Moisture–Microbial Interaction Amplifies N2O Emission Hot Moments Under Deepened Snow in Grasslands
Funding: This work was supported by the National Natural Science Foundation of China (32125025, 32330066, 32101307, 32301359).
ABSTRACT
Freeze–thaw-induced N2O pulses could account for nearly half of annual N2O fluxes in cold climates, but their episodic nature, sensitivity to snow cover dynamics, and the challenges of cold-season monitoring complicate their accurate estimation and representation in global models. To address these challenges, we combined in situ automated high-frequency flux measurements with cross-ecoregion soil core incubations to investigate the mechanisms driving freeze–thaw-induced N2O emissions. We found that deepened snow significantly amplified freeze–thaw N2O pulses, with these ~50-day episodes contributing over 50% of annual fluxes. Additionally, freeze–thaw-induced N2O pulses exhibited significant spatial heterogeneity, ranging from 3.4 to 1184.1 μg N m−2 h−1 depending on site conditions. Despite significant spatiotemporal variation, our results indicated that 68%–86% of this variation can be explained by shifts in controlling factors: from water-filled pore space (WFPS), which drove anaerobic conditions, to microbial constraints as snow depth increases. Below 43% WFPS, soil moisture was the overwhelmingly dominant driver of emissions; between 43% and 66% WFPS, moisture and microbial attributes (including denitrifying gene abundance, nitrogen enzyme kinetics, and microbial biomass) jointly triggered N2O emissions pulses; above 66% WFPS, microbial attributes, particularly nitrogen enzyme kinetics, prevailed. These findings suggested that maintaining higher soil moisture served as a trigger for activating microbial activity, particularly enhancing nitrogen cycling. Furthermore, we showed that hotspots of freeze–thaw-induced N2O emissions were linked to high root production and microbial activity in cold and humid grasslands. Overall, our study highlighted the hierarchical control of WFPS and microbial processes in driving freeze–thaw-induced N2O emission pulses. The easily measurable WFPS and microbial attributes predictable from plant and soil properties could forecast the magnitude and spatial distribution of N2O emission “hot moments” under changing climate. Integrating these hot moments, particularly the dynamics of WFPS, into process-based models could refine N2O emission modeling and enhance the accuracy of global N2O budget prediction.
1 Introduction
Nitrous oxide (N2O) is a long-lived and potent greenhouse gas, and its increasing concentrations over the past 150 years have directly contributed to global warming (Edenhofer et al. 2014). Despite extensive efforts to quantify global N2O sources and sinks using both bottom-up and top-down approaches, considerable uncertainty persists in estimating N2O emissions largely due to the extremely high spatial and temporal nature of N2O fluxes (Syakila et al. 2010). A major challenge stems from limited observations of “hot moments” of N2O emission, particularly during freeze–thaw periods. These episodic events could account for more than half of annual N2O emissions in temperate ecosystems (Wolf et al. 2010) yet remain underrepresented in measurements due to the technical and logistical challenges of cold-season monitoring (Butterbach-Bahl and Wolf 2017; Tian et al. 2020). Overlooking freeze–thaw-induced N2O emissions could result in an underestimation of global terrestrial N2O emissions by up to 30% (Del Grosso et al. 2022; Wagner-Riddle et al. 2017). With projected changes in winter snowfall in mid- to high-altitude regions (Gottlieb and Mankin 2024; Peng et al. 2010), the uncertainties related to N2O emission pulses during these hot moments are expected to intensify (Jia et al. 2021; Wolf et al. 2010), highlighting the urgent need to elucidate the mechanisms driving the occurrence and magnitude of these events.
Conventionally, the pulses of N2O emission during freeze–thaw periods were attributed primarily to the physical release of N2O trapped beneath frozen soil layers during winter. However, growing evidence suggests that microbial processes, particularly denitrification, play a far more critical role in driving freeze–thaw N2O pulses (Risk et al. 2013; Wang et al. 2023; Wolf et al. 2010), although these pulses may also result from nitrification (Griffis et al. 2024). Denitrification involves denitrifying organisms performing anaerobic respiration by stepwise reduction of NO3− to N2, a process driven by four metalloenzymes encoded by multiple functional genes that are widespread among bacteria and archaea in soil (Lycus et al. 2018; Nadeau et al. 2019). This process is highly sensitive to subtle changes in electron acceptor and donor availability, redox conditions, and the abundance of denitrifiers. At a microscale, the melting of snow during freeze–thaw periods increases soil moisture, creating anoxic soil conditions conducive to denitrification (Holst et al. 2008). The process is further amplified by the release of carbon (C) and nitrogen (N) substrate triggered by soil aggregate disruption (Ruan and Robertson 2017) or microbial cell lysis (Schimel et al. 2007), as well as N-related functional genes activation and expression (Jusselme et al. 2016; Nemeth et al. 2014; Wang et al. 2023).
Beyond microscale processes, large-scale variations in climate, soil properties, and vegetation strongly influence soil organic matter mineralization rates, ultimately regulating the availability of mineral N and C substrates for denitrification during freeze–thaw events (Vestgarden and Austnes 2009). These factors collectively shape the magnitude of freeze–thaw-induced N2O emissions across different ecoregions. While localized interactions between microbial communities and environmental conditions could produce discrete “hot spots” of N2O emissions, such phenomena are often inadequately captured in large-scale assessments due to the difficulty of obtaining spatially representative observations during winter. This limitation contributes to significant gaps in our understanding of terrestrial N2O flux dynamics, underscoring the need for integrated approaches that bridge micro- and macroscale processes.
Grasslands occupy approximately 40% of the Earth's land surface (Knight et al. 2024), with a significant portion experiencing long periods of winter snow cover, particularly in temperate and high-latitude regions (Peng et al. 2010). Although several studies have examined the effects of freeze–thaw cycles on N2O emissions in both field and laboratory settings in grasslands (Holst et al. 2008; Wu et al. 2020; Yao et al. 2010), a crucial factor remains largely overlooked: the impact of climate change on snow regime. While many regions experience a decline in snow cover, nearly 16% of Earth's land surface, particularly in Eurasia, has seen an increase in winter snowfall and extended snow cover duration (Peng et al. 2010; Pulliainen et al. 2020). Snow not only plays a pivotal role in regulating grassland productivity (Li et al. 2020) but also significantly influences winter soil processes. The depth and duration of snow cover directly affect freeze–thaw dynamics and, consequently, N2O production (Jia et al. 2021, 2022). Evidence from temperate grasslands has shown that deeper snow dramatically amplifies freeze–thaw N2O pulses by over 15-fold (Jia et al. 2021; Wolf et al. 2010). However, these studies are predominantly derived from single-site experiments, leaving a gap in understanding how snow regime changes interact with plant, soil, and microbial characteristics across diverse ecoregions. This lack of regional-scale insights hampers our ability to comprehensively assess how snow dynamics shape N2O emissions during freeze–thaw events and contributes to the uncertainty surrounding the global N2O budget.
To address these critical gaps, we conducted two complementary studies to assess the effects of snow depth on freeze–thaw-induced N2O emissions. We first used automated flux chambers to determine N2O fluxes over a complete year at a site scale in a temperate steppe and compared N2O fluxes between ambient snow and deepened snow. Second, recognizing the logistical challenges of in situ investigations during freeze–thaw periods at the regional scale, we implemented a snow manipulation microcosm incubation experiment using intact soil cores from 12 sites along a 1500 km transect across arid and semiarid grasslands in China. This experiment examined the effects of four snow depth treatments to capture variations in environmental and microbial drivers of N2O fluxes. Our study aims to elucidate the effects of future winter snowfall changes on N2O fluxes during freeze–thaw periods. We hypothesize that (1) deepened snow can increase freeze–thaw-induced N2O emissions, contributing disproportionately to annual fluxes; (2) this emission pattern is driven by changes in substrate level, soil aeration status, as well as soil microbial function; and (3) freeze–thaw N2O emissions will exhibit a large variability across this region as reflected by the climate and intrinsic soil properties.
2 Materials and Methods
2.1 Site-Scale Snow Manipulation Experiment
A snow manipulation experiment was conducted at a temperate grassland at the Inner Mongolia Grassland Ecosystem Research Station (IMGERS, 43°38′ N, 116°42′ E, 1200 m above sea level) of the Chinese Academy of Sciences. The region experiences a semiarid continental climate, with a mean annual temperature (MAT) of 0.9°C and mean annual precipitation (MAP) of 334 mm (Chen et al. 2019), approximately 10% of which occurs during wintertime (Li et al. 2020; Zuo et al. 2019). Over the past four decades, mean snow depth has increased across Inner Mongolia (Huang et al. 2016; Peng et al. 2010), including at our study site (Li et al. 2020). The soil at the study site was classified as Calcic Chernozems, with a loamy-sand texture. The grassland plant community was dominated by perennials, including Leymus chinensis (Trin.) Tzvel., Stipa krylovii Roshev., Artemisia frigida Willd. Sp. Pl., and Potentilla chinensis Ser.
The field experiment employed a randomized block design to account for shading, with two treatments: ambient snow and deepened snow (Figure S1a,b). Starting in 2013, a polyethylene mesh snow fence, measuring 1.25 m (height) × 100 m (length), was installed perpendicular to the prevailing northwest wind during wintertime to increase snow accumulation in the deepened snow plots. The snow fence was removed in late March of the following year. Three replicate plots, each measuring 8 × 4 m, were established along the snow fence for each treatment (Figure S1c). A buffer zone (> 20 m) was designed between ambient snow and deepened snow to minimize edge effects caused by the snow fence (Figure S1c).
2.2 Measurement of In Situ N2O Fluxes
From June 2018 to May 2019, surface N2O fluxes were measured using an automatic chamber system (Figure S1c,d). This system consisted of six opaque, automated gas flux chambers (28.5 cm diameter, 16.2 cm height; SC-21 N1, LICA, Beijing, China) connected to a multiplexer (SF3500 Soil Greenhouse Gas Flux Monitoring System, LICA, Beijing, China). The multiplexer enabled dynamic chamber deployment and directed gas samples to an off-axis integrated cavity output spectrometer (LGR-913-0055, ABB Inc., Quebec, Canada) for high-precision measurement of greenhouse gas concentrations. The chambers were randomly assigned to the ambient plots (n = 3) and deepened snow plots (n = 3; Figure S1c). Each chamber had a fixed polyvinyl chloride collar (PVC, 19.2 cm inner diameter, 10 cm height) which was inserted 3 cm into the soil. During wintertime, to make the automated chambers work effectively, snow was carefully excavated around the chambers (Figure S1c). This reduced the effective snowpack depth above the chambers, but was necessary to achieve a proper seal and maintain data quality. We acknowledged that this localized disturbance might influence the microenvironment around the chambers. However, due to the flat topography of the site, snowmelt in the soil was well connected, ensuring that soil moisture beneath the chambers remained similar to the surrounding area during the freeze–thaw period. Additionally, as the same protocol was consistently applied across all treatments and plots, comparisons between treatments remained valid.
Where V represents the chamber volume (cm3), and Pav, Wav, and Tav indicate the average air pressure (kPa), average water mole fraction (mmol mol−1), and average air temperature (°C), respectively. R is the ideal gas constant (8.314 J mol−1 K−1), and S is the surface area (cm2) covered by the chamber. dC'/dt (μmol−1 s−1) is the slope of linear regression of dry N2O concentrations (C′) over time, which are corrected by the water vapor content. M is the molecular mass (g mol−1) of N atoms of N2O. The annual flux was estimated based on the trapezoidal interpolation between measured fluxes and sampling intervals (e.g., Luo et al. 2022; Matson et al. 2017).
2.3 Regional-Scale Sampling
Twelve study sites were selected along a 1500-km southwest–northeast transect across Inner Mongolia in northern China (Figure S1e). The transect spans longitudes from 107°42′ E to 122°30′ E and latitudes from 38°54′ N to 50°12′ N, with MAT ranging from −2°C to 8°C, and MAP varying from 201 to 488 mm (Fick and Hijmans 2017). The aridity index (AI), defined as the ratio of precipitation to potential evapotranspiration, was obtained from WorldClim2 as well (Fick and Hijmans 2017), and varied from 0.13 to 0.55, with higher values indicating moister soil conditions. Vegetation types along the transect, from west to east, included desert steppe, typical steppe, and meadow steppe. The soils, primarily arid, sandy, brown loessials rich in calcium, were classified as Kastanozem in the FAO classification system (Cheng et al. 2009).
At each site, a 20 × 20 m plot was established, with four 1 × 0.5 m subplots randomly selected and spaced 5 m apart. To assess the role of plant function on the spatial variability of freeze–thaw-induced N2O emissions and water retention capacity, aboveground biomass (AGB) and belowground biomass (BGB) were measured in each subplot. AGB was measured by harvesting all plant material, while BGB was determined by excavating roots using soil cores (7 cm in diameter and 5 cm in depth). Additionally, 0–5 cm soil samples were randomly collected by a soil corer (3 cm diameter) in each subplot and immediately sieved through a 2-mm sieve. These soil samples were divided into two parts: one was air-dried for physiochemical analysis, and the other was stored at −80°C for DNA extraction. Furthermore, after removal of the aboveground biomass, four intact soil cores (7 cm in diameter and 5 cm in depth) were collected from each subplot, capped at both ends to limit disturbance during transport, and stored at −20°C for a further incubation experiment. The intact soil cores were not sieved or otherwise treated to preserve natural soil structure and minimize potential losses of N2O emissions. This ensured that gas diffusion and microbial processes remained consistent with in situ conditions, reducing the risk of introducing artifacts for the subsequent experiment.
2.4 Microcosm Freeze–Thaw Experiment
To estimate how changes in winter snow depth could influence freeze–thaw-induced N2O emissions at the regional scale, we conducted a microcosm incubation experiment using intact soil cores collected from the 12 study sites. For each site, we established four snow depth treatments: 0, 8, 16, and 28 cm, based on historical snow depth data in this region (Li et al. 2020; Ying et al. 2019). Each treatment had four replicates. Due to practical challenges in collecting and preserving natural snow for laboratory use, crushed ice was utilized as a surrogate. Importantly, as snow transitions into ice during the melting process in the field, the use of crushed ice provided a reasonable approximation of snowmelt dynamics. We used the measured average snow water equivalent (0.16 ± 0.03) by Li et al. (2020) at IMGERS in 2017 to convert the four snow depths into corresponding water equivalents, that is, 0, 49, 98, and 172 g. The snow meltwater was then frozen to produce crushed ice to simulate the gradual melting of snow. Hereafter, we referred to crushed ice as “simulated snow.” To accommodate the simulated snow, a PVC tube (6 cm height, 7 cm diameter) was attached to each soil core (Figure S1f), and waterproof tape was used to secure the joint to prevent water leakage during the simulated snow melting. To minimize the artifacts during the freeze–thaw period, we wrapped 2-cm-thick insulation material around the sidewall of each soil core to reduce the extreme temperature fluctuations that may occur (Henry 2007). Each soil core was placed on a support above a 200 mL jar to collect the leachate during thawing. This setup resulted in 192 microcosms (4 treatments × 4 replicates × 12 sites).
Prior to the start of the experiment, the intact soil cores were placed into an incubator at 5°C for 3 weeks to stabilize N2O fluxes. To better approximate natural conditions, the frequency and duration of freeze–thaw cycles were set based on the previously mentioned snow manipulation experiment at IMGERS (Jia et al. 2022). Three periods were designated (Figure S2): (1) a freezing period, during which soil samples were immediately frozen at −10°C for 1 week after snow application; (2) a subsequent freeze–thaw period, featuring 12 h of daylight at +7°C and 12 h of night at −10°C for 1 week; and (3) a thawing period, during which soil samples were thawed at +7°C for 10 days. The freeze–thaw and thawing periods were repeated, with the duration reduced to 3 and 5 days, respectively. This cycle was designed to mirror the temperature variations observed in the field. Soil temperature at each depth in incubators was recorded using iButton temperature data loggers (DS1922L, Maxim Dallas Semiconductor Corp., USA) during the incubation period (Figure S2).
Where dc/dt is the slope of the linear regression for N2O concentrations (c, mg m−3) over time (t, h). V indicates the volume of the headspace (m3) of glass jar. P and T are atmospheric pressure (Pa) and air temperature (°C) in the glass jar, respectively. R is the ideal gas constant (8.314 J mol−1 K−1). M is the mole mass (g mol−1) of N atoms of N2O, and Acore is the surface area of the soil core (m2).
2.5 Analysis of Soil Properties
General soil characteristics (pH, total N, SOC, silt, sand, and clay content) in the top 5 cm were determined according to standard methods (Table S1). Briefly, air-dried soil subsamples sieved through a 2-mm sieve were used to measure soil pH at a soil-to-water ratio of 1:2.5. For determination of soil C and N concentrations, 30 mg of soil was ground by ball mill (Retsch MM400, Haan, Germany) and treated with hydrochloric acid (HCl), followed by analysis using an element analyzer (Vario EL III; Elementar Analysensysteme GmbH, Hanau, Germany). Another air-dried soil subsample was fractionated into sand, silt, and clay using the laser diffraction method (Mastersizer 3000, Malvern Panalytical Inc., UK) (Faé et al. 2019). All particle size analysis results were described as a percentage of the oven-dried soil by weight.
After completing the microcosm incubation experiment, we immediately sampled soil cores and sieved the soil through a 2-mm sieve. Each soil sample was split into three subsamples. The gravimetric water content was determined by oven drying the soil sample at 105°C for 24 h and expressed as WFPS using the bulk density for each site and the mineral soil particle density of 2.65 g cm−3. Microbial biomass carbon (MBC) and nitrogen (MBN) were determined using the chloroform fumigation–extraction method (Brookes et al. 1985). About 10 g of fresh soil was fumigated for 24 h, followed by extraction with 0.5 M K2SO4. MBC and MBN were calculated as the difference in the extractable C and total extractable N between the paired fumigated and unfumigated soils, divided by kC and kN factors of 0.45 and 0.68, respectively (Shen et al. 1984). Extractable organic C and extractable N concentrations were measured using a total organic carbon analyzer (TOC-L-CPN, Shimadzu, Japan). Mineral N (NH4+, NO3−) and dissolved organic C (DOC) concentrations were measured by 2 M KCl using continuous flow injection colorimetry (SEAL Analytical AA3, SEAL Analytical GmbH, Norderstedt, Germany) and a total organic carbon analyzer, respectively.
2.6 DNA Extraction and SmartChip Analysis
DNA was extracted from the soil samples using the FastDNA Spin Kit (MP Biomedicals, USA) according to the manufacturer's instructions. The quality of DNA was assessed by ultraviolet absorbance (ND1000, NanoDrop, Thermo Fisher Scientific, USA). DNA concentration was measured using a QuantiFluor dsDNA Kit (Promega, USA).
High-throughput qPCR-based SmartChip technique (Su et al. 2022; Zheng et al. 2018; Zhu et al. 2017) was used to quantify the abundances of denitrifying genes (narG, napA, nirS, nirK, and nosZ) and nitrifying genes (amoA and amoB) in each soil sample. The choice of primers is listed in Table S2. The bacterial 16S rRNA (515 F/907 R) gene was used as the reference. The mixing system contained 3.1 μL of DNA sample, 24.8 μL of mix enzyme (LightCycler SYBR Green I), and 3.1 μL of denitrifying and nitrifying gene primers. The homogenates were transferred into a SmartChip using a MultiSample NanoDispenser and then quantified with a real-time PCR system (WaferGen, Biosystems, USA). The thermal cycle of qPCR conditions was initial denaturation at 95°C (10 min), followed by 40 cycles of denaturation at 95°C (30 s), annealing at 58°C (30 s), and extension at 72°C (30 s). A threshold cycle of less than 31 was used for later analysis. To ensure reproducibility, each qPCR reaction was conducted in three replicates. Relative and absolute gene copy numbers of a denitrifying gene, a nitrifying gene, and the 16S rRNA gene were calculated as follows (Zhu et al. 2017):
Relative gene copy number RGFun = ,
Where AGFun and RGFun indicate absolute and relative functional gene abundance, respectively. AG16S and RG16S indicate absolute and relative 16S rRNA gene copy number, respectively.
2.7 Bacterial 16S rRNA Gene Sequencing and Data Processing
We used the primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) to amplify the variable V3V4 region of the 16S rRNA gene for identification of bacterial communities (Mori et al. 2014). The gene was amplified, purified, quantified, pooled, and sequenced on an Illumina Novaseq6000 platform. For the demultiplexed paired-end 16S sequences, bioinformatic processing was performed using QIIME 2 version 2022.8 (Bolyen et al. 2019). In brief, primers were trimmed using Cutadapt (Martin 2011). Sequences were then denoised, dereplicated, and chimera filtered using DADA2 (Callahan et al. 2016). Reads were trimmed to retain only high-quality sequences based on the quality plots. Subsequently, filtered sequences were grouped into amplicon sequence variants (ASVs). Taxonomy was assigned to the curated ASVs using q2-feature-classifier against the SILVA release 138 reference database trained for the specific gene region (Bokulich et al. 2018; Quast et al. 2013). We removed chloroplasts and mitochondria ASVs, as well as singletons and doubletons. We used the functional annotation tool of the prokaryotic taxa (FAPROTAX) database to analyze the functional groups of bacteria in the soil (Louca et al. 2016). The bacterial ASVs were compared with dataset obtained by FAPROTAX (script version 1.2).
2.8 Fluorometric Enzyme Assays
The activities of six soil hydrolytic enzymes (α-glucosidase, β-glucosidase, β-xylosidase, cellobiohydrolase, leucine aminopeptidase, and N-acetyl-β-D-glucosaminidase) targeting C and N substrates in soils were measured using a modified method described by German et al. (2011). Briefly, fresh soil samples (2 g) were homogenized in 120 mL of 25 mM maleate buffer with pH 6.0 using a hand blender. The homogenates (125 μL) were mixed with 125 μL fluorometric substrate in microplates and incubated in the dark for 4 h at 25°C. Each assay microplate contained homogenate control, substrate control, and standard (4-methylumbelliferone). Following incubation, fluorescence was measured at 365 nm excitation and 450 nm emission by a fluorometer (BioTek Synergy H1 microplate reader, Winooski, VT, USA). Enzyme activities were calculated from these fluorescence values and expressed as the rate of substrate converted in nmol g1 dry soil h−1. To determine the maximum reaction velocity (Vmax), enzyme activities and substrate concentrations were fitted to the Michaelis–Menten model using nonlinear regression analysis. It is noteworthy that the experimental Vmax is an apparent kinetic parameter, not the actual value. The apparent Vmax indicates the maximum potential enzyme activity and enzyme concentration (Peng et al. 2023). The Vmax of organic C- and N-degrading enzymes was expressed as the sum of Vmax for α-glucosidase, β-glucosidase, β-xylosidase, and cellobiohydrolase, and the sum of Vmax for leucine aminopeptidase activity and β-N-acetylglucosaminidase, respectively.
2.9 Statistical Analysis
Shapiro–Wilk's test and Levene's test were used to assess the normality and homogeneity of variance for N2O fluxes measured both in situ at the IMGERS site and in the microcosm experiment. Log10 transformations were performed where necessary to meet model assumptions. Linear mixed-effect models were used to assess the differences between/among snow treatments, with snow treatments as fixed effects and sampling day and replicates as random effects (Crawley 2012). Relationships between net N2O fluxes and tested variables were explored using a linear regression model for each treatment across all sites.
A multimodal inferencing approach (Anderson and Burnham 2002) was used to determine the relative importance of soil and microbial variables in explaining variation in net N2O fluxes at different simulated snow depths across the 12 study sites. Variables with high collinearity (variance inflation factor, VIF > 5) were excluded through collinearity diagnostics. Multiple generalized linear regression models were generated for all possible combinations of variables using the dredge function in the MuMIn package. The best-supported model for each simulated snow depth was selected based on the lowest Akaike information criterion corrected (AICc) value. The relative importance of each candidate variable in the best-supported model was assessed using proportional marginal variance decomposition (pmvd) through the calc. relimp function in the relaimpo package (Feldman 2005).
Principal component analysis (PCA) was applied to microbial variables to reduce redundancy (Figure S3) and aggregate their effects on net N2O fluxes. Microbial attributes were expressed as an aggregated value derived from the first principal component (PC1) scores, with higher PC1 scores indicating greater microbial function (i.e., greater microbial biomass, enzyme activities, and functional genes, Figure S4). We then fit predictions of net N2O fluxes using WFPS and the new aggregated microbial attributes (PC1 score) as predictors. To evaluate the model's term, we performed model comparisons (Table S3) by separately fitting (i) a linear model with linear interaction terms for the two predictors and (ii) a nonlinear model with the same linear predictors plus a quadratic term for WFPS (WFPS2). The nonlinear model for net N2O fluxes had a lower AIC value (Table S3), indicating better model performance and supporting the inclusion of quadratic WFPS (WFPS2). To integrate the effects of WFPS and microbial attributes, we used the fitted models to predict net N2O fluxes (Table S4). Two-dimensional contour plots were used to visualize how freeze–thaw-induced N2O fluxes varied simultaneously with WFPS and microbial attributes, with observed net N2O fluxes overlaid.
Model performance was evaluated by comparing modeled and observed net N2O fluxes, assessing their agreement by goodness-of-fit measures. The modeled values and observed net N2O fluxes in the microcosm experiment are close to the 1:1 reference line (Figure S5a). The goodness of fit was then summarized by linear regression. The modeled and observed net N2O fluxes are highly correlated (R2 = 0.68, root mean square error = 0.34, Figure S5a). This evaluation verifies that the model can perform well for data prediction. Residual diagnostics were conducted to validate model assumptions. The normality assumption of the model residuals is verified by Figure S5b, supporting log10-transformed net N2O fluxes. Then we used a broken-line regression model (segmented method) in the segmented package (Muggeo 2008) to identify possible thresholds of WFPS, and thus visualize the significant interaction effects of WFPS and microbial attributes on net N2O fluxes.
Redundancy analysis (RDA) was conducted using the vegan package to evaluate the effects of climate, soil properties, and plant on microbial attributes among the three types of steppes and their individual effects based on the rdacca.hp package (Lai et al. 2022). All statistical analyses were conducted in R 4.3.0, and significance was considered at p < 0.05.
3 Results
3.1 Snow Depth Drives Freeze–Thaw-Induced N2O Emissions
Our high-frequency in situ measurements showed that deeper snow significantly increased N2O emissions compared to ambient snow throughout the year (p < 0.03; Figure 1a,b). Specifically, deeper snow increased freeze–thaw-induced N2O emissions (p = 0.008; Figure S6). The maximum pulse of N2O emissions under deeper snow (252.4 μg N m−2 h−1) was nearly ninefold of that in ambient snow (29.5 μg N m−2 h−1; Figure S6). Remarkably, N2O emissions during the brief 46-day freeze–thaw period accounted for 45% and 57% of annual fluxes in deepened and ambient snow, respectively (Figure 1b).
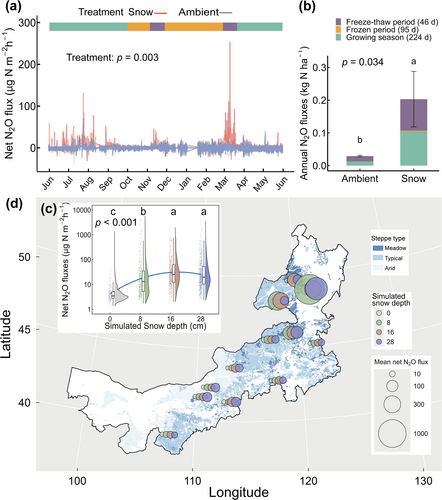
Across all soil collected, freeze–thaw-induced N2O emissions nonlinearly increased with simulated snow depths (p < 0.02; Figure 1c; Figure S7a). Notably, significant spatial variability was observed between sampling sites in N2O emissions with simulated snow depth, ranging from 3.4 ± 0.2 to 1184.1 ± 243.9 μg N m−2 h−1 (Figure 1d). Emissions were lowest in arid steppes (3.7 ± 0.1~37.6 ± 4.7 μg N m−2 h−1), followed by typical steppes (5.7 ± 0.5~94.9 ± 29.4 μg N m−2 h−1), and were highest in meadow steppes (20.1 ± 10.3~302.2 ± 67.5 μg N m−2 h−1) across all simulated snow depths (p < 0.03; Figure S7b). A similar pattern with N2O emissions was observed between WFPS and simulated snow depths (p < 0.03; Figure S8).
3.2 Controls of Ex Situ Net N2O Fluxes
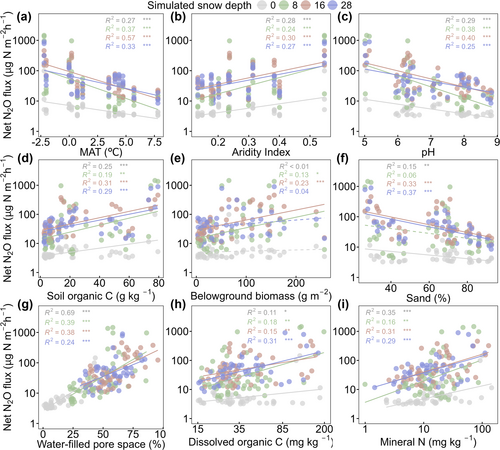
At each simulated snow depth, net N2O fluxes were negatively correlated with MAT, pH, and sand content (Figure 2a,c,f). In contrast, positive correlations were observed with AI, SOC, BGB, WFPS, and C and N availabilities (Figure 2b,d,e,g–i), with particularly strong correlations between net N2O fluxes and WFPS. Substrate availability and Vmax of organic C- and N-degrading enzymes were increased with WFPS (Figure S9).
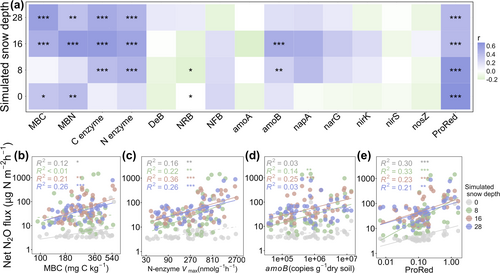
Net N2O fluxes were positively correlated with MBC and MBN, Vmax of organic C- and N-degrading related enzymes, amoB gene abundance of ammonia-oxidizing bacteria involved in nitrification, and the ratio of N2O production to reduction genes (ProRed: N2O production genes consisted of narG- and napA-encoding nitrate reductase, and the N2O reduction gene consisted of nosZ-encoding N2O reductase), although the strength of these correlations varied across simulated snow depths (Figure 3a–e).
3.3 Relative Importance of Controls of Ex Situ Net N2O Fluxes
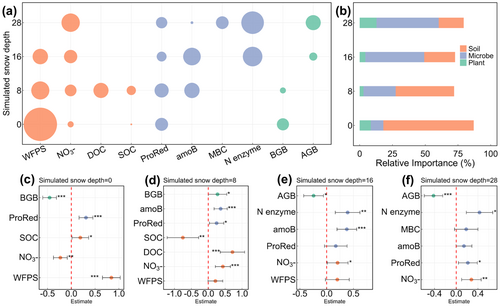
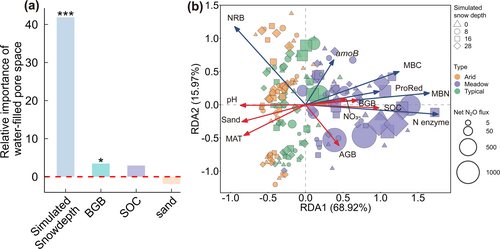
Under snow-free conditions, WFPS emerged as the overwhelmingly important predictor, with NO3−-N, SOC, ProRed, and BGB collectively explaining 86% of total variation in net N2O flux (Figure 4a–c). Under shallow snow conditions, soil and microbial factors together explained 68% of their total variation, of which abiotic factors accounted for over 50% (Figure 4b,d). In contrast, microbial factors, particularly Vmax of N-related enzymes and bacterial amoB gene abundance, dominated N2O emissions at 16 and 28 cm of simulated snow depth (Figure 4b,e,f). Notably, the contribution of soil physiochemical properties to the explained variance decreased from 80% to 24% with the increase in simulated snow depth (Figure 4b). Specifically, the importance of WFPS diminished sharply from 65% to being excluded from the model, while microbial predictors rose from 17% to 60% (Figure 4b).
Two thresholds for WFPS, 43% and 66%, were identified as key transitions for N2O emissions (Figure 5a). A significant interaction was observed between WFPS and microbial attributes (Figure 5b,c), aggregated values of microbial predictors based on PCA (Figure S3). Specifically, in Phase 1 (WFPS < 43%), soil moisture was the overwhelming factor triggering N2O emission. In Phase 2 (43% ≤ WFPS ≤ 66%), both microbial attributes and soil moisture jointly control emissions (Phase 2). Finally, in Phase 3 (WFPS > 66%), microbial attributes acted as the sole constraints on N2O emissions (Figure 5a,b,d).

3.4 Factors Driving Hotspots of N2O Emission Across Ecoregions
Given the joint control of N2O pulses by WFPS and microbial attributes, we further analyzed the factors shaping their spatial patterns to identify potential N2O hotspots. In addition to simulated snow depths, WFPS was strongly influenced by BGB and SOC (Figure 6a). The spatial heterogeneity of microbial attributes was mainly shaped by MAT, pH, BGB, SOC, and sand content (Figure 6b; Table S5), collectively accounting for 35% of the variance. Thus, in cold and humid grassland ecosystems with relatively low pH, high root biomass and microbial activity promoted the formation of hotspots for freeze–thaw-induced emissions, where N2O pulses were 1–2 orders of magnitude higher than those in arid steppes.
4 Discussion
By integrating high-frequency automated N2O flux measurements with cross-ecoregion microcosm incubations spanning 1500 km across diverse grassland ecosystems, we explored how snow regime shifts affected the spatial patterns and controls of freeze–thaw-induced N2O emission “hot moments.” Aligning with previous findings (Abalos et al. 2016; Wolf et al. 2010), we found that the spring freeze–thaw period (~50 days) accounted for nearly 50% of annual N2O fluxes, supporting our first hypothesis and highlighting the importance of accurately quantifying these transient events in N2O budget estimations. Notably, these emissions during the freeze–thaw period increased nonlinearly with simulated snow depth (Figure 1c), driven by phase transitions defined by specific WFPS thresholds (Figure 5). These thresholds marked a shift in the primary controls of N2O emissions, from soil physiochemical properties under shallow snow conditions to microbial properties at greater simulated snow depths. This hierarchical framework of biotic and abiotic controls not only challenges the conventional view that microbial activity is negligible at low temperatures but also provides new mechanistic insights into the critical role of winter climatic conditions in greenhouse gas accounting.
4.1 Controls of Freeze–Thaw N2O Emission Hot Moments
Freeze–thaw N2O pulses are driven by both the physical release of trapped N2O and microbial processes (Risk et al. 2013). While we could not directly quantify the relative importance of the two concurrent processes, previous studies suggest that physical release is less significant than microbial production during freeze–thaw period (Risk et al. 2014; Teepe et al. 2001). This was evidenced by our findings that N2O emissions were significantly accelerated with simulated snow depths (Figures 1c and 5) despite similar soil temperatures during the freeze period among these treatments (Figure S2). When the soil is dry, the ice crystals form without disrupting the soil matrix (Bullock et al. 1988), potentially trapping N2O due to the limited gas diffusion; in contrast, the presence of snowmelt water can facilitate the freeze–thaw expansion and contraction of ice, destabilizing soil aggregates and releasing nutrients from plant and microbial detritus within them (Bullock et al. 1988; Ruan and Robertson 2017). This could improve nutrient accessibility for nitrifiers and denitrifiers, thereby driving de novo N2O production (Wang et al. 2023). Thus, while physical release occurs during initial thawing, microbial processes, which remain active at low temperatures, sustain N2O production, particularly during the thaw phase when conditions favor denitrification (Risk et al. 2013).
Additionally, the soil's physicochemical structure, particularly the arrangement of organic residues within soil pores, could play a critical role in triggering N2O emission “hot moments” during freeze–thaw cycles (Groffman et al. 2009; Kim et al. 2022; Kravchenko et al. 2017). As snowmelt is absorbed by organic residues such as plant litter or organic particles, localized anaerobic hotspots conducive to denitrification are created (Kim et al. 2022; Kravchenko et al. 2017). High moisture levels within organic residues not only facilitated microbial metabolisms, leading to oxygen consumption, but also restricted oxygen inflow. Consequently, organic residues served as hubs for multiple processes (i.e., decomposition, nitrification, and denitrification), triggering substantial N2O emissions (Butterbach-Bahl et al. 2013). Indeed, our results showed that freeze–thaw-induced N2O emissions increased significantly with SOC and belowground plant C input (Figure 2d,e). Additionally, as simulated snow depth increased, WFPS also rose (Figure S8), facilitating the diffusion of substrates to surviving microbes within soil pores (Sorensen et al. 2016). This rise in WFPS could reactivate dormant microbes and enhance enzyme activities related to C and N cycling (Figure S9), thereby promoting greater N2O emissions.
Furthermore, we found that changes in the proportion of producers (narG/napA) and consumers (nosZ) of N2O played a critical role in determining N2O emissions at all simulated snow depths (Figure 3a,e). This emphasized the critical role of nitrate-reduction denitrifiers, instead of nitrite-reduction denitrifiers (nirS- and nirK-type denitrifiers), in regulating N2O emissions during freeze–thaw cycles in arid and semiarid grasslands. While most molecular studies of bacterial denitrification focus on nitrite reductase (Bothe et al. 2000), which catalyzes the first key step that results in the formation of a gaseous intermediate (Groffman et al. 2006), the role of nitrate reductase should not be ignored, especially in arid regions (Krichels et al. 2023). NarG denitrifiers can survive and remain active in arid environments, potentially favoring NO3− reduction to N2O upon wetting (Krichels et al. 2023). Under oxygen-limited conditions, NO3− reduction may serve as an alternative pathway for energy conservation (Wang et al. 2023). Collectively, the development of anaerobic microenvironments, combined with moisture-induced variation in soil C and N substrates, enzyme activities, and specific soil functional genes, synergistically influenced freeze–thaw N2O pulses.
4.2 Interactive Effects of WFPS and Microbes on Freeze–Thaw N2O Emissions
Our analysis revealed that abiotic and biotic factors interacted hierarchically to regulate freeze–thaw N2O emissions, with their relative importance shifting across different conditions (Figure 4), supporting our second hypothesis. This shift in control patterns was strongly dependent on WFPS thresholds (Figure 5), aligning with a global study that emphasizes moisture thresholds as pivotal in determining ecological processes (Zhang et al. 2023). Below 43% WFPS (Phase 1), typical of snow-free soil conditions or soils with low capacity to retain snowmelt, WFPS was the most decisive environmental trigger for freeze–thaw N2O emissions (Figure 4a). Low soil water content enhances oxygen availability in soil (Wei et al. 2023), making the conditions unfavorable for denitrifying activities and anaerobic denitrification (Wu et al. 2014; Yao et al. 2010), consequently constraining N2O emissions.
As WFPS continued to increase to 43%–66% with greater simulated snow depths (Phase 2), we noticed an abrupt decline in the importance of WFPS, whereas the role of microbial properties became more pronounced (Figures 4 and 5), suggesting that once anaerobic conditions were established, soil microbial properties became paramount. At WFPS levels exceeding 66% (Phase 3), close to the optimal 70%–80% range for denitrification (Davidson et al. 2000), microbial control became dominant. These high soil moisture conditions created strongly anaerobic conditions with low gas diffusivity, facilitating N2O reduction to N2 (Davidson et al. 2000). In the two phases, soils with enhanced microbial N cycling functions, particularly those with higher N-cycling enzyme activities (Figure S9), greatly influenced the magnitude of the N2O pulses (Figure 4e,f). These findings emphasize the complex interplay between soil moisture and microbial attributes in regulating N2O. Specifically, maintaining higher soil moisture levels was a prerequisite for controlling soil microbial activities and triggering freeze–thaw N2O emission “hot moments,” a pattern also reflected in the Cannon model proposed by Zhang et al. (2024).
4.3 Characteristics of Hotspots for Freeze–Thaw-Induced N2O Emissions
The climate and soil properties determined plant productions as well as microbial properties (Figure 6b), together controlling the spatial patterns of N2O emissions during the freeze–thaw period across different ecoregions. Compared to warm and dry steppes, cold and humid meadows harbored higher microbial biomass, N enzyme kinetics, and greater abundance of denitrifying genes (Figure S4), all of which indicated enhanced microbial N cycling. In these regions, higher root production not only enhances root C inputs (Phillips et al. 2011) but also increases WFPS by promoting soil porosity through root growth and soil aggregation (Figure 6a). This combination of high WFPS and greater plant C input (Figure 6b) facilitates the formation of plant residue-induced microscale hotspots of N2O production and emissions (Kravchenko et al. 2017). Consequently, these microscale hotspots aggregated into large-scale hotspots, contributing to 4 to 21-fold greater freeze–thaw-induced N2O emissions in cold and humid grasslands than warm and dry steppes, which supported our third hypothesis. In addition, deepened snow also promotes aboveground biomass production (Deng et al. 2023), which can accumulate a thick snow cover (Essery and Pomeroy 2004; Wolf et al. 2010), further enhancing N2O emissions during the freeze–thaw period.
Soil properties, particularly soil pH and texture, were also key modifiers for spatial variability of freeze–thaw N2O emissions (Figure 2c,f). The relationship between N2O and pH is multifaceted, as pH regulates both N-related enzyme activity (Sinsabaugh et al. 2008) and denitrifier community composition (Liu et al. 2014; Qiu et al. 2024). Consistent with the global study by Sinsabaugh et al. (2008), our results showed that N-related enzyme activity decreased with increasing pH from 5 to 9 (Figure 6b). This suggested that low pH enhanced N mineralization by increasing enzyme activities, thereby improving substrate availability and promoting N2O emissions. Furthermore, pH can indirectly affect N2O emissions by shaping the denitrifier community (Qiu et al. 2024), thus regulating the spatial variability of freeze–thaw N2O emissions.
Soil texture determines SOC formation and water-holding capacity (Rawls et al. 2003), thereby shaping N2O emissions (Figure 6). As noted by Kravchenko et al. (2017), the interaction between soil water and organic residues within soil pores is a critical driver for the occurrence and activities of N2O hotspots (Figure 6b). Specifically, sandy soils in our study area, typically found in high-pH, warm regions, have large soil pores that result in rapid drainage and limited substrate retention, constraining microbial processes and reducing N2O production. By contrast, clay-rich soils, generally found in low pH and cold regions, exhibited enhanced moisture retention, accumulation of organic residue, and favorable enzymatic conditions, fostering an environment conducive to the formation of N2O emission hotspots (Kim et al. 2022; Kravchenko et al. 2017). Therefore, these textural and climatic interactions jointly shaped the spatial variability of freeze–thaw N2O emissions under climate change.
4.4 Implications for N2O Modeling and Emission Accounting
Understanding the mechanisms that control the occurrence and the magnitude of freeze–thaw induced N2O “hot moments” is crucial for reducing uncertainty in N2O accounting (Butterbach-Bahl and Wolf 2017; Wagner-Riddle et al. 2017). To our knowledge, this is the first study to combine in situ high frequency N2O automated measurements with cross-ecoregion soil core incubations to explore the effects of snow regime shift on freeze–thaw-induced N2O emissions. Our findings revealed a shift in the controlling factors for N2O emissions, from WFPS, which governed the establishment of anaerobic conditions, to microbial constraints with increasing simulated snow depth. The hierarchical control exerted by WFPS and microbial properties explained much of the high temporal and spatial variability of N2O fluxes during freeze–thaw periods (Figure 5).
Building on this framework, we highlighted the potential to refine the parameterization in process-based models, enabling them to better capture the dynamic responses of freeze–thaw-induced N2O fluxes to changing environmental conditions. For example, integrating WFPS thresholds to categorize N2O emission drivers at different WFPS levels into model algorithms could enhance their predictive accuracy. Since WFPS is relatively easy to measure, and key microbial attributes driving N2O emissions can be estimated using climate and soil physicochemical properties, these insights could streamline the identification of N2O hotspots during freeze–thaw periods. Incorporating the hierarchical interactions between WFPS and microbial properties into predictive models would provide better tools for accurate N2O accounting and management of N2O emissions in response to climate change.
While our study provides valuable insights into freeze–thaw N2O pulses, several limitations must be acknowledged. Controlled simulations with standardized temperature fluctuations and truncated snow cover duration may introduce uncertainties when extrapolating findings to natural field conditions. Additionally, the limited sampling sites may restrict the generalization of findings across broader climatic and ecological gradients. Future studies should focus on multisite experiments that simultaneously monitor key variables (e.g., soil temperature, moisture, and microbial community) to better capture the complexity of freeze–thaw processes. Moreover, advanced techniques are needed to overcome the challenges of field monitoring during freeze–thaw periods. High-frequency automated chambers, coupled with isotopic tracing and integrated sensor networks measuring soil thermal and hydric conditions, can offer a comprehensive, multidimensional dataset, improving both N2O emission quantification and our understanding of the underlying mechanisms. This is crucial for improving N2O accounting, especially given that more than 50% of the continental land areas in the Northern Hemisphere experience freeze–thaw cycling (Chen et al. 2022).
Author Contributions
Jie Luo: conceptualization, data curation, formal analysis, investigation, methodology, validation, writing – original draft, writing – review and editing. Yong Peng: data curation, investigation. Zhou Jia: data curation, investigation. Yuntao Wu: data curation, investigation. Yuxuan Gao: data curation, investigation. Nairsag Jalaid: data curation, investigation. Xingming Zhang: data curation, investigation. Heng Ge: data curation, investigation. Bowen Qing: data curation, investigation. Hongyi Chen: data curation, investigation. Yanxin Zhan: data curation, investigation. Ping Li: investigation, methodology. Lingli Liu: conceptualization, funding acquisition, methodology, project administration, resources, supervision, writing – review and editing.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (32125025, 32330066, 32101307, and 32301359). We thank Wei Liu, Xiaoqin Zhu, Li Zhang, and other members of the Analytical and Testing Center for their assistance in the laboratory analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in Plant Data Center, Chinese Academy of Sciences at https://www.plantplus.cn/en/doi/10.12282/plantdata.1670. The sequence data generated in this study have been deposited in the National Genomics Data Center under accession number PRJCA034979.




