The End of an Era? Trends in Abundance and Reproduction of Australian Southern Right Whales (Eubalaena australis) Suggest Failure to Re-Establish Pre-Whaling Population Size
Funding: The lead author was funded by a research scholarship through the University of Tasmania and supplemented with the John Bannister Memorial Top-Up scholarship. Aerial surveys from 1978 to 2014 were funded by the Commonwealth Department responsible for the Environment, including latter periods administered through the Australian Marine Mammal Centre, except in 2009 when funds were obtained through The Island Foundation. Between 2015 and 2024, the aerial surveys were funded under the Australian Government's National Environmental Science Program through the Marine Biodiversity Hub and Marine and Coastal Hub.
ABSTRACT
The large-scale exploitation of whale populations in the whaling era led to the near extirpation of large whales all over the world. This must have had major repercussions for marine ecosystems globally. Consequent changes to those ecosystems and physical environments create uncertainty around whether present-day conditions are adequate to support full recovery of pre-whaling population sizes. Combined with potential effects of anthropogenic stressors, the future viability of exploited whale populations is questioned. This migrating species was left near extinction from whaling and has shown slow, yet steady, recovery in recent decades. Here, we collate abundance data from aerial surveys performed along the Australian coast between 1976 and 2024, covering 2250 km of coastal habitat, to study the recovery trajectory of Australian southern right whales (Eubalaena australis). We describe temporal trends in abundance, reproduction and growth of the western sub-population. Our study reveals that despite previously displaying exponential growth, and a present population size still residing far below pre-whaling levels, annual births have started declining since 2016 and annual abundances of unaccompanied individuals have dropped by 66%. Our results suggest the end of an era of this population's recovery, highlighting that an initial period of steady recovery does not guarantee successful re-establishment of previous abundance levels.
1 Introduction
Population crashes are a naturally occurring phenomenon in ecosystem dynamics but have become more frequent as a result of human exploitation and through various indirect effects of anthropogenic stressors (De Vos et al. 2015; Hoffmann et al. 2010). If, and to what extent, a population succeeds in growing once relieved from its driver decline, depends on a complex interplay between the intrinsic characteristics of a species (e.g., life history), population characteristics (e.g., demographic structure) and its interaction with the external (biotic and abiotic) environment (Gårdmark et al. 2003; Lotze et al. 2011). Given the dynamic nature of ecosystem conditions, populations' carrying capacities (the population size that can be sustained by present ecosystem conditions) may have changed and no longer match former population sizes (Del Monte-Luna et al. 2004). Hence, whether in the case a population does succeed in growing, former population sizes can be fully restored, is strongly dependent on whether present ecosystem conditions are still adequate to support pre-collapse abundance levels (Gårdmark et al. 2003; Lotze et al. 2011).
The southern right whale (SRW, Eubalaena australis) provides a textbook examples of population crashes, induced by intense whaling that targeted the species worldwide (Clapham 2016; Laws 1977; Romero et al. 2022). The name ‘right whale’ literally stems from being considered ‘the right whale to kill’. With an estimated 150,000 whales killed in the Southern hemisphere between 1790 and 1980 (Jackson et al. 2008), Southern right whales (SRW, Eubalaena australis) were one of the first whale species to be hunted to near extinction (Clapham 2016; Laws 1977; Romero et al. 2022). By the 1920s there were only an estimated couple hundred individuals remaining worldwide: the species was considered virtually extinct (Bannister 1986, 1990; Ling and Aitken 1981).
SRWs are migratory mammals with a circumpolar distribution in the Southern Hemisphere, between latitudes 20° S and 60° S (Kenney 2018). The migration of this species is tightly linked to their reproductive cycle: during austral winter, individuals migrate to coastal areas in lower latitudes where females gestate and use shallow coastal waters to nurse their young (Best et al. 2003; Carroll et al. 2011; Sprogis et al. 2024). Coastal aggregation areas are found around South Africa, Brazil, Argentina, Chile/Peru, Australia and New-Zealand (Bannister et al. 1997; Best et al. 2003; Carroll et al. 2015, 2020; Patenaude et al. 2007). In summer, SRWs reside in productive feeding grounds in higher latitudes, located between 40° and 65° south (Bannister et al. 1999; Carroll et al. 2011; Corkeron and Connor 1999; Rowntree et al. 2008; Sprogis et al. 2024), where their diet consists of euphausiids, including Antarctic krill (Euphausia superba), and other zooplankton, particularly copepods (D'Agostino et al. 2018, 2023; International Whaling Commission 2001; Tormosov et al. 1998). Apart from females with their calf, other individuals visit coastal regions too, but it is unsure whether there is consistency in what demographic group(s) and/or fraction of the population these ‘unaccompanied individuals’ (adults sighted in coastal areas not accompanied by a calf) represent (Best et al. 2003). It is hypothesised they might be visiting coastal waters for mating, but yearlings, sexually immature, skip-breeders and females that still need to gestate or had a failed pregnancy, may be part of this group too (Best et al. 2003).
In 1985, a global moratorium on whaling was implemented by the International Whaling Commission (IWC) (1978, 1986). Whale stocks have thereafter shown significant recovery post-whaling, but there is large variation in recovery rates between species, regions and populations (Baker and Clapham 2004; International Whaling Commission 2001; Tulloch et al. 2019; Zerbini et al. 2019). SRW recovery rates have been relatively slow compared to other whale species, such as humpback whales (Megaptera novaeangliae) (International Whaling Commission 1978; International Whaling Commission 1986; International Whaling Commission 2001; Noad et al. 2019; Tulloch et al. 2018). This is largely due to SRWs' relatively long reproduction cycle (Best 1994; Hamilton et al. 1998; Lockyer 1984; Zerbini et al. 2010). In healthy conditions, females are assumed to have their first offspring at the age of seven or eight, followed by 3-year calving intervals (period between subsequent calves) (Bannister 2001; Best 1994, 2001; International Whaling Commission 2001). Their 3-year calving cycle includes 1 year of gestation, 1 year largely spent nursing and 1 year of recovery allowing females to replenish body fat stores (Best 1994, 2001). Because SRWs are capital breeders that fast while nursing their calf, females are heavily reliant on the body fat storage built during summer (Best 1994; Lockyer 1984). Failure to acquire sufficient body fat reserves causes failed pregnancies, reduced calf survival and longer post-nursing recovery (Christiansen et al. 2018; Germishuizen et al. 2024; Leaper et al. 2006; Seyboth et al. 2016). Hence, deviations from the assumed standard calving interval are commonly linked to poor maternal body condition and limited food resources (International Whaling Commission 2013; Vermeulen et al. 2023).
Despite being one of the first whale species to receive legal protection (in 1935) the onset of SRW recovery was delayed until the 1970s (Bannister 1986, 1990; Ling and Aitken 1981). This is likely because illegal offshore whaling continued to exploit the species into the 1960s–1970s (Romero et al. 2022; Tormosov et al. 1998) but may have additionally been influenced by post-whaling demographic structure (Best 1994; Hamilton et al. 1998; Lockyer 1984; Zerbini et al. 2010). Nevertheless, SRW populations have substantially grown post-whaling: in 2009, the global SRW population was estimated to consist of 12,000–15,000 individuals, which was estimated to represent approximately 10% of their estimated pre-whaling abundance (International Whaling Commission 2013). There are strong differences in the status and growth rates of SRW populations (Arias et al. 2018; Bannister 2001; Best 2001; Brandão et al. 2023; Charlton et al. 2022; Findlay et al. 2017; International Whaling Commission 2001; Romero et al. 2022; Smith et al. 2023; Stamation et al. 2020; Vermeulen and Wilkinson 2023; Watson et al. 2021). For example, SRW populations in New Zealand (Carroll et al. 2013), South Africa (Brandão et al. 2018, 2023), Argentina/Brazil (Cooke et al. 2001; Groch et al. 2005) and southwest Australia (Bannister 1990, 2001) showed signs of relatively steady growth during the first few decades post-whaling (International Whaling Commission 2013), whereas abundances in Chile–Peru and southeast Australia (Stamation et al. 2020) remain small. Recently, various studies have flagged declining growth rates and increased mortality at major SRW aggregation areas (Cooke et al. 2001; Crespo et al. 2019; Findlay et al. 2017; Germishuizen et al. 2024; van den Berg et al. 2019; Vermeulen et al. 2023), and in Australia, increased inter-annual variation in birth rates was recently observed, potentially signalling similar trends (Smith et al., 2021). Provided these regions account for the majority of the global SRW stock (International Whaling Commission 2013; International Whaling Commission 2001), this has raised concerns about the further recovery of the SRW populations as well as their resilience under projected climatic changes (Agrelo et al. 2024; Albouy et al. 2020; Burnell 2001; Jackson et al. 2016; Sousa et al. 2021; Tulloch et al. 2018, 2019).
Here, we investigate the coastal abundance and reproductive success of the western Australian SRW sub-population, which constitutes the majority of the Australian SRW population (Smith et al. 2021). We collate 49 years of aerial survey data (1976–2024), capturing a period wherein this population grew from being virtually extinct, to an estimated couple thousand individuals. Hence, capturing a crucial period in the population's recovery. Our objective is to gain better insight into the population dynamics of SRWs post-whaling recovery. We assess temporal trends in abundance, growth rate, population size and calving intervals. We aim to contribute to future research on the general factors, processes and mechanisms that may support and/or jeopardise the recovery of animal populations after a substantial decline, which the presented study may help unravel.
2 Methods
2.1 Study Area
In this study, we collate and analyse Southern right whale abundance data ranging from 1976 to 2024, a total of 49 years of data. Our study area (Figure 1) spans the coastline from Augusta (Cape Leeuwin, Western Australia) to Ceduna (South Australia): covering more than 2250 km of coastline within the Biologically Important Area for SRWs (DCCEEW 2024). This study area includes all three large established coastal aggregation areas of the Australian SRW population (Doubtful Islands Bay, Israelite Bay, Head of Bight; Figure 1) (Bannister 2017; Charlton et al. 2019; Commonwealth of Australia 2012). This is a uniquely extensive spatial and temporal data coverage for research on baleen whales.
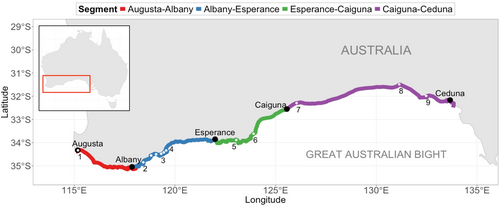
The Australian SRW populations consist of an eastern and western Australian population (also referred to as ‘southwestern’) (Bannister et al. 2011; Commonwealth of Australia 2012; DCCEEW 2024). These two sub-populations show some degree of genetic differentiation and have been found to display varying levels of growth, but their connectivity remains debated (Bannister 2017; Carroll et al. 2011). The western population is assumed to represent the majority of Australian SRWs, with a recent population estimate of more than 2000 individuals, whereas the eastern sub-population is estimated to consist of only a couple 100 individuals (International Whaling Commission 2013; Bannister 2017; Smith et al. 2021, 2023; Stamation et al. 2020). Our study area includes all aggregation areas attributed to the western Australian sub-population: (1) Flinders Bay; (2) Hassell Beach; (3) Cheyne, Wray, Dillon and Bremer Bays; (4) Doubtful Island Bay; (5) Yokinup Bay; (6) Israelite Bay; (7) Twilight Cove; (8) Head of Bight and (9) Fowlers Bay (Figure 1) (Bannister 2017; Charlton et al. 2019; Commonwealth of Australia 2012). Hence, our study area presumably covers all areas used as calving grounds by the western Australian sub-population.
2.2 Data Collation
2.2.1 Aerial Surveys
The uniquely extensive spatiotemporal extent of this study was achieved by curation and collation of SRW abundance records derived from aerial surveys conducted in Australia between 1976 and 2024 (Bannister 2001). These surveys were specifically aimed at monitoring SRW abundances. Some of the data have previously been analysed, but only as separate data sets (Bannister 2001). To date, these data have not been combined due to differences in flight paths, frequency as well as the annual spatial coverage, which are resolved in this study (see Section 2.2).
Aerial surveys were performed with single engine aircrafts (e.g., Cessna 172). Although no fixed transect line is defined, the aircraft remained within one nautical mile off the coast, flying parallel to the coastline at circa 1000 ft altitude. Human observers, positioned on both sides of the aircraft, searched the coast and whale sightings were recorded. At minimum, the number of individuals, their class (cow–calf pair, unaccompanied individual, juvenile) and the time of observation was recorded. Upon sightings of SRWs, the aircraft descended to 500 ft to capture a photograph of the whales' head with a hand-held camera (for photo identification, not used in this study), whereafter search is continued. In recent years, GPS locations of individual whales have been recorded, but this information is unavailable for the majority of the survey records included in the presented study. Survey flights were only performed on days when weather conditions are sufficient (e.g., wind speeds below 15 knots and good visibility) to support whale detection. All survey flights were deployed by the same organisation, and the flight crew (consisting of a pilot, photographer and two observers) has been largely consistent.
Annual surveys consisted of a series of outbound flights in the eastern direction (e.g., starting in Augusta, ending in Ceduna) performed on subsequent days, presuming appropriate weather conditions. The same coastal area is monitored again during the return journey of the aircraft (in western direction). Combined (out plus back) this series of flights is generally performed within a time span of 8 days. Further survey details are provided by Bannister (2001) and in Supporting Information S1.
The total spatial coverage and number of flights have varied between and within years. Initially, the surveys covered the coastline from Augusta to Israelite Bay (Western Australia), but in 1983, it was extended eastwards to expand from Augusta to Caiguna as a response to increased sightings around there (Bannister 2001). Since 1993 to date, the coastline between Augusta and Ceduna has been surveyed (Figure 1). From 1976 to 2007, aerial surveys were performed multiple times a year. From 2007 onwards, frequency was reduced to one series of survey flights (out and back), annually, monitoring from Augusta to Ceduna. The most extensive spatial extent was always surveyed between August and mid-September: coinciding with peak calf abundance (Bannister 1990; Charlton et al. 2022; Charlton et al. 2019). A detailed overview of survey flights is provided in Supporting Information S1.
The search area during survey flights covers the coastal area between the coastline and 1.5 km offshore. Because cow–calf pairs (SRW mothers with their young) reside almost exclusively in the shallow coastal waters within 1 km from the coast, the number of undetected cow–calf pairs along the surveyed coastline is assumed negligible (Best et al. 2003; Charlton et al. 2019; Elwen and Best 2004; Rayment et al. 2015; Renault-Braga et al. 2018). The (local) coastal residence time of cow–calf pairs ranges from 2 to 3 months, and various studies have shown the abundance of calves to be relatively consistent during peak abundance, occurring mid-August to mid-September (Bannister 1990, 2001; Burnell and Bryden 1997; Charlton et al. 2019, 2022; Kemper et al. 2022; Rowntree et al. 2001). Because all calving grounds of the western sub-population are included in our study area, the total abundance of calves observed during the aerial surveys (assuming total study area coverage) performed during this peak calf abundance is assumed to provide an accurate representation of the total annual number of births of the southwestern Australian sub-population (Bannister 2001; Best et al. 2003). Presented abundances of unaccompanied individuals should be interpreted with more caution, as these animals are more likely to move around between aggregation areas and possibly further offshore.
2.2.2 Data Collation and Pre-Processing
Integration of abundance data from 1976 to 2024 requires careful consideration of differences in, among others, the level of detail of observation records, as well as in annual spatial coverage, locations of start and end locations of flights and annual monitoring frequencies. Variability in the spatial extent of the survey area (see Section 2.1) as well as flight paths (departure and landing locations) prevents direct comparison of SRW abundances per flight or per year, and information on sightings locations (e.g., GPS-locations) was incomplete, hence unaccommodating to deal with this variation (e.g., using spatially explicit models). The surveys are not designed as a distance sampling study (e.g., no strictly standardised transect line, strip width or flight altitude). Resultingly, sightings probability and survey effort cannot be calculated (Bannister 2001), and sightings locations and times are not consistently recorded. On occasion, only total abundances (per class) were available instead of individual sightings records, and return-flights before 1993 did not record whales observed during the outward flight.
To collate the data while ensuring maximum spatiotemporal coverage of our analyses, we introduced coastal ‘segments’, splitting the study area into four segments (Figure 1). Segments are a derivative of the routes most flown (e.g., Augusta–Albany), but the observed number of whales on a segment can also consist of the sum of SRW observations from multiple flights (but never partial flights). For example, the number of individuals sighted from Caiguna to Nullarbor and from Nullarbor to Ceduna was summed to form segment Caiguna–Ceduna (between Caiguna and Ceduna the location of stops was highly variable, see Supporting Information S1). Flights were only collated if the complete set of flights covering the segment was performed on the same day and did not survey the same aggregation area in duplicate. Details are provided in Supporting Information S1.
What's more, outwards and return flights along the same segment cannot be considered independent samples if performed with only a few days in between. This was solved by deriving the maximum abundance per segment per month per year (maximum between the out and back surveys). Only those maximum abundances were used for further analyses. We truncated the data set to include observations only made within the peak calf abundance, to prevent overfitting and minimise potential statistical biases potentially created by differences in annual survey frequency. This has minimal impact on the results within the context of this study, as within-year variation in calf pair abundance is known to be negligible from August to mid-September (see Section 2.1) (Bannister 2001; Charlton et al. 2019).
2.3 Statistical Analysis
The abundance of calves and unaccompanied individuals was analysed separately, juveniles not presented due to small sample size (see Supporting Information S1). For modelling, we expressed the abundance data as the number of individuals per 100 km surveyed segment, to accommodate variation caused by the variable lengths of the segments. Segment lengths were calculated using a fixed length for each segment, derived from spatial line objects approximating the line of flight, drawn in QGIS (QGIS Development Team 2024). Standardised lengths of the segments are 349 km (Augusta–Albany), 532 km (Albany–Esperance), 485 km (Esperance–Caiguna) and 890 km (Caiguna–Ceduna).
We applied two types of regression models (details in 2.3.1 and 2.3.4), using Bayesian inference applied using the R-INLA package (Lindgren and Rue 2015). Bayesian inference was preferred over common frequentist approaches primarily because of its flexibility in the application of more complex model structures (Zuur 2012; Zuur et al. 2009). For all presented models, a Gaussian probability distribution with identity link function was applied. To avoid problems with pseudo-replication, we applied mixed models with factor segment as a random effect. We performed model selection and validation using protocols established by Zuur (2012) and Zuur et al. (2017). Model performance was evaluated based on Deviance Information Criterion (DIC) and Watanabe-Akaike's Information Criterion (WAIC) (Van Der Linde 2005; Watanabe and Opper 2010; Zuur et al. 2017).
2.3.1 Temporal Trends
We established an interpolated temporal trend for the total number of calves over the entire study area for the entire study period by using predicted values from the fitted GAMMs. Predicted values were generated by simulating 1000 values per segment, ranging from 1976 to 2024, and taking the mean of these values per segment per year. Predicted values were converted back to absolute abundances (counts, using segment lengths). A temporal trend for the entire study area was generated by taking the sum of the predicted values, per year, of the four segments.
2.3.2 Annual Growth Rate
2.3.3 Population Size
We additionally used the fitted GAMMs to estimate the current size of the western Australian SRW population based on the modelled calf abundance in the most recent year (2024). We calculated population estimates using the ‘conversion factor’ established by the International Whaling Commission (2013). This approach to estimating SRW population size uses an estimated number of mature females in a focal year based on the sum of calves observed in the last 3 years. Instead of taking the sum of the number of observed calves, we used the GAMM output (Figure 2) multiplied by three. This approach assumes a 3-year calving interval, which may result in an underestimation of the number of mature females, provided indications of a lengthening of calving intervals (see Results and Germishuizen et al. 2024). For this reason, we repeated the calculations using an estimated number of mature females derived by multiplying the estimated number of calves in 2024 by five instead of three. This assumed that all mature females in the population breed in 5-year intervals; hence, it should be considered a highly pragmatic estimate. We calculated what percentage of the pre-whaling population size these current estimates reflect, assuming a pre-whaling population size of 15,000 for the southern and western Australian population combined (Dawbin 1986; International Whaling Commission 2001). Note this pre-whaling estimate we use here, reflects a conservative estimate of pre-whaling abundances, as more recent studies have suggested former SRW population sizes in Australia to have been at least 30,000 (Carroll et al. 2015; Jackson et al. 2016). The population estimation approach used here is for approximation purposes only and should not be usedsubstitute more complex population modeling approaches.
2.3.4 Calving Intervals
In the absence of photo-identification data, the relationship between the abundance of calves and the abundance of calves in previous years can be used as indicators of changes in calving interval and age of first parturition. Note that the results of these GLMMs are indicative and do not substitute quantifications of calving intervals resulting from photo-identification studies. While photo identification can be used to validate these modelling results, it also has limitations whereby females do not always return to the coastal calving areas each year and may not always be ‘available’ to be photographed in calving areas with a calf (i.e., related to a sampling bias).
3 Results
3.1 Survey Data
The pre-processed data set ranges years 1976–2024, distinguishing four segments and contains records of 14,790 SRWs. It consists of 158 unique segment-year combinations for which abundance data is available: 42 years for Augusta–Albany, 47 years for Albany–Esperance, 44 years for Esperance–Caiguna and 25 years for Caiguna–Ceduna. The maximum number of calves observed (sum of individuals observed across the four segments) was 278 in 2018, and the maximum number of unaccompanied individuals observed was 311 in 2015. We found that the relative contribution of the segments is stable over time for calves, including in years with extremely low calf abundances. Further details are explained in Supporting Information S1.
3.2 Temporal Trends
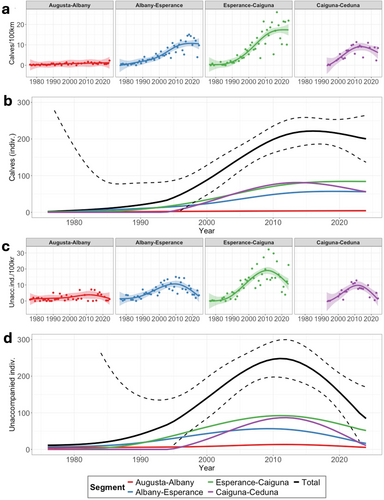
The annual abundance of calves showed strong growth from 1976 to at least 2010 (Figure 2). Throughout the study period 1976–2024, we found relative growth rates for calf abundances that ranged from 28.8% to −1.95% (Table 1). The mean instantaneous ROI ranged from 0.155 to −0.002. We found that the growth is exponential until at least 2000 (details in Supporting Information S1), indicating unconstrained growth, but there is a decline in the growth rates over time that becomes particularly apparent from 2005 onwards. We observed an increase in the inter-annual variation in calf abundances starting around the same time the exponential growth slowed down. The temporal trendline shows that since approximately 2017, the ‘peak’ years do not sufficiently compensate for the extreme low calf abundance in other years to continue the trend of increase (Figure 2), which is confirmed by the negative growth rates in this period (Table 1). At its peak in 2016, the modelled abundance of calves across the entire study area was 222 (CI 186–258) individuals. This has slightly declined to 200 (CI 137–264) individuals in 2024 (Figure 2). The growth rate has clearly declined over time and has been negative since 2017.
| Period | Relative annual increase (%) (CAGR) | Instantaneous rate of increase (ROI) | ||||
|---|---|---|---|---|---|---|
| Mean | Minimum | Maximum | Mean | Minimum | Maximum | |
| 1976–1984 | 20.8 | 15.2 | 28.8 | 0.155 | 0.068 | 0.337 |
| 1985–1994 | 13.7 | 12.4 | 14.8 | 0.047 | 0.036 | 0.062 |
| 1995–2004 | 15.2 | 9.9 | 21.2 | 0.034 | 0.020 | 0.055 |
| 2005–2014 | 4.9 | 1.4 | 8.7 | 0.009 | 0.003 | 0.017 |
| 2015–2024 | −0.7 | −1.9 | 0.8 | −0.002 | −0.004 | 0.001 |
- Note: Relative annual increase rates (CAGR) can be interpreted as the relative, percentual, year-to-year increase in the abundance of calves. Instantaneous rate of increase (ROI) represents the slope of the linear increase of the natural log of annual abundances.
The temporal trend for the abundance of unaccompanied individuals reveals a similar trend but appears to proceed calves by approximately 5 years. At the modelled peak in 2011, the number of unaccompanied individuals across the entire area was 248 individuals (CI 197–299), which dropped to an estimated 84 (CI −1 to 170) individuals only in 2024 (Figure 2). This is a decline of 66.1% in 13 years.
3.3 Population Size
Using the IWC conversion factor and standard assumption of a 3-year calving interval (International Whaling Commission 2013), we found an estimated population size of 2346 (CI 1619–3120) individuals for the western Australian population. This would reflect 16% (CI 11%–21%) of a conservative abundance estimate for the Australian population pre-whaling. When assuming 100% of mature females have calved in 5-year intervals in recent years, this results in a pragmatic population size estimate of 3940 individuals (CI 2699–5148) which would represent 26% (CI 18%–35%) of that pre-whaling abundance.
3.4 Cyclic Patterns
To understand whether there may have been a change in calving rates, we looked for differences in relationships between the abundance of calves and SRW abundances in prior years, pre- and post-2011 (whereafter the growth rate of calves levelled off and unaccompanied individuals' abundance started declining). Based on the output and performance of the GLMMs, we found positive linear relationships between the observed calf abundances and the abundance of calves on the same segment 3, 4, 5 and 8 years prior (Table 2). When analysing data from before 2011 separately, we found positive relationships with the abundance of calves 3, 4 and 7 years prior. This matches expectations for a population with a calving interval of 3–4 years and first parturition at 7 years. The relationship with calfY3, calfY4 and calfY7 became stronger when modelled for data before 2011 compared to all data. Post-2011, we only observed an effect on the abundance with calves 8 years prior. This relationship was stronger than the relationship found with calfY8 when modelling all data (Table 2). Only pre-2011, we found a positive relationship between the abundance of unaccompanied individuals and calves in the same year (unaccY0). We did not find any other relationship between calf abundances and the abundance of unaccompanied individuals.
| Data | Covariate | Mean | 95% Credible interval | dDIC | dWAIC |
|---|---|---|---|---|---|
| All | calfY8 | 0.72 | 0.575–0.868 | 73.4 | 73.3 |
| calfY4 | 0.46 | 0.310–0.618 | 28.4 | 26.9 | |
| calfY5 | 0.44 | 0.283–0.597 | 24.7 | 24.0 | |
| calfY3 | 0.50 | 0.304–0.706 | 20.1 | 16.7 | |
| Before 2011 | calfY7 | 1.15 | 0.920–1.380 | 71.3 | 72.0 |
| calfY3 | 0.83 | 0.631–1.037 | 49.8 | 48.3 | |
| unaccY0 | 0.38 | 0.279–0.482 | 40.9 | 40.3 | |
| calfY4 | 0.61 | 0.407–0.820 | 28.1 | 24.7 | |
| calfY8 | 0.68 | 0.367–0.990 | 16.6 | 13.9 | |
| 2011 Onwards | calfY8 | 0.81 | 0.582–1.033 | 32.6 | 32.6 |
- Note: The difference in DIC and WIAC reflects the improvement of model performance by including the covariate compared to the null model, where a larger difference indicates stronger improvement. The mean represents the slope of the fitted linear relationship.
4 Discussion
4.1 Calf Abundances
Throughout the Southern hemisphere, multiple SRW populations have been showing evidence of an initial period of substantial growth post-whaling (Arias et al. 2018; Bannister 2001; Best 2001; Brandão et al. 2023; Charlton et al. 2022; Findlay et al. 2017; International Whaling Commission 2001; Romero et al. 2022; Smith et al. 2023; Stamation et al. 2020; Vermeulen and Wilkinson 2023; Watson et al. 2021). Despite this, recent southern right whale population estimates are still nowhere near pre-whaling abundances, neither globally nor regionally (Carroll et al. 2014, 2015; Renault-Braga et al. 2023; Zerbini et al. 2019). Along the southwestern Australian coastline, annual sightings of the species grew from incidental in the 1970s (Bannister 1986; Ling and Aitken 1981) to hundreds of individuals annually in the last decade (Figure 2). From 1980 until the early 2000s, the annual abundance of calves in this population increased exponentially, indicating unconstrained population growth (Figure 2) (Bannister 2001), but over time the growth rate has decreased (Table 1).
Our results reveal that the annual abundance of calves in the western population of Australian SRWs has stopped growing around 2016–2017 and has started to decline. The halted growth in calf abundances we observed mid-2010s aligns with recent observations of other SRW populations such as Argentina (Crespo et al. 2019) and South Africa (Findlay et al. 2017), where the birth rates have plateaued. In addition, the increased inter-annual variation in calf abundances we observed in the studied Australian population matches observations in South Africa (Vermeulen and Wilkinson 2023). We observed increasingly large inter-annual variation since the early 2000s (Figure 2), and our statistical model reveals that in recent years, the ‘low’ years are no longer compensated by the calf abundance in other years. This is causing the halted growth trend and more recently the onset of a decline in annual calf abundances.
We estimate the current (2024) western Australian SRW population to range between 16% and at most 26% (pragmatic) of a conservative pre-whaling SRW abundance estimated for the entire Australian population (Dawbin 1986). Note that these percentages were to be halved if one were to assume more recent estimates (Carroll et al. 2015; Jackson et al. 2016). Assuming a 5-year calving interval (resulting in the 26%) is likely to result in an overestimate of the population size, as a mixture of 3-, 4- and 5-year intervals is more realistic (as observed by Germishuizen et al. (2024), discussed in 4.2). The present eastern Australian sub-population accounts for less than 300 individuals (Stamation et al. 2020), adding less than 2% to this estimate. Hence, the growth of this SRW population is being constrained at levels well below their pre-whaling abundance.
4.2 Calving Intervals
We additionally found indications of lengthened calving intervals in recent years. We found a positive linear relationship between the observed abundance of calves and 3, 4, 7 and 8 years prior (Table 2), matching expectations for a population with calving intervals ranging from 3 to 4 years and an age of first parturition of 7–8 years, as was previously reported for Australian SRWs (Bannister 2001; Charlton et al. 2019; International Whaling Commission 2013). The lack of similar relationships post-2011 suggests that the regular 3- to 4-year calving cycle has broken down, likely being smeared across 3-, 4-, and 5-year intervals, similar to what was observed in South Africa (Germishuizen et al. 2024). The shifts in calving intervals observed in our data are consistent with recent studies in both South Africa (Germishuizen et al. 2024) and a local study at Head of the Bight (Charlton et al. 2022), which indicated a decline in the relative occurrence of 3-year calving intervals since 2010 and an increase in the incidence of both 4- and 5-year intervals.
Calving intervals with durations greater than 3 years are assumed to be the result of failed pregnancies and ‘skipped’ years (International Whaling Commission 2013). The occurrence of ‘skip-breeding’ is widely observed in capital and seasonal breeders, including various pinnipeds (Beltran et al. 2021; Griffen 2018) as well as birds (Barboza and Jorde 2002; Orlando et al. 2024; Spendelow and Nichols 1989) and terrestrial mammals (Eiler et al. 1989; Pellew 1983). When, on occasion, a substantial fraction of a population is subject to skipping due to external drivers (e.g., climatic events such as heatwaves), this may cause larger inter-annual variation in birth rates (Beltran et al. 2021; Griffen 2018; Reed et al. 2015). Hence, lengthened calving intervals are not surprising given the large inter-annual variability observed in recent years (Figure 2).
Changes in calving intervals have repercussions for population estimates. This is well exemplified by the large difference between our conservative and pragmatic population estimates (Section 3.3), with the sole difference being the assumption of either 3- or 5-year calving intervals. As changes in the duration of reproduction cycles directly alter the per capita population growth rate, lengthened calving intervals can explain a slow-down in calf abundances. All other things being equal, a sudden shift in the population from a (100% occurrence of) 3-year calving interval to a (100% occurrence of) 4-year interval would theoretically directly cause a 25% decline in annual calf abundances. Consequently, the decline in calf abundances in recent years (Figure 2) may partially be explained by changes in calving intervals. It can be argued that if survival rates are sufficiently high, the population can still grow nevertheless stable average (i.e., plateauing) birth rates. However, given the strong reliance of SRW reproductive success on female body condition and the relationship between body condition and survival rates, it is likely that increased mortality rates have accompanied the recent shifts in calving intervals within this population (Christiansen et al. 2018; Del Monte-Luna et al. 2004; Germishuizen et al. 2024; Sigurjónsson et al. 1990; Vermeulen et al. 2023). Furthermore, changes in the number of annual births will have a lingering effect in the population: it could translate into a reduced stock of reproductive females, which would translate into a further decrease in the annual number of births in the future.
4.3 Unaccompanied Individuals
The abundance of unaccompanied individuals increased until 2011 but underwent a substantial decline of 66.1% from 2012 to 2024 throughout the entire study area. This supports the observed onset of a decline in annual calf abundances. Interestingly, calves have outnumbered unaccompanied individuals since 2018, similarly as observed in South Africa (van den Berg et al. 2019; Vermeulen and Wilkinson 2023). The modelled peak calf abundance occurs approximately 5 years after the modelled peak abundance of unaccompanied individuals. It is unsure if calf abundances will decline as much as unaccompanied individuals in the upcoming years because the demographic composition of the group of animals that are observed as unaccompanied individuals, the ecological relationship between these two groups is uncertain. Unaccompanied SRW individuals may comprise reproductively immature animals, as observed in previous studies identifying this cohort as pre-pubertal animals, including ages ranging from 2 to 5 years (Best et al. 2003). Provided the strong link between neonatal survival and maternal body condition (Christiansen et al. 2018; van Aswegen et al. 2024), a large-scale reduction of female body condition would reduce reproduction and survival rates. Observed decreases in maternal body condition recently observed in South Africa (Vermeulen et al. 2023) support this hypothesis. This would subsequently reduce the recruitment rate of new mothers relative to the number of births. If the abundance of unaccompanied individuals serves as an indicator of recruitment of mature females, this would explain why the temporal trend in calf abundances appears to follow unaccompanied individual abundances with a few years lag. This would translate into a further decline in calf abundances in the next few years and account for the lag between the similar temporal trends for calves and unaccompanied individuals.
Some uncertainty resides around how the observed abundances of unaccompanied individuals in our study relate to population dynamics as well as how accurate the surveys capture the number of unaccompanied individuals visiting the coast. The reduced abundance of unaccompanied individuals could reflect altered coastal residence time or altered migratory behaviour, such as extended stays in high latitudes in response to poleward shifts of feeding grounds (Lameris et al. 2021; van Weelden et al. 2021). Although the absolute abundances of unaccompanied individuals should, for now, be interpreted with caution, a clear decrease is observed in recent years, which matches temporal trends observed elsewhere. Continued monitoring of the demographic composition of unaccompanied individuals and their movement patterns is required to provide information about the causal mechanisms driving the observed decline.
4.4 Shifting Baselines?
The presented results could reflect the establishment of a new equilibrium population size for the Australian SRW western population, well below pre-whaling levels. Whales play a key role in shaping marine ecosystems through various mechanisms, including nutrient transfer, trophic interactions and enhancing ecosystem stability: their removal must have significantly impacted the functioning of marine ecosystems worldwide (Ainley et al. 2010; Albouy et al. 2020; Baker and Clapham 2004; Clapham 2016; Clapham et al. 2008; Kinzig et al. 2006; Ratnarajah et al. 2014; Roman et al. 2014, 2025; Roman and McCarthy 2010; Savoca et al. 2021; Tulloch et al. 2019). Because a substantial change in the abundance of a single species or species group (in the case of whaling) has repercussions for the entire ecosystem, population crashes can induce ecosystem collapse (Clapham et al. 2008; Renner et al. 2024; Scheffer et al. 2001). Enabled by positive feedback loops, the state of an ecosystem can be pushed beyond a threshold, whereafter the system loses its inherent ability to recover to previously stable conditions (Gårdmark et al. 2003, 2015; Roman et al. 2025; Scheffer et al. 2001; Scheffer and Jeppesen 1998). Thereafter, additionally favorable circumstances are required for the ecosystem to transition back to a state wherein conditions are sufficient for the crashed population to recover to former abundance levels (Gårdmark et al. 2003, 2015; Roman et al. 2025; Scheffer et al. 2001; Scheffer and Jeppesen 1998). A recent example of this is provided by a mass mortality event in a population of common murres (Uria aalge), which was found to have induced persistent ecosystem changes that are preventing recovery of pre-crash population levels (Renner et al. 2024). Similarly, in response to altered ecosystem conditions driven by whale populations' collapse, the ecosystem may have established a new equilibrium, now jeopardising the re-establishment of pre-whaling abundances.
Environmental changes or other anthropogenic stressors could also be jeopardising the re-establishment of pre-whaling abundances. Both the lengthened calving interval and increased inter-annual variation we found in the western Australian population suggest increasingly frequent exposure to high exposure levels to some external stressor in the months prior. Increased occurrence of skip-breeding is generally found as a response to increased food scarcity and stress (Gowan et al. 2019; Griffen 2018; Reed et al. 2015; Smout et al. 2020), which mechanism is expected to negatively affect SRW population dynamics under present and future climatic changes. There have already been indications that SRW populations elsewhere are affected by climate-related variability (Agrelo et al. 2024; Leaper et al. 2006; Seyboth et al. 2016; van den Berg et al. 2019). For example, survival probability and calf abundances have been found to correlate with sea ice extent, available krill densities, Oceanic Nino Index and Antarctic Oscillation patterns (Leaper et al. 2006; Seyboth et al. 2016; van den Berg et al. 2019; Agrelo et al. 2024). In response to shifting prey distributions and availability driven by climate change, migratory marine mammals may also change the timing of their annual migration, such as by extending their stay at foraging and/or calving grounds (Germishuizen et al. 2024; Grose et al. 2020; Lameris et al. 2021; Sousa et al. 2021). Shifts in both the location of feeding grounds and foraging strategy have already been observed in the South African SRW population (van den Berg et al. 2019, 2021). This can negatively affect SRWs directly through energy acquisition mechanisms and may additionally increase interspecific competition (Tulloch et al. 2019). Similar mechanisms have already been observed in other Southern Ocean megafauna species (de Little et al. 2007; Lameris et al. 2021; McMahon and Burton 2005; Volzke et al. 2021, 2024), and the life history traits, foraging strategy and complex reproductive cycle of SRWs are assumed to make them particularly vulnerable to the negative population-level effects of these mechanisms under projected climate scenarios (Albouy et al. 2020; Burnell 2001; Forcada et al. 2008; Jackson et al. 2016; Ross et al. 2023; Sousa et al. 2021; Tulloch et al. 2019).
4.5 Limitations
This study was based on aerial survey data (whale sightings) that were collected in a relatively consistent manner from 1976 to 2024, offering a uniquely extensive spatiotemporal coverage of SRW abundances. The surveys are not performed as a distance sampling study (e.g., no strictly standardised transect line, strip-width or flight altitude) and sightings probability cannot be calculated (Bannister 2001). Supported by previous studies and model validation (see Supporting Information S1), we therefore work with the assumption that the survey captured an accurate representation of the annual calf abundance in the study area (see Methods). Therefore, we are confident the surveys captured an accurate representation of annual calf abundances. Unaccompanied individuals are less bound to single aggregation areas than mothers with their calves and likely move around between aggregation areas. Local studies have observed mean residence times of 2 days and high day-to-day variation in the abundance of unaccompanied individuals (Charlton et al. 2019), which suggests that there is locally not a similar peak abundance as for calves. Therefore, presented abundances of unaccompanied individuals should be interpreted with more caution. We urge future survey efforts to adhere to higher sampling frequencies (e.g., surveying > 3 times a year) and further investigate within-season abundance trends at larger spatial scales. This will not only increase the confidence around temporal trends modelled but also support our understanding of SRWs' ecological behaviour.
The presented population estimates should be interpreted with caution. Estimates of the current proportion of pre-whaling abundances are likely an overestimation, provided the application of a conservative pre-whaling population estimate and uncertainty around calving intervals. The lack of quantitative information on recent calving interval ratios for the Australian populationcreates additional uncertainty around population estimates. In this study, we accommodated this uncertainty by basing our estimate on two extreme scenarios (3- and 5-year calving intervals). Nevertheless, even our most pragmatic population estimate clearly shows that the abundance of the Australian SRW population is presently nowhere near its pre-whaling size. The population estimation approach used here is for approximation purposes only: we applied it for the purpose of demonstrating that under either scenario the current population remains well below what it was prior to whaling.
There is large variation between whale species' recovery trajectories (e.g., Noad et al. 2019; Romero et al. 2022; Stewart et al. 2023; Tulloch et al. 2019; Zerbini et al. 2010) and response to climate change and other anthropogenic stressors (e.g., Burek et al. 2008; Grose et al. 2020; Hetem et al. 2014; Lameris et al. 2021; Moore and Huntington 2008; Simmonds and Eliott 2009; Sousa et al. 2021; Trathan et al. 2007; Tulloch et al. 2019; van Weelden et al. 2021). For SRWs and other species with long life spans, late sexual maturity and low reproductive output, population-level effects only become identifiable over decadal timescales. Hence, inter-species comparison of recovery trajectories and limiting factors heavily relies on the availability of long-term data. This highlights the unique value of the presented study and the importance of long-term monitoring efforts.
5 Conclusion
The Southern Ocean ecosystem was turned into an uncontrolled in situ experiment when commercial whaling in the 19th and 20th centuries left the majority of great whale populations severely exploited to near extinction (Clapham 2016; Laws 1977). Whalers managed to drive southern right whale populations, and later most other whale populations, to near extinction within a decade. Our long-term study reveals that annual calf production of the western Australian SRW population has stopped growing and has declined since approximately 2016–2017, while the population is still well below its pre-whaling levels. This underlines the urgency of increasing our understanding of the causal mechanisms and driving factors behind the constrained growth of SRW populations. Continued monitoring and further research into the various potential natural and anthropogenic factors potentially limiting the recovery of SRW populations will be crucial for directing future conservation efforts.
The collection of data that were collated in this study provide a unique opportunity to assess long-term trends in reproductive output of the western Australian SRW population. Investigating populations' recovery supports our knowledge of the general factors, processes and mechanisms that support the re-establishment of substantial, viable population sizes after population crashes, which is essential for effective conservation efforts. Our findings underline that an initial period of steady recovery does not guarantee that a population will continue to grow until they re-establish pre-disturbance levels and highlights the complexity of populations' recovery and the difficulty of interpreting population-level observations in a changing ocean. Despite uncertainty surrounding the driving mechanisms behind our findings, they are reason for concern about the fate of Australian southern right whales, leaving us with one burning question: does this mark the end of an era of southern right whale recovery?
Author Contributions
Anne Grundlehner: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, resources, software, validation, visualization, writing – original draft. Joshua N. Smith: conceptualization, funding acquisition, writing – review and editing. John L. Bannister: conceptualization, data curation, funding acquisition. Virginia Andrews-Goff: conceptualization, writing – review and editing. Madeleine Brasier: conceptualization, writing – review and editing. Micheal C. Double: conceptualization, writing – review and editing. Stuart P. Corney: conceptualization, writing – review and editing.
Acknowledgements
This study is dedicated to John L. Bannister who sadly passed away before the presented analyses were conducted, but had the vision to initiate the aerial surveys that lay the basis of the presented study, when right whales were still rare in Australian waters. He had the tenacity to ensure the aerial surveys continued over many decades. We pay our respects to his dedicated effort. We are grateful to the Western Australian Museum for supporting the aerial surveys throughout many years, and the people who have contributed to the realisation and continuation of the annual aerial surveys in any shape or form. In particular, the aerial surveys could not have been undertaken without the enthusiasm and persistent hard work of the pilots (the late John Bell, Julie Biser and Jenny Schmidt) and observer/photographers (Ray Smith, Alan Murdoch and Andrew Halsall). We would like to thank Simon Wotherspoon for his engagement in methodological discussions. Open access publishing facilitated by University of Tasmania, as part of the Wiley - University of Tasmania agreement via the Council of Australian University Librarians.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data used the results presented in the main article are available from Dryad at https://doi.org/10.5061/dryad.9s4mw6mth.




