Global patterns and drivers of species and trait composition in diatoms
Abstract
Aim
To examine species and trait composition in stream diatoms along environmental, climatic and spatial gradients and to ascertain if the use of different levels of biological organization is beneficial for investigating global environmental changes and the role of history in structuring communities.
Location
Global cover with datasets from the Antilles, France, Finland, New Zealand, La Réunion and the United States.
Methods
We related diatom species composition, guild composition, total richness and richness across guilds to environmental, climatic and spatial variables. We used non-metric multidimensional scaling (NMDS) with environmental variable fitting, redundancy analysis (RDA) with variation partitioning, analysis of similarities and linear mixed models as statistical tools.
Results
Species composition differed significantly among the study regions, while the differences in guild composition were less pronounced. US and French streams shared a large number of species, whereas islands shared only a few species with continents. For species composition, all predictors showed significant relationships with diatoms but pH, longitude, annual temperature and precipitation had the strongest impact. Variation partitioning revealed that the local environment outperformed climatic and spatial variables. For guild composition, there was a substantial overlap across regions in NMDS. The results from RDA demonstrated, however, that guild composition was better explained than species composition, especially by environmental variables. Both species and guild richness were significantly correlated with most predictors. Notably, species richness scaled positively with latitude.
Main conclusions
Diatom species and guild composition varied substantially in response to local environment and climatic and spatial variables indicating both environmental and historical effects. Species composition discriminated the geographical regions better, while guild composition detected the environmental gradients better. This emphasizes the need to examine different levels of organization to gain a deeper understanding of the roles of environment versus history in structuring communities. These findings suggest that diatom species distributions are under strong microevolutionary constraints. Conversely, guild distributions are less dependent on historic factors and are driven primarily by the environment, which makes them better suited for research on global environmental change.
Introduction
Microorganisms are the most abundant and diverse group of life on Earth, with many important roles in biogeochemical cycling of materials and elements (Falkowski et al., 2008). Therefore, it is surprising that the spatial ecology of microorganisms has only very recently become the focus of our attention (Potapova & Charles, 2002; Van der Gucht et al., 2007; Fuhrman et al., 2008). One of the reasons may be that the exhaustive sampling of microorganisms is notoriously difficult at large spatial scales and detailed distribution maps for microbes are therefore very scarce (Bru et al., 2011). However, during the past few years, microbial biogeography has seen major advances in both theory and practice (reviewed by Soininen, 2012). Nevertheless, some of fundamental questions still remain.
An ongoing debate over the spatial distribution of unicellular organisms has centred on two fundamentally opposing views. The first view is based on an argument that due to large population densities and small sizes, microorganisms are cosmopolitan, exhibiting global metapopulation dynamics (Finlay, 2002). This hypothesis would entail that microbial communities are mainly under local environmental control, i.e. their distributions are controlled by current ecological factors acting at a local scale rather than by history, large-scale climatic factors or dispersal limitation (Van der Gucht et al., 2007). The second major view posits that microorganisms exhibit biogeographical patterns similar to those of larger organisms, and their communities are thus regarded as being under both local (current ecological factors) and regional control (historical, dispersal-related and evolutionary factors). This second major view is supported by many recent large-scale studies (Hillebrand et al., 2001; Green et al., 2004; Martiny et al., 2006; Langenheder & Ragnarsson, 2007; Vyverman et al., 2007; Fuhrman et al., 2008; Passy, 2009; Bottin et al., 2014).
In addition to species composition, ecologists have recently begun investigating trait composition too because it reflects the functional response of communities to environmental gradients (McGill et al., 2006). A trait-based approach facilitates the quantification of patterns and processes along environmental and temporal gradients, making science more predictive. In algal research, traits have been used in plankton studies of marine (Edwards et al., 2013a) and freshwater environments (Reynolds et al., 2002; Edwards et al., 2013b). A growing number of investigations in the algal benthos have also adopted a trait-based approach. The high diversity of algal sizes and growth habits is reduced to three ecological guilds. These guilds – low profile, high profile and motile – trade off tolerance to nutrient limitation and disturbance for a better ability to acquire resources (Passy, 2007; Passy & Larson, 2011). Species in the low-profile guild are tolerant and of short stature with an unfavourable position in the biofilm understorey because of small body size and/or horizontal growth. In contrast, species in the high-profile and the motile guilds are sensitive to resource scarcity but due to specialized habits, i.e. extended form or motility, they can secure a more beneficial position in the biofilm, facilitating resource sequestration. Thus, the high-profile guild occupies the overstorey while the motile guild can move freely throughout the biofilm. The differential distribution of the guilds in the biofilm generates differential susceptibilities to disturbance, i.e. lowest in the low-profile guild but highest in the high-profile guild. The guild system is now broadly accepted because of its ability to distinguish spatial and temporal variability in resources (macronutrients and light) and disturbance (physical and chemical) (Berthon et al., 2011; Lange et al., 2011; Stenger-Kovács et al., 2013; Hlúbiková et al., 2014). However, guild distribution has been examined at comparatively small scales; its underlying environmental and spatial drivers at large scales are still poorly understood.
Here, our aim is to identify global patterns and drivers of stream diatom composition and richness at a species and a guild level. We thus use both traditional taxonomic composition and a functional perspective based on species traits. We examine if these community properties are solely affected by local environmental variables and climate or alternatively by geographical location, which would suggest that continents have distinct diatom species pools, constrained by historical factors. We expect guilds to track environmental gradients more closely than species composition, given their strong response to resource and stress gradients. Additionally, guilds have distinct body sizes, i.e. smaller in the low-profile but larger in the motile and high-profile guilds (Rimet & Bouchez, 2012). As body size affects diatom distribution (Heino & Soininen, 2006; Passy, 2012), we further predict a greater influence of spatial factors on guild than on species composition. In addition, we examine whether the main drivers of diatom diversity are consistent across species and guild levels.
Material and Methods
Datasets
We included diatom datasets from the Antilles, France, Finland, New Zealand, La Réunion and the United States with a total of 4437 study sites, sampled for diatoms and water chemistry. Datasets are described in detail in Table 1. After cleaning of diatom samples with acid or hydrogen peroxide, about 400–600 diatom frustules per sample were identified using a light microscope. The main diatom identification keys were Krammer & Lange-Bertalot (1986−1991) and Lange-Bertalot (1995−2015, 2000–2013).
| Study region | No. of sites | Years of sampling | Spatial extent in latitude and longitude (km) | Other details |
|---|---|---|---|---|
| Antilles | 132 | 2009–13 | 200/100 | 71 study sites in Martinique and 61 sites in Guadeloupe |
| France | 2748 | 1992–2009 | 960/920 | Water sampling was done 30 days before and after diatom sampling |
| Finland | 197 | 1986–2001 | 1100/300 | See Soininen et al. (2004) for details |
| New Zealand | 104 | 2006 | 1200/800 | See Astorga et al. (2014) for details |
| La Réunion | 55 | 2010 | 50/50 | Sampling covered nearly all island watersheds |
| United States | 1201 | 1993–2009 | 3000/5500 | Water sampling was done from 1993 to 2011 |
We considered four local environmental variables [pH, conductivity (μS cm−1), total phosphorus (mg l−1) and water temperature (°C)] available for all regions except New Zealand (without total phosphorus) and Finland (without water temperature). The four variables used are among the strongest local predictors of freshwater diatom distribution (Soininen, 2007). We also included four climatic variables [annual precipitation (mm), seasonality in precipitation (%), annual temperature (°C)and annual temperature range (°C)] drawn from the WorldClim database (Hijmans et al., 2005). Climate data covered the years 1950–2000 and average values were included in the analyses. Spatial predictors were latitude and longitude. Longitude was cosine transformed to account for the spherical shape of the Earth, making distances between regions more realistic. Appendix S1 in the Supporting Information gives pairwise correlations among local, climatic and spatial variables (Pearson r < 0.6 in all cases). All variables were standardized (mean = 0, variance =1) before analysis.
Diatom guilds
We assigned 1607 diatom species to guilds (low profile, high profile and motile) according to Lecointe et al. (1993), Passy (2007) and Rimet & Bouchez (2012). Following Rimet & Bouchez (2012), we also included a planktonic guild of species that inhabit the biofilm as a result of sedimentation from the plankton. We further distinguished as separate guilds acid-tolerant and nitrogen-fixing species, which have an advantage under acid and nitrogen limitation stress, respectively. Acid-tolerant forms (acidophilous and acidobiontic species in Van Dam et al., 1994) thrive in pH < 7. Nitrogen fixers (the members of the genera Denticula, Epithemia and Rhopalodia) rely on cyanobacterial endosymbionts to fix atmospheric nitrogen. All species (except for 83 species without determinations due to the lack of information about guilds) were assigned to guilds and the proportion of all species and the number of species detected in each guild was recorded for each sample.
Data analyses
We drew global maps to visualize the patterns in species and guild composition. Because the number of samples differed between regions, we randomly selected 50 samples from each region 999 times and computed mean species and guild richness to compare the study regions. Analysis of similarities (ANOSIM; Clarke, 1993) with Bray–Curtis distance and 999 permutations was used to test for significant differences in species and guild composition among study regions. The indicator value method (IndVal) (Dufrene & Legendre, 1997) identified the best indicator species for each region. A Mantel test with 999 permutations was used to test for correlation between dissimilarity matrices of species composition and guild composition.
We then analysed the global patterns in species and guild composition with non-metric multidimensional scaling (NMDS) followed by environmental factor fitting to examine the major structure of the data without any constraints. Redundancy analysis (RDA) with variation partitioning was performed to explore the biotic responses to the local environmental, climatic and spatial factors and to determine the fractions explained by individual predictor sets and their covariance (Borcard et al., 1992). We used a Hellinger transformed presence–absence matrix (Legendre & Gallagher, 2001) for species composition, and the occurrence of species in each guild for guild composition. Because water temperature values were not available for the Finland dataset this variable was excluded from variation partitioning analyses. We chose to keep total phosphorus in these analyses as a metric for trophic state, thus excluding New Zealand sites. All analyses were performed in R (R Core Team, 2014) with the Vegan package (Oksanen et al., 2013).
Finally, we used linear mixed models (package lme4 in R, Bates et al., 2014) with power-transformed diatom and environmental data (Ives, 2015) to examine global patterns in total diatom richness and richness within each guild separately. We used a Gaussian distribution because variables were transformed to better approximate normality. Study region was included as a random variable to account for spatial autocorrelation and any unknown factors related to the datasets (e.g. sampling design, field and laboratory methods, etc.). Also, we computed R2 values following Nakagawa & Schielzeth (2013): R2m, which is the variance explained by the fixed effects only, and R2c, the variance explained by both fixed and random effects. Mixed models were performed with all explanatory variables and their quadratic terms, except for water temperature which was excluded from this analysis due to the lack of measurement in Finland (see also above).
Results
Variability of water chemistry and climatic variables
Finnish streams had the lowest pH values with a median of 6.9, while all the other study regions had alkaline pH values with a median of nearly 8.0 (Appendix S2). Sites in France and in the Antilles had the highest total phosphorus concentrations, followed by streams in the United States and La Réunion island. Finnish streams had the lowest total phosphorus concentrations. Conductivity was the lowest in Finnish and New Zealand streams, but the highest in French and US streams. There were also notable differences in climatic variables among regions. For example, sites in New Zealand and in the Antilles had the highest mean precipitation while annual temperature was the highest in the Antilles and La Reunion island sites (Appendix S3).
Patterns in species and guild composition
Species composition differed sharply between the six study regions (ANOSIM, R = 0.437, P = 0.001). However, according to our global map, US and French streams, having some similarity in latitude, shared a large number of diatom species, whereas islands shared only a few species with the continental datasets (Fig. 1). IndVal analysis identified many species that were significantly confined to a certain study region (Appendix S4). All datasets had relatively similar proportions of the different guilds (Fig. 1), with the motile guild being overall the most species rich. However, according to ANOSIM, study regions also differed significantly (R = 0.208, P = 0.001) in guild composition. Mantel testing revealed that dissimilarities based on species composition and trait composition were significantly related (Mantel R = 0.589, P = 0.001).
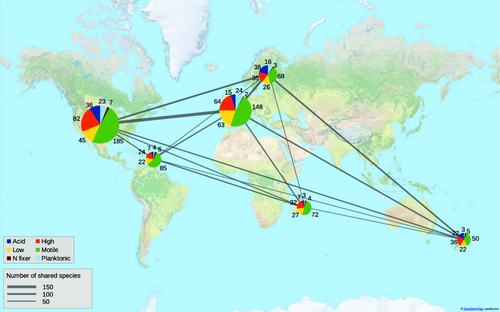
Map of mean sample species richness in study regions, with the number of shared species and occurrence of species in each guild. To control for differences in gamma diversity due to sampling effort, mean species richness and the number of shared species were calculated using 50 sites for each data set, chosen at random 999 times. Line widths indicate the mean number of shared species between the two focal regions and circle size is proportional to the mean sample species richness in each region. Numbers indicate the mean number of species belonging to each guild. Key: Acid, acid tolerant species; High, high-profile species; Low, low-profile species.
Drivers of species composition
In the NMDS analysis for species composition, local environmental, spatial and climatic variables all showed significant relationships with diatom composition (Table 2, Fig. 2). Based on R2-values, pH had the strongest relationship with species composition among the local variables (Table 2). From the spatial variables, longitude had a stronger relationship with species composition than latitude on the first two axes (Table 2a). Among the climatic variables, annual temperature and precipitation had the strongest effects followed by temperature range on the first two axes (Table 2a). Along NMDS axis 1, species composition was mainly related to pH, conductivity, longitude and temperature range (Fig. 2a, Table 2a). Along this axis, streams in Finland and La Réunion island had notably different communities compared with other regions. NMDS axis 2 represented primarily a temperature and latitude gradient, separating sites in the Antilles from other regions (Fig. 2a). NMDS axis 3 captured the variation in latitude, precipitation, temperature and conductivity. Along this axis, streams in the Antilles and La Réunion island had distinctly different species composition compared with other regions (Fig. 2c).

Non-metric multidimensional scaling (NMDS) plot for axes (a) 1 and 2, (b) 1 and 3, and (c) 2 and 3 using species composition. Environmental variables are also shown with arrows. The stress value of the analysis is 0.19. Key: Ann prec, annual precipitation; Ann temp, annual temperature; Conduc, conductivity; Prec sea, precipitation seasonality; TP, total phosphorus; Temp range, temperature range; Wat temp, water temperature; X, longitude; Y, latitude; AN, the Antilles; FI, Finland; FR, France; NZ, New Zealand; RE, La Réunion; US, United States.
| (a) Axes 1 and 2 | ||||
|---|---|---|---|---|
| NMDS1 | NMDS2 | R2 | P | |
| pH | 0.853 | 0.522 | 0.35 | 0.001 |
| Total phosphorus | 0.662 | −0.749 | 0.09 | 0.001 |
| Conductivity | 0.999 | −0.004 | 0.11 | 0.001 |
| Water temperature | 0.635 | −0.773 | 0.17 | 0.001 |
| cos X | 0.856 | 0.518 | 0.13 | 0.001 |
| Y | −0.119 | 0.993 | 0.08 | 0.001 |
| Annual precipitation | −0.502 | −0.865 | 0.13 | 0.001 |
| Annual temperature | 0.257 | −0.966 | 0.19 | 0.001 |
| Temperature range | −0.884 | 0.467 | 0.09 | 0.001 |
| Precipitation seasonality | −0.253 | −0.968 | 0.05 | 0.001 |
| (b) Axes 1 and 3 | ||||
|---|---|---|---|---|
| NMDS1 | NMDS3 | R2 | P | |
| pH | 0.959 | 0.282 | 0.24 | 0.001 |
| Total phosphorus | 0.987 | 0.158 | 0.03 | 0.001 |
| Conductivity | 0.872 | 0.490 | 0.15 | 0.001 |
| Water temperature | 0.900 | −0.436 | 0.07 | 0.001 |
| cos X | 1.000 | −0.031 | 0.08 | 0.001 |
| Y | −0.176 | 0.984 | 0.20 | 0.001 |
| Annual precipitation | −0.227 | −0.974 | 0.24 | 0.001 |
| Annual temperature | 0.346 | −0.938 | 0.17 | 0.001 |
| Temperature range | −0.653 | 0.758 | 0.18 | 0.001 |
| Precipitation seasonality | −0.120 | −0.993 | 0.04 | 0.001 |
| (c) Axes 2 and 3 | ||||
|---|---|---|---|---|
| NMDS2 | NMDS3 | R2 | P | |
| pH | 0.873 | 0.488 | 0.14 | 0.001 |
| Total phosphorus | −0.960 | 0.281 | 0.06 | 0.001 |
| Conductivity | −0.117 | 0.993 | 0.05 | 0.001 |
| Water temperature | −0.963 | −0.271 | 0.12 | 0.001 |
| cos X | 1.000 | 0.013 | 0.04 | 0.001 |
| Y | 0.459 | 0.888 | 0.26 | 0.001 |
| Annual precipitation | −0.483 | −0.876 | 0.31 | 0.001 |
| Annual temperature | −0.685 | −0.728 | 0.31 | 0.001 |
| Temperature range | 0.385 | 0.923 | 0.12 | 0.001 |
| Precipitation seasonality | −0.705 | −0.709 | 0.08 | 0.001 |
In RDA, study regions were also clearly separated by pH, conductivity and annual temperature, which were the most important contributors to axes 1 and 2, respectively (Appendix S5). Variation partitioning of species composition revealed that local environment had the strongest pure effect, followed by climate and spatial variables (4%, 2% and 1% of the variation, respectively) (Appendix S6). The undetermined fraction was high (90%).
Drivers of guild composition
In the NMDS analysis of guild composition, local environmental variables, climatic and spatial variables all showed significant relationships with guilds (Fig. 3, Appendices S7 & S8). However, these relationships (R2 values) were overall weaker than the respective relationships for species composition. Guild composition was mainly related to total phosphorus, annual temperature and water temperature along NMDS axis 1, while NMDS axis 2 represented mostly a gradient in pH, latitude, longitude and precipitation. The acid-tolerant species, most of which were found in the Finland dataset, were best separated from the other guilds.
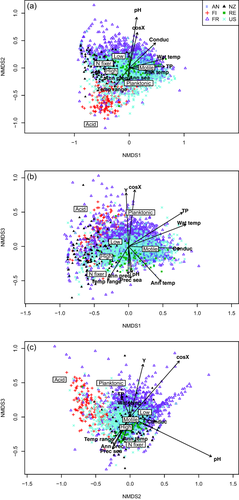
Non-metric multidimensional scaling plot for axes 1 and 2 (a), 1 and 3 (b), and 2 and 3 (c) using guild composition. Environmental variables are also shown with arrows. The stress value of the analysis is 0.07. See Figs 1 & 2 for key.
In RDA, axis 1 was mainly related to total phosphorus, temperature and conductivity and axis 2, to pH (Appendix S9). Variation partition further revealed that local environmental variables had the strongest effect on guild composition (14% of variation explained), exceeding that on species composition (Appendix S6). The undetermined fraction (74%) was lower than for species composition.
Species and guild richness
According to the linear mixed models, diatom species richness (power transformed; see Appendix S10 for details) was significantly (P < 0.001) related to all included variables except temperature range (Table 3, Appendix S11). Species richness had a negative relationship with pH and conductivity, whereas the relationship with total phosphorus was positive. Notably, species richness scaled positively with latitude.
| Total species | Acid tolerant | High profile | Low profile | Motile | Nitrogen fixer | Planktonic | |
|---|---|---|---|---|---|---|---|
| Intercept | 7.923*** | −8.792** | 3.472 | 8.536 (*) | 5.518** | −1140.114*** | −1.054*** |
| pH | −0.119** | −1.198*** | 0.031 | 0.252*** | −0.146*** | 22.247** | −0.006*** |
| pH2 | −0.022 | 0.310*** | −0.031*** | −0.140*** | −0.036* | −11.286** | 0.002*** |
| Total phosphorus | 0.412*** | 0.166 | −0.043* | −0.078** | 0.605*** | −57.390*** | 0.012*** |
| (Total phosphorus)2 | 0.099*** | 0.063 | −0.003 | 0.027 | 0.152*** | −7.186 | 0.001 |
| Conductivity | −0.142*** | −1.170*** | −0.169*** | −0.102** | 0.118** | 13.371 (*) | −0.009*** |
| Conductivity2 | −0.079** | 0.216*** | −0.037*** | −0.016 | −0.138*** | −16.783*** | 0.001 |
| cos X | −3.807*** | −1.851 | 0.717 | 0.298 | −5.546*** | 162.840*** | −0.177*** |
| cos X2 | −1.589*** | −1.222 | 0.141 | −0.287 | −2.032*** | 118.950*** | −0.065*** |
| Y | 3.213*** | 0.711 | 0.502*** | 1.439*** | 3.118*** | −113.622** | 0.082*** |
| Y2 | 0.544*** | −0.483 | −0.038 | −0.994*** | 0.629** | 21.508* | 0.013 (*) |
| Annual precipitation | −0.545*** | 0.202 | −0.282*** | −0.412*** | −0.342*** | 39.462** | −0.009*** |
| (Annual precipitation)2 | 0.083*** | −0.045 | 0.018 (*) | 0.056*** | 0.067*** | −37.825*** | 0.002* |
| Annual temperature | 0.809*** | −0.236 | −0.078 (*) | 0.154* | 0.996*** | −17.729 | 0.021*** |
| (Annual temperature)2 | 0.120*** | 0.119* | 0.070*** | 0.120*** | 0.083** | 13.790*** | −0.003*** |
| Temperature range | −0.110 | −1.633*** | −0.194*** | −0.252** | 0.190* | 20.701 | −0.009** |
| (Temperature range)2 | −0.001 | 0.129 | −0.031 | −0.058 | −0.073 | 20.594** | 0.008*** |
| Precipitation seasonality | −0.184*** | 0.286* | −0.063** | 0.013 | −0.207*** | 12.728 | −0.004* |
| (Precipitation seasonality)2 | 0.024 | −0.232*** | −0.008 | −0.038 | 0.064* | −21.920*** | 0.000 |
| d.f. | 3931 | 3931 | 3931 | 3931 | 3931 | 3931 | 3931 |
| AIC | 15043 | 21726 | 9786 | 13350 | 14227 | 57111 | −11424 |
| R2m | 0.39 | 0.23 | 0.28 | 0.11 | 0.55 | 0.23 | 0.45 |
| R2c | 0.58 | 0.59 | 0.78 | 0.97 | 0.83 | 0.23 | 0.82 |
- Values are model coefficients.
- *** P < 0.001; ** P < 0.01; * P < 0.05; (*)P < 0.1.
- Abbreviations: X, longitude; Y, latitude; R2m, variance explained by fixed effects only, R2c, variance explained by fixed and random effects; AIC, Akaike information criterion.
- For readability and comparability with richness plots (Appendix S11), signs of coefficients have been changed when necessary according to the sign of the power transformation (see Appendix S10).
The richness of acid-tolerant species decreased with pH and conductivity, as expected. The richness of motile taxa increased with total phosphorus, while high-profile, low-profile and nitrogen-fixing species decreased with total phosphorus. Planktonic taxa showed a positive relationship with total phosphorus and latitude. The explained variances by fixed and random effects were typically higher in the models for guilds than those for total species, while fixed effects were largely comparable between guilds and total species.
Discussion
Diatom species composition varied substantially at continental scales in response not only to local environmental variables but also to large-scale climatic and spatial factors. Although the correlations between local and climatic variables were relatively moderate (all r < 0.6; Appendix S1), we cannot rule out completely the potential influence of climate and geographical location on some important local environmental variables not included in our analyses. Even if the local effect on diatoms is stronger than the spatial or climatic effects globally (Appendix S6), our findings suggest that diatom composition seems to be shaped to a notable extent by historical factors as well, consistent with an evolved understanding of microorganisms as having a distinct biogeography (Martiny et al., 2006; Vyverman et al., 2007; Verleyen et al., 2009; Bennett et al., 2010).
The present results thus indicate that the filtering of diatom species to a local site takes place at both large (i.e. influence of climate and location) and local scales (influence of water chemistry). Thus, unicellular taxa appear to have distinct regional or continental species pools, similar to larger organisms. Martiny et al. (2006) suggested that, at continental scales, the distance effect should override any effects of the local environmental variables for microbes, while at intermediate scales (i.e. 100–3000 km), microbial communities should be structured both by the local environment and by distance. However, our results are consistent with Verleyen et al. (2009) and suggest that local environment is highly important for diatoms at continental scales as well. This is because water chemistry has notable continental patterns too (Appendix S2) and such variability in local factors should be considered explicitly when examining microbial communities at a global scale. Among the local variables, pH and conductivity emerged as the most important variables for diatom composition in agreement with an earlier report (Soininen, 2007). In contrast, total phosphorus had a somewhat weaker relationship with diatom composition in NMDS, perhaps due to smaller among-region variation than in conductivity.
This is the first global study of diatom guild composition, adding novel insights to the emerging field of functional biogeography (Violle et al., 2014). Based on findings at small spatial scales (Passy, 2007), we expected that guild composition would track both environmental and distance factors at continental scales better than species composition, due to the strong relationship between traits and environmental gradients and the correspondence between guilds and diatom sizes. Constrained ordination confirmed these predictions – guilds had stronger responses to all sets of predictors, but especially to local environmental factors. A contributing factor to the better discriminative power of guilds was their low regional fidelity, as revealed by NMDS. Acid-tolerant species were the only group with distinct distribution in Finnish streams, characterized by cold climate and acidic waters with low conductivity and nutrient concentrations. The other guilds did not display any characteristic distributional patterns. Thus, unlike species, which showed relatively dissimilar distributions across continents, guilds exhibited a substantial overlap, suggesting that species are the subject of stronger microevolutionary processes whereas guilds are more driven by environmental gradients. The prevalence of microevolutionary processes among diatom species agrees with the findings of notable between-species variation in distributions within diatom genera along spatial and environmental gradients (Weckström et al., 1997; Soininen & Niemelä, 2002). These findings have important implications for studies of global environmental change. They indicate that species composition due to regional specificity is not as suitable as guild composition for gauging environmental changes that occur at a global scale, such as global warming, eutrophication, acidification and brownification (i.e. the increasing levels of dissolved organic carbon and iron in surface waters).
We show that global diatom species richness is driven jointly by the local environment and climatic and geographical factors. Furthermore, we report a significant increase in diatom richness with latitude, opposite to the nearly universal poleward decline of richness, observed across all organismal groups (Hillebrand, 2004). Remarkably, an earlier study of US streams also documented a deviation from the latitudinal diversity gradient, whereby the greatest diatom richness was found in subtropical (c. 25°) and temperate regions (c. 50°), but the lowest at mid-latitudes (e.g. between 35° and 40°; Passy, 2010). This deviation was attributed to the corresponding latitudinal distributions of landscape and water chemistry factors, including wetlands, dissolved organic carbon and iron supply. It was proposed that wetlands export organically bound and bioavailable iron and thus promote diatom biodiversity in streams (Passy, 2010). Given that diatom richness in the present study increased at low pH and the lowest pH was detected in Finland where streams are humic-rich due to the large presence of wetlands, we conclude that it is possible that wetlands may at least partly control stream diatom biodiversity globally.
Of the local variables, pH had an overall negative trend with species richness, which disagrees with earlier findings by Heino et al. (2010) and shows that ion-poor waters may have globally higher diatom diversity. However, as also suggested by Pither & Aarssen (2005), the highest local species richness values were actually found in circumneutral waters (Appendix S11). We also found that richness increased with total phosphorus, confirming that a high supply of nutrients increases the niche dimensionality of the algal benthos, as opposed to terrestrial environments where richness declines with nutrient enrichment (Passy, 2008). Proportional guild richness generally behaved as predicted, for example the acid-tolerant guild decreased with pH, the motile and the planktonic guilds increased with total phosphorus, while the low-profile, high-profile and nitrogen-fixer guilds decreased with total phosphorus. Planktonic species proliferate in large rivers, which tend to be eutrophic. Nitrogen and phosphorus concentrations are correlated in rivers (Passy, 2012), so the decline of nitrogen fixers at high phosphorus concentrations is most likely a byproduct of higher nitrogen concentrations, where nitrogen fixers are at a competitive disadvantage and become displaced by non-nitrogen-fixing forms (Larson et al., 2015). The decrease of proportional richness of the high- profile guild with total phosphorus was unexpected, given prior reports showing that nutrient enrichment stimulated this guild (Passy, 2007; Passy & Larson, 2011; Stenger-Kovács et al., 2013). Considering that the high-profile guild is most sensitive to physical disturbance and not well adapted to growth on unstable silty substrates characteristic of eutrophic higher-order streams, it is possible that its lower proportional richness at high total phosphorus is in fact a consequence of other habitat factors and not directly related to nutrient levels. Considering the ambiguous behaviour of the high-profile guild we do not recommend its use in future assessments of eutrophication. Conversely, the low-profile and motile guilds, which are widespread and abundant, are good indicators of nutrient enrichment and deserve more extensive use in biomonitoring.
In conclusion, diatom species and guild composition varied substantially at continental scales in response to local environmental variables, large-scale climatic and historical (spatial) factors. Species composition discriminated better the geographical regions, while guild composition tracked better the environmental gradients. This emphasizes the need to examine different levels of organization to gain a deeper understanding of the roles of environmental, climatic, regional and historic factors in structuring biological communities. We therefore advocate broader implementation of functional approaches in global change research but stronger reliance on traditional taxonomical analyses for biogeographical investigations.
Acknowledgements
We are grateful to Thibaut Feret for his comments on data analyses and Sébastien Boutry, Julie Gueguen and François Delmas for help in data recovery. We also thank the French National Office of Water (ONEMA) and Asconit for technical and financial support in gathering the data, and two anonymous referees for their constructive comments.
References
Biosketch
Janne Soininen in an associate professor in spatial environmental research in University of Helsinki. He is interested in large-scale community ecology and especially in the distribution of small aquatic organisms.




