A time for every season: soil aggregate turnover stimulates decomposition and reduces carbon loss in grasslands managed for bioenergy
Abstract
A primary goal of many next-generation bioenergy systems is to increase ecosystem services such as soil carbon (C) storage and nutrient retention. Evaluating whether bioenergy management systems are achieving these goals is challenging in part because these processes occur over long periods of time at varying spatial scales. Investigation of microbially mediated soil processes at the microbe scale may provide early insights into the mechanisms driving these long-term ecosystem services. Furthermore, seasonal fluctuations in microbial activity are rarely considered when estimating whole ecosystem functioning, but are central to decomposition, soil structure, and realized C storage. Some studies have characterized extracellular enzyme activity within soil structures (aggregates); however, seasonal variation in decomposition at the microscale remains virtually unknown, particularly in managed ecosystems. As such, we hypothesize that temporal variation in aggregate turnover is a strong regulator of microbial activity, with important implications for decomposition and C and nitrogen (N) storage in bioenergy systems. We address variation in soil microbial extracellular enzyme activity spatially across soil aggregates and temporally across two growing seasons in three ecosystems managed for bioenergy feedstock production: Zea mays L. (corn) agroecosystem, fertilized and unfertilized reconstructed tallgrass prairie. We measured potential N-acetyl-glucosaminidase (NAG), β-glucosidase (BG), β-xylosidase (BX), and cellobiohydrolase (CB) enzyme activity. Aggregate turnover in prairie systems was driven by precipitation events and seasonal spikes in enzyme activity corresponded with aggregate turnover events. In corn monocultures, soil aggregates turned over early in the growing season, followed by increasing, albeit low, enzyme activity throughout the growing season. Independent of management system or sampling date, NAG activity was greatest in large macroaggregates (>2000 μm) and CB activity was greatest in microaggregates (<250 μm). High microbial activity coupled with greater aggregation in prairie bioenergy systems may reduce loss of soil organic matter through decomposition and increase soil C storage.
Introduction
Bioenergy production has been heralded as a means to develop and implement perennial agroecosystems which could potentially meet multiple land-use goals including harvestable (and marketable) bioenergy feedstock, reduction of soil erosion, increased biodiversity, and retention of soil carbon (C) and nutrients including nitrogen (N) (Tilman et al., 2009). Reconstructed, multispecies, native grasslands have gained interest as bioenergy feedstock production may provide additional incentive to integrating the critically endangered tallgrass prairie ecosystem into a working agricultural landscape (Fargione et al., 2009). Recent work has indicated that perennial systems, including native multispecies grasslands, can provide more ecosystem services, including soil C retention, than annual row-crop systems (Robertson et al., 2011; Gelfand et al., 2013). However, there is a tremendous amount of variability between studies in the degree to which these prairie systems deliver desired ecosystem services (Brye et al., 2002; Culman et al., 2010; James et al., 2010; Jarchow et al., 2015). If perennial bioenergy systems are to be utilized strategically in the landscape, it is critical to understand the ecological mechanisms responsible for long-term changes in soil ecosystem processes (Tiemann & Grandy, 2015).
As the drivers of decomposition, soil microorganisms are at the center of untangling variation in ecological services provided by perennial bioenergy systems. Microorganisms drive the transfer of energy and nutrients from decaying organic material back into the living components of ecosystems (Falkowski et al., 2008). In soil, the heterogeneous distribution of organic matter, microorganisms, extracellular enzymes, water, and redox potential regulates ecosystem-level decomposition and nutrient mineralization (Ettema & Wardle, 2002). However, most field studies of soil processes driven by microorganisms do not consider microbe-scale spatial and temporal distributions of resources that directly influence microbial processes. For example, structural homogenization of soil samples and extrapolation of soil microbial activity measured once or twice in a growing season may limit estimates and predictions of microscale factors that affect C and N storage within and between bioenergy cropping systems. Incorporation of spatial and temporal variation in soil conditions is essential due to its profound influence on the microbial habitat and the realized microbial activities that drive the cycling of carbon (C) and nutrients, including nitrogen (N) at the ecosystem scale.
Investigating factors affecting soil microbial activity in bioenergy feedstock production systems is critical to understanding and quantifying long-term processing and storage of soil organic matter. Soil organic matter storage and cycling is regulated in part by microbial communities that obtain nutrients and energy by excreting extracellular enzymes into their environment, and absorbing the products of the enzymatic reaction. Many extracellular enzyme assays have been developed for the laboratory, enabling scientists to examine a suite of enzymes involved in decomposition of common plant tissue components including cellulose, hemicellulose, chitin, and lignin (Tabatabai & Bremner, 1969; Marx et al., 2001; Sinsabaugh, 2010). These assays have been used to measure plant litter decomposition rates (Sinsabaugh et al., 1993) and quantify linked nutrient cycles (Schimel & Weintraub, 2003; Sinsabaugh et al., 2008). When considered together, enzymes activities can be integrated to examine the feedback between the environment and microbial metabolism driving C and N cycling in soils (Sinsabaugh et al., 2009). Extracellular enzyme activity responds to shifts in climate patterns (Brzostek et al., 2012; Zeglin et al., 2013), ecosystem management (Štursová & Baldrian, 2011; Bowles et al., 2014), and nutrient addition (Allison et al., 2010; Mineau et al., 2014). However, only a few previous studies have measured soil enzyme activity at a scale more appropriate for microorganisms that produce these enzymes (Allison & Jastrow, 2006; Dorodnikov et al., 2009; Bailey et al., 2012, 2013) and only a couple in managed agroecosystems (Schutter & Dick, 2002; Lagomarsino et al., 2012).
Soil structure is a major component of soil C protection because it influences microbial access to soil organic carbon. In perennial grass production systems, soil structure is less disturbed than row-crop systems, which along with abundant roots and mycorrhizal associations can promote soil aggregation (Angers & Caron, 1998; Jastrow et al., 1998), a critical component of soil structure. Soil aggregates influence soil organic matter storage and decomposition in many ways. Organic matter becomes physically enmeshed within larger aggregates and therefore protected from decomposition by soil organisms outside the aggregate (Six et al., 2000). Within macroaggregates, organic matter and soil particles fuse, forming microaggregates. Macroaggregates naturally turn over in soils, falling apart in response to major rainfall events, freeze–thaw cycles, and reforming through physical and chemical forces from roots, fungi, earthworms, and bacteria (Oades, 1993). In contrast, water-stable microaggregates can persist for decades to centuries (Jastrow, 1996). Soil aggregate turnover can lead to fluctuations in bulk density on short timescales, which influences not only microbial access to organic matter, but also calculations of soil C storage (Lee et al., 2009). Macroaggregate disruption releases formally protected organic matter to decomposition by extracellular enzymes and uptake by plants and microorganisms. From a microbial perspective, the dynamics of soil macroaggregate turnover result in dramatic changes in available substrate, and gas and water diffusion, which influence ecosystem-level cycling of C and N (Sollins et al., 1996; Schimel & Schaeffer, 2012).
Evaluations of cycling and storage of soil C and N in bioenergy systems can be strongly influenced by seasonal variation (Brye et al., 2002). This is likely due in part to fluctuations in soil microbial biomass and activity across the temperate cycling of seasons (Waldrop & Firestone, 2006; Yao et al., 2011). Observations from alpine and forest ecosystems support a generalized model of temporal partitioning of N assimilation and mineralization between microbes and plants (Schmidt et al., 2007). Mechanisms contributing to seasonal variation in microbial nutrient and C cycling include microbial community succession (Monson et al., 2006), physiological adjustments (Hargreaves & Hofmockel, 2014), and availability of plant litter and root exudates (Kaiser et al., 2011). However, previous work has not explicitly considered the role of soil physical habitat, including changes in habitat, in these temporal patterns. In addition to physical habitat constraints, resources such as energy (e.g., C) and nutrients also fluctuate seasonally. In managed systems in general and bioenergy systems specifically, nutrients are often added to increase aboveground plant production. Thus, inorganic fertilizer additions, often performed early in the growing season, can also affect seasonal availability of nutrients such as N and associated cycling of C. Explicit understanding of spatial and temporal controls on microbial cycling of coupled C and N in agroecosystems and managed grasslands will further improve our understanding of when plant and microbes immobilize nutrients, optimizing primary production in bioenergy systems and concomitantly reducing loss of nutrients to surrounding aquatic ecosystems.
In this study, we examined spatial and temporal drivers of soil extracellular enzyme activity in three ecosystems managed for aboveground biomass production at a single experimental site: Zea mays L. (corn) row-crop agroecosystem, restored diverse tallgrass prairie, and fertilized restored tallgrass prairie. These three systems represent high N, low C inputs in corn; moderate N, high C inputs in fertilized prairie; and low N, high C inputs in unfertilized prairie. Row-crop agroecosystems are also less aggregated than restored prairies (Bach et al., 2010), allowing comparison of soil physical habitat among the management systems. We hypothesized that unfertilized prairie systems would support highest C and N cycling enzyme activity, which would peak late in the growing season due to greater root inputs and plant senescence (Dietzel, 2014). Nitrogen addition in fertilized prairies would suppress extracellular enzyme activity overall (Ramirez et al., 2012) and shift seasonal peaks to earlier in the season due to greater cool-season (C3) plant cover (Jarchow & Liebman, 2013). Corn systems were expected to have the lowest extracellular enzyme activity, peaking at plant senescence due to minimal root biomass and high concentrations of inorganic N. Among soil aggregates, we predicted that extracellular enzyme activity would reflect fresh inputs with greatest activity in large macroaggregates (>2000 μm) and least in microaggregates (<250 μm).
Materials and methods
Study site
Soil was collected during the growing seasons of 2011 and 2012 from the Iowa State University Comparison of Biofuel Systems (COBS) experimental site in Boone County, IA [41°55′14.42″N, 93°44′58.96″W; refer to Jarchow & Liebman (2013)]. Soils consisted of loams in the Nicollet (fine-loamy, mixed, superactive, mesic Aquic Hapludoll) and Webster (fine-loamy, mixed, superactive, mesic Typic Endoaquoll) series with <3% slope. Sand content ranged from 27% to 53% across the site, and clay content ranged from 17% to 32%. We sampled three experimental bioenergy production treatments: no-till continuous corn (Zea mays L.; fertilization rates determined by spring soil nutri-ent analysis), planted tallgrass prairie, and fertilized planted tallgrass prairie (84 Kg N ha−1 yr−1). Both planted prairie systems were planted in 2008 with the same seeding mixture of 31 native species and are harvested annually for bioenergy feedstock production. Four replicate blocks contain one 27 × 61 m plot of each planting treatment in a randomized complete block design (total n = 12). In 2011, the COBS site received 457 mm of precipitation between May and August, with 70% falling in May and June. Precipitation in May and June was 25 mm and 50 mm greater than the 60-years average, respectively. Precipitation in July and August was 50% and 83% of the 60-years average. In 2012, only 250 mm of precipitation fell between May and August, and an additional 88 mm fell in September and October. This was half of the 60-years mean precipitation in all months, except October, which was only 5% less than the long-term mean. Soil physical properties at each sampling date are reported in Table 1.
| Ecosystem | Gravimetric water content | pH | Soil temperature (°C) | Bulk density (g soil cm−3) | Aggregate mean weighted diameter (mm) |
|---|---|---|---|---|---|
| Sampling date | |||||
| Corn | |||||
| 2011-May | 0.21 ± 0.01 | 6.5 ± 0.1B | 16.8 ± 0.3 | 1.57 ± 0.03 | 3.7 ± 0.1A |
| 2011-June | 0.12 ± 0.01 | 6.4 ± 0.1B | 18.6 ± 0.3 | 1.66 ± 0.03 | 3.7 ± 0.1A |
| 2011-July | 0.13 ± 0.01 | 6.6 ± 0.1B | 22.5 ± 0.3 | 1.39 ± 0.03 | 2.6 ± 0.1B |
| 2011-August | 0.18 ± 0.01 | 7.1 ± 0.1A | 21.5 ± 0.3 | 1.43 ± 0.03 | 2.9 ± 0.1B |
| 2012-May | 0.17 ± 0.01A | 5.9B | 15.9 ± 0.9 | 1.21 ± 0.04 | 3.2 ± 0.1 |
| 2012-July | 0.12 ± 0.01BC | 6.5AB | 21.8 ± 0.2 | 1.16 ± 0.04 | 2.9 ± 0.1 |
| 2012-August | 0.11 ± 0.01C | 6.6A | 19.3 ± 0.1 | 1.12 ± 0.04 | 3.0 ± 0.1 |
| 2012-September | 0.11 ± 0.01C | 6.7A | 13.9 ± 0.6 | 1.24 ± 0.04 | 2.8 ± 0.1 |
| 2012-October | 0.16 ± 0.01AB | 6.6A | 1.22 ± 0.04 | 2.9 ± 0.1 | |
| Fertilized prairie | |||||
| 2011-May | 0.19 ± 0.01 | 7.2 ± 0.1 | 14.1 ± 0.1 | 1.51 ± 0.03 | 3.6 ± 0.1B |
| 2011-June | 0.12 ± 0.01 | 7.2 ± 0.1 | 17.6 ± 0.2 | 1.64 ± 0.03 | 4.3 ± 0.1A |
| 2011-July | 0.16 ± 0.01 | 7.1 ± 0.1 | 20.8 ± 0.1 | 1.26 ± 0.03 | 3.2 ± 0.1B |
| 2011-August | 0.19 ± 0.01 | 7.3 ± 0.1 | 20.6 ± 0.2 | 1.30 ± 0.03 | 3.3 ± 0.1B |
| 2012-May | 0.11 ± 0.01B | 7.1 | 16.0 ± 0.3 | 0.89 ± 0.04B | 3.4 ± 0.1 |
| 2012-July | 0.15 ± 0.01AB | 7.4 | 21.3 ± 0.3 | 1.10 ± 0.04A | 3.4 ± 0.1 |
| 2012-August | 0.13 ± 0.01B | 7.3 | 18.9 ± 0.2 | 1.06 ± 0.04AB | 3.7 ± 0.1 |
| 2012-September | 0.11 ± 0.01B | 7.2 | 14.2 ± 0.4 | 1.05 ± 0.04AB | 3.4 ± 0.1 |
| 2012-October | 0.19 ± 0.01A | 7.5 | 1.15 ± 0.04A | 3.5 ± 0.1 | |
| Prairie | |||||
| 2011-May | 0.19 ± 0.01 | 7.4 ± 0.1 | 15.1 ± 0.2 | 1.54 ± 0.03 | 3.4 ± 0.1B |
| 2011-June | 0.12 ± 0.01 | 7.2 ± 0.1 | 18.6 ± 0.2 | 1.64 ± 0.03 | 4.1 ± 0.1A |
| 2011-July | 0.15 ± 0.01 | 7.0 ± 0.1 | 23.2 ± 0.3 | 1.27 ± 0.03 | 2.8 ± 0.1B |
| 2011-August | 0.17 ± 0.01 | 7.3 ± 0.1 | 21.4 ± 0.3 | 1.39 ± 0.03 | 2.8 ± 0.1B |
| 2012-May | 0.10 ± 0.01C | 7.6 | 17.3 ± 0.4 | 0.99 ± 0.04B | 3.1 ± 0.1 |
| 2012-July | 0.15 ± 0.01AB | 7.4 | 22.9 ± 0.4 | 1.20 ± 0.04A | 3.2 ± 0.1 |
| 2012-August | 0.12 ± 0.01BC | 7.3 | 20.1 ± 0.4 | 1.13 ± 0.04AB | 3.2 ± 0.1 |
| 2012-September | 0.11 ± 0.01BC | 7.2 | 14.6 ± 0.5 | 1.16 ± 0.04AB | 3.1 ± 0.1 |
| 2012-October | 0.18 ± 0.01A | 7.3 | 1.19 ± 0.04A | 3.3 ± 0.1 | |
- Soil temperature is average of temperature at 5 cm and 10 cm below soil surface. Different letters denote statistical difference between means of sampling date within a management system in each year (α = 0.05). Plain text letters represent differences within the corn management system, bold letters represent differences within the fertilized prairie system, and italic letters represent differences within the unfertilized prairie system.
Soil sampling
Soils were sampled from the top 10 cm of soil using a 5.5-cm-diameter slide-hammer soil coring device (Giddings Machine Company, Windsor, CO, USA). Three intact soil cores were collected from each plot at each sampling time and placed in plastic bags, stored on ice, and transported to the laboratory. The equivalent dry mass of each core was used to calculate bulk density. Each core was gently broken up along natural points of weakness and passed through an 8-mm sieve, removing large roots and rocks. Replicated cores were combined into one composite sample for each plot. A 10-g subsample of soil was removed immediately and dried at 105 °C for 24 h to determine field fresh gravimetric water content. The remaining soil was prepared for soil aggregate isolation utilizing an optimal moisture approach to standardize soil moisture content and minimize disturbance to microbial communities (Bach & Hofmockel, 2014). Briefly, sieved soils were placed in closed, sterilized plastic containers and dried to approximately 10% gravimetric water content at 4°C and subjected to the following fractionation procedure.
Aggregate fractionation
Approximately 500 g of cold-dried soil was place on a stack of sieves including 2000-μm-, 1000-μm-, and 250-μm-mesh openings. The stack was bolted to a circular sieve shaker intended for soil particle analysis (CSI Scientific, Forks Township, PA, USA) and shaken at 200–250 rpm for 3 min. Soil was gently removed from each sieve and weighed to determine the mass distribution of aggregates into the following fractions: large macroaggregates (>2000 μm), medium macroaggregates (1000–2000 μm), small macroaggregates (250–1000 μm), and microaggregates (<250 μm). Total mass acquired in each aggregate fraction was used to calculate the mean weighted diameter (MWD) of soil aggregates in each sample (Van Bavel, 1949). Subsamples of each aggregate fraction were saved to determine postsieving gravimetric water content, extracellular enzyme activity, and total C and N. Enzyme subsamples were frozen immediately until analysis was performed (2–4 months).
Soil enzyme analysis
Extracellular soil enzyme assays were modified from Marx et al. (2001) and DeForest (2009). Briefly, 1 g of frozen soil aggregates was suspended in 125 mL sodium acetate buffer with pH adjusted to median of soils. Slurries were pipetted into 96-well black microplates, and enzyme activities were determined by adding 4-methylumbelliferyl (MUB)-linked substrates for N-acetyl-glucosaminidase (NAG) and β-glucosidase (BG), β-xylosidase (BX), and cellobiohydrolase (CB) for a final concentration of 40 μM. Assays were incubated in the dark for 2 h, reactions stopped with 10 μL 0.5 M NaOH, and solution optical density determined fluorometrically at 450 nm on a microplate reader (BioTek, Winooski, VT, USA). In 2012, slight modifications were made to the enzyme assay protocol to reduce variability between analytical replicates (Hargreaves & Hofmockel, 2015). Enzyme concentrations were increased to 400 μM and soil enzyme slurries were incubated in 5-mL tubes before being transferred to 96-well plates for fluorometric analysis. Reanalysis of a subset of 2011 samples with the modified protocol showed no changes in relationships detected in the original dataset. Original enzyme activity of 2011 samples was greater than that of rerun samples, indicating enzyme activity had degraded in the additional 18 months of storage at −20 °C, so we proceeded with the original 2011 and 2012 datasets despite changes made in the assay protocol, and no direct statistical comparisons were made between the 2011 and 2012 data. For all samples, absolute potential enzyme activity (nmol h−1 g−1 dry aggregates) was calculated and reported as described by German et al. (2012).
Aggregate C & N
Total C and N were determined for each aggregate fraction and whole soil at each sampling date. A subsample of isolated aggregates was dried at 60 °C for 48–60 h, ground, and dry-combusted in a Thermo Flash 1112 CN Analyzer (Thermo Corp., Lakewood, NJ, USA).
Statistical analyses
Potential enzyme activities and total C and N of aggregate fractions were analyzed using a modified split-plot anova, which accounts for the nonindependence of aggregate and plot (Briar et al., 2011). Management system (corn, prairie, or fertilized prairie) was considered the main effect and aggregate fraction a subplot factor (e.g., repeated measure). For all variables in the full model, intra-annual responses differed between 2011 and 2012; therefore, analysis of complete 2011 and 2012 datasets was performed independently. A full factorial model between aggregate fraction, management system, and sampling date within each year was run for aggregate enzyme activity; among the four enzymes, the only detectable interaction was between sampling date and management system. Thus, aggregate fraction, management system, sampling date, and the management system by sampling date interaction were included as fixed effects with block as a random effect. Soil bulk density and aggregate MWD were measured at the plot level, so we used a mixed model with management system, sampling date, and their interaction as fixed effects and block as a random effect. Means from aggregate fractions and ecosystems were compared using Tukey's honestly significant difference (α = 0.05). Natural log transformations were performed to satisfy assumptions of normality when needed. Models were run using PROC MIXED in sas v. 9.2 (SAS Institute, Cary, NC, USA). To address the relationship between soil aggregation and extracellular enzyme activity, aggregate MWD was correlated with enzyme activity using a mixed model, accounting for the effect of sampling date and management system as random terms. A marginal r2 value was calculated to quantify the variation explained by MWD that was not explained by sampling date or management system (Nakagawa & Schielzeth, 2013). Analyses were performed using the lmer function in the lme4 package (v1.1-7) (Bates et al., 2014) and rsquared.glmm function (Lefcheck, 2014) in r (v3.1.0).
Results
Soil aggregate distribution and turnover exhibited seasonal fluctuations with interannual variation in the timing and magnitude of turnover. In 2011, both prairies had greatest mean weighted aggregate diameter (MWD) in June, and corn was most aggregated in May and June (P ≤ 0.002, Table 1). Increases in MWD were driven by increases in the mass of soil present in the large macroaggregate (>2000 μm) fraction and concomitant decreases in the small macroaggregate (1000–2000 μm) fraction, which exhibited the inverse seasonal patterns within management system (P = 0.0001; Fig. 1). Mass of soil in the microaggregate fraction also increased with decreases in large macroaggregates (P < 0.0001), but accounted for a very small percentage of total soil mass. These patterns indicated that large macroaggregates were regularly disrupted across the growing season, releasing small macroaggregates and microaggregates, and reformed between sampling dates (Fig. 1). In 2011, these large macroaggregate disruption events coincided with precipitation events and seasonal changes in soil bulk density. Bulk density in all management systems was greatest in June and decreased by 6%, 20%, and 26% in May, August, and July, respectively (P = 0.0001; Table 1).
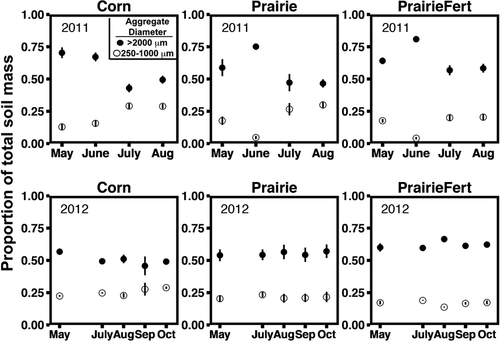
Reductions in the mass of large macroaggregates and MWD in 2011 generally corresponded with seasonal increases in potential extracellular enzyme activity (Table 2). Similar to the soil physical measures, extracellular enzyme activity exhibited seasonal trends that interacted with management system (P = 0.0001). For example, peak N-acetyl-glucosaminidase (NAG) and cellobiohydrolase (CB) activity occurred in July in fertilized and unfertilized prairie (Figs 2 and 3), coincident with turnover, but in corn maximum activity for these enzymes occurred in August. Increased potential enzyme activity at the same time as reduced aggregation may indicate that release of organic matter from disrupted large macroaggregates could stimulate enzyme activity. Organic resources (C concentration) within soil aggregate varied between fraction size regardless of sampling date or management system. Microaggregates contained the least total C and large macroaggregates the most (P < 0.0001, Fig. 4). In contrast, total N did not vary between aggregate fractions. As such, the aggregate C : N ratio fluctuated across fractions, driven by C concentration. Aggregate C : N ratios decreased from 13.2 to 12.2 as aggregate size fraction decreased from large macroaggregates (>2000 μm) to microaggregates (P < 0.0001, Fig. 4). Extracellular enzyme activity was positively correlated with aggregate C concentration for C-cycling enzymes (Table 3), but the relationship was not as strong as sampling date and management system effects.
| Enzyme | P | Marginal r2 | Slope |
|---|---|---|---|
| 2011 | |||
| N-Acetyl-glucosaminidase | NS | ||
| β-Glucosidase | 0.007 | 0.10 | −133.79 |
| Cellobiohydrolase | 0.03 | 0.11 | −33.06 |
| β-Xylosidase | 0.0001 | 0.19 | −35.36 |
| 2012 | |||
| N-Acetyl-glucosaminidase | 0.0008 | 0.16 | 78.82 |
| β-Glucosidase | <0.0001 | 0.29 | 603.20 |
| Cellobiohydrolase | <0.0001 | 0.30 | 40.98 |
| β-Xylosidase | <0.0001 | 0.25 | 77.22 |
| P-value | Marginal r2 | Slope | |
|---|---|---|---|
| 2011 | |||
| N-Acetyl-glucosaminidase | NS | ||
| β-Glucosidase | <0.0001 | 0.09 | 13.2 |
| Cellobiohydrolase | 0.01 | 0.03 | 2.14 |
| β-Xylosidase | 0.001 | 0.05 | 2.26 |
| 2012 | |||
| N-Acetyl-glucosaminidase | 0.02 | 0.004 | 0.68 |
| β-Glucosidase | NS | ||
| Cellobiohydrolase | NS | ||
| β-Xylosidase | NS | ||
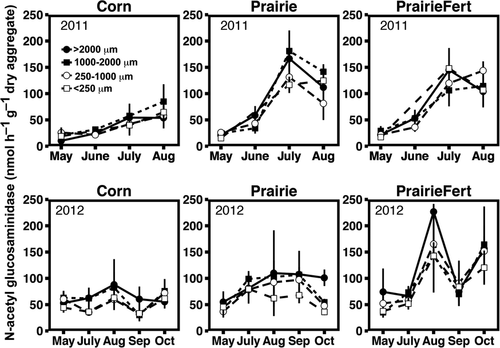
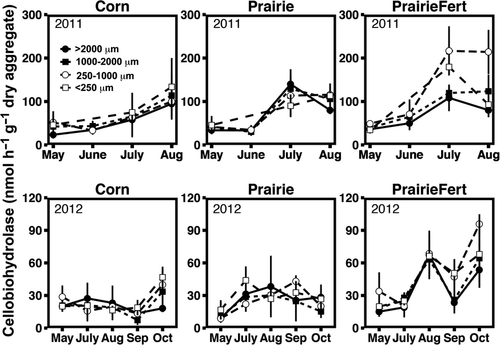
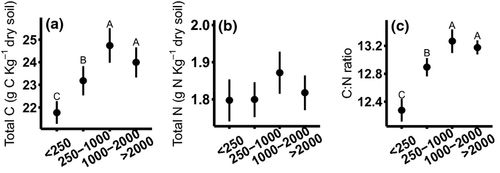
In 2012, we also observed seasonal fluctuations in soil physical and biological responses, but extreme drought affected timing and magnitude of some of these responses. Aggregate turnover was minimal, but detectable seasonal changes in aggregation depended on management system. Fertilized prairie had greatest MWD in August and least MWD in May and July, converse to corn (P = 0.0001, Table 1). Again, fluctuations in bulk density generally corresponded with changes in aggregation. Bulk density in both prairie systems was greater in October 2012 than May (P = 0.02, Table 1) and was consistent across the growing season in corn. Drought conditions that affected fluctuations in soil physical factors also contributed to unique seasonal trends in extracellular enzyme activity in 2012. Overall, there was considerably less potential enzyme activity in 2012, but all enzyme activities again exhibited a management system by sampling date interaction (P ≤ 0.0001). Enzyme activity in unfertilized prairies was least in May, but similar at all other sampling dates, and fertilized prairie systems exhibited very high enzyme activity in August and October, much higher than the other systems at those sampling dates. In corn systems, NAG activities were consistent across the entire growing season (Fig. 2), and C-cycling enzyme activity (BG, CB, BX) was greatest in October (Fig. 3). Potential extracellular enzyme activity correlated positively with aggregate MWD in 2012 (Table 2), corroborating the 2011 evidence that soil aggregation independently influences enzyme activity. The contrasting direction of this correlation in 2012 is likely a result of minimal changes in MWD and the strong influence of drought conditions on enzyme activity. Unlike 2011, enzyme activity also varied consistently among aggregate fractions. In large macroaggregates, NAG activity was 30–40% greater than in microaggregates (P < 0.02). In contrast, CB activity was 22% greater in microaggregates than in large macroaggregates (P = 0.03). Differences in potential enzyme activity among aggregate fractions were likely related to available substrate resources. Total C, total N, and C : N ratio differed consistently among aggregate fraction in the same manner observed in 2011 (P < 0.0001; Fig. 4). Aggregate total C was weakly positively correlated with the potential activities of NAG (Table 3), and when potential enzyme activities were expressed per g C in each aggregate fraction, C-specific enzyme activity did not differ between aggregate fractions (Table S1).
As described above, management system effects on soil physical and biological factors interacted with sampling date. The fact that the magnitude, and in some cases direction, of difference between management systems was not consistent at multiple sampling dates underscores the limitations of extrapolating data from a couple or even single sampling date to estimate ecosystem functioning among bioenergy management systems. Interestingly, when data from the entire growing season were considered together, consistent differences in some soil physical and biological measures between management systems emerged in both 2011 and 2012. In both years, fertilized prairie had the greatest aggregate MWD (Table 4) and corn had the greatest soil bulk density (Table 4). Fertilized prairie also supported greater extracellular enzyme activity than unfertilized prairie and corn (Table 4). Thus, with two full years of growing season data, fertilized prairie bioenergy systems were supporting greater enzyme activity and greater soil aggregation than unfertilized prairie and corn, despite greater root biomass inputs from unfertilized prairie (Jarchow et al., 2015).
| Unfertilized prairie | Fertilized prairie | Corn | |
|---|---|---|---|
| 2011 | |||
| Aggregate mean weight diameter (MWD; mm)*** | 3.29 ± 0.16B | 3.62 ± 0.12A | 3.26 ± 0.14B |
| Bulk Density (g/cm3)*** | 1.46 ± 0.02B | 1.42 ± 0.02B | 1.51 ± 0.02A |
| N-Acetyl-glucosaminidase (nmol h−1 g−1 dry aggregate)*** | 85 ± 8A | 81 ± 7A | 41 ± 4B |
| Cellobiohydrolase (nmol h−1 g−1 dry aggregate)* | 76 ± 7B | 93 ± 9A | 66 ± 8B |
| β-Glucosidase (nmol h−1 g−1 dry aggregate)* | 301 ± 30A | 364 ± 31A | 232 ± 23B |
| β-xylosidase (nmol h−1 g−1 dry aggregate)** | 46 ± 5B | 72 ± 12A | 26 ± 2C |
| 2012 | |||
| Aggregate mean weight diameter (MWD; mm)*** | 3.19 ± 0.08B | 3.48 ± 0.04A | 2.98 ± 0.06C |
| Bulk density (g cm−3)*** | 1.13 ± 0.03B | 1.04 ± 0.02C | 1.19 ± 0.02A |
| N-acetyl-glucosaminidase (nmol h−1 g−1 dry aggregate)*** | 75 ± 5B | 100 ± 8A | 56 ± 4C |
| Cellobiohydrolase (nmol h−1 g−1 dry aggregate)* | 25 ± 2B | 43 ± 3A | 22 ± 2B |
| β-Glucosidase (nmol h−1 g−1 dry aggregate)** | 382 ± 32B | 660 ± 41A | 386 ± 28B |
| β-Xylosidase (nmol h−1 g−1 dry aggregate)NS | |||
- *P < 0.05, **P < 0.01, ***P < 0.001, NSP>0.05.
- Different superscript letters denote statistically different groups between management systems (across the row).
Discussion
Soil microbial decomposition processes in perennial reconstructed tallgrass prairie and annual corn row-crop bioenergy systems were affected by management and seasonal weather patterns in part through the distribution and turnover of soil aggregate habitats (Fig. 1, Table 1). Many factors contribute to seasonal sifts in extracellular enzyme activities, including temperature, water availability, plant growth, and root exudation. Differences in seasonal patterns among the three bioenergy feedstock production systems suggest that system-specific management factors, such as crop selection, fertilizer addition, and soil disturbance (e.g., wheel traffic, planting), clearly have an impact above and beyond the influence of temperature and water inputs. By focusing on soil microbial habitats (aggregates), we found seasonal disruption of large macroaggregates corresponded with increases in potential extracellular enzyme activity, especially in 2011, in the absence of drought. This implies that seasonal shifts in weather and weather-related ecosystem properties (e.g., soil moisture) can influence soil physical structure and enzyme activity on very short timescales (weeks). In turn, management practices can affect soil microbial habitats (aggregation) and potential activities (enzyme activity), and changes at this microbial scale may inform long-term changes in ecosystem parameters such as total soil C. In the present study, fertilized prairie supported greater soil aggregation and enzyme activity, further illuminating the connection between microbial activity and physical habitat. This trend only became evident when considering multiple sampling dates, but was consistent in 2011 and 2012, indicating the importance that temporal variation in soil physical and biological factors can have on estimates of whole ecosystem processes. These are important insights as scientists seek to evaluate the ability of bioenergy systems to provide ecosystem services presently and into uncertain future climate scenarios.
Seasonal dynamics in soil aggregation are affected both by weather differences between the sampling years and by bioenergy system management. Dry periods increase aggregate stability (Dimoyiannis, 2009) and precipitation events can disrupt macroaggregates, which is consistent with our observations. In both years, corn systems experienced aggregate disruption between May and July sampling dates, with slight aggregate rebuilding in August. These fluctuations are consistent with previous estimates of 27 days for macroaggregate formation in agroecosystems (Plante & McGill, 2002). The lack of precipitation in 2012 likely contributed to the minimal disruption of large macroaggregates in that growing season. Furthermore, observed aggregate turnover contributed to seasonal changes in soil bulk density, underscoring that bulk density is not a static soil measure, which can have profound effects when scaling ecosystem processes to an aerial basis (Lee et al., 2009). Increased aggregation and reduced magnitude of turnover events in both prairie systems support previous work emphasizing the role of root networks and microbial activity in stabilizing soil aggregates (Tisdall & Oades, 1982; Six et al., 2004).
Microbial activity, as measured by potential extracellular enzyme activity, exhibited seasonal peaks that corresponded with aggregate disruption, particularly in 2011. Measurements of potential enzyme activity may not be realized to the same extent in the field but do reflect biological investment in enzyme production in response to soil resources and conditions. Release of labile organic matter formally protected in large macroaggregates coincided with enhanced enzyme activity and may have led to the subsequent formation of new large macroaggregates later in the growing season (Jastrow et al., 1998; Six et al., 2006). In addition to soil structural changes, soil moisture also fluctuated seasonally in a similar pattern (Table 1). Changes in soil moisture likely played an additional role in seasonal enzyme activity fluctuations because increased soil moisture can stimulate enzyme activity (Waldrop & Firestone, 2006; Zeglin et al., 2013). Observed increases in enzyme activity in all management systems in October 2012 are likely driven by the partial release from drought conditions.
In addition to aggregate turnover and moisture fluctuations in the soil environment, differences in plant communities likely contribute to management-specific seasonal responses. Simultaneous work at this field site found that unfertilized prairie systems have the greatest aboveground biomass production and standing root biomass (Jarchow et al., 2015), but we observed greater enzyme activity in fertilized prairie. This suggests that N availability is stimulating enzyme production beyond root-derived substrates (Yao et al., 2011). In addition, fertilized prairie systems at this study site have greater cover of cool-season (C3) plants than unfertilized prairie systems (Jarchow & Liebman, 2013). Thus, peaks in C-cycling enzyme activity in fertilized prairie systems in July 2011 and 2012 may be driven by fine root turnover and senescence of cool-season plants and higher quality root tissue (Craine et al., 2003). Additionally, mesocosm work with cool-season grasses showed that drought conditions could stimulate extracellular enzyme activity (Sanaullah et al., 2011), which may contribute to the spikes in activity observed in fertilized prairie in 2012. Sustained high levels of enzyme activity in unfertilized prairie systems into August and September could be the result of maximum warm-season (C4) root growth and exudation, consistent with previous studies of microbial biomass in temperate grasslands (Lynch & Panting, 1982; Garcia & Rice, 1994). Conversely, low availability of root C substrates likely limited enzyme activity in N-rich corn systems (Hargreaves & Hofmockel, 2014), and extracellular enzyme activity increased with annual corn plant growth. Furthermore, heavy allocation of plant-fixed C to growth and grain production in corn may limit microbial access to substrates including root biomass and exudates, resulting in lower enzymatic potential in corn.
Management system differences in enzyme activity and soil aggregation are not evident at every sampling date. By evaluating the entire growing season, we are able to more accurately quantify differences in C and N cycling between these three bioenergy management systems. Soil C and N storage is difficult to measure in general as changes over a few years are very small relative to the size of the total soil C pool, and especially in fertile mollisols as in our study. Aggregate turnover and potential enzyme activity together may represent sensitive measures of biological potential for decomposition (nutrient release) and long-term soil C storage (Tiemann & Grandy, 2015). In diversified perennial grassland systems, the greater aggregation and reduced severity of turnover, but high enzyme activity, may signify substantial release of soil C and N to support biota coupled with healthy soil structure that increases C storage (or reduces SOM loss). Furthermore, coupling N fertilization with grassland establishment in fertilized prairie enhanced microbial biomass, enzyme activity, and soil aggregation despite greater root biomass in unfertilized prairie (Jarchow et al., 2015). In fact, a parallel study of whole soil enzyme activities and C and N pools at this experimental site shows similar differences between these management systems, indicating the observed aggregate-level data reported here are contributing to realized ecosystem-scale differences (Bach & Hofmockel, 2015).
Regardless of management system and sampling date, differences in extracellular enzyme activity are consistent across aggregate fractions. Greater activity of NAG in large macroaggregates and elevated C-cycling enzyme activity (BG, CB) in small macroaggregates and microaggregates are consistent with previous work (Bailey et al., 2012). Extracellular enzyme activity within soil aggregate fractions is generally positively correlated with aggregate C content (Table 3) in all management systems and across all sampling dates. Furthermore, C-specific enzyme activities do not vary between aggregate fractions (Table S1). Despite having a positive correlation with aggregate C concentrations within each fraction, between-aggregate comparisons revealed that C-cycling enzymes such as CB exhibit greater activity in the microaggregate fraction, which have lower C concentrations than large macroaggregates. This may seem counterintuitive, but a couple of potential interpretations exist. First, microbes and enzymes may be stabilized on the surface of microaggregates (Bailey et al., 2012), where greater surface area may support a larger microbial community per volume than large macroaggregates. Another contributing factor to increased CB and BG activity in microaggregate fractions may be C : N stoichiometry. Lower C : N ratios in microaggregates may increase cellulase activities as more N may be allocated toward enzyme production, as observed by Nie et al. (2014). This change in C : N ratio across aggregate fractions is consistent between management systems and is not affected by inorganic fertilizer additions. Likewise, greater C : N ratio in large macroaggregates may be contributing to elevated NAG activity. Microbes may be investing in greater NAG production to meet N demands for decomposition of increased organic matter in large macroaggregates. Additionally, NAG cleaves both a glucose and amine group from more complex molecules, so increased NAG enzyme activity may be attributed to increased demand for both N and C in large macroaggregates (Fansler et al., 2005). Although the pattern of enzyme activity differences among aggregate fractions is consistent among the management systems, the distribution of aggregate fractions varies by bioenergy system. Thus, bioenergy management system effects on soil aggregation and aggregate turnover may be a key factor contributing to grassland systems' ability to retain soil C and N to a greater extent than row-crop corn.
There are two key conclusions that can be drawn from this dataset. Firstly, soil structure (aggregates) may affect extracellular enzyme decomposition of organic matter by mediating access to substrate quantity and quality. Secondly, ecosystem type and management affect seasonal turnover of aggregate fractions and fluctuations in extracellular enzyme activity, which results in greater microbial stabilization and physical protection of soil organic matter in prairie systems, especially fertilized prairies. Soil aggregation can play an important role mediating microbe–substrate interactions in ecosystems. Further consideration of ecosystem processes such as extracellular enzyme activity at the aggregate scale could expand our limited understanding of soil ecosystem processes in a wide range of habitats. In turn, management system and weather strongly influenced soil aggregate turnover. These data indicate that seasonal dynamics in extracellular enzymes may influence ecosystem-level C and N cycling and that physical soil structure may be an overlooked contributor to these short-term seasonal dynamics within managed bioenergy systems.
Acknowledgements
This work was supported by a grant from the Agriculture and Food Research Initiative of the USDA National institute of Food and Agriculture (NIFA), grant number # 20116700330364 (KSH), and an Iowa State University Plant Science Institute Fellowship to EMB. Giselle Narvaez, Becca Luzbetak, Kira Murray, Erin Frankson, Austin Putz, Eric Asbe, Megan Barthalomew, Dade McBrayer, and Mark Anthony provided assistance in the field and laboratory. Richard Cruse, Sarah Hargreaves, Alison King, Ryan Williams, Brian Wilsey, and an anonymous reviewer provided valuable feedback on drafts of the manuscript. Authors declare no conflict of interests.




