Critical-weight-range marsupials in northern Australia are declining: a commentary on Fisher et al. (2014) ‘The current decline of tropical marsupials in Australia: is history repeating?’
Abstract
Many mammal species are declining in parts of Australia's tropical savannas, for reasons that are not yet well defined. A recent paper (Fisher et al., 2014, Global Ecology and Biogeography, 23, 181–190) suggested that the primary cause is predation by feral cats, with the main evidence presented being a purported over-representation of small species amongst the marsupials that have contracted in range (‘small body size signifies high current extinction risk’). However, a review here of the information presented in that paper shows that no marsupial species smaller than 100 g has shown range contraction in northern Australia, and that most (15 of 17) declines are of species in the ‘critical weight range’ (35 g to 5.5 kg).
The availability of increasingly large data sets, computational power and sophisticated statistical programs has spawned a suite of analyses of factors associated with decline and extinction. There are now many such analyses that have considered the causes and patterning of the decline of the Australian mammal fauna (e.g. Cardillo & Bromham, 2001; Chisholm & Taylor, 2007; McKenzie et al., 2007). Fisher et al. (2014) also explored the causes of the current decline in the mammal fauna of northern Australia. The argument offered by Fisher et al. (2014) is that there is a disparity in size range for declining native marsupials between tropical northern and southern Australia, with small species far more likely to be undergoing range contraction in northern Australia. Fisher et al. (2014) then inferred that this was because native mammals in southern Australia were detrimentally affected by predation by both feral cats Felis catus and the introduced red fox Vulpes vulpes, whereas only the former is present in northern Australia. I have five concerns with the Fisher et al. (2014) analysis and conclusions.
Body Size Of Species With Declining Ranges In Northern Australia
Fisher et al. (2014) asserted that ‘in recent tropical Australian marsupial declines … small body size signifies high current extinction risk as measured by proportional decline in range’, and that there was no critical weight range (‘CWR’) effect in northern Australia. The CWR is a pattern previously reported elsewhere in Australia whereby lighter (< 35 g) and heavier (> 5.5 kg) native mammals were less prone to decline and extinction than mammals in the weight range 35 g to 5.5 kg (Burbidge & McKenzie, 1989). Fisher et al. (2014) took their response variable, proportional range decline, from digitized maps of original and current range in Van Dyck & Strahan (2008), and those data are presented in their Appendix S1.
Here, I re-present those data in Fig. 1, and for all species < 100 g in Table 1. These data show that there has been no range decline for the 20 species in the weight class < 35 g nor for the few species in the 35–100 g weight class (Tables 1 & 2). Species with the greatest proportional range contractions were medium-sized marsupials (particularly in the weight class 100–5500 g). A total of 15 of 49 northern Australian species in the CWR have declined in range; whereas only two of the 30 northern Australian species not in the CWR have declined in range. This difference in proportion of declining species to their respective species tallies is highly significant (P < 0.01; Fisher's exact test). The only two declining species in northern Australia that are not in the CWR formerly occurred mostly in southern Australia. One species (Macropus parryi) remains common, and the proportional decline value (0.9991) given by Fisher et al. (2014) for the other species, northern populations of Lasiorhinus krefftii, appears to be inconsistent with the source Fisher et al. (2014) cited, given that the map in Van Dyck & Strahan (2008) shows no decline in the species’ very marginal range in northern Australia.
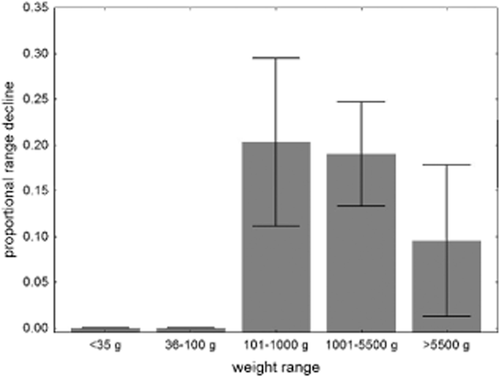
Relationship between ‘proportional range decline’ and weight for all mammal species in northern Australia, with data derived entirely from Fisher et al. (2014). Values shown are means with standard errors as whiskers. The number of species in each weight range class are: < 35 g (18), 36–100 g (3), 101–1000 g (15), 1001–5500 g (31), > 5500 g (12).
| Species | Mass (g) | Proportional range decline | Decline rank |
|---|---|---|---|
| Planigale ingrami | 4 | 0.00 | 0 |
| Planigale maculata | 10 | 0.00 | 1 |
| Sminthopsis youngsoni | 10 | 0.00 | 0 |
| Acrobates pygmaeus | 12 | 0.00 | 0 |
| Sminthopsis bindi | 13 | 0.00 | 2 |
| Sminthopsis murina | 14 | 0.00 | 0 |
| Sminthopsis crassicaudata | 15 | 0.00 | 0 |
| Sminthopsis archeria | 15 | ||
| Sminthopsis butleri | 16 | 0.00 | 2 |
| Pseudantechinus ningbing | 18 | 0.00 | 0 |
| Pseudantechinus mimulusa | 18 | ||
| Sminthopsis leucopus | 19 | 0.00 | 0 |
| Antechinomys laniger | 20 | 0.00 | 0 |
| Sminthopsis macroura | 20 | 0.00 | 0 |
| Pseudantechinus bilarni | 25 | 0.00 | 1 |
| Antechinus adustus | 28 | 0.00 | 0 |
| Cercartetus caudatus | 30 | 0.00 | 0 |
| Antechinus bellus | 34 | 0.00 | 3 |
| Antechinus flavipes | 34 | 0.00 | 0 |
| Sminthopsis virginiae | 34 | 0.00 | 1 |
| Notoryctes caurinusa | 40 | ||
| Sminthopsis douglasi | 50 | 0.00 | 1 |
| Antechinus leo | 54 | 0.00 | 0 |
| Notoryctes typhlopsa | 55 | ||
| Antechinus godmani | 61 | 0.00 | 0 |
| Weight range | No. of species in weight range | No. of species declining (%) |
|---|---|---|
| < 35 g | 18 | 0 (0) |
| 35 g to 5.5 kg (‘CWR’) | 49 | 15 (30.6) |
| > 5.5 kg | 12 | 2 (16.7) |
- CWR, critical weight range.
Fisher et al. (2014) did not specify how they applied the terms small, medium and large, but a general convention (e.g. Johnson, 2006) is to equate the CWR with medium size, and hence small is < 35 g and large is > 5500 g. Another approach is to consider the weight of mammals eaten by feral cats. In a dietary analysis in north-eastern Australia, Kutt (2012) noted that cats preyed selectively on ‘small mammals (< 10 g, 50–100 g)’. Accordingly, if predation by feral cats was the primary factor driving decline, it might be most evident in the decline of species < 100 g. Of course, this latter supposition is not necessarily valid: if smaller species occurred at higher densities and/or had higher reproductive output, they may cope better with having a larger number of individuals eaten than for medium-sized species.
In some analyses, Fisher et al. (2014) used another distributional decline response variable, a ‘decline rank’ based on expert opinion. Much of the scoring of ‘decline rank’ derives from a rating given in a pamphlet by Fitzsimons et al. (2010) although the information presented there is qualitative (e.g. ‘some decline’) and includes only 30 of the 83 northern marsupial species considered by Fisher et al. (2014). Eight of those 30 qualitative statements of decline are ‘Uncertain’. Of the other two main sources cited in Fisher et al.'s Appendix S2 as the basis of their decline-rank index, Woinarski et al. (2011) provided no measure of geographic contraction, and information on the extent of range decline given in IUCN (2010) was not comprehensive and mostly was not quantitative. Much of the information on which the variable ‘decline rank’ was based referred specifically to local decline in abundance from a small number of sites rather than to any contraction in distributional range. For example, for Antechinus bellus, the small northern species accorded the highest value for this index (i.e. the most extensive decline) by Fisher et al. (2014), their stated evidence of range decline is the study of Woinarski et al. (2010), which described population decline in one conservation reserve, and there is no evidence that the geographic range of this species has contracted (Woinarski & Oakwood, 2008). Similarly, there is no evidence of contraction in the geographic ranges of at least four (Planigale maculata, Sminthopsis bindi, Sminthopsis virginiae and Sminthopsis douglasi) of the other six small (< 100 g) north Australian species given a non-zero decline rank by Fisher et al. (2014). Fisher et al. (2014) have inconsistently confounded measures relating to local population decline and to decrease in geographic range.
Study Boundaries
Fisher et al. (2014) compared patterns of decline in northern and southern Australia, with this division crisply defined by the Tropic of Capricorn (23°26′ S). While this makes for an easy circumscription and a clear demarcation, it makes little ecological sense, and partly obscures what turns out to be a principal focus, that of comparing marsupial declines in areas with foxes and those without foxes. This boundary bisects the vast deserts of central Australia, notwithstanding the spatial cohesion of their mammal fauna and threats (e.g. Burbidge et al., 1988). Furthermore, and notwithstanding their statements that ‘foxes are absent from the tropics’ and ‘absent from the north’, much of the area that they define as northern or tropical Australia is within the range of the introduced red fox. For example, West (2008) indicated that c. 1.4 million km2 of Australia north of the Tropic of Capricorn is occupied by the red fox, about 24% of its Australian range. Thus, many marsupial species characterized as northern Australian in Fisher et al. (2014) have distributions that are entirely or mostly within the range of the red fox.
Furthermore, in comparing the historic fate of mammals in northern and southern Australia, Fisher et al. (2014) incorrectly stated that the 24 Australian mammal extinctions ‘have been restricted to southern Australia’. However, based on mapping used as their source (Van Dyck & Strahan, 2008), I show in Table S1 in the Supporting Information that of the 27 mammal extinctions in Australia since European settlement, five species were restricted to small islands and at least seven of the extinct species that formerly occurred on mainland Australia had distributions that mainly occurred within, or extended well into, northern Australia (e.g. Burbidge et al., 1988).
Temporal Context
Fisher et al. (2014) asserted that the Australian mammal extinctions were a largely historic phenomenon: ‘in the second half of the 19th century … 24 species went extinct’. Instead, since European settlement, most mammal extinctions in Australia occurred in the 20th century (e.g. Johnson, 2006). Burbidge et al. (1988) described a gradational pattern of decline in central Australian mammals (including many species spanning the tropical divide), culminating in extinctions for many species between the 1940s and 1960s.
Fisher et al. (2014) also indicated that marsupial declines in northern Australia have occurred mostly since the 1950s or 1970s. This may be true for some areas (e.g. Kakadu), but there is substantial evidence that the timing has varied among locations, with earlier decline in lower-rainfall areas of northern Australia (e.g. Kitchener, 1978; McKenzie, 1981; Ziembicki et al., 2013). Furthermore, the sparse subfossil record in northern Australia (e.g. Cramb & Hocknull, 2010; Start et al., 2012) indicates a far more substantial decline in the mammal fauna that most likely did not begin in 1950 or 1970.
Completeness of the Native Mammal Fauna
Fisher et al. (2014) limited their considerations of mammal decline in Australia to marsupials, notwithstanding the broadly comparable pattern of declines of the diverse Australian native rodent fauna (e.g. McKenzie et al., 2007; Burbidge et al., 2008). For northern Australia, this assemblage includes about 40 species, spanning a broad ecological, size and life-history range. There has been no suggestion that the magnitude of rodent decline in northern Australia is different from that of marsupials, or that the causes of such decline may be different. If the reason for exclusion was taxonomic confounding of analysis with ecological and life-history parameters, phylogenetic supertrees (e.g. Hanna & Cardillo, 2013) provide a better solution than simply discarding much of the species complement.
A consequence of this exclusion of a large component of the mammal fauna is that some of the interpretation of Fisher et al. (2014) ultimately hinges on very few species. For example, Fisher et al. (2014) reported that for north Australian marsupials ‘there was a 15-fold difference in mean mass between species that had the greatest declines in range size and species that had stable range sizes’. However, the category of largest range decline comprises just two species. Generalization from such a small set of species is questionable, particularly if some of those species (such as Dasyurus hallucatus) are responding idiosyncratically to particular factors.
As another example, Fisher et al. (2014) concluded that ‘tropical species with more carnivorous diets declined more than species with herbivorous diets’. However, because of the relatively small pool of species, this conclusion was based on only four carnivorous and three omnivorous marsupial species showing decline in northern Australia, with notably divergent patterns of decline. Furthermore, most of the declining species in northern Australia are not carnivorous, and a simple tabulation shows that the average proportional range decline score for carnivorous species is not greater than for species in other diet categories (Table 3). These analytical problems are compounded further because Fisher et al. (2014) made errors in the categorization of species diets. For example, they classified (as described in their Appendix S1) the obligate specialized folivore Petauroides volans as having a diet of ‘nectar, gum and insects’, but classified Petaurus norfolcensis (which eats nectar, gum and insects) as a folivore.
| Diet | No. of species declining (%) | No. of species not declining | Mean proportional range decline score |
|---|---|---|---|
| Grass, leaves | 7 (19) | 29 | 0.11 |
| Grass, seeds, fungi | 3 (75) | 1 | 0.42 |
| Nectar and some invertebrates | 3 (23) | 10 | 0.12 |
| Invertebrates and vertebrates | 4 (15) | 22 | 0.12 |
Alternative Explanations of Decline
Fisher et al. (2014) is explicit in a principal objective: ‘we aim to test plausible causes of recent declines in range’; however, the study provides no systematic comparison of the explanatory power of alternative hypotheses. Other than predation by feral cats, such potential causes include changed fire regimes, habitat degradation and resource depletion due to livestock and feral herbivores, disease and, for one species in particular, poisoning by the introduced cane toad Rhinella marina (and interactions between these factors), with this set of potential drivers notably different from the factors most affecting tropical mammals elsewhere in the world (Woinarski et al., 2011). There is substantial evidence for effects of fire (e.g. Pardon et al., 2003; Woinarski et al., 2004; Legge et al., 2008) and grazing (e.g. Woinarski & Ash, 2002; Kutt & Woinarski, 2007; Legge et al., 2011) on the abundance of marsupial species in northern Australia, including population viability modelling that predicted extinction under some fire regimes (Firth et al., 2010).
Conclusions
Fisher et al. (2014) provided one consistent data set on range decline in Australian marsupials. Those data indicate that there is no decline of small marsupials (< 100 g) in tropical Australia, but indicate a pattern of increased decline in marsupials with weights between 100 and 5500 g. Hence, the data presented in Fisher et al. (2014) are inconsistent with their interpretation. Their invalid conclusion that there is no CWR effect amongst declining marsupials in northern Australia may divert attention from the real priority needs for conservation action. Of course, the CWR effect is a mathematical pattern rather than an explanation or a pre-ordained ticket to extinction: the task remains to identify, and ameliorate, the cause(s) of decline.
Well-considered field research based on manipulation of a small set of plausible competing causal factors is far more likely to provide incisive results relating to causality (and hence to prioritization of conservation management response) than is inference drawn from modelling, especially when the latter fails to assess or test alternative hypotheses of decline (Cardillo & Meijaard, 2012).
Acknowledgements
I thank the handling editor Dr Fleishman, Dr Aaron MacNeil and an anonymous referee for helpful comments.




