages and different biogeographical origins (Irvine & Armstrong, 1990; Osunkoya, 1996).
Continent-wide trends for aridification from at least the Miocene (20 Ma) and the expansion–contraction dynamics of rain forest vegetation as a consequence of the major climatic oscillations of the Quaternary (c. 3 Ma) to the present have caused extensive contraction of the rain forest flora across the continent (Hill, 1994; Kershaw et al., 2007; Kooyman et al., 2013, 2014). These major contractions of the Quaternary are likely to have resulted in the loss of specialized plant pollinators and constrained the plant–animal pollinator relationships to mostly small generalist pollinators. Previous studies have suggested that the small white pollination syndrome in similar floras could have emerged as a consequence of the paucity of larger and more specialized pollinators (Carpenter et al., 2003). Ecological filtering in fruit size and type within Australia has also been demonstrated previously in relationship to floristic interchange between the Sunda and Sahul floras (Yap et al., 2018).
4.4 Colour and size characteristics diverge between life-forms
Our study shows that overall, large flowers are more colourful than small flowers. In particular, climbing species (vines) have more large and colourful flowers than free-standing species. Climbers preferentially occupy the canopy (Schnitzer & Bongers, 2002) and may attract different pollinators from subcanopy to canopy tree species, resulting in a different pollination syndrome and more conspicuous flowers (Ewango, 2010). Climbers in Australian rain forest, such as species in Apocynaceae (the most abundant family of climbing species in our study) and Bignoniaceae, are known to be pollinated by butterflies, bees and wasps (William & Adam, 1994) that visit more conspicuous and colourful flowers than the generalist small beetles, flies and thrips associated with small whitish flowers (Armstrong & Irvine, 1989). In addition, climbers have a higher proportion of red fleshy fruits that tend to be preferred by birds over other colours (Schaefer & Schmidt, 2004).
4.5 Similar temporal events elicit different trait shifts across the same cohort of species
Despite sharing biogeographical histories and being subjected to similar ecological filtering (reflecting abiotic factors) and selective processes, the size and colour traits of the fruits and flowers of the extant rain forest flora of Australia differed significantly. Differences between fruit and flower colours under similar selection pressures were previously identified in a global study of fruit and flower colour diversity based on high-resolution reflectance data (Stournaras et al., 2013). That study found little indication of phylogenetic conservatism and suggested that constraints resulting from the chemical properties of pigments probably limit the diversity of both fruit and flower colour and might also explain the smaller global colour diversity of fruits. Using human-visible colour allocations, our results confirm the lack of phylogenetic signal, but in contrast to previous findings, indicate a higher percentage of colour diversity in fleshy fruits than flowers. This suggests either fundamental differences in reflectance versus human-visible colour data (ultraviolet-reflecting flowers potentially being lumped into the human white category) or a geographically specific signal in the selected traits. In addition, the inclusion in previous studies of dry fruits that lacked colour variation should have influenced only the percentages of coloured fruits and not the overall colour representation. However, the influence of the different data types, and in this case human-visible colours only, on the findings presented remains an open question until comparative data become available.
All the Australian rain forest species have had to survive severe changes in environmental conditions, most notably the climatic fluctuations that caused habitat contractions. The result of those habitat contractions was the retreat of rain forest to refugia in the wettest places. Depending on the topography and proximity to the coast, the rain forest refugia varied in size and distribution through time, with both the distance between remnant patches and the effective population sizes of local species changing with them (Kooyman et al., 2013; Mellick et al., 2012; Rossetto, McPherson, et al., 2015). For plants, these processes impacted on the fitness and viability of populations, the between-refugia dynamics and the potential for expansion and recolonization (Rossetto, Crayn, Ford, Ridgeway, & Rymer, 2007; Rossetto et al., 2008; 2009). Those same processes also filter the range of fauna available for pollination and seed dispersal (Rossetto, Kooyman, et al., 2015). At larger scales, and across salt-water barriers, similar types of constraints operate in relationship to the continental exchange between the floras of Sunda and Sahul (Kooyman et al., 2019; Yap et al., 2018).
Within that scenario, and regardless of the size or colour of fleshy fruits, a lack of dispersers does not prevent the localized persistence of species through self-replacement (close to adult trees). Previous research has demonstrated a strong connection between available fauna and the dispersal, distribution and survival of large-fruited species (Rossetto et al., 2008; Rossetto, Kooyman, et al., 2015), in addition to the selective filtering processes and traits that lead to occupation and persistence in refugial habitats (Rossetto & Kooyman, 2005).
However, for preferentially outcrossing species (such as most Australian rain forest trees; Sjöström & Gross, 2006), if pollinators are absent or the population is too small, successful reproduction is significantly constrained, and this can potentially translate to rapid localized extinctions. Within that scenario, selection for smaller, paler flowers by (say) small generalist beetle pollinators could result in relatively rapid selection (of a labile trait) for small pale flowers. We showed that floral homogeneity was not constrained to Sahul-derived lineages but extended to those of Sunda origins (Figure 6). This convergence suggests that domination by small white flowers is most likely to represent a combination of ecological filtering and selection and evolutionary constraints. Embedded in that is the idea that lineages with ancestral states inclusive of small white flowers might respond in a relatively rapid manner to selection pressures for small white flowers, resulting in more rapid convergence (Sauquet et al., 2017).
4.6 Conclusion
The Australian rain forests have persisted despite massive contractions in area, severe fragmentation and isolation. These processes have shaped the distribution of species and traits. Some species tracked preferred habitats as a consequence of inherent conservatism and an inability to change, whereas other species adapted to changing environments and the associated shifts in their interactions with pollinators and dispersers. In the present study, we have highlighted how a combination of adaptation and retention of traits can result in convergence across multiple lineages with different biogeographical histories (e.g., small white flowers). However, it also showed how the responses of plant species to selective pressures imposed by the different animals involved in pollen and fruit dispersal can result in substantially different outcomes for fruits and flowers for the same species subjected to the same temporal processes and disturbances.
ACKNOWLEDGMENTS
The authors would like to acknowledge the contribution of S. Yap for the Sunda and Sahul data set, Peter D. Wilson for providing the map included in Figure 1, and two anonymous reviewers for their valuable comments on an earlier version of this manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHORS CONTRIBUTIONS
C.E.L.D., R.M.K. and M.R. conceived the ideas and designed the methodology; R.M.K. compiled and collected the original trait data, while C.E.L.D. added fruit and flower colour fields; C.E.L.D. analysed the data and generated figures; and R.M.K. coordinated aspects of the writing. All authors contributed equally to the manuscript, including reviews of drafts, and gave final approval for publication.
Open Research
DATA AVAILABILITY STATEMENT
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.6hdr7sqwm
REFERENCES
BIOSKETCHES
Chloé E. L. Delmas is a researcher at INRAE (French National Institute for Agricultural Research and Environment) and was previously a visiting scholar in Sydney (NSW, Australia) undertaking postdoctoral research on plant trait evolution and plant–animal interactions. Her current research focus in ecology and evolution is on the interaction of grapevines with biotic and abiotic factors.
Robert M. Kooyman is a Research Fellow at Macquarie University and a Research Associate at the Royal Botanic Gardens, Sydney and Missouri Botanical Garden. His current research focus is on plant traits, evolutionary ecology, biogeography and neo- and palaeoecology in the rain forests of Malesia, Australia and Madagascar.
Maurizio Rossetto is the principal research scientist at the Royal Botanic Gardens, Sydney and leads the Evolutionary Ecology Unit. His current research focus is on the evolution, biogeography, population ecology and geospatial genetic structure and diversity of the Austral–Asian flora.




