California forests show early indications of both range shifts and local persistence under climate change
Abstract
Aim
Forest regeneration data provide an early signal of the persistence and migration of tree species, so we investigated whether species shifts due to climate change exhibit a common signal of response or whether changes vary by species.
Location
California Floristic Province, United States; mediterranean biome.
Methods
We related Forest Inventory and Analysis (FIA) data from 2000−07 for 13 tree species to high-resolution climate and geographical data. Using methods from invasion ecology, we derived indices of species-specific regeneration overlap and central tendency change (range-wide global indicators) based on kernel density estimation of presence and absence of regeneration. We then built regeneration surfaces to identify areas of occurrence of high regeneration (regeneration hotspots, local indicators) in both geographical and climate space for 13 common tree species.
Results
Differences between presence and absence of regeneration in forests varied in magnitude across species, with little evidence that tree regeneration is shifting to higher latitudes and elevations, the expected geographical fingerprint of climate change. We also identified potential topographic mediators of regeneration dynamics. Multiple regeneration hotspots were found for many species, suggesting the influence of non-climatic factors on regeneration. Differences between the presence and absence of regeneration in geographic and climate spaces were not always congruent, suggesting that shifting climate space and range area are not entirely coupled.
Main conclusions
The distributions of regeneration in Californian forests show diverse signals, not always tracking the higher latitudinal–elevation fingerprint of climate change. Local regeneration hotspots are common in our analysis, suggesting spatially varying persistence of forest linked to natural and anthropogenic disturbances. Our results emphasize that projections of tree range shifts in the context of climate change should consider the variation of regeneration drivers within species ranges, beyond the overall climate signal.
Introduction
Climate change is expected to drive substantial changes in species distributions that may be hard to detect given the need for long-term data over large areas to tease apart the many factors affecting the distribution and abundance of species (Parmesan, 2006). In the case of sessile, long-lived organisms, such as trees, early indicators of species range change may be detectable using ‘life stage for time’ substitution: juveniles are used as a proxy of recent climate trends and may manifest systematically different distributions from adults (Lenoir et al., 2009; Zhu et al., 2012, 2014; Bell et al., 2014; Monleon & Lintz, 2015).
Examining patterns of seedling and sapling recruitment within the current distribution of adults incorporates the effects of local forest structure and seed supply on regeneration patterns. For example, Dobrowski et al. (2015) showed that forest structure can buffer the impacts of climate change on tree species and associated distribution shifts (Lloret et al., 2012, reviewed stabilizing processes). It is therefore crucial to assess signals of forest range shifts in the presence of potential buffering mechanisms.
We would expect that, under directional climate change, the distribution of forests with regeneration would be different from that of forests without regeneration (Fig. 1a). The overall magnitude and directionality of the displacement between regenerating and non-regenerating forests could therefore provide a general picture of displacement – referred to as range-wide or global indicators hereafter.
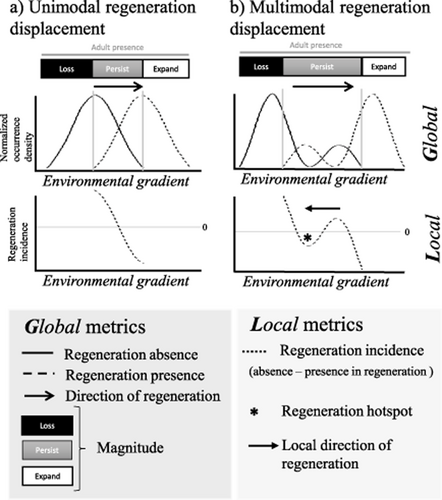
Conceptual scheme of global and local regeneration dynamics. A signal of climate change is expected to displace the density of occurrence of regeneration versus non-regeneration in forests along an environmental gradient (dotted lines versus solid lines in top panels of a and b). We can identify global measures of regeneration dynamics (a) as loss in regeneration (Loss; no regeneration within the distribution of conspecific adults), persistence (Persist; both presence and absence of regeneration), and expansion (Expand; presence of regeneration at all sites where adults are present). Moreover we can identify the directionality of the change (arrows in global dynamics; top panels) by connecting the median of the two density distributions. Nevertheless, the signal of regeneration may be affected by many other factors (e.g. disturbance, other climate variables, management) creating a multimodal regeneration distribution along the environmental gradient (b). We can identify local hotspots of regeneration (*) using the relative regeneration density of occurrence (dotted line in bottom graphs), the difference between non-regeneration and regeneration frequencies. In a unimodal response (a) where all forests shift regeneration in the same direction on the environmental gradient we could find only one local regeneration hotspot, whereas if the response is more complex (e.g. multimodal; b), we could identify several local hotspots of regeneration whose directionality (arrows) may or may not coincide with the global shift.
Most studies predict or document a poleward or uphill shift of distributions as species track changing temperature regimes (Walther et al., 2002; Parmesan & Yohe, 2003; Hickling et al., 2006). The migration hypothesis sensu Zhu et al. (2014) posits that we should thus expect tree regeneration to behave in the same manner. Another plausible scenario – the turnover hypothesis sensu Zhu et al. (2014) – suggests instead that regeneration may occur preferentially in high-productivity (warm, wet) sites that also promote increased population turnover due to higher rates of mortality, recruitment and growth. These two hypotheses are not mutually exclusive, as higher productivity (and turnover) could be found at higher elevations due to warming and increased availability of water. Nevertheless, in water-limited systems (such as California) the turnover hypothesis may be better applied to hydrological rather than temperature controls on regeneration. Distinguishing between these two hypotheses, and thus the mechanisms driving range shifts, is key to generalizing the fingerprint of climate change on forests.
Beyond these hypotheses, other factors operate concurrently along climate and geographical gradients, such as forest management (Vayreda et al., 2013) in conjunction with other disturbances and biotic interactions (Carnicer et al., 2014; Serra-Diaz et al., 2015) and local adaptations (Aitken et al., 2008). These more local drivers may transform a unimodal signal of regeneration shift in response to climate change (Fig. 1a) into a more complex set of signals with localized areas of relatively high regeneration, or regeneration, hotspots (Fig. 1b; local indicators hereafter). The identification of local hotspots is useful because early indicators of resilient populations can have implications for species conservation under climate change (Franklin et al., 2013; Hannah et al., 2014; Tingley et al., 2014). Global and local regeneration-based indicators of the effects of climate change on tree species distributions are needed to better capture the dynamics of species range shifts beyond a unified direction within a species' range.
In this study we assess shifts in the presence–absence of regeneration for 13 tree species in the California Floristic Province (CAFP) within the climate and geographical spaces typically used to detect the fingerprint of climate change – defined respectively by the precipitation/temperature and elevation/latitude axes – to identify signals of range shift and local persistence under climate change. We applied methods used in invasion ecology to derive global and local indicators to answer the following questions: (1) are there differences between regeneration and adult distributions; (2) do these differences convey an overall loss, persistence or expansion of forest regeneration; and (3) are signals of regeneration in climate and geographic spaces related to non-climatic drivers?
Methods
Study region and study species
Our study region was the US portion of California Floristic Province (CAFP; Fig. S1 in Supporting Information), a hotspot of plant biodiversity in a Mediterranean climate region (Myers & Mittermeier, 2000). Long-term, regional changes in tree species distributions and abundances in this region have also been associated with changes in land use (Thorne et al., 2008) and fire regimes (Dolanc et al., 2014). We selected 13 species from the pool of tree species that are either endemic to (entire range within) or that have most of their range within the latitude and elevation of the CAFP, as well as the more widespread Abies concolor. This selection (Table 1) ensured that the analysis of global and local signals was based on the majority or entirety of the species range and that there were sufficient observations to implement the interpolation techniques used.
| Species | Mean elevation of plots (m) | Percentage (no.) of plots with regeneration | Nhotspots CLIMATE | Nhotspots GEOGRAPHY |
|---|---|---|---|---|
| Coast live oak, Quercus agrifolia | 418 | 46.2 (49) | 3 | 2 |
| Blue oak, Quercus douglasii | 492 | 22.4 (44) | 2 | 3 |
| California bay laurel, Umbellularia californica | 494 | 68.9 (115) | 4 | 6 |
| Grey pine, Pinus sabiniana | 628 | 28.1 (34) | 7 | 5 |
| Tanoak, Notholithocarpus densiflorus | 684 | 89.8 (298) | 7 | 8 |
| Interior live oak, Quercus wislizeni | 747 | 70.2 (106) | 4 | 6 |
| California black oak, Quercus kelloggii | 1000 | 46.6 (229) | 7 | 5 |
| Canyon live oak, Quercus chrysolepsis | 1021 | 80.3 (425) | 6 | 3 |
| Sugar pine, Pinus lambertiana | 1887 | 39.3 (165) | 7 | 7 |
| Incense cedar, Calocedrus decurrens | 1441 | 66.2 (351) | 7 | 4 |
| White fir, Abies concolor | 1835 | 68.7 (495) | 7 | 5 |
| Jeffrey pine, Pinus jeffreyi | 2010 | 31.8 (92) | 8 | 7 |
| Red fir, Abies magnifica | 2285 | 61.7 (108) | 7 | 6 |
| Average | 5.84 | 5.15 | ||
| Standard deviation | 1.91 | 1.77 |
Forest sampling and climate data
We analysed geolocated field measurements from the Forest Inventory and Analysis (FIA) programme of the US Department of Agriculture (USDA) Forest Service (2008) to examine the distribution of juveniles (regeneration) versus conspecific adults in the CAFP. The FIA data yield a large, well-distributed sample of forest conditions (Appendix S1). A FIA plot consists of four circular, contiguous 168-m2 macroplots where trees > 12.5 cm diameter are measured and four nested microplots where trees of 2.5–12.5 cm are measured; a plot fits within an area of c. 65 m × 65 m. Some of us (W.W.D., R.K.M.) had access to true plot locations – as opposed to coordinates intentionally altered for privacy (Food Security Act of 1985, 7 U.S.C. 2276(d)) – as part of ongoing studies of the spread and impacts of the forest disease sudden oak death (Rizzo et al., 2005; Meentemeyer et al., 2011). This high spatial precision allowed us to overlay FIA data with high-resolution downscaled climate data. We used data from 2001−07 for 7879 FIA plots in the CAFP (Appendix S1).
The FIA sampling procedure distinguishes different tree size classes. In this study, we defined regeneration as those plots with individuals < 12.7 cm diameter at breast height (d.b.h.) present; this approximately matched regeneration to establishment conditions in the second half of the 20th century (12.7 cm d.b.h. corresponded to 48.42 ± 7.37 years on average across our species; see Appendix S2 for size–age regressions), while trees > 12.7 cm d.b.h. are likely to have established under prior conditions. This approach was used to detect effects of climate change and land-use change (e.g. fire management, logging practices) where changes occurred or accelerated in the second half of the 20th century (e.g. Keeley et al., 1999; Boisvenue & Running, 2006).
We obtained estimates of mean annual temperature (MAT) and precipitation (PRECIP) within the CAFP for historical climate (1950−2000) from the PRISM dataset (Daly et al., 1994) downscaled to 90 m using a spatial gradient and inverse distance squared weighting approach (Flint & Flint, 2012; see Franklin et al., 2013, for a full description of the dataset and methods). These downscaled values were extracted for the true FIA plot locations.
Global indicators: regeneration distribution in climate and geographical space
We defined forest regeneration for a given species as the presence of any juvenile size class (< 12.7 cm d.b.h.) along with conspecific adults in a FIA plot. Conversely, a plot with adults present but juveniles absent would be considered non-regenerating forest. By only considering regeneration where there were adults in the overstorey we ensured that a seed pool was present and that regeneration was not confounded with range shifts dependent on processes such as dispersal. While dispersal-driven range shifts are also important, the focus of our study was forest persistence. We applied a framework originally developed to characterize niche dynamics in space and time for invasive species (Guisan et al., 2014) to investigate displacement in forest regeneration in environmental and geographical space. We estimated regeneration dynamics using the techniques provided by Broennimann et al. (2012) for niche estimation and niche overlap, and the methodology developed by Petitpierre et al. (2012) to identify niche dynamics. All analyses were conducted using R (R Development Core Team, 2014).
Estimating global regeneration dynamics: persistence, expansion and loss
Regeneration and non-regeneration density of occurrence was estimated in a two-step process (full methods and code are available at Broennimann et al., 2012). First, forest plots were placed in 1000 × 1000 gridded bidimensional spaces defining either the climate (MAT, °C; PRECIP, mm) or the geographical space (ELEVATION, m; LAT_Transformed, a unit-less transformation of latitude derived to avoid publishing true plot locations). Climate niche space provided information on regeneration displacement along climate gradients, whereas geographical space helped evaluate geographical indicators expected from a warming fingerprint (higher elevation and latitude). Mean annual temperature and precipitation have been used in previous analysis of the turnover hypothesis (Zhu et al., 2014) and are highly correlated with the group of 19 bioclimatic variables (Appendix S2) widely used in species distribution studies (Booth et al., 2014). Second, kernel density estimation was applied to the gridded environmental space using forest plot occurrences. The result was a normalized density distribution in environmental space (Fig. 2a, b). These steps were carried out for regenerating and non-regenerating forests. This approach enabled direct comparison of densities without depending on different sample sizes (see Broennimann et al., 2012, for an extended methodological explanation).
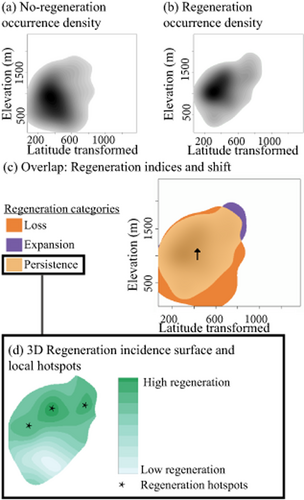
Overview of methods. Incidence surfaces of non-regeneration (a) and regeneration (b) are built in geographical or environmental space. These regeneration surfaces are overlaid, and indices of regeneration are calculated. The central tendency of changes in the distribution of regeneration is estimated by connecting the centroid of no-regeneration and regeneration incidence surfaces (black arrow). Local regeneration hotspots are calculated from three-dimensional surfaces within the regeneration persistence (d), estimated as the difference between surfaces in (a) and (b).
Regeneration indices, magnitude and direction
We used three indices to describe regeneration dynamics based on the overlap between density values (Fig. 2c). The overlap was calculated using the 0.95 quantile of the non-regeneration and regeneration distributions to avoid spurious effects of the kernel density smoothers at the margins of the distribution. The indices included: (1) regeneration loss: the presence of non-regenerating forests alone; this is linked to the extinction debt of the species – adults are still present but local extinction (extirpation) is expected due to lack of regeneration; (2) regeneration persistence: both regenerating and non-regenerating forests overlap –other factors potentially drive regeneration patterns; and (3) regeneration expansion: the presence of regenerating forests alone – potential direction of species range change. These categories were inspired by Petitpierre et al. (2012), but were renamed to better describe regeneration dynamics. We calculated the index for each dynamic as the proportion of density of occurrence of regenerating forests in the categories of regeneration expansion and persistence, and the proportion of occurrence density of non-regeneration in the regeneration loss category. As in Petitpierre et al. (2012), the regeneration expansion and persistence indices sum to one because they encompass all regeneration, whereas regeneration loss is only based on forest non-regeneration density values.
As a global indicator of regeneration displacement, we estimated the direction and magnitude of the vector connecting the center of gravity of the regeneration and non-regeneration density distributions (Broennimann et al., 2012; Guisan et al., 2014). This procedure was carried out for both climate and geographical space. Because our study area is topographically complex, topographic moisture could potentially buffer deteriorating (warmer, drier) climate conditions. We therefore also compared the direction of the shifts in climate and geographical space to the species topographic position. We computed the topographic wetness index (TWI; Moore et al., 1991) using SAGA GIS (http://www.saga-gis.org/) from a 100-m resolution digital elevation model. A higher value of TWI indicates potential accumulation of soil moisture, which could imply regeneration conditions less constrained by atmospheric climate variables, and therefore not following the migration hypothesis of climate change. We calculated the TWI for each species by averaging TWI values across all plots where it was present.
Local indicators
Local regeneration hotspots
Hotspots are defined as locations in the bidimensional spaces with a high prevalence of regeneration. Two steps were carried out to identify local hotspots: (1) building a three-dimensional regeneration surface, and (2) identifying basins of high regeneration through morphometric analysis. In the regeneration surface, the x and y dimensions refer to the environmental or geographical space (MAT-PRECIP or LAT_Trans-ELEVATION, respectively) and the z dimension corresponds to the measure of regeneration incidence (the difference between the density of occurrence of forest regeneration and non-regeneration) (Figs 1 & 2d). Negative values of regeneration incidence indicate preferred areas for regeneration, whereas in areas with positive values regeneration is less prevalent. In the resulting ‘terrain’ of species regeneration, valleys (low values) indicate high incidence of regeneration and ridges (high values) indicate less regeneration (Fig. 2d).
In the second step, we identified local hotspots through morphometric analysis of the regeneration surface (Figs 1 & 2d). We used three criteria to identify regeneration hotspots based on the topographic convergence index (TCI), which we calculated from the regeneration incidence surface (z; see above). TCI is widely used in surface analysis to describe the degree of concavity and convexity in a plane while accounting for drainage properties (Wilson & Gallant, 2000). We first selected cells with TCI below quantile 0.01 to identify valleys with a high incidence of regeneration. Subsequently, we selected cells within these patches where the incidence of regeneration was larger than the incidence of non-regeneration cells. Finally, we only included hotspots of at least 3 pixels × 3 pixels to filter the potential noise of isolated pixels. This filtering process retained only those local dynamics exhibiting a clear pattern of regeneration or non-regeneration.
We analysed whether the prevalence of local regeneration hotspots was related to characteristics of our species and plots. Specifically, for each species we computed Pearson's correlation coefficients between the number of hotspots and the total number of plots (e.g. range size); the amount of regeneration displacement (movement index of the global indicator); and the percentage of plots with signs of natural disturbance or forest management. For these, we characterized each plot by the presence/absence of natural disturbance (e.g. fire, drought) or evidence of management (e.g. clearcuts or other fuels treatments, etc.), based on information in the FIA data.
Results
Regeneration expansion in climate and geographical space was found for all species, although regeneration persistence was dominant over regeneration loss and expansion (Fig. 3a). That is, both regenerating and non-regenerating forests largely overlap in climate and geographical space. The species with the greatest regeneration expansion were California bay laurel and coast live oak, whereas the species with the greatest regeneration loss were California blue oak in climate space and red fir and canyon live oak in geographical space. We found no clear evidence that regeneration expansion was consistently more or less prevalent than regeneration loss (Fig. 3b). For more than half of the species (seven), regeneration loss was more prevalent, whereas for three species expansion was more prevalent. For the other three species (red fir, grey pine, tanoak) the pattern was dependent on whether climate or geographical space was being analysed; larger losses were identified in geographical space whereas larger expansions were identified in climate space.
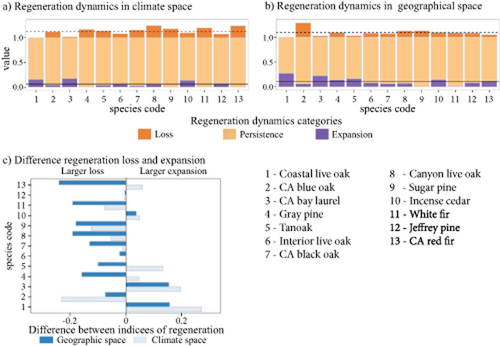
Global indicators of regeneration niche and regeneration geography dynamics across 13 tree species in the California Floristic Province (numbers correspond to the key at the bottom of the figure). (a) Regeneration loss, regeneration persistence and regeneration expansion. Value is the proportion of regeneration presence (density values) in the categories of persistence and expansion and regeneration absence (density values) in the loss category. Dotted and solid horizontal lines indicate the mean value of regeneration loss and expansion, respectively. (b) Differences between regeneration loss and expansion. See Table 1 for species scientific names; numbers correspond to rank mean elevation of species occurrence (low to high elevation). CA, California. Species are ordered by increasing mean elevation (Table 1).
Changes in the geographical distribution of regeneration were not consistent among species, and generally not congruent with the climate change migration hypothesis (Fig. 4a). Only two species (14%) showed a signal of regenerating at both higher latitude and elevation, consistent with expected climate change migration patterns. Fewer than half of the species exhibited regeneration at higher elevations (five out of thirteen; 38%), while the rest of the species analysed presented a global indicator of regeneration shifting to lower elevation (eight out of thirteen; 61%), and the majority of them also showed a shift to lower latitudes (Fig. 4a).
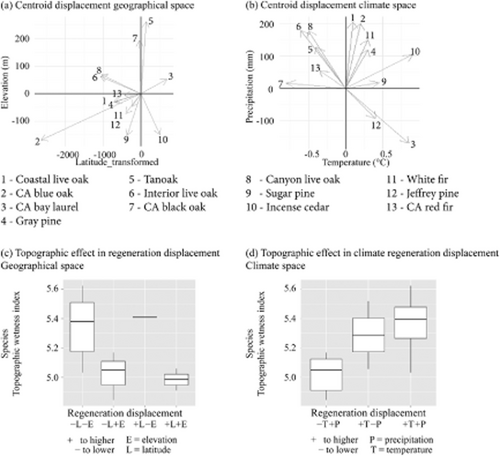
Global indicator shifts (centroid displacement between adult and juvenile distribution) in regeneration climate space (a) and regeneration geography (b). Regeneration shifts dynamics as a function of topographic wetness index for climate (c) and geographical (d) space. See Table 1 for species scientific names; numbers correspond to rank mean elevation of species occurrence (low to high elevation). Species are coded by number and ordered by increasing mean elevation (Table 1). Lat_transformed, latitude transformed variable; CA, California.
Global indicators of regeneration dynamics in climate space highlight the variability among species (Fig. 4b). The majority of the species analysed are regenerating in areas of higher precipitation than their adult distributions (11 out of 13 species; 85%) while 8 out of 13 (61%) are regenerating at warmer temperatures. Interestingly, the two highest-elevation species exhibited the counterintuitive dynamic of regenerating at lower precipitation and higher temperature. Overall, the regeneration climate shifts are mixed with regard to competing hypotheses of the effects of climate change on regeneration: Six species (46%) showed patterns congruent with the turnover hypothesis – regenerating at higher temperature and precipitation – whereas five (39%) showed patterns congruent with climate change migration hypothesis – regenerating at higher precipitation but lower temperature; two species showed neither pattern.
When patterns of regeneration dynamics were compared with the topographic wetness index (TWI; Fig. 4c and 4d), species regenerating in locations with higher temperatures than adult distributions were also found in sites with higher TWI, which could be offsetting the evapotranspiration demands of higher temperatures through greater topographically mediated water availability (Crimmins et al., 2011). Such topographic buffering can also be inferred in geographical space; species regenerating at lower (typically warmer) elevations were found in sites with higher TWI than species regenerating at higher elevations. This is especially noticeable for species regenerating at lower latitudes, where higher temperatures were also expected.
Several local regeneration hotspots were identified for all species. On average, we found five local hotspots in geographical space (Table 1) and nearly six for climate space. These numbers are relatively high, given that a unidirectional change of regeneration would detect one or no hotspots (Fig. 1; e.g. regeneration moving as one block through an environmental space). The number of local regeneration hotspots varied among species; for some the number of local hotspots was low (e.g. blue oak had two for climate and three for geography), while for others it was relatively high (e.g. Jeffrey pine had eight for climate and seven for geography). The correlation between the number of hotspots in the regeneration climate space and the regeneration geography was 0.45 (Kendall's τ), suggesting less than perfect correspondence between the two spaces (climate and geography).
The number of hotspots was correlated with the percentage of plots with forest management for both climate (0.66) and geographical space (0.44), but only weakly correlated with the percentage of disturbed plots (0.35 for climate and −0.33 for geographical space; Table 2). Species range size and the number of hotspots was correlated (0.51), but only for climate space (Table 2). We found very little correlation between number of hotspots and the percentage regeneration of the species or the regeneration expansion index (Table 2).
| No. of plots | Percentage of regeneration | Regeneration expansion index | Percentage of treated plots | Percentage disturbed plots | |
|---|---|---|---|---|---|
| No. of regeneration hotspots climate space | 0.51 | 0.14 | –0.35 | 0.65 | 0.15 |
| No. of regeneration hotspots geographical space | –0.01 | 0.22 | 0.03 | 0.44 | –0.33 |
Discussion
Are there signals of regeneration displacement?
We detected differences between forests with and without regeneration in both climate and geographical space (Fig. 3a), although the main signal was the overlap in both. Regeneration expansion was as prevalent as loss in climate space; whereas regeneration loss was clearly more prevalent in geographical space. These results were somewhat surprising because we expected high levels of regeneration loss in both climate and geographical spaces given that lagged tree responses to changes in climate are expected to lead to an extinction debt (Bertrand et al., 2011). Our results suggest that higher-elevation species are experiencing larger loss than lower-elevation species, especially in geographical space (Fig. 3b). Nevertheless, the dominance of regeneration persistence, in both climate and geographical dimensions, may be indicative of buffering mechanisms to climate change that may slow rates of species distribution shifts (Lloret et al. 2012; Dobrowski et al., 2015). However, this overlap could also indicate that other factors may have had a recent, strong influence on regeneration dynamics (see below).
Generally, regeneration dynamics in both geographical and climate space agreed, but some species did exhibit opposite dynamics (Fig. 3b). The regeneration dynamics for these cases showed that regeneration expansion in climate space exceeded regeneration loss in geographical space (e.g. tanoak, grey pine, red fir). Our interpretation is that these species are occupying new portions of climate space while their geographical range shrinks. This distinction is important, because it warns of simultaneous range reduction and shifting, unlike an overall range reduction that would tend to diminish both climate and geographical space or a range shift that would imply comparable regeneration expansions in climate and geographical space. While examining a more complete geographical space (latitude, longitude and elevation) might further disentangle these dynamics, our results highlight the importance of analysing both climate and geography. In areas of complex terrain, for example mountain ranges oriented in different directions with regard to prevailing winds and a strong rainshadow effect, typical correlations of climate and geography can be decoupled. For example, Loarie et al. (2008) shown that in California movement to higher elevations often means taking a southward path.
It would be misleading to interpret our results of large areas of regeneration persistence as meaning high levels of regeneration in forests (Table 1). The methods used here aim to describe shifts in regeneration niche. For example, coast live oak shows a large regeneration expansion index but fewer than half of the total plots recorded any regeneration in the last c. 50 years (Table 1).
Are global indicators of forest regeneration consistent with a climate change fingerprint?
We found slightly more evidence for the turnover (6 species of 13 studied, 46%) than the climate change migration (five species, 39%) hypothesis. Similarly, mixed results were also found by Zhu et al. (2014), although they found even higher support for the turnover hypothesis in eastern forests of the United States (77%). Nevertheless, three of our studied species did not clearly fall into either of the two categories since they were regenerating at higher temperatures but lower precipitation, thereby making a straightforward attribution to higher turnover rates difficult. We argue, however, that these species could also be included in the turnover hypothesis because TWI analysis suggested higher potential availability of water via topographically mediated hydrology (Fig. 1c).
Our results concur with findings from other studies that for some species juveniles seem to be concentrating at lower elevations (Lenoir et al., 2010; Crimmins et al., 2011; Rabasa et al., 2013). Regeneration tended to occur at lower elevations for the majority of our study species as well (9 out of 14, 65%; Fig. 4a). Our analysis of TWI revealed that counter-intuitive responses in regeneration, such as shifts to lower-elevation warmer and/or drier macroclimatic conditions could be compensated via topoclimatic effects on temperature such as cold air pooling and topographic shading (Dingman et al., 2013; Curtis et al., 2014) and/or water balance effects (Lenoir et al., 2010; Crimmins et al., 2011).
For the interior western United States, with its water-limited semi-arid to arid climates, Bell et al. (2014) reported regeneration loss at the northernmost part of their study area. Our results concur with this finding because the majority of our species were also showing more regeneration at lower latitudes (Fig. 4b). Our results partially agree with Monleon & Lintz (2015) for those species that were analysed in both studies. On the other hand, our studies disagree with respect to California bay laurel, where they predicted regeneration at lower temperatures and we detected regeneration at higher temperatures. In addition, previous work from Lenihan et al. 2008 and Dobrowski et al. (2015) projected expansion of hardwoods of the mixed hardwood conifer belt (tanoak, California black oak, bay laurel, canyon live oak and interior live oak) under climate change – species we found to be regenerating at higher elevations. Nevertheless, direct comparisons are difficult due to different methods used. We restricted our analysis to regeneration in the presence of adults and we used a different characterization for juveniles.
All in all, results show that a single common signal of range dynamics in a broad-scale analysis is largely absent (Rabasa et al., 2013). Such species-specific patterns were also found by Monleon & Lintz (2015), although their signal of regeneration at colder temperatures is more evident for the northernmost species of the US Pacific coast. Interestingly, some of the tree species that have historically occurred together (coast live oak–tanoak; incense cedar–sugar pine–black oak; blue oak–grey pine) are showing distributional shifts in opposite directions, suggesting the potential for reorganization of biota into novel assemblages, in agreement with the shifts in vegetation composition during the 20th century that have been described in the region (Thorne et al., 2008; Dolanc et al., 2014). Indeed, expecting a common fingerprint when rates of change in climate are heterogeneous (Flint & Flint, 2012; Dobrowski et al., 2013) may be a rather oversimplified hypothesis (VanDerWal et al., 2012) and potentially misleading for management purposes at the species-specific level. Recent analyses of California plants and animals also suggests individualistic upslope and downslope shifts in species elevation in response to climate change (Rapacciuolo et al., 2014), and Serra-Diaz et al. (2014) projected different bioclimatic velocities – the pace at which species and populations are exposed to climate change – among some of the species of this study.
Local indicators and the heterogeneity of regeneration
The existence of regeneration hotspots reveals a complex regeneration landscape across current species ranges and may add information to the signal of the range-wide global dynamics. Such multimodal behaviour has also been identified in patterns of seedling density and adult density (Fig. 6 in Monleon & Lintz, 2015; to a lesser extent in Figs 2 & 3 in Rabasa et al., 2013). Indeed, the complex landscape of regeneration patterns may be due to many drivers, including legacy effects (Nowacki & Abrams, 2014), land-use dynamics (Syphard et al., 2011; Dolanc et al., 2014), change in successional trajectories resulting from altered severity and frequency of disturbance (Syphard et al., 2009), and local adaptation (Sork et al., 2010). Also, spatially structured biotic interactions can overshadow the fingerprint of climate change and have a strong effect on species distributions (Lenoir et al., 2009; Serra-Diaz et al., 2015). For example, Gworek et al. (2007) found a significant effect of seed-caching rodents on survival of Jeffrey pine seedlings, obscuring a clear pattern of species migrating northwards, which is consistent with the results of this study. Overall, species-specific abilities to track climate change are diverse, and therefore the main drivers may shift and interact depending on the context (Bertrand et al., 2011; McLaughlin & Zavaleta, 2013). Nevertheless, we found that disturbance and silvicultural treatments were related to the number of local regeneration hotspots (Table 2), emphasizing that we need to better understand the synergies between human-mediated natural disturbances, land management and climate change in forested landscapes.
Our methods should be understood as a first step in picturing the varying local responses of regeneration. Indeed, the study of local maxima and minima using kernel density estimation of regeneration allowed us to detect nuanced differences between populations within species ranges. However, further work is necessary to distinguish signal and noise in regeneration using such techniques. For instance, it is possible that regeneration was not detected in the plots. In addition, spurious effects may be found at the edges of the distribution, which are also are very interesting locations in terms of climate change. Improved methods for detecting regeneration hotspots could also benefit from the potential connections between regeneration hotspots by dispersal. Incorporating more detailed demographic variables for hotspot detection (such as basal area or recruitment intensity as in Zhu et al., 2014) could help identify drivers along different gradients, especially if interactions between soil and climate variability were included in the analysis (Bertrand et al., 2012; Ibáñez et al., 2014). Land managers could further benefit from hotspot analyses carried out in latitude–longitude space so that regeneration hotspots could be identified explicitly (see Appendix S3).
Arguably, analysis of the distribution of regeneration hotspots reflects the distribution of the plots, the orientation of predominant mountain ranges, the species ranges and other attributes of their distributions. We found no systematic bias in the methods since there is a low correlation between the number of plots, regeneration, regeneration dynamics and local regeneration hotspots, and one would expect that any bias in the distribution of plots should be similar for both regeneration and no-regeneration samples.
Deepening our understanding of early signals: the timing and the drivers
Both regeneration dynamics and the relationship between adults and juveniles may provide an indicator of species range resilience or shift (Lenoir et al., 2009; Zhu et al., 2012; Woodall et al., 2013; Bell et al., 2014; Monleon & Lintz, 2015), but several limitations of our approach should be acknowledged explicitly. First, the age of juveniles was identified based on a size–age relationship (< 12.7 cm d.b.h. in our case). Presumably, the time spent in the juvenile size class depends on species and site productivity, and thus we may expect smaller individuals classified as juveniles to be more prevalent in drier sites than wetter sites. Also, most studies classify juveniles using the same criterion across species, despite different species having different growth rates. Instead, Monleon & Lintz (2015) applied a statistical threshold to separate seedlings and adults. We chose a threshold that could generally distinguish individuals that established during the last 50 years of rapid climate change; but making the link between the exact age of juveniles and microclimatic site conditions remains a challenge.
For this study we adapted relatively simple, although extensively used, climate descriptors, namely mean annual temperature and mean annual precipitation (see Garcia et al., 2014, for further dimensions of climate change). Patterns of regeneration are more proximally driven by bioclimatic variables other than average temperature and precipitation, for example water deficit, seasonality of temperature and moisture (Flint & Flint, 2012). We envision that future work will test the overlap of juveniles and adults using multiple variables related to climate change (see Blonder et al., 2014, for an n-dimensional technique), including extreme events. Indeed, the large overlap of regeneration and non-regeneration spaces suggests that other factors shape regeneration dynamics, but could also point to relatively stability of the range. Interestingly, Benavides et al. (2015) found trade-offs between survival and growth of juveniles for several species: recruits were abundant in the upper elevations of the range but had higher growth rates at lower elevations. This apparent trade-off could eventually stabilize species ranges (Benavides et al., 2015).
Our results suggests that signals of regeneration shifts are present but not widespread, and importantly the directions of such changes are species specific and do not always concur with the upslope or northward migration expected due to recent warming. Regeneration distributions for tree species also show a direct anthropogenic signal of forest management and disturbance. Conservation managers are likely to need to be able to adapt large-scale mitigation plans to observed local-scale patterns rather than assuming a uniform pattern of how climate change may affect species biogeography. The results of this study suggest that macroecology in the Anthropocene will have to synthesize spatially varying drivers of those factors shaping the future distribution of species, rather than using a global fit to detect range change.
Acknowledgements
J.M.S.-D., J.F., A.D.S. and F.W.D. received funding support from the National Science Foundation's (NSF) Macrosystems Biology Program, Collaborative award EF-1065864 to F.W.D. and EF-1065826 to J.F., and thank A. Flint and L. Flint for climate downscaling as well as other collaborators (A. Hall, L. Hannah, I. McCullough, M. Moritz, M. North, K. Redmond, H. Regan, V. Sork, L. Sweet). R.K.M. and W.W.D. were supported by funding from the USDA Forest Service Pacific Southwest Research Station and the NSF (EF-0622770) as part of the joint NSF-NIH Ecology of Infectious Disease program. J.M.S.-D. acknowledges support from GRUMETS (SGR 2014 1491). We also thank B. Petitpierre for sharing the R code necessary to apply the methods in this paper.
References
Josep M. Serra-Diaz is interested in unveiling the drivers of species distribution shifts under climate change using complementary mechanistic and correlative approaches, as well as the explicit consideration of ecological processes in spatial ecology.




