Converting carbon dioxide to high value-added products: Microalgae-based green biomanufacturing
Abstract
Today's world faces the dual pressure of carbon dioxide (CO2) emission reduction and an energy crisis. Microalgae, which can use solar energy to convert CO2 to organic matter, have emerged as a promising and renewable cell factory for producing nutrients, biofuels, and various high value-added compounds (HVACs). They possess numerous advantages, such as high photosynthetic efficiency, fast growth rate, and use of agro-industrial waste and nonagricultural land for cultivation. Microalgae can also effectively remove eutrophic elements (e.g., nitrogen and phosphorus) from wastewater and atmospheric pollutants (e.g., SOx and NOx) from flue gas, thus providing great environmental benefits. However, microalgae-based production often faces low productivity, limiting applicability in industrial settings. Genetic and metabolic modifications of certain microalgal strains have proven effective in improving productivity. Here, we review the latest developments regarding the microalgae-based production of platform compounds, biofuels, and other HVACs. Although still in the early exploration stage, the rapid development of gene editing tools, a deeper understanding of the metabolic pathways of microalgae and their regulatory mechanisms, and further optimization of cultivation procedures and photosynthetic efficiency can eventually enable the launch of microalgae-based biomanufacturing for green industrial production. Therefore, this technology is strategically important for solving the current energy crisis problems of excessive CO2 emissions and environmental pollution. This review provides information about the advancement and development of microalgae-based production over the past two decades and discusses possible future directions in the field.
1 INTRODUCTION
To date, the global economy and population continue to grow; however, the current growth rates can lead to the exhaustion of our nonrenewable natural resources, including oil, coal, and natural gas, within 20 years if no changes are made (Herrington, 2021). Moreover, carbon dioxide (CO2) emissions from fossil fuel combustion for energy production have become a dominant cause of global warming and harmful environmental effects (Gustafson et al., 2020). For example, according to the Global Energy Review (2021) published by The International Energy Agency (IEA, 2021), worldwide CO2 emissions have continuously increased since the 1990s. As the global economy is gradually recovering from the COVID-19 pandemic, it has been observed that CO2 emissions in 2021 have increased by 6% compared to those in 2020. In addition to CO2, fossil fuel combustion generates significant emissions of common air pollutants, such as NOx (encompassing nitric oxide [NO] and nitrogen dioxide [NO2]), sulfur dioxide (SO2), and particulate matter, posing a significant threat to global public health (Oliveri Conti et al., 2017; Wong et al., 2014).
Indeed, the tremendous pressure on each nation in the world to reduce fossil fuel emissions has prompted the discovery and development of clean and renewable energy resources, including, but not restricted to, solar power, wind power, hydropower, and biofuels. The latter includes the macroalgae-based production of biofuels, which possesses the unique benefit of converting CO2 to organic carbon, consequently reducing the greenhouse effect.
Carbon capture, utilization, and storage (CCUS) are increasingly integrated into different government policies to mitigate climate change (Schenuit et al., 2021). The CCUS methods include physical, chemical, and biological carbon sequestration. Biological sequestration, considered the most promising method to alleviate global greenhouse effects (Smith, 2016; Song et al., 2017), utilizes the photosynthesis of plants to convert CO2 to organic carbon, which is stored long term in plant biomass or the soil. Microalgae, mainly composed of cyanobacteria, green algae, and diatoms, are a class of unicellular or simple multicellular photosynthetic microorganisms widely distributed in freshwater and marine systems. Although microalgae account for less than 1% of the biomass of all photosynthetic organisms, they are the most photosynthetically efficient species, producing more than 50% of the atmospheric oxygen on earth (Chapman, 2013). In addition to their beneficial function of carbon sequestration/oxygen production, microalgae have attracted much attention in the biomanufacturing field because of their capability to synthesize a broad range of compounds due to their enormous biodiversity (Cardozo et al., 2007; Ratha & Prasanna, 2012).
The conventional production of fuels and bulk chemicals, which, to date, heavily rely on petroleum refining, faces significant problems such as safety during production, environmental pollution, and shrinking supplies of oil and natural gas. In place of petrochemicals, biomanufacturing is a new manufacturing mode that breaks away from the route of petrochemical industry, and has the typical characteristics of low carbon, recyclable, green, and clean (Chen & Wang, 2022). Microalgae can use solar energy to fix CO2 and convert C1 compound into organic matter, which can further generate a variety of metabolites through a variety of metabolic pathways and then produce a variety of biofuels and fine chemicals through genetic engineering modification. Therefore, microalgae have attracted much attention in recent years as “green cell factories” (Machado & Atsumi, 2012). Compared with other microorganism chassis cells, microalgae possess several advantages as cell factories; for example, they are more photosynthetically efficient due to their simple structure and highly operable genes, and they have a mature genetic operating system that allows convenient gene editing to improve the yield and simultaneously to reduce the generation of byproducts (Chen & Wang, 2021; Naduthodi et al., 2021; Ng et al., 2020; Poliner et al., 2018). Furthermore, microalgae have a fast growth rate and strong environmental adaptability and can be cultivated using agro-industrial wastes on coastal or saline-alkaline lands that are unsuitable for agricultural production (Chen et al., 2020; Ge et al., 2018; Zhang et al., 2014). Therefore, microalgae-based biomanufacturing is an economically efficient and environmentally friendly green sustainable technology.
In this review, we first summarize the recent advancement and development in microalgae-based production of platform chemicals, biofuels, and high value-added compounds (HVACs), as well as the key bottlenecks limiting the industrial application of this technology. We then discuss the opportunities to use this technology in synthetic biology (enabled by the development of this technology) and potential strategies to improve its economic efficiency and commercial feasibility.
2 PLATFORM CHEMICALS
Platform chemicals are small organic compounds that serve as building blocks to produce various HVACs; for example, propylene glycol, isoprene, and 3-hydroxyalanine are all such building blocks. Till now, approximately 330 million tons of platform chemicals are produced annually from oil-, coal-, and natural gas-based raw materials worldwide (Pachapur et al., 2016). However, the production of platform chemicals by chemical synthesis faces various challenges, such as high-energy consumption, complex production processes, and the use of toxic catalysts that cause environmental safety concerns. In addition, the diminishing petrochemical resources only escalate the problem. In this regard, biosynthesis is considered a more energy-efficient, safer, and renewable alternative to chemical synthesis; thus, it has become an increasingly popular technology for platform compound production. In 2004, the United States Department of Energy identified 12 biomass-derived chemicals as potential platform compounds (Werpy & Petersen, 2004). The global market for bio-based platform chemicals is expected to grow at an annual rate of 8% between 2021 and 2031, topping 23 billion US dollars by 2031 (FactMr, 2021).
With increased environmental awareness, the demand for bio-based platform chemicals to produce degradable polymers is growing fast. Notably, microalgae are considered one of the major resources for producing such bio-based platform compounds; therefore, they have attracted much attention globally in recent years.
2.1 Propanediols
Propanediols are used as building blocks for producing unsaturated polyesters, epoxy resin, and polyurethane resin. Compared with their conventional production by catalytic conversion of petroleum derivatives, the biosynthesis of propanediols has the advantages of mild conditions and low environmental pollution (Lee et al., 2015). To achieve the photosynthetic production of 1,3-propanediol from CO2, Hirokawa and colleagues introduced genes encoding enzymes involved in glycerol synthesis and metabolism into the cyanobacterium Synechococcus elongatus PCC 7942. The engineered strain produced 1,3-propanediol under photosynthetic conditions, reaching a titer of 288 mg/L after 14 days of cultivation (Hirokawa, Dempo, et al., 2017). The same research team subsequently improved the yield of 1,3-propanediol by optimizing the expression of the introduced genes and utilizing gene disruption guided by in-silico simulation of metabolic flux distribution. This further-engineered strain achieved a titer of 1220 mg/L, which was a more than 4-fold improvement in productivity (Hirokawa, Maki, & Hanai, 2017; Hirokawa, Matsuo, et al., 2017).
Cyanobacteria-based 1,3-propanediol production relies on enzymes, such as glycerol dehydratase, introduced from anaerobes. However, the highly oxidative intracellular environment of cyanobacteria is incompatible with the oxygen-sensitive glycerol dehydratase. To solve this problem, Liu et al. constructed a 1,3-propanediol synthetic cassette containing genes from the facultative anaerobe Klebsiella pneumoniae and integrated it into the chromosome of the nitrogen-fixing cyanobacterium Anabaena sp. PCC 7120 by homologous recombination. The engineered strain accumulated 46.0 mg/L of 1,3-propanediol in the presence of heterocysts, which was 1.7-fold higher than that with no heterocysts (Liu et al., 2018). Thus, the production of 1,3-propanediol in Anabaena was effectively improved by insulation of the oxygen-sensitive synthetic pathway within heterocysts.
Li and colleagues also have reported the photosynthetic production of 1,2-propanediol from CO2 using genetically engineered S. elongatus PCC 7942 (Li & Liao, 2013). A product titer of up to 22 mg/L was achieved with the introduction of the Escherichia coli genes encoding methylglyoxal synthase (mgsA), glycerol dehydrogenase (gldA), and aldehyde reductase (yqhD). The modified PCC 7942 was further engineered by introducing two secondary alcohol dehydrogenase (sadh) genes in place of gldA to minimize the accumulation of the pathway intermediate acetol, resulting in a product titer of ~150 mg/L (Li & Liao, 2013). The highest productivity for the photosynthesis of this compound was achieved by David and colleagues in 2018 (David et al., 2018). A synthetic pathway comprising mgsA, yqhD, and alcohol dehydrogenase (ADH) was introduced into Synechocystis sp. PCC 6803 and optimization of the cultivation conditions led to a product titer of almost 1 g/L in the stationary phase.
2.2 Isoprene
Isoprene, an important base material found in animals and plants, is a key intermediate compound for producing rubber and various adhesives. It is most readily obtained as a byproduct of the thermal cracking of naphtha or oil. In 2013, Matos and colleagues compared the renewable production of isoprene on a 10-ton scale by autotrophic microalgae and heterotrophic bacteria, and they concluded that the photosynthetic production by microalgae had a greater value in terms of material, energy, and economic efficiency (Matos et al., 2013). In 2016, Gao et al. reported the photosynthetic production of isoprene using genetically engineered S. elongates (Gao et al., 2016). The authors selected the methylerythritol phosphate pathway over the mevalonate pathway for isoprene synthesis based on the carbon efficiency and the precursor driving force. Moreover, using dynamic flux analysis and metabolite profiling, Gao and colleagues engineered the biosynthetic pathway with the stepwise introduction of isoprene synthase (ISPS), 1-deoxy-d-xylulose 5-phosphate synthase, isopentenyl pyrophosphate isomerase (IDI), an IDI–ISPS fusion protein, and 4-hydroxy-3-methylbut-2-enyl-diphosphate synthase. The engineered strain accumulated a titer of 1.26 g/L isoprene in 21 days, which was a significant increase from earlier reports that used photosynthetic microorganisms (Gao et al., 2016).
In addition, Chaves et al. reported in 2018 an even higher yield of photosynthetic isoprene production in engineered Synechocystis sp. PCC 6803, by which the cellular levels of the reactant dimethylallyl-diphosphate and the enzyme ISPS were simultaneously enhanced. The synergy between the two modifications resulted in an isoprene-to-biomass production ratio of 12.3 mg/g, the highest verifiable yield reported to date (Chaves & Melis, 2018). Besides propanediol and isoprene, microalgae have been used to convert CO2 to other platform chemicals, including olefins, carboxylic acids, and other alcohols, such as 2,3-butanediol (Table 1). Thus, microalgae are considered a clean, green, and renewable resource for producing platform chemicals with great potential in the chemical industry.
| Platform compound | High value-added polymer | Microalgal strain | Final yield (mg/L/day) | Reference |
|---|---|---|---|---|
| 1,3-Propanediol | Polyester, polyether, polyurethane | Synechococcus sp. PCC 7942 | 61 | Hirokawa, Maki, and Hanai (2017) |
| Synechococcus sp. PCC 7120 | 2.3 | Liu et al. (2018) | ||
| 1,2-Propanediol | Polypropylene glycol | Synechococcus sp. PCC 7942 | 15 | Li and Liao (2013) |
| Synechocystis sp. PCC 6803 | 100 | David et al. (2018) | ||
| Isoprene | Rubber | Synechocystis sp. PCC 6803 | 60 | Gao et al. (2016) |
| Synechocystis sp. PCC 6803 | 1.7 | Chaves et al. (2017) | ||
| Ethylene | Polyethylene, polystyrene, PVC, polyester | Synechocystis sp. PCC 6803 | 2104 | Veetil et al. (2017) |
| Synechocystis sp. PCC 6803 | 19.2 | Durall et al. (2020) | ||
| 3-Hydroxypropionic acid | Poly-3-hydroxypropionic acid | Synechocystis sp. PCC 6803 | 139.5 | Wang, Chen, and Zhang (2016) |
| Synechococcus sp. PCC 7942 | 329.5 | Lan et al. (2015) | ||
| 2,3-Butanediol | Resin | Synechococcus sp. PCC 7942 | 54.36 | Kanno and Atsumi (2017) |
| Synechococcus sp. PCC 7942 | 100 | Nozzi et al. (2017) |
3 BIOFUELS
Biofuels are considered an emerging renewable energy replacement for fossil fuels and can be produced from diverse biological sources. Compared with the first-generation biofuels from agricultural sources and the second-generation biofuels from nonfood feedstock, the third-generation biofuels from microalgae show several advantages, such as high photosynthetic efficiency, high oil content, fast growth rate, short harvesting cycle, and usage of nonagricultural land (Chowdhury & Loganathan, 2019). Therefore, microalgae are considered the most promising resource for future energy production. Biofuels are produced from microalgae in solid (e.g., biochar), liquid (e.g., biodiesel, biocrude, and bioethanol), or vapor states (e.g., biohydrogen and biogas) (Chen et al., 2020). In particular, the solid biochar is mainly produced by high-temperature carbonization of bloom cyanobacteria salvaged from lakes or rivers, which does not involve the usage of microalgae as cell factories for biosynthesis. Here, we mainly discuss and review the related progress in the synthesis of liquid and vapor biofuels using microalgae as a cell factory.
3.1 Biodiesel
The oil productivity of microalgae greatly exceeds that of the best oil-producing crops, making microalgae the only feasible source of renewable biodiesel that may potentially supply our global needs for transport fuels (Chisti, 2007). Microalgal oil can also be converted to commercially available biodiesel using processing techniques such as extraction, separation, and methyl esterification. However, the oil content of the cell biomass is the key factor determining the economic efficiency of microalgae-based biofuel production (Chisti, 2007). It can be enhanced by modifying the cultivation conditions, such as culturing under nutrient deprivation or optimizing the light exposure, temperature, and pH of the culture medium (Singh et al., 2016). Paradoxically, some oil-enhancing techniques may also cause decreased cell growth and photosynthesis, leading to reduced productivity (Ran et al., 2019). The mechanisms by which microalgae respond to external stimuli are not fully understood. Thus, managing the conflict between biomass production and oil accumulation remains a major challenge in microalgal oil production.
The rapid advancement in “algal omics” technologies can help us better understand the mechanisms that control microalgal gene regulation and energy metabolism, as well as their protein functions and interactions (Guarnieri & Pienkos, 2015). In recent years, genetic and metabolic engineering of lipid biosynthetic pathways in microalgae have emerged as effective oil-enhancing strategies (Ran et al., 2019; Singh et al., 2016). The main approaches include upregulation, downregulation, mutation, and/or overexpression of endogenous genes or introduction of foreign genes to increase the efficiency of the relevant oil-producing pathway (Table 2). The combined application of genetic and metabolic engineering and synthetic biology might promote the development of microalgae as an economically efficient renewable resource for high-quality biodiesel production.
| Microalgae | Engineering methodology | Results | Reference |
|---|---|---|---|
| Phaeodactylum tricornutum | Overexpression of endogenous MDH | 250% increase in oil content | Xue et al. (2015) |
| Chlamydomonas reinhardtii | Introduction of GPAT from Lobosphaera incise | 50% increase in TAG | Iskandarov et al. (2015) |
| Thalassiosira pseudonana | Knockdown of endogenous lipase Thaps3_264297 | 230% increase in oil content | Trentacoste et al. (2013) |
| Chlorella spp. | Introduction of AtNADK3 | 110.4% increase in oil content | Fan et al. (2015) |
| C. reinhardtii | Knockout of endogenous PLA2 | 274% increase in oil content | Shin et al. (2019) |
| Nannochloropsis | Mutation of endogenous TPS | 34% increase in FAME content, 75% increase in FAME yield | Ryu et al. (2020) |
- Abbreviations: AtNADK3, NAD(H) kinase 3 from Arabidopsis; FAME, fatty acid methyl ester; GPAT, glycerol-3-phosphate acyltransferase; MDH, malate dehydrogenase; PLA2, phospholipase A2; TAG, triacylglycerol; TPS, trehalose-6-phosphate synthase.
3.2 Biohydrogen
Microalgae can produce biohydrogen through fermentation processes (photofermentation, dark-fermentation, and combined photo and dark-fermentation) and photolysis (direct and indirect photolysis) (Goswami et al., 2021; Li et al., 2013). Although hydrogen productivity varies among different microalgal species and production processes, the low yield and high production cost are the bottlenecks for commercial-scale operations. To date, the common techniques used to increase productivity include bacteria–microalgae coupled production, the use of oxygen scavengers (e.g., sodium bisulfite), mixed cultivation of several microalgal species, immobilization of microalgal cells to improve light utilization, and optimization of bioreactor conditions (Chen et al., 2016; Lakatos et al., 2017; Ramanan et al., 2016; Wei, Yi, et al., 2017; Xu et al., 2020). In recent years, to boost the yield of hydrogen, genetic engineering has been utilized to modify the key enzymes involved in biohydrogen production; for example, the green algal hydrogenase, a critical enzyme catalyzing photolysis-dependent biohydrogen production, can be easily inhibited by oxygen via its gas tunnel. To promote hydrogen production, Yang et al. modified the amino acid residues around the gas tunnel through genetic engineering to prevent oxygen from accessing the active site of the enzyme; therefore, the hydrogen production in Chlorella sp. improved by up to 30-fold (Yang et al., 2019).
Furthermore, Yacoby and colleagues effectively increased the rate of hydrogen photoproduction by replacing the wild-type hydrogenase with a bioengineered ferredoxin-hydrogenase fusion protein to facilitate electron transfer from ferredoxin to hydrogenase (Yacoby et al., 2011). Appel and colleagues reported photosynthetic hydrogen production using a photosystem I-hydrogenase fusion in vivo, transferring the photosynthetic electrons from CO2 fixation to hydrogenase (Appel et al., 2020). Moreover, the ribulose 1,5-bisphosphate carboxylase oxygenase (Rubisco)-deficient Chlamydomonas reinhardtii showed greater hydrogen productivity than that of the wild type in sulfur-depleted culture, suggesting that the downregulation of the Rubisco-dependent Calvin cycle promoted the electron transfer to hydrogenase for sustained hydrogen production under sulfur deprivation (Hemschemeier et al., 2008).
In addition, many cyanobacterial strains have large antenna complexes to absorb sunlight for photosynthetic activities. In these strains, light saturation is a major cause of poor light conversion efficiency under outdoor conditions. In the green microalga Desmodesmus sp. S1, truncation of the light-harvesting antenna by engineering the quantity of photosystem II subunits alleviated light saturation and improved the solar energy conversion efficiency, which resulted in a fourfold to eightfold increase in the hydrogen production (Hu et al., 2020). Other bioengineering methods that have been used to increase microalgal biohydrogen productivity are summarized in Table 3.
| Microalgae | Methodology | Results | Reference |
|---|---|---|---|
| Chlamydomonas reinhardtii | Introduction of light-inducible miRNA targeting psbA | Increase in hydrogen yield | Wang et al. (2017) |
| C. reinhardtii | Introduction of POX from Escherichia coli and catalase from Synechococcus elongatus | 2-fold increase in hydrogen yield | Xu et al. (2011) |
| Chlorella sp. DT. | Knockdown of endogenous PsbO | 9-fold increase in hydrogen yield | Lin et al. (2013) |
| C. reinhardtii | Truncation of light-harvesting antenna | 6-fold increase in hydrogen yield | Kosourov et al. (2011) |
| Synechocystis sp. PCC 6803 | Use of inhibitors of the photosynthetic and respiratory electron transport chains | 30-fold increase in hydrogen yield | Burrows et al. (2011) |
| C. reinhardtii | Introduction of heat-inducible miRNA targeting psbA | 60% increase in hydrogen yield | Li et al. (2018) |
| C. reinhardtii | Δpgr5 mutant generated via DNA insertional mutagenesis | 2–5.5 times higher in hydrogen yield | Khosravitabar and Hippler (2019) |
- Abbreviations: POX, pyruvate oxidase; psbA, photosystem II protein D1 precursor; Psbo, photosystem II manganese-stabilizing polypeptide.
Carboxysomes are bacterial intracellular microcompartments that encapsulate enzymes (such as those involved in photosynthetic carbon fixation) into a polyhedral protein shell. Our research team constructed self-assembled carboxysome shells in E. coli by introducing a set of carboxysome shell protein-encoding genes and incorporated [FeFe]-hydrogenases within the empty shell through signal peptide-assisted delivery for producing hydrogen (Li et al., 2020). The oxygen-deprived microenvironment within the shell could facilitate hydrogen production by the encapsulated oxygen-sensitive hydrogenases, turning the engineered carboxysomes into efficient hydrogen-producing nanoreactors. Of note, although many advancements have been made in improving the efficiency of microalgal biohydrogen production, the current productivity levels are still inadequate for the industrialization and commercialization of such a technique. Hence, further improvements in yield and cost could lead this biotechnology to a commercial success.
3.3 Bioethanol
Bioethanol, the first biofuel produced through photosynthetic carbon fixation and the most successful biofuel so far, can be produced by yeast fermentation using microalgal carbohydrates as feedstock. The concentration of carbohydrates in microalgal cells can be boosted by modifying the cultivation conditions (such as cultivation under nutrient deficiency) (Braga et al., 2018; Zhu et al., 2014). Although ethanol is not a natural metabolite of microalgae, it can be produced in modified strains where an ethanol synthesis pathway has been introduced. To date, all photosynthetic pathways for ethanol production depend on the catalytic actions of pyruvate decarboxylase (PDC, encoded by pdc) and ADH (encoded by adh) (Luan et al., 2015). PDC converts the metabolite pyruvate to acetaldehyde, and (with either NADH or NADPH as a cofactor) ADH reduces acetaldehyde to ethanol. The facultative anaerobic bacterium Zymomonas mobilis has notable bioethanol-producing capabilities, outperforming yeast in certain circumstances (He et al., 2014). Thus, the PDC–ADH pathway in Z. mobilis (pdcZM–adhZM) is commonly used to create engineered microorganisms for bioethanol production. For instance, Gao et al. created the Synechocystis sp. PCC 6803 mutant strain with a significantly improved ethanol-producing efficiency (212 mg/L/day) by introducing pdcZM, which overexpressed the endogenous ADH (slr1192) and disrupted the poly-β-hydroxybutyrate synthesis pathway (Gao et al., 2012).
With regard to the industrialization potential, Algenol Biofuels and Joule Unlimited are two US biofuel companies holding a leading position in photoautotrophic ethanol production. The Algenol patent #WO2009098089 (filed August 13, 2009; Coleman et al., 2009) showed that the replacement of pdcZM with the pdc from Zymobacter palmae (pdcZP), which possesses a higher catalytic efficiency, resulted in a 10%–20% increase in ethanol yield. In addition, the replacement of adhZM with slr1192 and usage of the zinc-inducible promoter PziaA to drive the pdcZP-slr1192 expression further boosted the yield to 7.1 g/L in 30 days. Meanwhile, the Joule patent #US20120164705A1 (filed November 13, 2011, Reppas et al., 2012) reported the construction of a Synechocystis sp. PCC 7002 mutant by introducing pdcZM and two adh genes from different sources and disrupting the lipoic acid synthesis pathway. This engineered strain produced 5.62 g/L ethanol in 13 days. However, despite these advances, the microalgal bioethanol production is still in its infancy, and further research and development of bioengineering technologies are needed to make it a commercial success.
4 HIGH VALUE-ADDED COMPOUNDS
HVACs usually refer to secondary metabolites of animals, plants, or microorganisms with a high economic value. These compounds are often used in the cosmetic, food processing, and pharmaceutical industries. Microalgae naturally produce a variety of HVACs, including carbonates, alkaloids, carotenoids, terpenes, and steroid hormones (Bilal et al., 2017). Many microalgal species that possess favorable properties, such as fast growth, a well-understood genome and metabolic pathway, and mature genetic manipulation technology, have been used as chassis cells for the production of various HVACs. In recent years, guided by synthetic biology, several such chassis cells have been redesigned and produced by introducing foreign genes encoding complete synthetic pathways for the more efficient synthesis of HVACs. Table 4 summarizes the current development status of HVAC synthesis using engineered microalgal chassis cells. Lutein, p-coumaric acid, limonene, astaxanthin, and farnesene have all been efficiently produced by various engineered microalgal strains.
| Microalgae type | Strain | HVAC | Reference |
|---|---|---|---|
| Prokaryotic | Synechocystis sp. PCC 6803 | p-Coumaric acid | Gao et al. (2021) |
| Synechocystis sp. PCC 6803 | Lutein | Lehmann et al. (2021) | |
| Synechococcus elongatus UTEX 2973 | Limonene | Lin et al. (2021) | |
| Synechococcus sp. PCC 7002 | Astaxanthin | Hasunuma et al. (2019) | |
| Anabaena sp. PCC 7120 | Farnesene | Halfmann et al. (2014) | |
| Synechococcus sp. PCC 7942 | Limonene | Wang, Liu, et al. (2016) | |
| Eukaryotic | Chlamydomonas reinhardtii | Xylitol | Pourmir et al. (2013) |
| C. reinhardtii | Sesquiterpenoid | Lauersen et al. (2016) | |
| Phaeodactylum tricornutum | Docosahexaenoic acid | Hamilton et al. (2014) | |
| Bacillariophyta | Bisabolene | Tanaka et al. (2021) |
Compared with eukaryotic algae, the technologies related to prokaryotic algal biosynthesis are more mature. Currently, astaxanthin, p-coumaric acid, limonene, and lutein have been successfully synthesized using different cyanobacterial chassis cells (Table 4). Research on the synthetic biology of eukaryotic algae is still in the exploratory stage. Chlamydomonas reinhardtii is a model eukaryotic microalgal species widely studied with regard to synthetic biology and genetic engineering. For example, feeding engineered C. reinhardtii expressing the foreign VP28 protein to shrimp significantly controlled its white spot disease (Pham et al., 2021). In addition, the xylulose reductase derived from Neurospora crassa was codon-optimized and transferred into C. reinhardtii to obtain a xylitol yield of 0.38 g/L (Pourmir et al., 2013). Moreover, the plant Patchouli patchoulol synthase derived from Pogostemon cablin could synthesize sesquiterpenes in C. reinhardtii (Lauersen et al., 2016). Furthermore, the chloroplast system of C. reinhardtii was modified using synthetic biology technology to directly produce recombinant proteins such as cholera and malaria vaccine and immunotoxin (Gregory et al., 2013), as well as immunotoxins targeting B-cell tumors (Tran, Henry, et al., 2013; Tran, Van, et al., 2013). However, further development of C. reinhardtii as a green chassis cell has been hindered by the lack of efficient expression elements and comparative evaluation, as well as low transgene titers. Some scholars have systematically evaluated the existing expression elements of C. reinhardtii and created novel synthetic expression elements by promoter engineering, which significantly improved the efficiency of C. reinhardtii as a chassis cell for synthetic biology (Hu et al., 2021).
The synthetic biology of other models of eukaryotic algal species is under active development and is increasingly used to create chassis cells for biosynthesis. For example, the introduction of a highly active exogenous diacylglycerol acyltransferase into Nannochloropsis increased the activity of eicosapentaenoic acid (EPA) and reduced the role of EPA in the oxidative pathway, resulting in a 5-fold increase in the EPA content in triglycerides (Wei, Shi, et al., 2017). This is significant for developing high-quality edible oils using Nannochloropsis biomass as a raw material. The marine microalga Phaeodactylum tricornutum is another model organism of eukaryotic algae, which synthesizes the terpenoid fucoxanthin via the 1-deoxy-d-xylulose 5-phosphate synthase (Dxs) pathway. The expression plasmid of the Dxs gene was integrated into the genome of P. tricornutum, and the fucoxanthin content of the resulting Dxs transformant was 2.4 times that of the wild-type algal strain (Eilers et al., 2016). In addition, CRISPR technology has been applied in Dunaliella salina and Chlorella, Agrobacterium-mediated genetic transformation was achieved in Euglena gracilis, and intron-mediated gene expression was realized in Cyclotella spp. The development of these technologies has laid the foundation for the future construction of transformed microalgal cell factories for the industrial production of HVACs (Becker et al., 2021; Hu et al., 2021; Kim et al., 2021).
The selection of an appropriate algal cell chassis, either eukaryotic or prokaryotic, for the synthesis of a particular HVAC depends on the availability of an appropriate biosynthetic pathway, as well as the feasibility and efficiency of the construction of such a pathway via genetic engineering. The chassis cells, as the production vector, should also provide the substrates for synthesizing the target product. Once the chassis is selected, the enzymes and regulatory factors involved in the metabolic process can be modified to improve the production yield.
5 CONCLUSIONS AND FUTURE PROSPECTS
This review summarizes the potential usage of microalgae-based biomanufacturing at the industrial level and its advantages and shortfalls. Although this technology is still in the early exploration stage, it represents the future of green manufacturing. The rapid development of genetic and metabolic engineering tools for a better understanding of microalgal metabolic pathways and their regulatory mechanisms, as well as the further optimization of cultivation procedures and photosynthetic efficiency, can eventually launch microalgae as green renewable cell factories for industrial production.
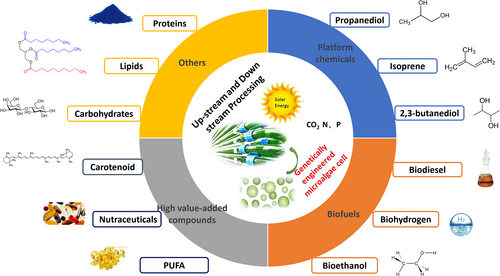
Acting as chassis cells, microalgae can utilize solar energy to convert CO2 to organic carbon directly and generate a variety of metabolites. The recent advances in microalgal genetic and metabolic engineering have helped to establish these photosynthetic microorganisms as promising cell factories for the production of biofuels, fine chemicals, and macromolecules such as proteins and nucleic acids (Figure 1). Compared with heterotrophic chassis cells, microalgae have the advantage of carbon fixation. Thus, microalgae-based biomanufacturing is considered a future solution to unsustainable chemical production and a remedy to mitigate the greenhouse effect. However, microalgae-based biomanufacturing often faces the challenges of low productivity and high costs for cell cultivation and product processing, limiting its applicability in industry. To tackle these challenges, it is critical to identify and overcome bottlenecks: from strain selection and engineering to cell cultivation, product extraction, and processing.
Thus, to construct a highly productive microalgal cell factory, novel designs and the establishment of an efficient synthetic pathway are needed by using accurate, stable, and efficient genetic engineering procedures. To achieve this aim, a comprehensive understanding of microalgal synthetic biology and the masterful utilization of microalgae-specific gene editing methods are key to ensuring successful strain construction. To the best of our knowledge, some gene editing methods used in microalgal engineering have inherent problems of low accuracy, high-frequency off-target modifications, and unstable transformants. The discovery of tailored solutions to these problems is a prerequisite for creating stable strains and sustainable biomanufacturing. In particular, traceless editing and large fragment operation require special attention when multiple rounds of gene editing are utilized together to create the desired strain. Furthermore, microalgae may synthesize a large pool of organic molecules through various metabolic pathways, which frequently crosstalk with each other. The specific synthetic pathway, engineered for the production of a target compound, can be influenced by other metabolic pathways competing for the same carbon or nitrogen sources. The productivity of the target pathway could be improved by metabolic engineering through improved and newly discovered strategies, such as enzyme modification or promoter engineering, to drive the metabolic flow to the target pathway. Therefore, a better understanding of the metabolic flow of microalgae and its regulation is essential to develop efficient microalgae-based production.
Furthermore, the feasibility of microalgae-based biomanufacturing depends on biomass accumulation and the yield of the target products. The relatively low biomass of microalgae is a significant disadvantage so far. The availability of the biomass may be improved by optimization of the cultivation procedure, sunlight exposure, and temperature and the use of an appropriate bioreactor. To date, efforts to improve biomass accumulation through genetic engineering have been relatively scarce. To move microalgal cell factories from the laboratory to industrial production, future work should focus on screening high-biomass, fast-growing strains, and the bioengineering of key pathways to enhance biomass accumulation and the product yield.
Finally, the current low light conversion efficiency of microalgae in laboratory culture (1%–2%) is insufficient for industrial production (Perin et al., 2019). The improvement in photosynthesis efficiency is a fundamental and essential step toward industrialization. The conventional techniques mainly rely on the design and optimization of the reactor system to enhance light absorption and utilization. Therefore, the development of novel bioengineering tools to modify microalgae as chassis cells will be a better approach to improving photosynthesis efficiency through strain engineering. For example, introducing foreign photosynthesis genes (such as chlorophyll genes) may result in an increase in light energy capture and a higher photosynthesis efficiency.
AUTHOR CONTRIBUTIONS
Zhongliang Sun contributed to the data curation, formal data analysis, methodology, validation, and writing of the original draft. Hui Chen contributed to the study design, manuscript writing, review, and editing. Liqin Sun contributed to the study design, manuscript writing, review, and editing. Qiang Wang contributed to the study conceptualization, manuscript writing, review, and editing.
ACKNOWLEDGMENTS
This project was supported in part by grants from the National Key R&D Program (#2021YFA0909600), the National Natural Science Foundation of China (#32170138, #32102819, and #31870041), the Henan University Science and Technology Innovation Team (#22IRTSTHN024), the Henan Provincial Natural Science Foundation (#212300410024), the Henan Provincial Science and Technology Tackling Project (#222102110131), and the 111 Program (#D16014).
CONFLICT OF INTEREST
The authors declare that no competing financial interests or personal relationships have influenced the work reported in this review.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




