Winter hardiness of Miscanthus (I): Overwintering ability and yield of new Miscanthus ×giganteus genotypes in Illinois and Arkansas
Abstract
Miscanthus ×giganteus (M×g) is an important bioenergy feedstock crop. However, biomass production of Miscanthus has been largely limited to one sterile triploid cultivar, M×g ‘1993-1780’, which we demonstrate can have insufficient overwintering ability in temperate regions with cold winters. Key objectives for Miscanthus breeding include greater biomass yield and better adaptation to different production environments than M×g ‘1993-1780’. In this study, we evaluated 13 M×g genotypes, including ‘1993-1780’, in replicated field trials conducted for three years at Urbana, IL; Dixon Springs, IL; and Jonesboro, AR. Entries were phenotyped for first-winter overwintering ability and plant hardiness (ratio of new tillers to old), yield in years 2 and 3, and first heading date, plant height, and culm number in years 1 and 2. We observed substantial variation for overwintering ability and biomass yield among the M×g genotypes tested and identified ones with better overwintering ability and/or higher biomass yield than ‘1993-1780’. Most entries at Urbana were damaged during the first winter, whereas few or no entries were damaged at Dixon Springs or Jonesboro. However, M×g ‘Nagara’ was entirely undamaged during the first winter and produced high biomass yields at Urbana (19.7 Mg/ha in year 2 and 20.9 Mg/ha in year 3), whereas M×g ‘1993-1780’ exhibited an overwintering loss of 29%, had severely damaged survivors (hardiness score of 25%), and reduced biomass yield (8.1 Mg/ha in year 2 and 16.2 Mg/ha in year 3), indicating that M×g ‘Nagara’ could be a better choice in hardiness zone 5 (average annual minimum air temperature of −23.3 to −28.9°C) or lower. In Dixon Springs, where M×g ‘1993-1780’ was undamaged by the first winter, it yielded highest among all the entries (21.6 Mg/ha in year 3), though not significantly higher than M×g ‘Nagara’ (18.2 Mg/ha in year 3).
1 INTRODUCTION
Miscanthus is a C4 perennial grass from East Asia and Oceania. As a biomass feedstock crop, Miscanthus can be used for the production of electricity, bioproducts, and liquid fuels such as ethanol (Clifton-Brown et al., 2017; Clifton-Brown, Schwarz, & Hastings, 2015; Somerville, Youngs, Taylor, Davis, & Long, 2010). Miscanthus ×giganteus (M×g) is an interspecific hybrid between M. sacchariflorus and M. sinensis and has been of primary interest for commercial biomass production (Hodkinson & Renvoize, 2001). We note that nothospecies, such as M×g, are defined by their parental species, but not by their ploidy or their parents’ ploidy (McNeill et al., 2012), so M×g can be diploid, triploid, or tetraploid. However, only one triploid genotype of M×g is currently grown for biomass. Though many named cultivars of triploid M×g have been described in North America and Europe, molecular marker data from Głowacka et al. (2015) suggest that they are all the same genotype, which we call M×g ‘1993-1780’ in reference to the accession number of the type specimen at Kew Royal Botanic Gardens Herbarium, or, at most, horticultural sports derived from the same genet (Głowacka et al., 2015; Hodkinson & Renvoize, 2001). In the United States, M×g ‘1993-1780’ is typically called M×g ‘Illinois’, as we obtained this genotype from Chicago Botanical Garden (Maughan et al., 2011). M×g ‘1993-1780’ is a natural hybrid between a tetraploid M. sacchariflorus and diploid M. sinensis that was exported from Yokohama, Japan (likely originating from 30–35°N latitude in Japan, and corresponding to USDA hardiness zones 8 or 9, with average annual minimum air temperatures of −1.1 to −6.7°C and −6.7 to −12.2°C, respectively) to Denmark in the 1930s and subsequent distributed throughout Europe and North America (Greef & Deuter, 1993; Hodkinson et al., 2002; Hodkinson & Renvoize, 2001; Linde-Laursen, 1993).
Dry biomass yields of M×g ‘1993-1780’ in North America and Europe have typically ranged from 13.7 to 37.8 Mg/ha (Arundale et al., 2014; Christian, Riche, & Yates, 2008; Clifton-Brown & Lewandowski, 2002; Clifton-Brown et al., 2001; Clifton-Brown, Stampfl, & Jones, 2004; Dohleman & Long, 2009; Dong et al., 2018a; Heaton, Dohleman, & Long, 2008; Heaton, Voigt, & Long, 2004; Kaiser, Clark, Juvik, Voigt, & Sacks, 2015; Lewandowski, Clifton-Brown, Scurlock, & Huisman, 2000). Although field trials of M×g ‘1993-1780’ have demonstrated its potential throughout Europe and North America, poor overwintering ability has been the major limitation of this genotype in temperate regions with cold winters (Clifton-Brown & Lewandowski, 2000; Kucharik, VanLoocke, Lenters, & Motew, 2013; Pude, 1998). In particular, M×g ‘1993-1780’ has exhibited consistently poor overwintering in USDA hardiness zone 4 (average annual minimum air temperature of −28.9 to −34.4°C) and colder environments and has had intermittent losses of stand in hardiness zone 5 (average annual minimum air temperature of −23.3 to −28.9°C) depending on the severity of a given winter (Clifton-Brown & Lewandowski, 2000; Heaton et al., 2008; Jørgensen, Mortensen, Kjeldsen, & Schwarz, 2003; Lewandowski et al., 2000).
During late autumn, temperate-adapted Miscanthus genotypes become dormant, with aboveground shoots that senesce and viable belowground rhizomes that resume growth in spring. Miscanthus is most sensitive to winter damage during the establishment year (i.e., first winter; Clifton-Brown & Lewandowski, 2000). Multiple agronomic and propagation methods have been tested on M×g ‘1993-1780’ for their effects on biomass yield and overwintering ability, including size of propagation materials, planting depth, planting time, and artificial induction of dormancy in autumn (Boersma & Heaton, 2014; Eppel-Hotz, Jodl, Kuhn, Marzini, & Munzer, 1998; Lewandowski, 1998; Mangold, Lewandowski, Xue, & Kiesel, 2018; Pude & Franken, 1998; Schwarz, Kjeldsen, Münzer, & Junge, 1998). Unfortunately, the sensitivity of M×g ‘1993-1780’ to low temperatures during winter dormancy has not been reliably mitigated by agronomic treatments. Moreover, until recent efforts to breed new M×g hybrids, it has not been possible to explore the potential of genetics to improve overwintering ability and biomass yield in M×g directly. However, genetic variation for its relatively high overwintering ability has been observed among and within M×g's progenitor species, M. sacchariflorus and M. sinensis (Clifton-Brown & Lewandowski, 2002; Clifton-Brown et al., 2001; Kaiser & Sacks, 2015; Yan et al., 2012), suggesting that selection of parents should be an effective strategy to produce M×g cultivars that are more winter-hardy than the genotype currently grown commercially.
Given that M×g ‘1993-1780’ is a sterile triploid that requires expensive vegetative propagation to establish a field (Christian, Yates, & Riche, 2005; Lewandowski, 1998; Słomka et al., 2012), it is economically critical that high crop productivity be consistently maintained over multiple years in order to amortize the high costs of establishment. Therefore, the establishment and maintenance of high stand densities are necessary to ensure consistently high yields and economic viability of M×g plantations. Furthermore, the development of a new crop based on a single genotype is highly risky, given that a virulent pest or disease would have the potential to cause widespread losses (Clifton-Brown & Lewandowski, 2002). Thus, a lack of genetic variation in M×g available to growers has been a key constraint to the development of this crop. New M×g cultivars with high yield potential and better adaptation to target biomass-production environments are needed. In this study, we evaluated adaptation and biomass yield of 13 M×g genotypes (nine triploids and four tetraploids) at two locations in Illinois (Urbana and Dixon Springs) and one location in Arkansas (Jonesboro). The objectives were to (a) quantify genetic variation for overwintering ability and biomass yield among these M×g genotypes, (b) compare relevant US production environments and assess interactions between M×g genotypes and these environments, and (c) identify M×g genotypes that are superior to the leading cultivar, ‘1993-1780’.
2 MATERIALS AND METHODS
2.1 Plant materials and experiment design
Fifteen Miscanthus genotypes were studied, including 13 M×g, one M. sinensis, and one M. sacchariflorus (Supporting information Table S1). Nine of the M×g genotypes were triploid, including the commercial standard ‘1993-1780’, four full-sibs from a cross made at the University of Illinois (10UI-032 series), three from wild-collected seed harvested in Kyushu, Japan (Ogi series; Nishiwaki et al., 2011), and a newer cultivar named ‘Nagara’ selected from a cross by M. Deuter at Tinplant (Deuter, 2011). Four of the M×g were tetraploid (PF series, kindly provided by New Energy Farms, Leamington, ON, Canada). Using molecular markers, Głowacka et al. (2015) determined that all of the triploid M×g in this study are genetically distinct. The two parents of the four 10UI-032 series triploid full-sib progeny, M. sacchariflorus 4× ‘Bluemel’ and M. sinensis ssp. condensatus ‘Cabaret’, were included as controls. M. sacchariflorus 4× ‘Bluemel’ was previously observed to be hardy in Urbana, IL, but M. sinensis ssp. Condensatus ‘Cabaret’ was previously found to be only marginally hardy in central IL (Kaiser & Sacks, 2015). Each genotype was vegetatively propagated by dividing rhizomes of stock plants and growing ramets in 38-cell trays (T.O. Plastics, Clearwater, MN, USA) in a greenhouse at Urbana, IL.
Field trials were established on May 13–14, 2013, at Urbana, IL (40.1°N, 88.2°W; Drummer silty clay loam); on May 21, 2013, at Dixon Springs, IL (37.6°N, 88.7°W; Grantsburg silt loam); and on May 1, 2013, at Jonesboro, AR (35.8°N, 90.7°W; Collins silt loam). Average annual minimum air temperatures from 1976 to 2006 were −26.1 to −23.3°C at Urbana, IL (hardiness zone 5b); −20.6 to −17.8°C at Dixon Spring (hardiness zone 6b); and −15.0 to −12.2°C at Jonesboro (hardiness zone 7b). For each location, the field trial was a randomized complete block design with four replications. Within each block, each genotype was planted in a four-row plot with eight plants per row. The spacing of plants between and within rows was 0.91 m. One border row of M×g ‘1993-1780’ was planted around each trial. Irrigation was applied as needed during the first year to ensure good establishment. In the spring of each year, 100.8 kg N/ha was applied. To control weeds, Atrazine at 2.8 kg/ha, S-metolachlor at 1.5 L/ha, and 2,4-D at 1.8 L/ha were applied (all values shown are for active ingredient). Hand-weeding was done as needed.
2.2 Data collection
The evaluation system to obtain phenotypic data is described in Table 1. To evaluate overwintering ability, survival data were taken on each plant in the autumn and spring bracketing the first and second winters of the trial (autumn 2013 and spring 2014; and autumn 2014 and spring 2015). Overwintering ability was calculated as 0 (plant was alive in previous year's autumn but was dead in current year's spring) or 1 (plant was alive in previous year's autumn and also regrew in current year's spring). For each trial location, data on air temperature (daily mean, maximum and minimum) and soil temperature at 10 cm below the surface of bare soil were compiled from nearby weather stations (Supporting information Data S1). To evaluate the effects of the 2013–2014 winter on the plants in greater detail than binary survival data would allow, we also recorded plant hardiness scores (the ratio of number of new tillers to number of previous year's tillers, recorded as percentage in an increment of 10%; Table 1) at Urbana (May 21, 2014) and Dixon Springs (June 3, 2014). At the warmest and most southern location, Jonesboro, plant hardiness score data were not taken because all genotypes survived and grew vigorously in 2014. Dry biomass yields were recorded in 2014 (year 2) and 2015 (year 3). Plants were also phenotyped for first heading date, plant height, and culm number in 2013 (year 1) and 2014 (year 2). In summary, a total of six traits were phenotyped (Table 1).
| Trait (unit) | Abbreviation | Trait description and evaluation system |
|---|---|---|
| Overwintering ability (Prpn) | OWA | Recorded if the plant was dead or alive. Data taken in late October and again during the last week of the following May. Dead plant was recorded as 0, and alive plant was recorded as 1. Missing plants were recorded as NA. Overwintering ability was then calculated from survival data as follows: 0 (plant was alive in previous year's autumn but was dead in current year's spring), 1 (plant was alive in previous year's autumn and also regrew in current year's spring). Data were taken on individual plants in each plot |
| Hardiness (%) | Hardiness | The ratio of the number of new live tillers to the number of tillers that grew in the previous year. Recorded in 10% increments, with a cap of 100%. Data were taken on each individual plant at Urbana (May 21, 2014) and Dixon Springs (June 3, 2014) |
| Yield (Mg/ha) | Yield | After dormancy in late autumn, individual rows of each four-row plot were harvested by cutting plants at 20 cm above the soil surface, and dried whole at 55°C, then weighed. For each genotype, yield per plot was calculated first by summing the dry weights of the four rows. Yield per hectare was estimated as: 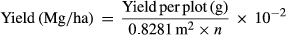 , where 0.8281 m2 is the unit area per plant (0.91 m × 0.91 m), n is number of living plants, 1 M×g = 106 g, and 1 ha = 104 m2 , where 0.8281 m2 is the unit area per plant (0.91 m × 0.91 m), n is number of living plants, 1 M×g = 106 g, and 1 ha = 104 m2 |
| Heading date (days) | HD | First heading date. Date on which the first inflorescence emerged ≥1 cm beyond the flag-leaf sheath. Data were taken on whole plots and were recorded weekly |
| Plant height (cm) | Ht | Length of the plant's tallest flowering culm from soil surface to tip of the panicle (awns excluded). If no panicles were present, length of the tallest culm from soil surface to highest part of the highest leaf. Data were taken on the middle four plants in each plot |
| Culm number (count) | Culm | Count of the number of culms per plant. Data were recorded on the middle four plants in each plo. |
2.3 Statistical analysis of phenotypic data
For each of the six traits studied, individual plot values were first calculated (Table 1). For overwintering ability and hardiness score, raw data were collected on individual plants within each of the four-row plots (32 plants per plot). As such, plot values for overwintering ability were calculated as the proportion of plants per plot that survived the winter, and hardiness score plot values were calculated by averaging hardiness scores within a plot of each plant that was alive during the spring. Yield raw data were taken for each row in the four-row plots, and yield plot values were calculated by dividing plot yield (sum of the four rows) over plot area (calculated based on the number of live plants per plot), which was then expressed as Mg/ha (Table 1). First heading date was recorded on a per plot basis. Data on plant height and culm number were phenotyped on the middle four plants within each plot, and plot values were calculated by averaging data of the middle four plants.
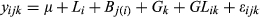
where y is the trait individual plot value, 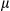 is the grand mean,
is the grand mean, 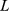 is location,
is location, 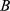 is block nested in location,
is block nested in location, 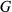 is genotype,
is genotype, 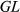 is genotype by location interaction, and
is genotype by location interaction, and  is error. Location and genotypes were fixed effects, and block nested in location was a random effect in the model. Genotype LS means at each field trial location were calculated using the linear model:
is error. Location and genotypes were fixed effects, and block nested in location was a random effect in the model. Genotype LS means at each field trial location were calculated using the linear model: 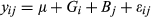 . Phenotypic correlations among traits were calculated in SAS CORR, and genetic correlations among traits were calculated using SAS GLM with the MANOVA option.
. Phenotypic correlations among traits were calculated in SAS CORR, and genetic correlations among traits were calculated using SAS GLM with the MANOVA option.
3 RESULTS
Large and highly significant differences among genotypes (13 M×g, one M. sinensis and one M. sacchariflorus) and locations (Urbana, IL; Dixon Springs, IL; and Jonesboro, AR) were observed for each of the six traits in all years measured (Figures 1 and 2 and Supporting information Table S2). Significant interactions between genotype and location were also observed for all traits in each year (Supporting information Table S2).
3.1 Winter hardiness
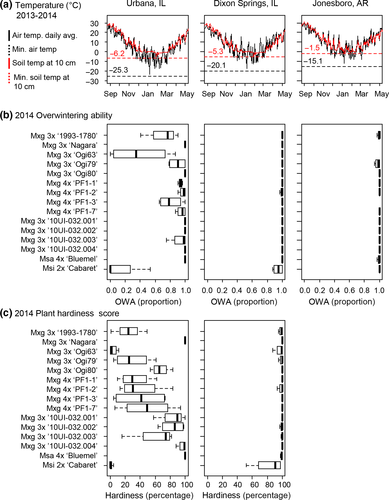
The first winter of the study (2013–2014) was especially cold in the northern US Midwest, with minimum air temperatures of −25.3°C in Urbana (the most northern of the three trial sites), −20.1°C at Dixon Springs, and −15.1°C at Jonesboro (Figure 1a). Moreover, minimum soil temperatures at 10 cm depth were −6.2°C at Urbana, −5.3°C at Dixon Springs, and −1.5°C at Jonesboro in January 2014 (Figure 1a). Consistent with the differences in minimum temperatures among the trial locations, fewer than half of the entries at Urbana had ≥0.95 proportion survival over the first winter, and two entries, M×g ‘Ogi63’ and M. sinensis ssp. condensatus ‘Cabaret’, had fewer than half their plants survive. In contrast to the losses at Urbana, at Dixon Springs, only ‘Cabaret’ had ≤0.95 proportion survival over the first winter, and at Jonesboro, all entries had ≥0.98 proportion survival (Figure 1b and Table 2). Similarly, hardiness scores of surviving plants averaged <70% for 10 out of 15 of the entries at Urbana, but were >98% for all entries at Dixon Springs and Jonesboro, except for M×g ‘Ogi63’ (96%) and M. sinensis ssp. condensatus ‘Cabaret’ (83%) at Dixon Springs (Figure 1c and Table 2). Thus, at Urbana, surviving plants of most entries were damaged and weak in the spring of 2014, whereas most plants of nearly all the entries were vigorous at Dixon Springs and Jonesboro.
| Entrya | Urbana, IL | Dixon Springs, IL | Jonesboro, AR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First-winter (2013–2014) OWA (Prpn) | First-winter (2013–2014) hardiness (%) | Year 2 (2014) yield (Mg/ha) | Year 3 (2015) yield (Mg/ha) | First-winter (2013–2014) OWA (Prpn) | First-winter (2013–2014) hardiness (%) | Year 2 (2014) yield (Mg/ha) | Year 3 (2015) yield (Mg/ha) | First-winter (2013–2014) OWA (Prpn) | Year 2 (2014) yield (Mg/ha) | Year 3 (2015) yield (Mg/ha) | |
| M×g 3× ‘1993-1780’ | 0.71 ± 0.11 | 25 ± 11.3 | 8.1 ± 1.7 | 16.2 ± 1.9 | 1.00 ± 0.00 | 98 ± 1.1 | 13.6 ± 1.2 | 21.6 ± 0.8 | 0.99 ± 0.01 | 6.1 ± 1.5 | 11.3 ± 1.2 |
| M×g 3× ‘Nagara’ | 1.00 ± 0.00 | 100 ± 0.1 | 19.7 ± 0.3 | 20.9 ± 1.0 | 1.00 ± 0.00 | 100 ± 0.0 | 17.7 ± 0.9 | 18.2 ± 1.6 | |||
| M×g 3× ‘Ogi63’ | 0.39 ± 0.21 | 4 ± 2.3 | 6.9 ± 2.1 | NA | 1.00 ± 0.00 | 96 ± 2.6 | 10.2 ± 1.5 | 9.7 ± 1.0 | |||
| M×g 3× ‘Ogi79’ | 0.90 ± 0.06 | 29 ± 10.9 | 11.7 ± 0.6 | 7.8 ± 1.5 | 1.00 ± 0.00 | 99 ± 1.1 | 9.7 ± 0.6 | 8.8 ± 0.6 | 0.98 ± 0.02 | 8.7 ± 1.0 | 11.5 ± 0.7 |
| M×g 3× ‘Ogi80’ | 1.00 ± 0.00 | 67 ± 5.4 | 16.0 ± 0.8 | 16.7 ± 0.8 | 1.00 ± 0.00 | 100 ± 0.0 | 13.2 ± 0.8 | 11.0 ± 0.3 | 1.00 ± 0.00 | 6.8 ± 1.6 | 9.6 ± 1.7 |
| M×g 4× ‘PF1-1’ | 0.94 ± 0.01 | 33 ± 9.5 | 13.3 ± 0.9 | 16.8 ± 0.5 | 1.00 ± 0.00 | 100 ± 0.0 | 16.0 ± 0.9 | 15.9 ± 1.2 | 1.00 ± 0.00 | 7.8 ± 1.3 | 12.2 ± 0.9 |
| M×g 4× ‘PF1-2’ | 0.97 ± 0.02 | 39 ± 13.4 | 10.6 ± 1.2 | 16.4 ± 1.1 | 0.99 ± 0.01 | 98 ± 1.4 | 12.9 ± 0.9 | 15.9 ± 0.9 | 1.00 ± 0.00 | 8.7 ± 2.0 | 11.1 ± 0.4 |
| M×g 4× ‘PF1-3’ | 0.80 ± 0.08 | 40 ± 16.3 | 10.4 ± 1.4 | 13.4 ± 1.1 | 1.00 ± 0.00 | 100 ± 0.0 | 15.4 ± 0.5 | 15.9 ± 0.9 | |||
| M×g 4× ‘PF1-7’ | 0.95 ± 0.03 | 50 ± 15.9 | 13.3 ± 0.6 | 17.9 ± 1.1 | 1.00 ± 0.00 | 100 ± 0.0 | 17.0 ± 0.5 | 16.7 ± 0.7 | 1.00 ± 0.00 | 9.4 ± 2.1 | 10.7 ± 0.4 |
| M×g 3× ‘10UI-032.001’ | 1.00 ± 0.00 | 84 ± 8.0 | 13.5 ± 0.5 | 16.9 ± 0.8 | 1.00 ± 0.00 | 100 ± 0.0 | 11.3 ± 1.5 | 15.4 ± 0.8 | 1.00 ± 0.00 | 8.0 ± 2.0 | 7.6 ± 1.1 |
| M×g 3× ‘10UI-032.002’ | 1.00 ± 0.00 | 84 ± 7.2 | 12.0 ± 1.0 | 14.7 ± 0.6 | 1.00 ± 0.00 | 99 ± 0.6 | 13.8 ± 0.5 | 13.3 ± 0.5 | 1.00 ± 0.00 | 8.2 ± 1.1 | 7.2 ± 1.1 |
| M×g 3× ‘10UI-032.003’ | 0.93 ± 0.06 | 61 ± 13.3 | 8.0 ± 0.7 | 12.0 ± 0.6 | 1.00 ± 0.00 | 100 ± 0.4 | 9.8 ± 0.5 | 9.1 ± 0.4 | 1.00 ± 0.00 | 8.0 ± 1.6 | 9.5 ± 1.6 |
| M×g 3× ‘10UI-032.004’ | 1.00 ± 0.00 | 97 ± 2.6 | 9.6 ± 1.0 | 10.0 ± 0.5 | 1.00 ± 0.00 | 100 ± 0.0 | 8.2 ± 0.6 | 6.8 ± 0.7 | 1.00 ± 0.00 | 5.8 ± 1.3 | 7.9 ± 0.4 |
| Msa 4× ‘Bluemel’ | 1.00 ± 0.00 | 100 ± 0.0 | 8.0 ± 0.5 | 13.8 ± 1.0 | 1.00 ± 0.00 | 99 ± 0.5 | 4.3 ± 0.6 | 8.8 ± 0.6 | 1.00 ± 0.00 | 1.7 ± 0.2 | 4.3 ± 0.5 |
| Msi 2× ‘Cabaret’ | 0.13 ± 0.13 | 1 ± 0.9 | 1.5 ± 0.0 | 1.4 ± 0.0 | 0.95 ± 0.03 | 83 ± 9.4 | 3.4 ± 0.3 | 7.3 ± 0.4 | 1.00 ± 0.00 | 3.9 ± 0.5 | 5.8 ± 0.7 |
Notes
- NA: not available, missing data.
- ‘Ogi63’, ‘Ogi79’, and ‘Ogi80’ were from wild-collected seeds harvested in Kyushu, Japan (Nishiwakiet al., 2011).
- PF series were from New Energy Farms (previously Pyramid Farms), Leamington, ON, Canada.
- Four entries were full-sibs from the cross 10UI-032 (M. sacchariflorus 4× ‘Bluemel’×M. sinensis ssp. condensatus ‘Cabaret’; the parents were included in the field trials as controls).
- ‘Nagara’, ‘Ogi63’, and ‘PF1-3’ were not planted in AR.
- a M×g: Miscanthus ×giganteus; Msa: Miscanthus sacchariflorus; Msi: Miscanthus sinensis.
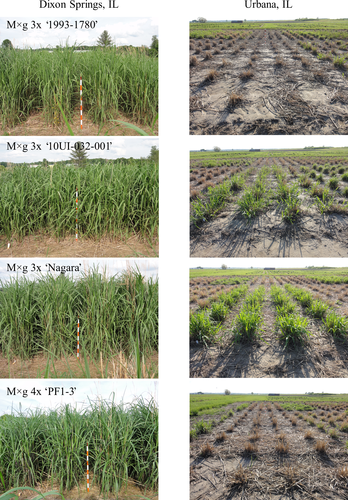
Notably, average proportion survival of the commercial triploid M×g standard, ‘1993-1780’, during the first winter (2013–2014) at Urbana, was only 0.71 and the hardiness score averaged only 25%, indicating that these plantings were severely damaged. In contrast to ‘1993-1780’, plots of the triploid M×g ‘Nagara’ were entirely undamaged (Figures 1 and 2 and Table 2). In this trial, only triploid M×g ‘10UI-032.004’ and its tetraploid M. sacchariflorus parent, ‘Bluemel’, survived the first winter in Urbana, comparable to that of ‘Nagara’ (Figures1b,c), which is remarkable considering that the other parent of the 10UI-032 cross, diploid M. sinensis ssp. condensatus ‘Cabaret’, was the least hardy entry in the study, with only 0.13 proportion survival and just 1% for its winter hardiness score (Figure 1b,c and Table 2). Interestingly, all four of the triploid M×g 10UI-032 full-sibs had high overwintering rates at Urbana (0.93–1.00), though their hardiness scores varied (61%–97%). Among the three triploid M×g Ogi series genotypes, collected as seed from wild plants in Kyushu in southern Japan (USDA hardiness zone 9b, average annual minimum air temperature of −1.1 to −3.9°C), one had low overwintering ability during the first winter at Urbana (‘Ogi63’: 0.39) but the other two entries had relatively high rates of winter survival (‘Ogi79’: 0.90; ‘Ogi80’: 1.00), yet hardiness scores indicated that all three were damaged to varying degrees (‘Ogi63’: 4%; ‘Ogi79’: 29%; ‘Ogi80’: 67%).
During the second winter of the study (2014–2015), minimum air temperatures were −26.4°C in Urbana (February 24, 2015, Supporting information Data S1) and −23.1°C at Dixon Springs (February 19, 2015), which were slightly lower than the first winter. At Jonesboro, minimum air temperature of the second winter was similar to the first winter (−14.7°C, 9 January 2015). However, minimum soil temperatures were higher at all three locations during the second winter than during the first winter: −3.8°C in Urbana (20 February 2015), −3.4°C at Dixon Springs (10 January 2015), and −0.7°C at Jonesboro (11 January 2015). In Dixon Springs and Jonesboro, all plants that were alive during the year 2 growing season also survived the second winter (Figure 3). In Urbana, only five entries (M×g ‘1993-1780’, M×g ‘Ogi79’, M×g ‘PF1-3’, M×g ‘PF1-7’, and M. sinensis ssp. condensatus ‘Cabaret’) had additional losses during the second winter (Figure 3), which was consistent with the warmer second winter, and better-established plants going into the second winter relative to the first winter.
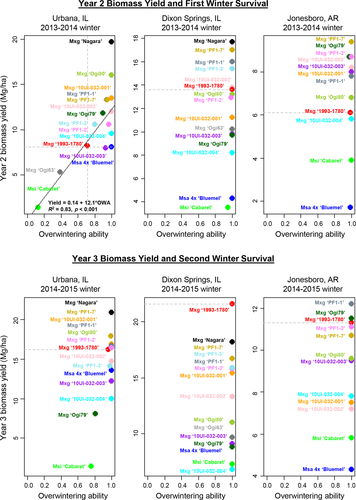
3.2 Biomass yield
The most winter-hardy M×g genotype, ‘Nagara’, produced the highest biomass yield at Urbana, with 19.7 dry Mg/ha in 2014 (year 2) and 20.9 Mg/ha in 2015 (year 3), whereas the severely winter-damaged M×g ‘1993-1780’ yielded only 8.1 Mg/ha in 2014 and 16.2 Mg/ha in 2015. At Urbana, ten M×g genotypes produced higher biomass yield than ‘1993-1780’ in year 2 (2014) and six entries yielded more than this control in year 3 (2015) (Figure 3 and Table 2). As indicated in Table 1, the biomass yield estimates in the main text were corrected for number of live plants in each plot, which can bias estimates upward if there are missing plants. Nevertheless, corrected yields for most entries that had lost many plants during the first winter at Urbana were much lower than expected for this location, and lower than in Dixon Springs, where few or no plants were lost over the winter (Figure 3 and Table 2). Thus, the yield estimates corrected for number of live plants are conservative assessments of yield reductions in plots that suffered stand losses over the winter, and these primarily represent reduced yields associated with lower vigor of the surviving plants. Estimates of biomass yield that were uncorrected for the number of missing plants per plot are provided in Supporting information Table S5; though uncorrected estimates can be biased downward, in plots that suffered stand losses, these primarily represent reductions in yield due to both loss of stand and reduced vigor of the surviving plants.
At Dixon Springs, where all M×g genotypes survived and regrew vigorously, five M×g genotypes including ‘Nagara’ produced higher biomass yield than ‘1993-1780’ in year 2 (2014), but none outperformed this commercial control in year 3 (2015; Figure 3 and Table 2). Nevertheless, in year 3 (2015) at Dixon Springs, yield of ‘Nagara’ (18.2 Mg/ha) did not differ significantly from that of ‘1993-1780’ (21.6 Mg/ha; Table 2). At Jonesboro, ‘Nagara’ was not included in the field trial, yet eight M×g genotypes outperformed ‘1993-1780’ in year 2 (2014) and two outyielded ‘1993-1780’ in year 3 (2015) (Figure 3 and Table 2). In Jonesboro, the highest yielding triploid M×g genotype in both year 2 (8.7 Mg/ha) and year 3 (11.5 Mg/ha) was ‘Ogi79’, yet this genotype was among the lowest yielding at Dixon Springs and Urbana (Figure 3 and Table 2). Though genotype × location interactions were highly significant, year 3 yields at Jonesboro were on average substantially lower than at Dixon Springs (Table 2). Of the genotypes tested at both locations, all of the seven highest yielding genotypes at Dixon Springs had substantially lower yields at Jonesboro (Table 2).
Interestingly, the cold-hardy parent of the four M×g full-sibs (10UI-032 series), M. sacchariflorus ‘Bluemel’, had greater year 3 biomass yield than the cold-sensitive male parent M. sinensis ‘Cabaret’ at only the northern locations, Urbana and Dixon Springs. However, at the southern location, Jonesboro, M. sinensis ‘Cabaret’ had overwintered well and outyielded M. sacchariflorus ‘Bluemel’ (Figure 3 and Table 2). Two of the full-sibs, ‘10UI-032.001’ and ‘10UI-032.002’, yielded more than either of their parents in year 3 at all three locations, indicating that they inherited the best characteristics of their divergently adapted parents (Figure 3 and Table 2).
3.3 Correlations between traits
At Urbana, where there was substantial variation in overwintering ability among the 13 M×g genotypes, we observed a strong positive genetic correlation between first winter (2013–2014) overwintering ability and hardiness score (0.81; Table 3). Heading date during the first growing season (2013) had a moderate genetic correlation with first winter hardiness score at Urbana (0.61; i.e., later flowering was associated with greater winter hardiness), but first-season plant height and culm number had negligible genetic correlations with first-winter hardiness score (Table 3). Biomass yield in year 2 (2014) at Urbana had moderate positive genetic correlations with first-winter overwintering ability (0.59; Table 3) and hardiness score (0.50). Notably, by limiting the correlation analysis to the nine entries that exhibited overwintering losses during the first winter at Urbana (OWA < 1.0; Table 2), a strong positive correlation was observed between year 2 (2014) yield and the first-winter (2013–2014) overwintering ability among these entries (R2 = 0.83; Figure 3). However, by the third year (2015), biomass yield at Urbana had only negligible genetic correlations with first-winter overwintering ability and hardiness score. Nevertheless, year 3 yields of the commercial standard M×g ‘1993-1780’ at Urbana (16.2 Mg/ha in 2015) were substantially lower than that observed in previous trials established during years with milder winters, when all plants overwintered successfully (23.0 Mg/ha in 2012, Kaiser et al., 2015). At Dixon Springs and Jonesboro, all M×g genotypes survived the first winter without damage. As such, estimates of correlations between overwintering ability or hardiness with other traits were possible only for the Urbana trial.
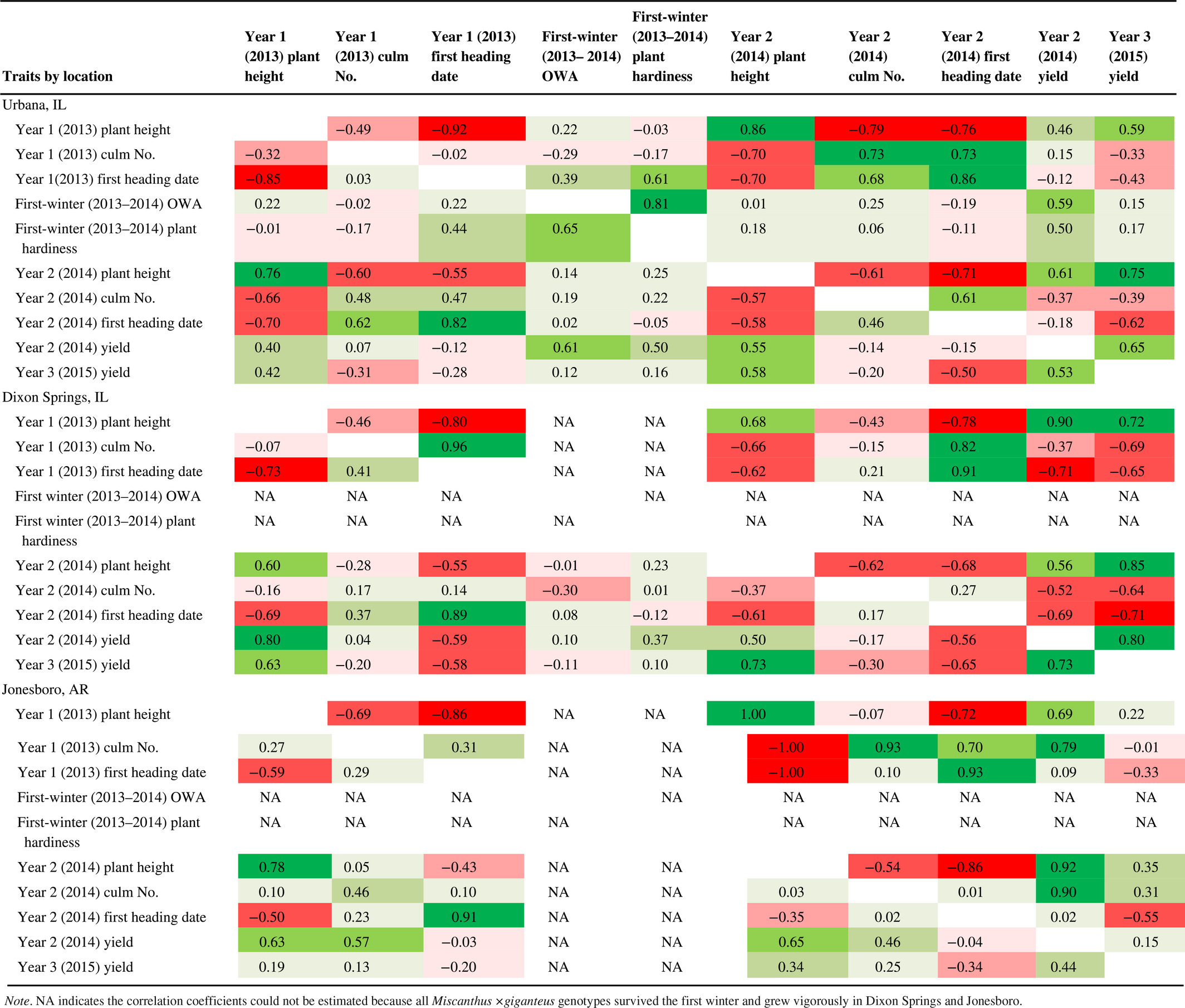
Plant height in year 2 (2014) had moderate to strong positive genetic correlations with year 2 yield at all locations (0.56–0.92) and with year 3 yield at Urbana and Dixon Springs (0.75 and 0.85, respectively); for Jonesboro, the genetic correlation between height and yield in year 3 was low (0.35). Genetic correlations between culm number in year 2 with yield in years 2 or 3 were negative and low in Urbana (−0.37 and −0.39 respectively), and negative but moderate in Dixon Springs (−0.52 and −0.64, respectively); in Jonesboro, the correlation was high but positive with year 2 yield (0.90) and low but positive with year 3 yield (0.31; Table 3). Most of the M×g genotypes evaluated produced more culms per plant than ‘1993-1780’ in year 2, with some entries exceeding twice the number of culms as the control cultivar (Supporting information Table S4). Year 2 heading date had moderate to high negative genetic correlations with year 2 plant height (−0.68 to −0.86) and year 3 yield (−0.55 to −0.71) at all three locations (Table 3), indicating that earlier flowering was associated with the taller and more productive M×g genotypes.
4 DISCUSSION
Most of the 13 M×g genotypes evaluated at Urbana were severely damaged (i.e., low survival rates or low hardiness scores for surviving plants) during their first winter (2013–2014), whereas at the warmer and more southerly trial locations of Dixon Springs and Jonesboro, nearly all of the genotypes were undamaged (Table 2 and Figures 1 and 2). M×g ‘1993-1780’ is currently the only genotype of Miscanthus grown commercially for biomass production in North America and Europe (Głowacka et al., 2015; Hodkinson et al., 2002; Lewandowski et al., 2016), yet first-year plants of this cultivar were maladapted to the 2013–2014 winter in Urbana, with an overwintering rate of only 0.71 and a hardiness score of just 25%. As a result of stand-losses during the first winter at Urbana, biomass yield of ‘1993-1780’ was low in year 2, with only 8.1 Mg/ha, and unable to fully recover, with yield of only 16.2 Mg/ha in year 3 (Table 2 and Figures 1-3). Commercial growers would consider this lackluster performance to be economically untenable because large gaps in a field would reduce yields, promote weeds, and be difficult to fill with replacement plants. Such a field would need to be plowed under and replanted, which would be a costly loss.
In most years at Urbana, new plantings of M×g ‘1993-1780’ survive well with little or no damage during the first winter. In our previous studies of M×g ‘1993-1780’ at Urbana, when temperatures were warmer than those observed during the current study, we observed no stand losses during the first winters, and third year yields were 7–8 Mg/ha greater than during the current study (Dong et al., 2018a; Kaiser et al, 2015). In contrast to the first-year plants in this study, in a parallel study (Dong et al., 2018b) over the cold 2013–2014 winter at Urbana, we observed that third-year plants of M×g ‘1993-1780’ had a high survival rate, but hardiness scores indicated that the plants had been damaged, with relatively few and weak late-emerging shoots. However, the third-year plants of M×g ‘1993-1780’ subsequently recovered by June (Dong et al., 2018b), confirming that mature field plantings of Miscanthus are more resilient to low-temperature stresses during the winter than first-year plants.
Previous controlled-environment studies have estimated the temperature at which 50% of isolated M×g ‘1993-1780’ rhizomes are killed (LT50) as ranging from −2.6°C to −4.4°C (Clifton-Brown & Lewandowski, 2000; Fonteyne et al., 2016; Peixoto, Friesen, & Sage, 2015), which might have led one to predict lower survival rates at the Urbana and Dixon Springs locations than was observed based on the minimum temperatures at 10 cm below bare soil for each site (−6.2 and −5.3°C at Urbana and Dixon Springs, respectively). However, this apparent discrepancy between results from the laboratory and the field could be accounted for by the insulation of belowground rhizomes from the plant's aboveground crown, and/or avoidance of cold by rhizomes that grew deep in the soil (i.e., more than 10 cm belowground). For example, under sod, the minimum temperature during the 2013–2014 winter at Urbana was only −2.2°C at 10 cm and −1.4°C at 20 cm. Regional modeling of soil temperatures indicated that large areas of the US Midwest can expect temperatures between −3.5 and −6.0°C at 10 cm below bare soil in most years (Kucharik et al., 2013), suggesting that first-year plantings of M×g ‘1993-1780’ would be at risk for winterkill or injury in this region and those with similar climates. Results of the current and previous field studies support the conclusion that insufficient winter hardiness for the establishment of M×g ‘1993-1780’ is an intermittent problem in hardiness zone 5 (average annual minimum air temperature of −23.3 to −28.9°C), and a consistent problem in hardiness zone 4 (average annual minimum air temperature of −28.9 to −34.4°C) and lower hardiness zones (Clifton-Brown & Lewandowski, 2000; Heaton et al., 2008; Jørgensen et al., 2003; Lewandowski et al., 2000). In order for cultivation of M×g in cold temperate environments to be commercially viable, greater winter hardiness is needed than found in the M×g ‘1993-1780’ genotype, which currently accounts for nearly all Miscanthus biomass area in North America and Europe (Głowacka et al., 2015; Hodkinson et al., 2002; Lewandowski et al., 2016).
Fortunately, two triploid M×g genotypes, ‘Nagara’ and ‘10UI-032.004’, and the tetraploid M. sacchariflorus ‘Bluemel’, a parent of ‘10UI-032.004’, survived fully and undamaged from the severe winter of 2013–2014 at Urbana (Table 2 and Figures 1-3). In particular, M×g ‘Nagara’ produced significantly higher biomass yield than M×g ‘1993-1780’ in both year 2 (19.7 Mg/ha in 2014) and year 3 (20.9 Mg/ha in 2015) at Urbana (Table 2 and Figure 3), suggesting ‘Nagara’ might be a better choice than ‘1993-1780’ for biomass production in hardiness zone 5 or colder; moreover, these high yields by ‘Nagara’ were similar to what we would have expected from ‘1993-1780’ had the winter been mild in Urbana, and were similar to the yields observed for both entries at Dixon Springs, where ‘1993-1780’ was undamaged by the first winter. Additionally, Dierking, Allen, Brouder, and Volenec (2016) observed that ‘Nagara’ had higher yields than ‘1993-1780’ in KY and the two genotypes yielded similarly in IN. Thus, these results demonstrate that it is possible to select and breed triploid M×g that has both superior winter hardiness than M×g ‘1993-1780’ and high-yield potential.
Some of the four tetraploid PF lines in this study exhibited great overwintering ability and biomass yield potential compared with M×g ‘1993-1780’, especially at Urbana trial (Figure 3 and Table 2). However, the potential risk of invasiveness via seed production from these fertile tetraploid M×g genotypes warrants care. Because Miscanthus is self-incompatible, if a single fertile genotype were to be planted as a monoculture, we would not expect such a planting to be a great risk for seed-production and dispersal. Moreover, when plant breeders have successfully selected Miscanthus populations that are fixed for nonshattering, then it should be possible to deploy fertile Miscanthus cultivars by direct-seeded plantings in production fields without risk of invasiveness, as this hallmark domestication trait will greatly limit the potential for seed dispersal. Until nonshattering cultivars are developed, however, we do not advise commercial release and production of fertile biomass cultivars of Miscanthus in environments where they are likely to generate viable seed and seedlings.
Substantial variation in overwintering ability and winter hardiness of M×g can be obtained by selection of parents and within M×g populations. The tetraploid M. sacchariflorus parent of ‘Nagara’, 93M0005064, was collected from along the banks of the Nagara River in Gifu Prefecture, Japan, and its M. sinensis parent was also of Japanese provenance but selected for adaptation to Saxony-Anhalt, Germany (hardiness zones 7b, average annual minimum air temperature of −15.0 to −12.2°C; Deuter, 2011). In contrast, Clifton-Brown et al. (2001) observed first-winter (1997-1998) survival for another tetraploid M. sacchariflorus collected along the Nagara River in Gifu Prefecture, EMI no. 5, to be only 50% in Sweden and 33% Denmark. Among the four full-sib progeny of the 10UI-032 M×g family, overwintering ability was uniformly high (0.93–1.00), but hardiness score varied from 61% to 97% (Table 2 and Figure 1), demonstrating substantial variation for adaptation to the 2013–2014 winter in Urbana within a single cross. One parent of the 10UI-032 cross was tetraploid M. sacchariflorus ‘Bluemel’, which Clark et al. (2018) determined belongs to the southern Japan genetic group, yet was one of the few fully hardy genotypes in the current study (overwintering: 1.00; hardiness: 100%). The other parent of 10UI-032 was M. sinensis ssp. condensatus ‘Cabaret’, which is indigenous to maritime southern Japan (hardiness zones 9b to 10a; average annual minimum air temperature of −3.9 to 1.7°C), was the least hardy genotype in this study (overwintering: 0.13; hardiness: 1% at Urbana; Table 2 and Figure 1). Given the great difference between the parents, and the high level of overwintering observed in all four 10UI-032 progeny, we conclude that alleles for winter survival came primarily from the tetraploid M. sacchariflorus parent and were dominant and/or additively exceeded a threshold needed to survive the 2013–2014 winter in Urbana. Similarly, hardiness score in the 10UI-032 progeny was skewed toward the tolerant tetraploid M. sacchariflorus parent (Table 2 and Figure 1c). Among the three triploid M×g Ogi series genotypes, which were collected as seed from wild plants in Kyushu, Japan (USDA hardiness zone 9b, average annual minimum air temperature of −3.9 to −1.1°C), overwintering and hardiness in Urbana ranged as low as 0.39% and 4%, respectively, for ‘Ogi63’, but for ‘Ogi80’, all plants survived and the hardiness score was a relatively high 67%, demonstrating that wild unselected M. sacchariflorus and M. sinensis plants from subtropical southern Japan can sometimes produce progeny with substantially greater winter hardiness than might be expected (Table 2 and Figure 1). Taken together, data from the current and prior studies suggest that there is considerable genetic variation for overwintering ability among and within M. sacchariflorus from Japan that can be exploited to breed more winter-hardy M×g. Moreover, there exist wild populations of M. sacchariflorus and M. sinensis that are adapted to natural environments with much colder winters (lower hardiness zones) than in Japan (Clark et al., 2014, 2015, 2016). Consequently, these can also be used to further improve winter hardiness of M×g.
Genetic correlations between biomass yield and other traits also have implications for future Miscanthus breeding. The positive correlations between overwintering ability and biomass yield were expected, especially among entries that exhibited overwintering losses (Figure 3). Both of these traits will be important for developing new M×g cultivars that are well-adapted to temperate regions with cold winters. Moreover, such genetic correlations between these two important traits could accelerate the breeding of improved cultivars. Intriguingly, a later first heading date was found to be correlated with better overwintering ability (0.39) and plant hardiness (0.61), suggesting an opportunity for indirect selection as early as the end of the first season.
In contrast to previous studies (Lim et al., 2014; Zhao et al., 2013), we observed that first heading date was negatively correlated with biomass yield and plant height across all three field trials (Table 3). Perhaps high-yielding M×g genotypes such as ‘Nagara’ might have a more rapid growth rate than other previously studied Miscanthus species, enabling the M×g genotypes to grow tall and accumulate biomass early in the growing season. In addition, plants often flower when they reach a certain size (Adds, Larkcom, & Miller, 2004; de Jong & Klinkhamer, 2005). Such a phenomenon was reflected in plant height in this study, in that the M×g genotypes that flagged earlier in this study were also taller relative to other M×g plants (Table 3). Under the high-vigor hypothesis, early flowering M×g genotypes would be desirable to the extent that this trait is associated with high-yield potential, but this would need to be balanced with the need to select for high overwintering ability in cold winter target environments.
The three field trials in our study demonstrated that considerable genotypic variation and genotype × environment interaction variation exist for overwintering ability and biomass yield in M×g. In particular, this study documented large phenotypic differences among triploid M×g entries that have been shown via molecular markers to differ genetically. Notably, some newly developed M×g genotypes exhibited substantially better overwintering ability than the commercial cultivar M×g ‘1993-1780’. In particular, M×g ‘Nagara’ had superior overwintering ability to M×g ‘1993-1780’ in Urbana, yet similarly high-yield potential in Dixon Springs. Dierking et al. (2016) also found that M×g ‘Nagara’ has high-yield potential. Thus, in USDA zone 5 or lower, ‘Nagara’ may be a safer choice for growers than M×g ‘1993-1780’, considering the high frequency of cold winters in these regions. The large differences we observed for overwintering ability among M×g genotypes have important implications for the development of Miscanthus as a biomass crop overall. Our finding that M×g ‘Nagara’ is substantially more winter-hardy than M×g ‘1993-1780’ (syn. ‘Illinois’) opens up large areas of the northern US Midwest, New England, Canada, and northern Europe to the opportunity for commercial biomass production of Miscanthus. Additionally, genetic variation for winter hardiness among M×g and within its parental species, M. sacchariflorus and M. sinenis, indicates that it will be feasible to breed new, improved M×g cultivars that are better adapted to the upper Midwest USA and other regions with similarly cold winters.
ACKNOWLEDGEMENTS
This work was supported by the Energy Biosciences Institute, HATCH project ILLU-802-311, the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2018-68005-27937, and the DOE Office of Science, Office of Biological and Environmental Research (BER), grant nos. DE-SC0006634 and DE-SC0012379. We thank Ben Baechle, Matt Conaster, and Jami Nash for assistance with the field trials. We thank New Energy Farms for providing the PF series M×g entries.




