Global meta-analysis of how marine upwelling affects herbivory
Abstract
Aim
Nutrient subsidies support high primary productivity, increasing herbivore abundance and influencing their top-down control of producers. Wind-driven upwelling events deliver cold nutrient-rich water to coastlines, supporting highly productive marine environments. Results from studies comparing ecological processes across upwelling regimes are mixed: some reveal weaker herbivory in upwelling regions, while others report a positive relationship between upwelling and herbivory. In this synthesis we examine the influence of upwelling on top-down control of producers across the globe.
Location
Global; marine ecosystems.
Time period
1978–2017.
Major taxa studied
Marine herbivores and algae.
Methods
We used data from herbivory studies focusing specifically on the influence of upwelling activity (upwelling studies), and a broader collection of herbivore exclusion studies dating back four decades. For the upwelling studies we compared herbivore effects between experiments replicated across sites for which upwelling conditions were described by the authors. Meanwhile, for the broader collection of experiments we used externally sourced oceanographic data to characterize upwelling activity, and examined how herbivory changed along a gradient of upwelling activity.
Results
Our results consistently reveal that upwelling weakens herbivore effects on producers. Herbivory was, on average, four times weaker in upwelling sites relative to sites under weak upwelling or downwelling regimes in studies that specifically examined upwelling. The analysis of the broader herbivory literature revealed a similar weakening influence of upwelling on herbivory; however, the effect size was smaller and varied across producer functional groups.
Main conclusions
Nutrient subsidies from upwelling events reduce top-down control by herbivores in coastal ecosystems; however, the negative relationship between upwelling intensity and herbivory is likely the result of a combination of co-occurring processes. First, increased primary production overwhelms consumption by herbivores. Second, cold water reduces herbivore metabolism and activity. Finally, surface currents associated with upwelling activity transport herbivore larvae offshore, decoupling secondary production from herbivory.
1 INTRODUCTION
Ecosystems are often connected by flows of organisms and materials that represent important resource subsidies, influencing trophic interactions and ecosystem function (Polis, Anderson, & Holt, 1997). The regulatory effects of resource flows have become a fundamental feature of ecological theory (Loreau & Holt, 2004), providing insights into connections between ecosystems across large spatial scales (Loreau, Mouquet, & Holt, 2003) and allowing for a more complete understanding of ecosystem function (e.g., Baxter, Fausch, & Saunders, 2005). Nutrient subsidies, in particular, can influence food-web structure and dynamics. Ecological theory predicts that producers are generally limited by their resources (Hairston, Smith, & Slobodkin, 1960), and primary productivity determines trophic connectivity and the ability of herbivores to reduce plant biomass (Fretwell, 1987). Nutrient imports can increase primary productivity, which in turn supports high secondary production, leading to strong consumption and top-down control of autotrophs (Shurin, Gruner, & Hillebrand, 2005). In this meta-analysis we examine how nutrient subsidies delivered by marine upwelling, a widely occurring oceanographic phenomenon, influence herbivory across the globe.
In marine systems, wind-generated upwelling events deliver deep, cold, nutrient-rich water to shallow coastlines. Upwelling activity supports some of the planet's most productive marine environments. In fact, the four major eastern boundary upwelling regions generate one fifth of the global fish catch (Fréon, Barange, & Arístegui, 2009). Coastlines exposed to strong upwelling activity are associated with high benthic algal growth rates, cover, nutrient content, and productivity (Blanchette, Broitman, & Gaines, 2006; Bustamante et al., 1995; Vinueza, Menge, Ruiz, & Palacios, 2014). While this demonstrates a positive influence of upwelling activity on marine producers, its influence on secondary production and top-down control may be more complex.
First, high benthic primary productivity in sites exposed to strong upwelling does not always translate into increased densities of benthic herbivores. While high food availability and quality in upwelling sites can increase the reproductive potential of herbivores (Pulgar et al., 2013), offshore currents transport larvae away from the coast, reducing invertebrate recruitment to sites under strong upwelling regimes (Blanchette et al., 2006; Broitman, Navarrete, Smith, & Gaines, 2001). Second, studies examining the effects of upwelling activity on herbivory have yielded mixed results. Experiments in Chile revealed that the strength of herbivore effects did not vary across contrasting upwelling regimes; however, per-capita herbivore effects (total grazer effect divided by the number of grazers) were stronger in upwelling sites (Nielsen & Navarrete, 2004). Similarly, experiments in New Zealand revealed that herbivore effects did not differ between the upwelled western coast and the downwelling eastern coast, but reported stronger herbivory during initial succession in upwelled areas (Menge et al., 1999). However, another study found weaker herbivore effects along the upwelled coast, but those authors examined the effects of herbivores on later successional stages potentially generating different conclusions (Guerry & Menge, 2017). A similar weakening effect of upwelling activity on herbivory was reported along the west coast of North America, but the authors also suggested that upwelling may indirectly strengthen benthic herbivory via shading effects from phytoplankton blooms (Freidenburg, Menge, Halpin, Webster, & Sutton-Grier, 2007). Less research has been devoted to understanding the influence of upwelling in tropical coasts; however, strong upwelling activity in the Galapagos Islands weakened grazer impacts (Vinueza et al., 2014). Such variation in outcomes among studies merits further analysis to identify general patterns of influence of upwelling on herbivory, and potential factors that could explain the variation in reported effect-sizes.
Here, we synthesize data from published experiments in a meta-analytic framework to examine the influence of upwelling on herbivore effects. First, we compare effect-sizes among replicated herbivore-exclusion experiments, designed specifically to examine the influence of upwelling events on herbivory. We then go beyond the upwelling literature, expanding the geographical scale past regions traditionally studied (i.e., western coast of South and North America, and New Zealand). For this, we relied on a broader set of published herbivore-exclusion experiments and examined how the strength of herbivory varies along a gradient of upwelling intensity defined by the Bakun upwelling index (BUI; Bakun, 1973). The temporal dynamics of upwelling activity can also influence the delivery of resources and larvae to coastlines (Menge & Menge, 2013). Thus, we incorporated both upwelling intensity and variability in our global analysis. By expanding our synthesis beyond field-specific studies we seek to reduce the ‘file-drawer effect’, a well-known issue with meta-analyses (Arnqvist & Wooster, 1995) wherein significant effects are more likely to be published than null results. As the studies in the broader collection of literature focused on a variety of research questions, publication biases should not be specifically associated with upwelling effects.
We test the general hypothesis that upwelling weakens the top-down effects of herbivores across broad geographical scales. Classic theory linking bottom-up and top-down control predicts that higher productivity linked to stronger upwelling activity should strengthen herbivore effects via increases in consumer abundance (Oksanen, Fretwell, Arruda, & Niemela, 1981). However, recent meta-analyses of marine herbivory reveal weak herbivore effects in productive systems (Burkepile & Hay, 2006; Hillebrand, 2002). Further, the offshore advection of larvae in upwelling sites may decouple herbivore larval production from recruitment (Blanchette et al., 2006; Broitman et al., 2001), likely weakening herbivore effects by limiting herbivore abundance. Thus, a combination of increased algal growth and reduced herbivore recruitment could lead to weak herbivore effects at upwelling sites. While our primary goal is to examine relationships between upwelling activity and herbivory, we also explore how upwelling intensity interacts and may covary with other factors known to influence productivity and top-down control (i.e., day length, temperature, turbidity, latitude, habitat type, and producer functional group).
2 METHODS
2.1 Marine herbivory literature search
We compiled studies that measured the response of marine producers to the removal or exclusion of herbivores in intertidal and subtidal environments by searching the Institute for Scientific Information's Web of Science using the following terms: (graz* OR herbiv*) AND (exclud* OR exclus* OR fenc* OR cage* OR remov*) AND (macrophyte* OR alga* OR seagrass* OR eelgrass* OR seaweed*). We also included studies cited by other meta-analyses of marine herbivory (Burkepile & Hay, 2006; Poore et al., 2012). To meet our criteria, authors must have reduced herbivore densities in exclusion treatments via manual removal, by installing cages or fences, or through chemical means such as copper-based paints and pesticides. At the end of each experiment, authors measured producer percent cover, biomass, density, or growth inside exclusion and unmanipulated (control) treatments. Lastly, all studies reported the mean producer abundance inside the exclusion and control plots, and their respective number of replicates and measure of variance. A list of the data sources is found in the Appendix and Supporting Information Appendix S2.
2.2 Response variable and moderators
We calculated herbivore effects as:  where
where 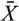 e is the mean producer abundance in the exclusion treatment, and
e is the mean producer abundance in the exclusion treatment, and  c is the mean abundance in the control treatment. Thus, yi measures the proportional change resulting from the experimental removal of herbivores, such that yi > 0 when herbivore removal results in an increase in producer abundance relative to the control, and yi < 0 when producer abundance is lower in the exclusion relative to the control. We obtained means and standard deviations from each study. When data were reported as time series, we used the data from the end of the experiment. If a procedural control was used to test for methodological artifacts, we recorded that treatment’s mean, deviation, and sample size. Effect-sizes calculated using the mean from the procedural control treatment as the denominator were strong predictors of the effect-sizes calculated using the control treatment means (ANOVA: F1,196 = 5.16, p < .05), suggesting that artifacts associated with the exclusion method had little effect on the outcome of experiments. Thus, we used the control means to calculate yi for the rest of our analysis.
c is the mean abundance in the control treatment. Thus, yi measures the proportional change resulting from the experimental removal of herbivores, such that yi > 0 when herbivore removal results in an increase in producer abundance relative to the control, and yi < 0 when producer abundance is lower in the exclusion relative to the control. We obtained means and standard deviations from each study. When data were reported as time series, we used the data from the end of the experiment. If a procedural control was used to test for methodological artifacts, we recorded that treatment’s mean, deviation, and sample size. Effect-sizes calculated using the mean from the procedural control treatment as the denominator were strong predictors of the effect-sizes calculated using the control treatment means (ANOVA: F1,196 = 5.16, p < .05), suggesting that artifacts associated with the exclusion method had little effect on the outcome of experiments. Thus, we used the control means to calculate yi for the rest of our analysis.
For each experiment, we recorded information regarding habitat type, herbivore type, and the method used to exclude them. If enough taxonomic information was provided, we also classified the producer according to functional groups proposed by Steneck and Dethier (1994). Light availability is a determinant of primary productivity, so we estimated the mean day length (MDL) in hours for the duration of each experiment using the ‘geosphere’ package (Hijmans, Williams, & Vennes, 2017) from the R statistical software environment (R Core Team, 2018), and used it as a covariate in the analysis. Water clarity may also influence light availability, so we obtained data for diffuse attenuation coefficients of the photosynthetically available radiation (KdPAR). The KdPAR provides an indicator of turbidity (Son & Wang, 2015), and is available through the National Oceanographic and Atmospheric Administration’s (NOAA’s) portal for remotely sensed oceanographic data (https://coastwatch.pfeg.noaa.gov/erddap/index.html).
We quantified the intensity and variation of upwelling at each experimental site using BUI data (Bakun, 1973) obtained from NOAA. The BUI reflects the water flux (cubic metres per second per 100 m of coastline) away from the coast (upwelling; positive values) or towards it (downwelling; negative values). NOAA generates upwelling indices worldwide at 0.5° intervals and a temporal resolution of 6 hr; we obtained the data using the “xtractomatic” package for R (Mendelssohn & Wilson, 2018). This index has been used to characterize upwelling activity in previous studies (e.g., Freidenburg et al., 2007; Menge et al., 1999; Menge & Menge, 2013); however, it is unreliable for locations in latitudes below 25°, complex coastlines, and small islands (Bakun & Agostini, 2001). We therefore excluded experiments that matched those criteria, as well as studies in estuaries to avoid confounding effects from terrestrial processes. To characterize upwelling regimes, including within year variation, we calculated the mean (BUIM) and standard deviation (BUISD) of the 6-hourly upwelling indices across 2 years following the initiation of each experiment.
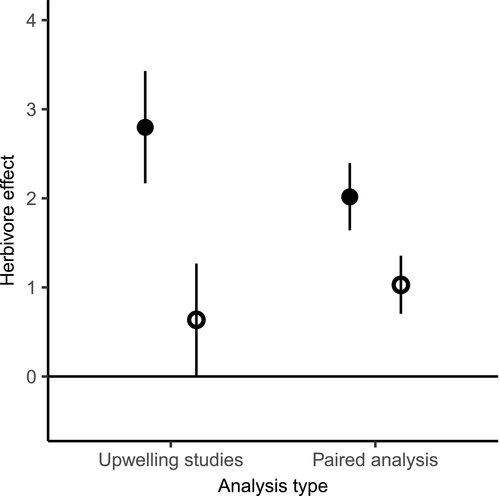
The global distribution of BUI data (Figure 1) reflects known geographical patterns of upwelling activity. The data predict a gradient of upwelling intensity along the west coast of North America (Figure 1), where upwelling increases in strength and frequency from Oregon to California (Huyer, 1983). The data also predicted strong upwelling activity in central Chile and along the western coast of South Africa, matching published descriptions of upwelling activity in those regions (Lutjeharms & Meeuwis, 1987; Montecino & Lange, 2009). Localized upwelling centres were also represented in the data, predicting positive BUIM values along the west coast of New Zealand's South Island (Menge et al., 1999), and in Brazil's Cabo Frio (Valentin, Andre, & Jacob, 1987). Although the BUI quantifies upwelling activity, it does not provide a measure of resource availability. To examine links between the BUI, productivity, and nutrient availability we obtained daily chlorophyll-a mean concentrations from the SeaWifs dataset, accessed via the ‘xtractomatic’ package. Data from SeaWifs was available from September 1997 to December 2010. We also recorded nutrient concentration data from publications when available, focusing on nitrate because it was the most commonly reported nutrient.

2.3 Statistical analyses
We used three analytical approaches to assess the robustness of our analysis and consistency of our inferences regarding upwelling's influence on herbivory. First, we focused on the upwelling literature, comparing herbivore effect-sizes between experiments replicated across contrasting upwelling regimes. We used a linear mixed effects (LME) model with each study's classification of upwelling regime as a fixed categorical factor, and the replicate measurements or sites within each study as a nested random factor. We also examined the fixed effect of the BUIM and BUISD on effect-sizes using a LME model with the same nested random factor described above.
For our second analysis, we examined the influence of BUIM and BUISD on herbivore effects reported by the broader set of herbivory studies, including upwelling studies. While this approach allowed us to broaden the scope of our analysis and reduce potential publication bias, it also led us to consider other factors that could alter the relationship between upwelling activity and herbivory. As a preliminary step, we used a LME with individual experiments as random factors to analyse the fixed effects of: producer functional group, herbivore type (macrograzer versus mesograzer), habitat type, substrate type (plate versus naturally available surface), and the season when the experiment was terminated. Based on that analysis we excluded levels of factors for which there were insufficient data to compare across upwelling regimes. We then ran a LME model to examine the fixed effect of BUIM and BUISD while considering the individual experiments as a random effect. We included MDL as a covariate because it is an important driver of primary productivity; however, we excluded sea surface temperature and nutrient concentrations as they are themselves influenced by upwelling activity. The KdPAR dataset's earliest available measurements date back to 2012, thus we were only able to examine the relationship between herbivore effects and the diffuse attenuation coefficient for a subset of the experiments (n = 14). We examined the effect of KdPAR on herbivore effects separately for that subset of experiments using a LME model with each experiment as a random effect and KdPAR as a continuous fixed variable.
For our third approach we used an alternative analysis to further control for variation introduced by the factors discussed above. We applied a nearest-neighbour algorithm, using the ‘optmatch’ package for R (Hansen, 2007), to pair individual experiments according to contrasting upwelling regimes (positive and negative BUIM), and equivalent producer functional group, grazer type, habitat type, and region. For each pair, one experiment was associated with positive mean BUIM and the other with negative values, but both experiments within each pair were associated with the same region, habitat type, producer functional group, and grazer type. We compared the effect-sizes between upwelling regimes using a Student's paired t test.
The BUI is a useful measure of upwelling activity, but it does not quantify the delivery of nutrient subsidies or primary productivity. Thus, we used a linear regression to examine relationships between log-transformed nitrate concentrations and the BUIM. The chlorophyll-a data did not meet assumptions of normality after transformations, so we used a nonparametric Spearman's rank correlation analysis to examine the effect of BUIM on chlorophyll-a concentrations. We performed all statistical analyses using the R statistical software. Mixed effects models for meta-analyses were generated using the “metafor” package (Viechtbauer, 2010). We estimated individual mean effect-sizes and 95% confidence intervals for fixed moderators using restricted maximum likelihood, and used those values in graphs depicting results.
3 RESULTS
3.1 Analysis of upwelling literature
Studies designed specifically to compare herbivore effects across upwelling gradients revealed the strongest results. Herbivore effects were on average four times weaker in sites exposed to upwelling activity relative to those where upwelling was weak or absent [LME: −2.20 (−2.34, −2.06); p < .001; Figure 2]. We also examined the relationship between BUIM and herbivory, which revealed a negative effect of upwelling intensity on herbivore effects [LME: −0.13; (−0.13, −0.11); p < .001; Figure 3a]. Notably, the BUI data we obtained coincide with the authors' classification of upwelling activity in their respective study sites. In other words, BUIM values from experiments conducted in ‘upwelling sites’ were consistently higher than values from experiments in ‘non-upwelling sites’ (Wilcoxon rank sum test: W = 11, p < .001), providing an additional validation for the metric of upwelling activity. While BUIM had an important effect on herbivory, effect-sizes were not influenced by BUISD [LME: −0.02; (−0.03, 0.01); p = .13]. Meanwhile, herbivore effects strengthened with longer days [LME: 9.03 (7.89, 10.16); p < .001]. We could not examine the relationship between KdPAR and herbivory for the upwelling literature because those experiments were conducted before the earliest KdPAR data were archived in NOAA's database: 2012.
3.2 Analysis of broader herbivory literature
We examined the effects of different factors known to influence herbivory prior to our main analysis of the broader literature. Herbivore effects varied among producer functional groups: leathery macrophytes, corticated foliose algae, foliose algae and microalgae experience the strongest effects from herbivores, meanwhile herbivore effects on crustose algae were weak and negative (see Supporting Information Appendix S1: Figure S1.1). There was also variation in effect-size among habitat type, wherein herbivore effects were strongest in rocky subtidal and intertidal reefs, and highly variable for subtidal soft sediments, which were underrepresented in the data (see Supporting Information Figure S1.2). Finally, macrograzers exerted stronger top-down control on producers when compared to mesograzers (see Supporting Information Figure S1.3), and effect-sizes did not vary significantly among experiments terminated in different seasons (see Supporting Information Figure S1.4). Based on those results we focused our subsequent analysis on the following reduced dataset: we excluded data on crustose algae (10 experiments) because changes in crustose cover following the exclusion of herbivores was often the result of competitive interactions with faster growing non-calcified algae, rather than consumption. We also removed experiments preformed in subtidal soft sediments because they were poorly represented in the data, and were only associated with negative BUIM values. Given that our analysis revealed that mesograzers exert weaker effects on producers than macrograzers, we analysed the data for the two consumer groups separately. The resulting dataset for macrograzers consisted of 81 studies, including 228 experiments spanning all continents (see Supporting Information Appendix S2; Figure 1). Meanwhile, the dataset for mesograzers consisted of 9 studies, including 11 experiments performed in North America, Australia, and New Zealand.
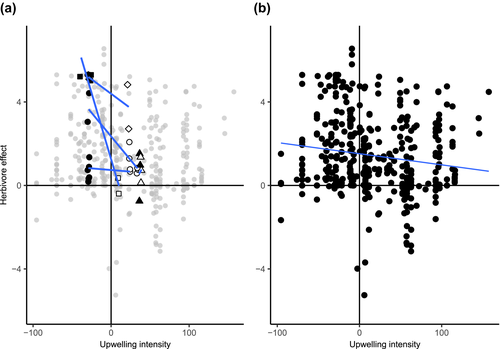
The analysis of the broader collection of herbivory experiments manipulating macrograzers revealed a negative influence of BUIM on herbivore effect-size [LME: −0.019 (−0.024, −0.014); p < .001; Figure 3b]. In nine experiments, changes in algal cover were indirectly caused by the exclusion of a predator, rather than the action of a grazer. We repeated the analysis without those data to remove potential bias and again found a significant negative effect of mean BUIM on herbivory [LME: −0.018; (−0.02, −0.01); p < .001]. Upwelling activity weakened herbivory on two functional groups in particular: foliose algae [LME: −0.06 (−0.07, −0.04); p < .001; Figure 4a] and corticated foliose algae [LME: −0.03 (−0.04, −0.01); p < .001; Figure 4b]. Neither the BUISD [LME: 0.002; (0.001, 0.006); p = .22], nor MDL [LME: 0.08 (−0.10, 0.27), p = .39] influenced herbivore effects among the broader collection of studies. The analysis of KdPAR’s influence on herbivore effects, however, revealed a nearly significant positive effect [LME: 11.97; (−1.64, 25.58); p = .06]. Our analysis of the mesograzer data did not reveal an effect of BUIM [LME: −0.0025 (−0.0140, 0.0089); p = .66] or BUISD [LME: −0.0006 (−0.0042, 0.003); p = .73] on herbivory. Longer days (MDL), however, were associated with stronger mesograzer effects [LME: 0.17 (0.041, 0.31); p < .01}. We could not examine the influence of KdPAR on herbivory by mesograzers because those data were only available for three experiments.
3.3 Analysis of paired experiments
Our third approach, that is pairing independent studies using a nearest-neighbour algorithm, yielded results consistent with the analysis of the upwelling literature: the effect of herbivores was significantly weaker in sites associated with positive BUIM values relative to sites associated with negative values (Student’s t test: mean difference= −0.99; t = −3.87; p < .001). While the two analytical approaches produced similar results, the analysis of the upwelling literature revealed a stronger effect of upwelling activity on herbivory than the pairing approach (Figure 2). The distribution of negative and positive upwelling values was uneven, and so was the distribution of experiments across grouping variables. Thus, this pairing procedure excluded multiple studies, and the resulting dataset was composed of 108 experimental pairs of high versus low upwelling sites.
3.4 Relationship between upwelling intensity and environmental parameters
Upwelling activity differentially influenced nutrient and chlorophyll concentrations. Across the studies that reported nutrient availability, nitrate concentrations increased with upwelling intensity (linear model: F1,22 = 23.14; R2 = .49; p < .01; Supporting Information Appendix S3: Figure S3.1). Chlorophyll concentrations followed the opposite trend, however, and decreased with higher BUIM (Spearman’s rank correlation: ρ = −.42, p < .001; Supporting Information Figure S3.2).
4 DISCUSSION
Our synthesis supports the hypothesis that upwelling activity weakens top-down control of marine producers. All three analytical approaches consistently reveal a negative relationship between upwelling intensity and herbivore effects. Studies designed to compare herbivory across upwelling gradients (upwelling studies) revealed the strongest effects of upwelling; however, the generality of those results is limited by low research effort and a narrow geographical breadth. By considering the broader herbivory literature and using open sourced oceanographic data (Bakun index), we were able to draw more general conclusions regarding the relationship between upwelling activity and top-down control by herbivores. This broader analysis also helped us address potential publication bias resulting from upwelling studies publishing null results at consistently lower rates than positive or negative results. While upwelling studies revealing the strongest effects could suggest the presence of a publication bias, this may be the result of careful site-selection and experimental replication by the authors of those studies. While we attempted to address potential publication bias within the upwelling literature, bias could still arise from the selective publication of significant herbivore removal effects over null results among the broader herbivory literature (i.e., studies not focused on upwelling). However, if weak or non-significant herbivore effects are more likely in strong upwelling conditions, but less likely to be published, then our results would be a conservative estimate of the effect of upwelling on herbivory.
While the different analytical approaches discussed above consistently revealed a weakening effect of upwelling on the effects of macrograzers, the analysis of the mesograzer data revealed an overall weak effect, which was not influenced by upwelling activity. This is consistent with previous experimental work revealing weak herbivore effects by mesograzers (Poore, Campbell, & Steinberg, 2009); however, the low number of studies manipulating those herbivores makes it difficult to draw general conclusions.
The weakening effect of upwelling intensity on top-down control revealed by our results is contrary to ecological theory, which predicts that increased primary productivity should support high herbivore densities, leading to stronger top-down control on autotrophs (Oksanen et al., 1981). Results from previous studies examining the influence of upwelling on trophic structure also reveal inconsistencies with theory linking top-down and bottom-up control: in Chile the cover of long-lived algae is highest in sites exposed to strong upwelling activity while the density of herbivores varies independently of oceanographic patterns (Broitman et al., 2001). Similarly, there are no consistent differences in herbivore densities between sites under contrasting upwelling regimes in South Africa or New Zealand (Bosman, Hockey, & Seigfreid, 1987; Guerry & Menge, 2017), and studies in California and central Chile reported higher densities in non-upwelling sites (Blanchette et al., 2006; Nielsen & Navarrete, 2004). Those contrasts with theoretical predictions can be explained by a combination of processes linked to upwelling activity. First, upwelled nutrients increase primary productivity and producer growth rates (Blanchette et al., 2006; Bustamante et al., 1995), overwhelming top-down control by herbivores. Second, the offshore advection of larvae by surface currents in strong upwelling areas limits consumer recruitment (Connolly, Menge, & Roughgarden, 2001; Gaines, Brown, & Roughgarden, 1985). Thus, increased primary productivity combined with reduced herbivore recruitment could explain the association between strong upwelling and weak herbivory. While offshore transport can decouple herbivore larval production from recruitment, higher food availability and quality in upwelling zones can support larger herbivores (Bosman, Hockey, & Siegfried, 1987; Pulgar et al., 2013). In turn, larger individuals may have stronger effects on producers relative to smaller individuals, suggesting that per-capita herbivore effects (i.e., herbivore effect divided by herbivore abundance) could be stronger in upwelling zones (as described by Nielsen & Navarrete, 2004). We lacked the data to examine this mechanism; however, a better understanding of how upwelling influences herbivore demography would generate valuable insights into linkages between oceanographic and ecological processes.
The advection of surface water away from the coast may also explain why nutrient and chlorophyll concentrations exhibit different patterns along an upwelling gradient. The offshore flow of water in upwelling centres allows deep water to rise to the surface, increasing the concentration of nutrients along the coast. This increase in nutrient availability should lead to high planktonic primary productivity; however, numerical responses by phytoplankton may lag behind the delivery of upwelled nutrients, and planktonic producers are swept offshore. Indeed, according to the intermittent upwelling hypothesis, the supply of phytoplankton is greatest in sites exposed to intermittent upwelling activity, and decreases with increasing upwelling frequency and intensity (Menge & Menge, 2013). That study focused on the phytoplankton–invertebrate sub-web in intertidal communities, concluding that resource supply and predation pressure should be strongest in sites exposed to intermittent upwelling. The authors also speculated that nutrient supply should increase along a gradient of persistent downwelling to persistent upwelling, while herbivore effects should decrease along the same gradient (Menge & Menge, 2013), consistent with our results. The intermittency index used in that study was based on ecological and environmental data that were collected previously by the authors in their study sites. Given our meta-analytical approach, we lacked the necessary information to calculate such an index.
In addition to increases in nutrient availability, upwelling events also reduce water temperature along coastlines, and cold upwelled water should decrease metabolic rates, dampening top-down control (Bruno, Carr, & O'Connor, 2015). Research shows that low temperatures indeed lead to reduced grazing rates (Polunin & Klumpp, 1992), and can weaken top-down control on producers (Kishi, Murakami, Nakano, & Maekawa, 2005). While cold temperatures linked to upwelling activity can reduce the activity of consumers (Sanford, 1999), it would be difficult to disentangle the relative influence of cooling and nutrient enrichment on top-down control as both occur simultaneously and are driven by the same process (i.e., upwelling). This is an important interaction to consider in future research as rising ocean temperatures should increase primary productivity and reduce the metabolic constraints imposed by cold upwelled waters (O'Connor, 2009), potentially altering food-web structure and dynamics (Bruno, Carr, & O'Connor, 2015).
The influence of climate change on upwelling activity goes well beyond warming oceans. Changes to coastal pressure gradients due to atmospheric greenhouse gas loading can increase the intensity and duration of equatorward winds and upwelling activity (Bakun, 1990). Stronger and more persistent upwelling can also reduce invertebrate recruitment to coastlines by increasing offshore larval advection (Iles et al., 2012), potentially leading to declines in consumer populations. Increases in greenhouse gas concentrations can alter oceanographic processes at even larger scales by increasing the frequency of El Niño-like conditions (Timmerman et al., 1999). Strong El Niño events can weaken and even cause the cessation of upwelling activity, leading to large declines in edible algal forms and herbivore populations (Vinueza, Branch, Branch, & Bustamante, 2006).
Our coverage of tropical regions was limited by low research effort and the lack of BUI data for low latitudes. Despite the lack of research near the equator, major eastern boundary upwelling regions extend into tropical latitudes where upwelling intensifies and becomes nearly constant (Bakun, 1990). Upwelling centres at low latitudes are ideal systems to examine the ecological processes structuring marine communities in the tropics. Seminal research suggested that shores near the equator are largely devoid of benthic algae due to the strong and persistent action of a diverse suite of herbivores (Menge & Lubchenco, 1981); however, a recent study in the Galapagos archipelago reveals that upwelling activity can relax that top-down control promoting higher algal cover (Vinueza et al., 2014). Warmer surface temperatures in the tropics may also lead to large thermal contrasts between cold upwelling centres and warm adjacent water masses. Such contrasts in environmental conditions raise questions regarding the adaptation of tropical herbivores to cold temperatures. Those questions are particularly intriguing in seasonal tropical upwelling areas, such as those located along the Central American isthmus (O'Dea, Hoyos, Rodíguez, De Gracia, & De Gracia, 2012), where surface water temperature can vary by 10°C or more (D'Croz & O'Dea, 2007).
Several variables other than upwelling intensity also explained significant variation in herbivore effect among studies. Mean day length had a positive influence on herbivore effects. We included that variable in our analysis to account for relationships between light availability and primary productivity, expecting weaker herbivore effects with higher primary productivity. The positive influence of day length on herbivore effects may instead reflect seasonal patterns of temperature and herbivore activity in which grazing rates are strongest during warmer months (Polunin & Klumpp, 1992), when days are longer. Turbidity also influences light availability in aquatic systems, and the nearly significant positive relationship between turbidity (KdPAR) and herbivore effects suggests that low light penetration may limit producer growth, leading to increased herbivory (Freidenburg et al., 2007).
The effect of herbivore removal varied among producer functional groups, and the strength of the interaction between upwelling intensity and herbivore effects varied accordingly. Sheet-like algae (foliose and corticated foliose groups) responded strongly to the removal of grazers, and herbivory on those forms was significantly reduced by upwelling activity. Low morphological complexity and a lack of structural defences of foliose and corticated foliose algae may increase their susceptibility to herbivory. However, their high productivity and growth rates may allow those algae to increase their cover rapidly, swamping herbivore effects when nutrient levels are high (Littler, Taylor, & Littler, 1983; Steneck & Dethier, 1994), such as in upwelling conditions. Leathery macrophytes also responded strongly to the exclusion of grazers, but upwelling did not weaken herbivory on that group. The data for leathery macrophytes were dominated by kelps (nearly 75%), which can store nutrients in the form of amino-acids enabling growth during nutrient-poor periods (Zimmerman & Kremer, 1986). As a result, kelps may respond weakly to increases in nutrient supply (Pfister & Van Alstyne, 2003). Thus, variation in the supply of upwelled nutrient subsidies may have little effect on kelp growth rates, leading to weak effects of upwelling activity on top-down control of kelps.
Our results demonstrate that upwelling activity reduces the strength of top-down control by herbivores. The seeming contradiction between ecological theory and our results suggests that the direction of the relationship between primary productivity and top-down control can depend on system-specific processes. In this case, the increases in primary productivity associated with upwelling activity may not generate the strong herbivore effects that theory predicts due to the recruitment and metabolic limitations imposed by the offshore advection of larvae and surface cooling in upwelling areas. Understanding how upwelling influences global patterns of productivity and top-down control of herbivores is crucial for predicting future changes to the structure and dynamics of marine ecosystems. Given the importance of upwelling systems for fisheries (Fréon et al., 2009) such changes could have serious repercussions to human societies.
ACKNOWLEDGMENTS
Funding for AJS was provided by McGill University's Biology Department and Neotropical Environment Option (NEO), the Biodiversity and Ecosystem Services Sustainability Program (BESS), and a Predoctoral Fellowship award from the Smithsonian Tropical Research Institute. This work would not have been possible without the thoughtful comments by Andrew Altieri, Andrew Hendry, Stephanie Bratkovics and Nicole Knight.
APPENDIX. A: DATA SOURCES
Aguilera, M. A., & Navarrete, S. A. (2007). Effects of Chiton granosus (Frembly, 1827) and other molluscan grazers on algal succession in wave exposed mid-intertidal rocky shores of central Chile. Journal of Experimental Marine Biology and Ecology, 349(1), 84–98.
Aguilera, M. A., Valdivia, N., & Broitman, B. R. (2015). Herbivore-alga interaction strength influences spatial heterogeneity in a kelp-dominated intertidal community. PLoS ONE, 10(9), e0137287.
Albrecht, A. S. (1998). Soft bottom versus hard rock: Community ecology of macroalgae on intertidal mussel beds in the Wadden Sea. Journal of Experimental Marine Biology and Ecology, 229, 85–109.
Anderson, M. J. (1995). Variations in biofilms colonizing artificial surfaces: Seasonal effects and effects of grazers. Journal of the Marine Biological Association of the United Kingdom, 75, 705–714.
Anderson, M. J. (1999). Distinguishing direct from indirect effects of grazers in intertidal estuarine assemblages. Journal of Experimental Marine Biology and Ecology, 234, 99–218.
Anderson, M. J., & Underwood, A. J. (1997). Effects of gastropod grazers on recruitment and succession of an estuarine assemblage: A multivariate and univariate approach. Oecologia, 109, 442–453.
Andrew, N. L., & MacDiarmid, A. B. (1991). Interrelations between sea-urchins and spiny lobsters in northeastern New Zealand. Marine Ecology Progress Series, 70, 211–222.
Andrew, N. L., & Underwood, A. J. (1993). Density-dependent foraging in the sea urchin Centrostephanus rodgersii on shallow subtidal reefs in New South Wales, Australia. Marine Ecology Progress Series, 99, 89–98.
Arrontes, J., Arenas, F., Fernandez, C., Rico, J. M., Oliveros, J., Martinez, B., … Alvarez, D. (2004). Effect of grazing by limpets on mid-shore species assemblages in northern Spain. Marine Ecology Progress Series, 277, 117–133.
Atalah, J., & Crowe, T. P. (2010). Combined effects of nutrient enrichment, sedimentation and grazer loss on rock pool assemblages. Journal of Experimental Marine Biology and Ecology, 388(1–2), 51–57.
Bazterrica, M. C., Silliman, B. R., Hidalgo, F. J., Crain, C. M., & Bertness, M. D. (2007). Limpet grazing on a physically stressful Patagonian rocky shore. Journal of Experimental Marine Biology and Ecology, 353, 22–34.
Berthelsen, A. K., & Taylor, R. B. (2014). Arthropod mesograzers reduce epiphytic overgrowth of subtidal coralline turf. Marine Ecology Progress Series, 515, 123–132.
Bertness, M. D., Crain, C. M., Silliman, B. R., Bazterrica, M. C., Reyna, M.V., Hildago, F., & Farina, J. K. (2006). The community structure of western Atlantic Patagonian rocky shores. Ecological Monographs, 76, 439–460.
Bertness, M. D., Leonard, G. H., Levine, J. M., Schmidt, P. R., & Ingraham, A. O. (1999). Testing the relative contribution of positive and negative interactions in rocky intertidal communities. Ecology, 80, 2711–2726.
Bessey, C., Heithaus, M. R., Fourqurean, J. W., Gastrich, K. R., & Burkholder, D. A. (2016). Importance of teleost macrograzers to seagrass composition in a subtropical ecosystem with abundant populations of megagrazers and predators. Marine Ecology Progress Series, 553, 81–92.
Boaventura, D., Alexander, M., Della Santina, P., Smith N. D., Re, P., da Fonseca, L. C., & Hawkins, S. J. (2002). The effects of grazing on the distribution and composition of low-shore algal communities on the central coast of Portugal and on the southern coast of Britain. Journal of Experimental Marine Biology and Ecology, 267, 185–206.
Bracken, M. E., Jones, E., & Williams, S. L. (2011). Herbivores, tidal elevation, and species richness simultaneously mediate nitrate uptake by seaweed assemblages. Ecology, 92(5), 1083–1093.
Bulleri, F., Russell, B. D., & Connell, S. D. (2012). Context-dependency in the effects of nutrient loading and consumers on the availability of space in marine rocky environments. PLoS ONE, 7(3), e33825.
Carter, S. K., VanBlaricom, G. R., & Allen, B. L. (2007). Testing the generality of the trophic cascade paradigm for sea otters: A case study with kelp forests in northern Washington, USA. Hydrobiologia, 579, 233–249.
Cervin, G., Lindegarth, M., Viejo, R. M., & Åberg, P. (2004). Effects of small-scale disturbances of canopy and grazing on intertidal assemblages on the Swedish west coast. Journal of Experimental Marine Biology and Ecology, 302, 35–49.
Christofoletti, R. A., Murakami, V. A., Oliveira, D. N., Barreto, R. E., & Flores, A. A. (2010). Foraging by the omnivorous crab Pachygrapsus transversus affects the structure of assemblages on sub-tropical rocky shores. Marine Ecology Progress Series, 420, 125–134.
Cook, K., Vanderklift, M. A., & Poore, A. G. (2011). Strong effects of herbivorous amphipods on epiphyte biomass in a temperate seagrass meadow. Marine Ecology Progress Series, 442, 263–269.
Cowen, R. K., Agegian, C. R., & Foster, M. S. (1982). The maintenance of community structure in a Central Califonia giant kelp forest. Journal of Experimental Marine Biology and Ecology, 64, 189–201.
Dethier, M. N. (1994). The ecology of intertidal algal crusts—Variation within a functional-group. Journal of Experimental Marine Biology and Ecology, 177, 37–71.
Dethier, M. N., & Duggins, D. O. (1988). Variation in strong interactions in the intertidal zone along a geographical gradient: A Washington-Alaska comparison. Marine Ecology Progress Series, 50, 97–105.
Dudgeon, S. R., Steneck, R. S., Davison, I. R., & Vadas, R. L. (1999). Coexistence of similar species in a space-limited intertidal zone. Ecological Monographs, 69, 331–352.
Duggins, D. O., & Dethier, M. N. (1985). Experimental studies of herbivory and algal competition in a low intertidal habitat. Oecologia, 67, 183–191.
Dunmore, R. A., & Schiel, D. R. (2003). Demography, competitive interactions and grazing effects of intertidal limpets in southern New Zealand. Journal of Experimental Marine Biology and Ecology, 288, 17–38.
Dye, A. H. (1995). The effects of excluding limpets from the lower balanoid zone of rocky shores in Transkei, South Africa. South African Journal of Marine Science, 15, 9–15.
Ebrahim, A., Olds, A. D., Maxwell, P. S., Pitt, K. A., Burfeind, D. D., & Connolly, R. M. (2014). Herbivory in a subtropical seagrass ecosystem: separating the functional role of different grazers. Marine Ecology Progress Series, 511, 83–91.
Ferreira, C. E. L., Goncalves, J. E. A., Coutinho, R., & Peret A. C. (1998). Herbivory by the Dusky Damselfish Stegastes fuscus (Cuvier, 1830) in a tropical rocky shore: Effects on the benthic community. Journal of Experimental Marine Biology and Ecology, 229, 241–264.
Fletcher, W. J. (1987). Interactions among subtidal Australian sea urchins, gastropods, and algae: effects of experimental removals. Ecological Monographs, 57, 89–109.
Franco, J. N., Wernberg, T., Bertocci, I., Jacinto, D., Maranhão, P., Pereira, T., & Tuya, F. (2017). Modulation of different kelp life stages by herbivory: Compensatory growth versus population decimation. Marine Biology, 164(8), 164–173.
Freidenburg, T. L., Menge, B. A., Halpin, P. M., Webster, M., & Sutton-Grier, A. (2007). Cross-scale variation in top-down and bottom-up control of algal abundance. Journal of Experimental Marine Biology and Ecology, 347, 8–29.
Gaines, S. D. (1985). Herbivory and between-habitat diversity: The differential effectiveness of defenses in a marine plant. Ecology, 66, 473–485.
Geller, J. B. (1991). Gastropod grazers and algal colonization on a rocky shore in northern California—The importance of the body size of grazers. Journal of Experimental Marine Biology and Ecology, 150, 1–17.
Guerry, A. D., & Menge, B. A. (2017). Grazer impacts on algal community structure vary with the coastal upwelling regime. Journal of Experimental Marine Biology and Ecology, 488, 10–23.
Harley, C. D. G. (2003). Abiotic stress and herbivory interact to set range limits across a two-dimensional stress gradient. Ecology, 84, 1477–1488.
Harley, C. D. G. (2006). Effects of physical ecosystem engineering and herbivory on intertidal community structure. Marine Ecology Progress Series, 317, 29–39.
Hill, N. A., Blount, C., Poore A. G. B., Worthington, D., & Steinberg P. D. (2003). Grazing effects of the sea urchin Centrostephanus rodgersii in two contrasting rocky reef habitats: effects of urchin density and its implications for the fishery. Marine and Freshwater Research, 54, 691–700.
Hori, M., Noda, T., & Nakao, S. (2006). Effects of avian grazing on the algal community and small invertebrates in the rocky intertidal zone. Ecological Research, 21, 768–775.
Johnson, C. R., & Mann K. H. (1986). The importance of plant defence abilities to the structure of subtidal seaweed communities: The kelp Laminaria longicruris de la Pylaie survives grazing by the snail Lacuna vincta (Montagu) at high population densities. Journal of Experimental Marine Biology and Ecology, 97, 231–267.
Johnson, L. E. (1992). Potential and peril of field experimentation—The use of copper to manipulate molluscan herbivores. Journal of Experimental Marine Biology and Ecology, 160, 251–262.
Keuskamp, D. (2004). Limited effects of grazer exclusion on the epiphytes of Posidonia sinuosa in South Australia. Aquatic Botany, 78, 3–14.
Kim, J. H. (1997). The role of herbivory, and direct and indirect interactions, in algal succession. Journal of Experimental Marine Biology and Ecology, 217, 119–135.
Lever, M. A., & Valiela, I. (2005). Response of microphytobenthic biomass to experimental nutrient enrichment and grazer exclusion at different land-derived nitrogen loads. Marine Ecology Progress Series, 294, 117–129.
Lindberg, D. R., Estes, J. A., & Warheit, K. I. (1998). Human influences on trophic cascades along rocky shores. Ecological Applications, 8, 880–890.
Lotze, H. K., Worm, B., & Sommer, U. (2001). Strong bottom-up and top-down control of early life stages of macroalgae. Limnology and Oceanography, 46, 749–757.
Lubchenco, J. (1983). Littorina and Fucus: Effects of herbivores, substratum heterogeneity, and plant escapes during succession. Ecology, 64, 1116–1123.
Lubchenco, J., & Menge, B. A. (1978). Community development and persistence in a low rocky intertidal zone. Ecological Monographs, 59, 67–94.
Maneveldt, G. W., Eager, R. C., & Bassier, A. (2009). Effects of long-term exclusion of the limpet Cymbula oculus (Born) on the distribution of intertidal organisms on a rocky shore. African Journal of Marine Science, 31(2), 171–179.
Markel, R. W., & DeWreede, R. E. (1998). Mechanisms underlying the effect of the chiton Katharina tunicata on the kelp Hedophyllum sessile: Size escapes and indirect effects. Marine Ecology Progress Series, 166, 151–161.
Masini R. J., Anderson, P. K., & McComb, A. J. (2001). A Halodule-dominated community in a subtropical embayment: physical environment, productivity, biomass, and impact of dugong grazing. Aquatic Botany, 71, 179–197.
McSkimming, C., Tanner, J. E., Russell, B. D., & Connell, S. D. (2015). Compensation of nutrient pollution by herbivores in seagrass meadows. Journal of Experimental Marine Biology and Ecology, 471, 112–118.
Menge, B. A., Daley, B. A., Lubchenco, J., Sanford, E., Dahlhoff, E., Halpin, P. M., … Burnaford, J. L. (1999). Top-down and bottom-up regulation of New Zealand rocky intertidal communities. Ecological Monographs, 69, 297–330.
Miller, M. W., & Hay, M. E. (1996). Coral-seaweed-grazer-nutrient interactions on temperate reefs. Ecological Monographs, 66, 323–344.
Mrowicki, R. J., Maggs, C. A., & O'Connor, N. E. (2014). Does wave exposure determine the interactive effects of losing key grazers and ecosystem engineers? Journal of Experimental Marine Biology and Ecology, 461, 416–424.
Myers, J. A., & Heck, K. L., Jr. (2013). Amphipod control of epiphyte load and its concomitant effects on shoalgrass Halodule wrightii biomass. Marine Ecology Progress Series, 483, 133–142.
Nacken, N. & Reise, K. (2000). Effects of herbivorous birds on intertidal seagrass beds in the northern Wadden Sea. Helgoland Marine Research, 54, 87–94.
Nielsen, K. J. (2001). Bottom-up and top-down forces in tide pools: Test of a food chain model in an intertidal community. Ecological Monographs, 71, 187–217.
Nielsen, K. J., & Navarrete, S. A. (2004). Mesoscale regulation comes from the bottom-up: intertidal interactions between consumers and upwelling. Ecology Letters, 7, 31–41.
O'Connor, N. E., Donohue, I., Crowe, T. P., & Emmerson, M. C. (2011). Importance of consumers on exposed and sheltered rocky shores. Marine Ecology Progress Series, 443, 65–75.
Ojeda, F. P., & Muñoz, A. A. (1999). Feeding selectivity of the herbivorous fish Scartichthys viridis: Effects on macroalgal community structure in a temperate a rocky intertidal coastal zone. Marine Ecology Progress Series, 184, 219–229.
Perreault, M. C., Borgeaud, I. A., & Gaymer, C. F. (2014). Impact of grazing by the sea urchin Tetrapygus niger on the kelp Lessonia trabeculata in Northern Chile. Journal of Experimental Marine Biology and Ecology, 453, 22–27.
Petraitis, P. S. (1987). Factors organizing rocky intertidal communities of New England: Herbivory and predation in sheltered bays. Journal of Experimental Marine Biology and Ecology, 109, 117–136.
Phillips, N. E., & Hutchison, E. (2008). Grazer effects on algal assemblages and mussel recruitment in two different mid-intertidal communities in the Cook Strait, New Zealand. New Zealand Journal of Marine and Freshwater Research, 42, 297–306.
Poore, A. G. B., Campbell, A. H., & Steinberg, P. D. (2009). Natural densities fail to limit the growth of macroalgae or their epiphytes in a temperate algal bed. Journal of Ecology, 97, 164–175.
Preen, A. (1995). Impacts of dugong foraging on seagrass habitats: Observational and experimental evidence for cultivation grazing. Marine Ecology Progress Series, 124, 201–213.
Prince, J. (1995). Limited effects of the sea-urchin Echinometra mathaei (Deblainville) on the recruitment of benthic algae and macroinvertebrates into intertidal rock platforms at Rottnest Island, Western Australia. Journal of Experimental Marine Biology and Ecology, 186, 237–258.
Reynolds, P. L., Richardson, J. P., & Duffy, J. E. (2014). Field experimental evidence that grazers mediate transition between microalgal and seagrass dominance. Limnology and Oceanography, 59(3), 1053–1064.
Robertson, A. I., & Mann, K. H. (1982). Population dynamics and life history adaptations of Littorina neglecta Bean in an eelgrass meadow (Zostera marina L.) of Nova Scotia. Journal of Experimental Marine Biology and Ecology, 63, 151–172.
Robles, C. (1982). Disturbance and predation in an assemblage of herbivorous Diptera and algae on rocky shores. Oecologia, 54, 23–31.
Ruesink, J. L. (2016). Epiphyte load and seagrass performance are decoupled in an estuary with low eutrophication risk. Journal of Experimental Marine Biology and Ecology, 481, 1–8.
Russell, B. D., & Connell, S. D. (2005). A novel interaction between nutrients and grazers alters relative dominance in marine habitats. Marine Ecology Progress Series, 289, 5–11.
Saunders, B. J., Kendrick, G. A., & Harvey, E. S. (2015). Temperate territorial damselfish act like tropical damselfish, but have no measurable effect on algae within their feeding areas. Journal of Experimental Marine Biology and Ecology, 472, 107–118.
Scheibling, R. E. (1994). Molluscan grazing and macroalgal zonation on a rocky intertidal platform at Perth, Western Australia. Australian Journal of Ecology, 19, 141–149.
Scheibling, R. E., Kelly, N. E., & Raymond, B. G. (2009). Herbivory and community organization on a subtidal cobble bed. Marine Ecology Progress Series, 382, 113–128.
Sharpe, A. K., & Keough, M. J. (1998). An investigation of the indirect effects of intertidal shellfish collection. Journal of Experimental Marine Biology and Ecology, 223, 19–38.
Sjotun, K., Eggereidel, S. F., & Hoisaeter, T. (2007). Grazer-controlled recruitment of the introduced Sargassum muticum (Phaeophyceae, Fucales) in northern Europe. Marine Ecology Progress Series, 342, 127–138.
Sousa, W. P. (1979). Experimental investigations of disturbance and ecological succession in a rocky intertidal algal community. Ecological Monographs, 49, 227–254.
Sousa, W. P., Schroeter, S. C., & Gaines, S. D. (1981). Latitudinal variation in intertidal algal community structure: The influence of grazing and vegetative propagation. Oecologia, 48, 297–307.
Strain, E. M., Johnson, C. R., & Thomson, R. J. (2013). Effects of a range-expanding sea urchin on behavior of commercially fished abalone. PLoS ONE, 8(9), e73477.
Taylor, D. I., & Schiel, D. R. (2010). Algal populations controlled by fish herbivory across a wave exposure gradient on southern temperate shores. Ecology, 91, 201–211.
Tejada-Martinez, D., López, D. N., Bonta, C. C., Sepúlveda, R. D., & Valdivia, N. (2016). Positive and negative effects of mesograzers on early-colonizing species in an intertidal rocky-shore community. Ecology and Evolution, 6(16), 5761–5770.
Underwood, A. J. (1984). Vertical and seasonal patterns in competition for microalgae between intertidal gastropods. Oecologia, 64, 211–222.
Underwood, A. J. (1998). Grazing and disturbance: an experimental analysis of patchiness in recovery from a severe storm by the intertidal alga Hormosira banksii on rocky shores in New South Wales. Journal of Experimental Marine Biology and Ecology, 231, 291–306.
Valentine, J. P., & Johnson, C. R. (2005). Persistence of the exotic kelp Undaria pinnatifida does not depend on sea urchin grazing. Marine Ecology Progress Series, 285, 43–55.
Van Tamelen, P. G. (1987). Early successional mechanisms in the rocky intertidal: the role of direct and indirect interactions. Journal of Experimental Marine Biology and Ecology, 112, 39–48.
Vanderklift, M. A., & Kendrick, G. A. (2005). Contrasting influence of sea urchins on attached and drift macroalgae. Marine Ecology Progress Series, 299, 101–110.
Vitelli, F., Hyndes, G. A., Kendrick, A., & Turco, A. (2015). Turf-forming algal assemblages on temperate reefs are strongly influenced by the territorial herbivorous fish Parma mccullochi (Pomacentridae). Marine Ecology Progress Series, 523, 175–185.
Williams, S. L., Bracken, M. E., & Jones, E. (2013). Additive effects of physical stress and herbivores on intertidal seaweed biodiversity. Ecology, 94(5), 1089–1101.
Witman, J. D. (1987). Subtidal coexistence: storms, grazing, mutualism, and the zonation of kelps and mussels. Ecological Monographs, 57, 167–187.
Wootton, J. T., Power, M. E., Paine, R. T., & Pfister, C. A. (1996). Effects of productivity, consumers, competitors, and El Niño events on food chain patterns in a rocky intertidal community. Proceedings of the National Academy of Sciences USA, 93, 13855–13858.
Worm, B., & Chapman, A. R. O. (1998). Relative effects of elevated grazing pressure and competition from a red algal turf on two post-settlement stages of Fucus evanescens. Journal of Experimental Marine Biology and Ecology, 220, 247–268.
Worm, B., & Lotze, H. K. (2006). Effects of eutrophication, grazing, and algal blooms on rocky shores. Limnology and Oceanography, 51, 569–579.
Worm, B., Lotze, H. K., Hillebrand, H., & Sommer, U. (2002). Consumer versus resource control of species diversity and ecosystem functioning. Nature, 417, 848–851.
Zacher, K., Wulff, A., Molis, M., Hanelt, D., & Wiencke, C. (2007). Ultraviolet radiation and consumer effects on a field-grown intertidal macroalgal assemblage in Antarctica. Global Change Biology, 13, 1201–1215.
Open Research
DATA AVAILABILITY STATEMENT
The data used in the analysis are available online publicly through the Dryad archive.
REFERENCES
BIOSKETCHES
Andrew Sellers is a doctoral candidate at McGill University in Canada, and research fellow in the Smithsonian Tropical Research Institute (STRI). His work focuses on coastal marine ecology, with an emphasis on how large-scale oceanographic phenomena influence local-scale ecological processes, particularly on tropical shores.
Brian Leung is a mathematical ecologist at McGill University in Canada. His research focuses on large-scale ecological predictions, using mathematical, computational and statistical models, focusing on biological invasions, biodiversity change, and sustainability.
Mark Torchin is a marine ecologist at the STRI in Panama. His research is in coastal marine ecology with an emphasis on host–parasite and consumer interactions, infectious diseases and biological invasions.




