Meta-analysis reveals that the effects of precipitation change on soil and litter fauna in forests depend on body size
Philip A. Martin and Leonora Fisher should be considered joint first author.
Abstract
Anthropogenic climate change is altering precipitation regimes at a global scale. While precipitation changes have been linked to changes in the abundance and diversity of soil and litter invertebrate fauna in forests, general trends have remained elusive due to mixed results from primary studies. We used a meta-analysis based on 430 comparisons from 38 primary studies to address associated knowledge gaps, (i) quantifying impacts of precipitation change on forest soil and litter fauna abundance and diversity, (ii) exploring reasons for variation in impacts and (iii) examining biases affecting the realism and accuracy of experimental studies. Precipitation reductions led to a decrease of 39% in soil and litter fauna abundance, with a 35% increase in abundance under precipitation increases, while diversity impacts were smaller. A statistical model containing an interaction between body size and the magnitude of precipitation change showed that mesofauna (e.g. mites, collembola) responded most to changes in precipitation. Changes in taxonomic richness were related solely to the magnitude of precipitation change. Our results suggest that body size is related to the ability of a taxon to survive under drought conditions, or to benefit from high precipitation. We also found that most experiments manipulated precipitation in a way that aligns better with predicted extreme climatic events than with predicted average annual changes in precipitation and that the experimental plots used in experiments were likely too small to accurately capture changes for mobile taxa. The relationship between body size and response to precipitation found here has far-reaching implications for our ability to predict future responses of soil biodiversity to climate change and will help to produce more realistic mechanistic soil models which aim to simulate the responses of soils to global change.
1 INTRODUCTION
Anthropogenic climate change is altering global precipitation patterns (Seager et al., 2018) and increasing the frequency and severity of extreme drought and precipitation events (Sun et al., 2007). Understanding the consequences of precipitation changes is particularly vital for forests, given their critical roles in the global carbon cycle (Walker et al., 2021) and in supporting global biodiversity (Benton et al., 2022). Impacts of precipitation changes on forests include increased tree mortality (Anderegg et al., 2019) and consequent increases in CO2 emissions (Doughty et al., 2015; Yang et al., 2018), and mixed effects on aboveground forest biodiversity (Fleming et al., 2021). However, the effects of disturbances on the biodiversity of soil and litter invertebrate fauna in forests remain poorly known (Pressler et al., 2019; Winding et al., 2020) despite its importance in regulating organic matter decomposition, nutrient cycling and plant health among other ecosystem functions (Handa et al., 2014; Nielsen et al., 2015).
Since soil moisture is a key limiting factor to the fitness and behaviour of many soil and litter fauna, precipitation changes may threaten the processes to which they contribute (Coyle et al., 2017). Precipitation changes and associated changes in soil moisture and distribution of water within the soil matrix can alter the movement of microfauna such as nematodes, and therefore their access to food sources, or the humidity in pores which represent the habitat of mesofauna such as Collembola (Coyle et al., 2017; Deckmyn et al., 2020). These changes can alter reproduction and mortality of a wide range of soil and litter fauna (Kardol et al., 2011; Singh et al., 2019; Wang et al., 2022). For example, extreme drought conditions can increase mortality for taxa such as Collembola (Wang et al., 2022) and Enchytraeidae (Maraldo et al., 2009). Nonetheless, while some studies have reported biodiversity losses as a result of precipitation reduction (Aupic-Samain, Santonja, et al., 2021; Chikoski et al., 2006; Lindberg et al., 2002; Santonja et al., 2017) others have reported increases (Homet et al., 2021; Lensing et al., 2005), with similarly mixed results for studies of precipitation increases (Chikoski et al., 2006; Frew et al., 2013; Landesman et al., 2011) making generalisation challenging.
One obvious reason for heterogeneity among studies of soil faunal responses to precipitation change is the magnitude of the precipitation change itself. Most studies of precipitation change represent manipulative experiments often using rain exclusion devices for reduction treatments or irrigation for precipitation increases, with ambient conditions used as a control. Meta-analyses have failed to find a consistent relationship between the magnitude of precipitation changes and changes in either the abundance or taxonomic richness of soil fauna (Peng et al., 2022). This could in part reflect diverging responses in taxonomic groups to precipitation changes (Coyle et al., 2017). Functional traits, morphological, physiological or phenological features measurable at the individual level (Violle et al., 2007), might offer a tractable way to disentangle some of these differences.
There are many traits that could influence soil faunal responses to precipitation change. Here we focus on three. First, taxa that inhabit the litter layer are likely to be more exposed to extreme fluctuations in moisture, and thus to respond more strongly than taxa inhabiting deeper, more buffered soil horizons (Fraser et al., 2012). Second, the presence of an exoskeleton and a cuticle layer that helps to reduce water loss and may hence render arthropods less prone to desiccation than soft-bodied annelids such as Enchytraeidae (Evans, 2008; Singh et al., 2019). Third, body size relates to microhabitat preferences and therefore dependence on water availability. For example, microfauna, such as nematodes, inhabit water films, so may be particularly vulnerable as they are essentially aquatic organisms (Vandegehuchte et al., 2015), mesofauna, such as Collembola, are sensitive to changes in soil moisture because they are confined to existing air-filled pore spaces (Wang et al., 2022) while macrofauna can create their own pore spaces (Lavelle et al., 2002) and are more capable of avoidant behaviour such as burrowing to deeper depths (Gerard, 1967). Therefore, increasing body size likely yields greater resistance to changes in precipitation.
In addition to functional traits, the characteristics of forest ecosystems could also influence the responses of soil and litter fauna to precipitation changes. For example, in relatively arid regions that have regularly been exposed to drought conditions in the past, soil and litter fauna communities would have been subject to environmental filtering, which selects for combinations of functional traits that govern species' ability to persist in the local environment (Balmford, 1996; Kraft et al., 2015). This means that we might expect greater reductions in the abundance of soil and litter fauna in humid forests following precipitation reductions than in relatively arid forests. Equally, we might expect greater increases in abundance in arid forests following precipitation increases. Integrating information on functional traits and ecosystem characteristics into research syntheses should allow for a mechanistic understanding as to why soil faunal responses to precipitation are heterogeneous.
Alongside a lack of understanding of between-study variability, it is also unclear how study design impacts study results. Meta-research in ecology has shown that differences in experimental and sampling designs can have large impacts on the accuracy of estimates of biodiversity change (Christie et al., 2019, 2020; Spake et al., 2021). But methodological robustness is rarely assessed in ecological meta-analyses (Pullin et al., 2022). In addition to methodological robustness, it is also unclear whether experimental studies employ realistic future precipitation scenarios. This is a particularly serious issue, given existing concerns about the use of unrealistic precipitation manipulations in global change experiments which may result in under- or overestimations of impact (Korell et al., 2020; Kröel-Dulay et al., 2022).
To address these knowledge gaps, we carried out the first meta-analysis of the effects of precipitation changes on soil and litter fauna in forests. In this study, we address three questions: (1) What are the impacts of precipitation changes on the abundance and diversity of forest soil and litter invertebrate fauna? (2) What are the major determinants of the impacts of precipitation changes on abundance and diversity? and (3) What are the major biases in studies of the impacts of precipitation change on forest soil and litter invertebrate fauna?
For question 1, we hypothesised that precipitation reductions cause declines in the abundance and diversity of soil and litter fauna, whereas additional precipitation causes an increase in abundance and diversity (H1, Figure 1a). For question 2, we tested five hypotheses: (i) an increased magnitude of changes in precipitation amplifies changes in abundance and diversity (H2, Figure 1b); (ii) the effect of precipitation magnitude is further amplified for organisms found in litter compared to soil dwellers (H3, Figure 1c), (iii) for organisms without an exoskeleton compared to those with an exoskeleton (H4, Figure 1d), (iv) for organisms with smaller body sizes (H5, Figure 1e) or (v) that organisms in moist forests are more sensitive to reductions in precipitation, while organisms in drier forests are sensitive to increases in precipitation (H6, Figure 1f). There were no hypotheses for question 3.
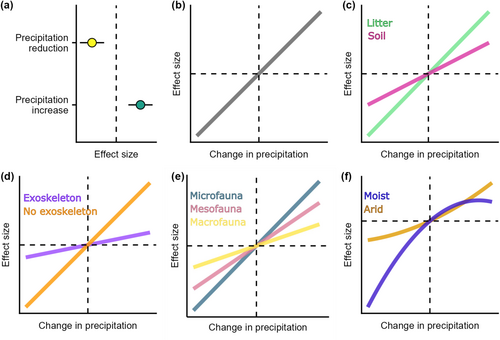
2 MATERIALS AND METHODS
2.1 Searches and screening
This study focuses on the impacts of precipitation changes on forest soil and litter invertebrate fauna in field settings. We formally defined these as PECOS elements (Table 1, Grames et al., 2019). More precise definitions of these elements can be found in the Methods S1. This study follows guidelines for synthesis in environmental management (Collaboration for Environmental Evidence, 2018). We chose to focus on precipitation change because although there are numerous primary studies investigating its impact there is currently a lack of robust synthesis. We did not focus on the impacts of other important global change drivers such as temperature change due to existing meta-analyses on this topic (Goncharov et al., 2023; Peng et al., 2022).
| PECOS element | Description |
|---|---|
| Population | Soil and litter fauna found in forest ecosystems. We defined these as invertebrates which spend a significant proportion of their life in litter and/or soil, excluding ants. Details of the selected taxonomic groups are in Table S1 |
| Exposure | Reductions and increases in precipitation |
| Comparison | Any comparison between forests that vary in the frequency or intensity of precipitation that they are subject to. This comparison may be spatial or temporal |
| Outcomes | Abundance, biomass, and diversity of soil and litter fauna |
| Space | Studies carried out in the field. All types of forest and woodland are considered relevant |
As part of a previous systematic map (Martin et al., 2021), we screened a large number of papers to identify those that assessed the impact of natural disturbances on soil and litter invertebrate fauna in forests (see Methods S1 for a summary of systematic map methods). From the systematic map we identified 320 articles that related to impacts of natural disturbances. On 22 February 2024, we updated our search by searching in three bibliographic platforms (Web of Science, Scopus, and Open Access Theses and Dissertations) to find studies on the impact of precipitation changes on soil and litter invertebrate fauna in forests published after our initial systematic map searches. Since different bibliographic platforms have different rules for the formatting of searches, we developed platform-specific searches (see Tables S2 and S3). By searching for unpublished grey literature as well as published, peer-reviewed literature, we aimed to minimise the risk of publication bias which could lead inaccurate estimates of disturbance impacts (Konno & Pullin, 2020). In addition to formal searches, we contacted expert researchers to help identify potentially relevant studies.
Once searches were complete, we downloaded all references found as .bib or .ris files and used the R package synthesisr to remove duplicate articles (Westgate & Grames, 2020). The bibfix package (Haddaway et al., 2021) was used to repair bibliographic files with incomplete data. Files were then uploaded to sysrev (Bozada et al., 2021)—an online tool that allows for screening and data extraction by review teams (see Martin, 2021). Article titles and abstracts were screened for relevance, and articles that met inclusion criteria were retained and their full text reviewed. To meet our eligibility criteria, studies needed to: (1) relate to soil and litter fauna in forests; (2) address the impact of changes in precipitation; (3) be field-based (i.e. not be carried out in greenhouses or mesocosms); (4) quantitatively assess soil fauna biomass, abundance or diversity; (5) have a comparison between sites that vary in the intensity or frequency of the precipitation that they were exposed to; (6) be written in English; (7) report measures of centrality (mean or median) for relevant litter or soil fauna outcomes.
At the title and abstract screening stage, in order to be retained, articles were expected to meet Criteria 1–3 and Criterion 5. At the full-text stage, Criteria 1–7 needed to be met in order for an article to be retained. At the full-text screening stage, we provided reasons for the exclusion of all articles that did not meet our inclusion criteria in accordance with ROSES guidelines (Haddaway et al., 2018; Figure S1). Despite being a multilingual team, we focussed only on English language literature because the inclusion of non-English language literature would have made the consistency check between reviewers challenging. We acknowledge that excluding literature written in non-English languages is a shortcoming that may lead to biases (Amano et al., 2021; Konno et al., 2020).
To ensure consistency, a random sample of 10% of titles and abstracts were screened by two team members, using our inclusion criteria. Any disagreements between the two people were discussed, and eligibility criteria were revised where appropriate. Cohen's Kappa scores were calculated to test the agreement between the two people (Cohen, 1960). If Kappa scores were below 0.6, another 10% of titles and abstracts were screened by the same two team members with the process repeated until Kappa scores were >0.6. The same process was repeated for the full texts of publications that met the inclusion criteria. After screening of titles and abstracts, inter-reviewer agreement was 98.7% and the Kappa score was 0.93. For full text screening, agreement was 100% and the Kappa score was 1.0. We found 1965 papers during our updated searches, 82 of which were retained after screening of titles and abstracts, and 38 of which were used for critical appraisal and data extraction. We used 430 comparisons between control and treatment groups extracted from these studies. The screening process is summarised in more detail in Figure S1.
2.2 Critical appraisal
Critical appraisal of studies to assess their methodological robustness is a vital part of synthesis (Collaboration for Environmental Evidence, 2018). We did this by assessing the following threats to the internal validity of a study based on Martin et al. (2020) (i) selection bias: when selection of study sites leads to a result that is systematically different to the target population; (ii) confounding: where systematic distortion of the effect of a treatment caused by mixing of the treatment of interest with other disturbances (e.g. plots where precipitation was manipulated were in plantations while control plots were in natural forests); and (iii) performance bias, differences that occur due to knowledge by researchers about treatment allocation. We therefore determined whether studies (i) consisted of both spatial (i.e. comparisons between control and treatment groups) and temporal comparisons (i.e. comparisons before and after a precipitation change), (ii) used randomisation to assign treatment and control units, (iii) avoided confounding factors and (iv) whether studies were manipulative experiments that allow determination of causality. We assigned studies an overall score of low, medium or high validity depending on the fulfilment of a priori criteria (see Table S5). These scores were later used in sensitivity analyses (see Section 2.4).
2.3 Data extraction and coding
We extracted data on the means, measures of variation and sample sizes for each relevant biodiversity measure both in the control and treatment groups. When data for more than one time period or site were presented in the same study, we extracted all available data. When variation around the mean was presented as standard errors, we converted it to standard deviation using the equation , where refers to the standard error and refers to the sample size. Where data were presented in the form of figures, we extracted this using the R package metadigitise (Pick et al., 2019). In total, 16% of studies lacked data on variation and 14% lacked data on sample sizes and so to avoid problems associated with excluding studies with missing data (Nakagawa & Freckleton, 2008) we chose to impute these values using the method of Nakagawa, Noble, et al. (2023). Using these data, we then calculated the log response ratio and its variance (Hedges et al., 1999) as implemented by Nakagawa, Noble, et al. (2023) for use as an effect size, which improves the accuracy and precision of meta-analyses especially when sample sizes are small.
Regarding explanatory variables and contextual data, we extracted information on the geographic location of studies, the perturbation type (precipitation reduction or precipitation increase), the magnitude of precipitation change (% change compared to control), the relevant taxonomic groups reported in a study, the size class of fauna (microfauna, mesofauna and macrofauna) based on Nielsen (2019), the kind of biodiversity outcome measured (abundance, Shannon–Weiner diversity or species richness), the sampling design of the study based on Christie et al. (2019, 2020), the sampling method (e.g. soil core, soil monolith and pitfall trap), the duration of study, the time after the beginning of perturbation in precipitation at which fauna was sampled, and whether the fauna sampled possessed an exoskeleton. Using study location data, we extracted information on site aridity index (defined as the ratio of mean annual precipitation over potential evapotranspiration) using the data of Zomer et al. (2022). We turned aridity index values into a categorical variable based on the recommended categories of Zomer et al. (2022), classifying sites with an aridity index >0.65 as humid, and those <0.65 as arid.
2.4 Statistical analyses
We used multilevel meta-analytical models with inverse variance weighting as implemented in the R package metafor (Viechtbauer, 2010). To examine the impacts of precipitation reduction or increase on soil and litter fauna abundance, taxonomic richness and Shannon–Wiener diversity index (Figure 1a), we built models with no modifiers that included study and site as nested random effects to account for the lack of independence between observations from the same study and site (Nakagawa, Noble, et al., 2023; Nakagawa, Yang, et al., 2023). We chose not to combine the different outcomes for diversity as doing so can blur responses and limit interpretability of results (Liu et al., 2023). We calculated the I2 statistic to estimate the percentage of the total variability in effect size values that was due to real heterogeneity. At this stage, we also performed two sensitivity analyses to test the impact of removing studies (i) that failed Geary's test of normality (Nakagawa, Noble, et al., 2023) and (ii) that we classified as having low validity in our critical appraisal.
To test our hypotheses about how precipitation change alters soil and litter fauna biodiversity, we ran models that included the percentage change in annual precipitation (Figure 1b). All of our other hypotheses involved interactions between changes in precipitation and other variables, and so we also ran models including interactions with the variables: (i) microhabitat (litter or soil, Figure 1c), (ii) presence of an exoskeleton (Figure 1d); (iii) body size of study taxon (Figure 1e) and (iv) aridity of the study forest (Figure 1f). To account for the potential impacts of publication bias, we included a model parameter representing the square root of the inverse of effective sample size (Nakagawa et al., 2021), to test the impact of the small-study effect, in which smaller studies have different—often larger—effect sizes when compared to larger studies. We also tested the potential of a decline effect, in which the effect sizes reported by studies declines over time (Koricheva & Kulinskaya, 2019; Nakagawa et al., 2021). Model selection was carried out using Akaike information criterion (AICc) adjusted for small sample sizes with models with a ΔAICc <2 considered to have similar support. We carried out model averaging for models with a ΔAICc <2 using the ‘zero method’ (Grueber et al., 2011) in the R package MuMIn (Barton, 2015) in order to produce model coefficients and associated statistics.
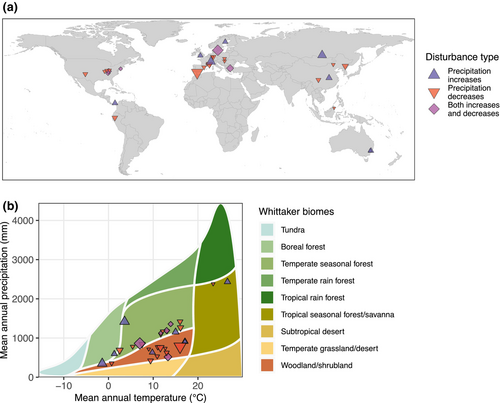
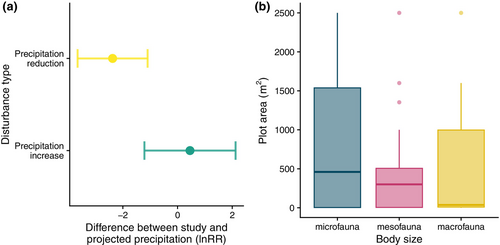
We assessed three different types of bias that may undermine the realism and accuracy of estimates of biodiversity changes as a result of precipitation change. To determine geographic biases, we plotted study locations on a map. We derived annual precipitation and temperature data from the geographic coordinates of the sites and used the R package plotbiomes to assess biases in the forest biomes that have been studied (Ștefan & Levin, 2018). Next, we assessed the similarity of the precipitation changes simulated in experiments to future projections of precipitation change in the same location. To do this, we calculated the change in precipitation imposed by experiments and used the R package geodata (Hijmans et al., 2023) to compare this to projected values for the same location for 25 climate models (ACCESS-CM2, ACCESS-ESM1-5, AWI-CM-1-1-MR, BCC-CSM2-MR, CanESM5, CanESM5-CanOE, CMCC-ESM2, CNRM-CM6-1, CNRM-CM6-1-HR, CNRM-ESM2-1, EC-Earth3-Veg, EC-Earth3-Veg-LR, FIO-ESM-2-0, GISS-E2-1-G, GISS-E2-1-H, HadGEM3-GC31-LL, INM-CM4-8, INM-CM5-0, IPSL-CM6A-LR, MIROC-ES2L, MIROC6, MPI-ESM1-2-HR, MPI-ESM1-2-LR, MRI-ESM2-0 and UKESM1-0-LL) for the period 2041–2060 for shared socio-economic pathway 245—a medium carbon emissions scenario. We used data from multiple climate models to account for potential differences between them. We compared the precipitation rate for experiments to those projected to occur under our selected emissions scenario for each experiment and model by calculating the log response ratio between experimental and projected precipitation. We then ran linear mixed effects models for each climate model with a random term for each study to assess whether experiments that reduced or increased precipitation were similar in precipitation rates to projected changes (as indicated by log response ratios not significantly different from zero). Since results were similar for almost all climate models (see Figure S6), we summarised them by calculating the median effect sizes and confidence intervals. Finally, given the importance of spatial scale when estimating biodiversity changes (Chase & Knight, 2013; Spake et al., 2021), we assessed the plot size at which treatments were applied in experiments for fauna with different body sizes to identify where there may be a mismatch between the mobility of taxa and the scale of experiments.
3 RESULTS
3.1 Description of data set
Our dataset comprises 38 primary studies, representing 46 sites and 430 effect sizes. Almost half of the sites were found in Europe (22 sites, 47%), followed by North America (10 sites, 22%) and Asia (7 sites, 15%), with relatively few in South America (4 sites, 8%) and Oceania (3 sites, 7%) and no sites in Africa. Acari were the most studied taxonomic group (33% of effect sizes), followed by Collembola (21%), Nematoda (7%) and Diplopoda (3%). The remaining 31 taxonomic groups for which we found data make up 37% of our effect sizes. The vast majority of effect sizes related to faunal abundance (329 effect sizes, 77%), with relatively few effect sizes for the Shannon–Wiener index (48, 11%) and taxonomic richness (53, 12%).
Most studies were experimental manipulations (87%) while there were relatively few observational studies (13%). The majority of studies used spatial comparisons (85%), with 10% using temporal comparisons, and only 4% using both temporal and spatial comparisons. Most effect sizes came from sampling carried out less than 3 years after initial precipitation changes (60%) while 15% of effect sizes were from samples >10 years after precipitation changes occurred. Following critical appraisal, only one study was classified as having high validity, 16 had medium validity and 16 had low validity. The major reasons for studies failing to achieve high validity were a lack of randomisation of treatments and risk of confounding variables. Samples were mainly taken during Autumn (132 effect sizes, 31%) or Summer (92, 21%), with relatively few in Winter (55, 13%) and Spring (38, 9%).
3.2 Impacts of precipitation change
Precipitation reductions led to a 35% reduction in soil and litter fauna abundance (Figure 2a, coefficient = −0.43, confidence intervals = −0.71, −0.17, p = .001, k = 185). There was significant between-study heterogeneity (Q = 2767, p< .001) and the proportion of this heterogeneity that was due between-study differences was high (I2 = 98%). Precipitation increases led to a 39% increase in abundance in soil and litter fauna (Figure 2a, coefficient = 0.33, confidence intervals = 0.06, 0.59, p = .016, k = 139). Variation in effect size was again significant (Q = 1636, p < .001) and a large amount of this was due to real heterogeneity (I2 = 78). For both precipitation reduction and increases, removing effect sizes that failed Geary's test of normality did not qualitatively alter the results (Table S6). However, in the case of precipitation reduction, removing studies with low validity markedly reduced the summary effect size (Table S6). Further investigation revealed that this was likely to be due to higher validity studies reducing precipitation in a more extreme manner.
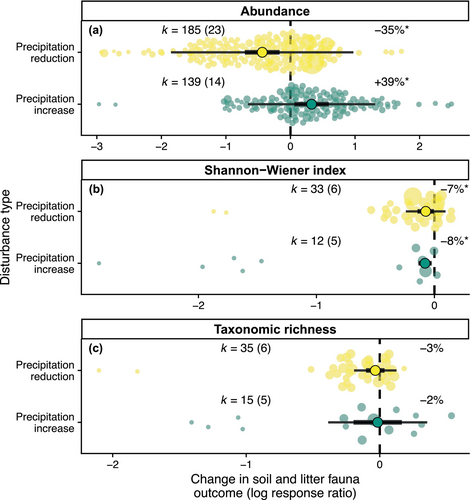
The impacts of precipitation changes on both Shannon–Wiener diversity and taxonomic richness were less pronounced than those seen for abundance. Shannon–Weiner diversity showed significant reductions with both decreased precipitation (Figure 2b, coefficient = −0.07, confidence intervals = −0.14, 0.00, p = .036, k = 33) and increased precipitation (Figure 2b, coefficient = −0.08, confidence intervals = −0.14, −0.02, p = .005, k = 12). Precipitation reduction reduced taxonomic richness by 3%, but this effect was not statistically significant (Figure 2c, coefficient = −0.03, confidence intervals = −0.10, 0.04, p = .345, k = 35). Precipitation increase also reduced taxonomic richness, this time by of 2%, but this effect was again not statistically significant (Figure 2c, coefficient = −0.02, confidence intervals = −-0.20, 0.17, p = .869, k = 15). Although most of these models indicated significant variability in effect sizes, the proportion of heterogeneity due to between-study differences was much lower than for the analyses of abundance (I2 = 0%–68%, Table S6). Removing effect sizes that failed Geary's test of normality did not qualitatively alter the results but again removing studies with low validity tended to lead to more extreme summary effect sizes (Table S6).
3.3 Drivers of precipitation change impact
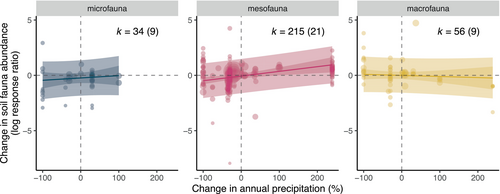
When investigating the reasons for differences in the impact of precipitation changes on abundance, the most parsimonious models included an interaction between the magnitude of precipitation change and organism body size as well as moderators to account for study size and whether effect sizes change over time (Table S7). Model averaging suggested that mesofauna abundance responded to changes in the magnitude of precipitation, while this was not the case for microfauna and macrofauna (Figure 3). Of the moderators only, the interaction between mesofauna and the magnitude of precipitation change was statistically significant (coefficient = 0.005, SE = 0.002, p = .001), while for microfauna and macrofauna, the slopes were much less steep and not statistically significant (Table S8). The most parsimonious models included the effects of study size and publication year, suggesting that larger studies showed more positive impacts on abundance and that the same was true for more recent studies (Figures S2 and S3, Table S8) but neither effect was statistically significant. We tested whether the impact of precipitation change differed between two of the most well-studied taxonomic groups, Collembola and Acari (Table S9). While we found that both groups showed a response to changes in precipitation magnitude, there was no statistically significant difference between the responses (Figure S4, Table S10).
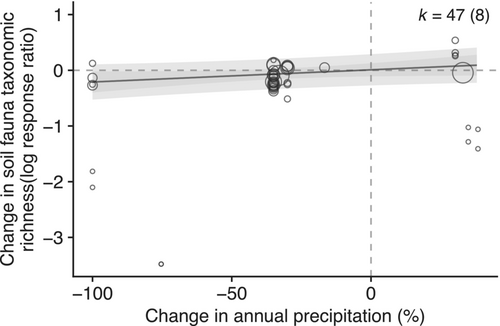
The most parsimonious models for changes in taxonomic richness included only the magnitude of precipitation change or the year of publication (Table S11). Model averaging showed a significant positive relationship with precipitation change (coefficient = 0.002, SE = 0.001, p = .0473, Figure 4, Table S12), indicating support for the impact of precipitation change magnitude. Again, more recent studies tended to have larger effect sizes, although this trend was not statistically significant (Table S12). Similarly, for Shannon–Wiener diversity the most parsimonious models included different combinations of the magnitude of precipitation change and/or the year of publication (Table S13). Model averaging showed a non-significant negative effect of precipitation magnitude on Shannon–Wiener diversity (coefficient = −0.002, SE = 0.004, p = .703, Table S14), and small non-statistically significant effects of study size and publication year (Figure S5).
3.4 Study biases
There are clear biases in the geographic distribution of studies, with a large number of studies carried out in western Europe and the United States, but relatively few in South America and Asia, and no studies found for Africa (Figure 5a). This translates to an underrepresentation of tropical forest biomes, with most studies carried out in temperate seasonal forests or woodland/shrubland biomes found in Mediterranean climates (Figure 5b). In addition to geographic biases, there were also a number of biases that could impact the validity of study results. First, studies of the effects of precipitation reduction reduced precipitation by 91% more than projected changes for the same location (Figure 6a, coefficient = −2.37, SE = 0.65, p = .004), while studies of precipitation increase increased precipitation by 58% more than projected changes, although this difference was not statistically significant (Figure 6a, coefficient = 0.455, SE = 0.846, p = .603). For more details of results for each of the 25 climate models used in this analysis see Figure S6. Second, the plots used for experimental manipulations tended to be small for studies of micro-, meso- and macrofauna (Figure 6b), with median areas of 460, 300 and 36 m2, respectively.
4 DISCUSSION
Our findings partially supported our hypothesis (H1), indicating that reductions in precipitation generally cause large decreases in the abundance of soil and litter fauna in forests, while precipitation increases have the opposite effect. However, impacts on taxonomic richness and Shannon–Wiener diversity were typically less pronounced. Changes in abundance depended on the magnitude of precipitation changes and taxa body size: mesofauna abundance changes were positively correlated with changes in precipitation, but there was little detectable effect of body size for either micro- or macrofauna. We found weak support for a positive correlation between changes in precipitation and changes in taxonomic richness but no support for this correlation for Shannon–Wiener diversity. Thus, the best supported of our hypotheses regarding the variability in response to precipitation changes was H5 that the impacts of precipitation change depended on taxa body size. However, there was only weak support for H2 that increased magnitude of changes in precipitation amplifies changes in abundance and diversity, and little support for the effects of fauna occupying litter or soil (H3), possessing an exoskeleton (H4) or whether sites were relatively humid or arid (H6) regarding the modification of impacts of precipitation changes. Additionally, our analyses to account for publication biases relating to study size and changes in effect sizes over time mean that these results are highly robust.
4.1 Impacts of precipitation change
Our results broadly agree with those of the meta-analysis by Peng et al. (2022), who found that impacts of precipitation change on the abundance of soil fauna in forests were much larger than for richness. However, our meta-analysis included more than three times as many primary studies relating to forests, and over twice as many effect sizes, indicating that our results represent an important advance in robustness. Our results also broadly mirrored those found by the recent meta-analysis of Bristol et al. (2023) who focussed solely on nematodes and showed a non-significant increase in abundance as a result of precipitation increases and a non-significant decrease as a result of precipitation reductions. In addition, unlike the synthesis of Goncharov et al. (2023) we found evidence for the impacts of precipitation change on mesofauna, while their study only found impacts of precipitation change on nematodes. In contrast to all previous similar meta-analyses on this topic (Blankinship et al., 2011; Goncharov et al., 2023; Peng et al., 2022), we found important evidence for nuanced effects of precipitation change.
The lack of pronounced changes in either taxonomic richness or Shannon–Wiener diversity was surprising, but one intriguing finding was that species richness changed little as a result of precipitation change, while Shannon–Wiener diversity was significantly reduced. This hints that, as suggested by others, some Oribatida and Collembola species become increasingly dominant when soil moisture is altered, reducing evenness (Meehan et al., 2020). In contrast with our findings for changes in abundance, we found relatively little support for the effect of changes in precipitation magnitude or species traits on taxonomic richness or Shannon–Wiener diversity. This could result from changes in local diversity as a result of perturbations often not reflecting those in community composition (Hillebrand et al., 2018; Zajicek et al., 2021). This occurs when there is turnover in the identity and abundance of species but no systematic change in the number of species (Hillebrand et al., 2018) as appears to be common for numerous human-impacted ecosystems (Dornelas et al., 2014; Vellend et al., 2013). This response differs from that observed in soil microbial communities under reduced precipitation, which not only show a reduced abundance of the active community but also show lower diversity and markedly different composition (Metze et al., 2023; Zhou et al., 2020). However, it is also possible that the apparent lack of effect is actually a result of different responses to precipitation changes across taxonomic or functional groups that we were unable to capture due to a lack of data.
4.2 Drivers of precipitation change impact
Our findings for changes in abundance suggest that water availability is a key constraint for many forest soil taxa (Aupic-Samain, Baldy, et al., 2021), but that impacts of soil moisture vary depending on organism body size. The effect of intense precipitation changes on mesofauna abundance is consistent with previous studies that suggested that this group can be particularly sensitive to environmental changes (Wu & Wang, 2019) and that high-intensity disturbances can have an enduring effect on ecological processes and hinder recovery (Nielsen & Ball, 2015). This could lead to reductions in the incorporation of leaf litter into soil, given that litter forms a major part of the diet for taxa such as Collembola and Oribatida (Potapov et al., 2022). The apparent lack of significant impact of precipitation change on micro- and macrofauna, while contradicting our expectations, could have a variety of causes.
Our results suggest that there is a hump-shaped relationship between the body size of soil and litter fauna and their sensitivity to precipitation changes, with micro- and macrofauna being relatively insensitive and mesofauna being highly sensitive. We hypothesise that this sensitivity is caused by three factors. First, differences in the ability to avoid predation. Under drier conditions, microfauna, such as nematodes, can become restricted to small pores (Erktan et al., 2020) that act as refuges from predatory mesofauna such as mites (Potapov et al., 2022) which are unable to access them. Mesofauna are confined to larger, air-filled pores (Erktan et al., 2020). In contrast to nematodes, this confinement does not protect them against predation, because meso- and macrofauna predators can move between soil layers in search of prey (Potapov et al., 2022). Thus, mesofauna remains subject to predation even in dry conditions.
Second, physical adaptations to dry conditions. Both micro- and macrofauna possess physical adaptations which aid them in drier conditions. Microfauna, such as nematodes, can go into anhydrobiosis when under drought stress (Landesman et al., 2011; Watanabe, 2006). Many macrofauna, such as spiders or millipedes, have thick exoskeletons which protect them against desiccation as well as being highly mobile, thus allowing them to move more easily to wetter soil patches. In contrast, many mesofauna, such as Collembola or Protura, have few physical adaptations to drought conditions and have limited mobility within the soil. As a consequence, they are likely subject to greater drought-induced mortality than micro- or macrofauna.
Third, availability of food sources. Soil and litter fauna feed on a wide variety of food, ranging from living or dead plant matter to soil bacteria and fungi, as well as other soil fauna (Potapov et al., 2022). Changes in the biomass and palatability of these different food sources as a result of precipitation reductions causes cascading bottom-up impacts throughout soil food webs. While reductions in the food sources for all soil and litter fauna seem likely in dry conditions, mesofauna may be more severely impacted than the other groups. For example, many Collembola species are fungivores and drier conditions may reduce the abundance and quality of fungi available to them. Drought conditions appear to favour mycorrhizal fungi that contain melanin in their cell walls (Fernandez & Koide, 2014; Pigott, 1982), making them resistant to decomposition thereby limiting food sources for detritivores (Fernandez et al., 2013; Fernandez & Koide, 2014; Malik & Haider, 1982). In addition, precipitation reductions can reduce saprotrophic fungi abundance, and important source of food for Collembola (Sanders et al., 2024). However, many micro- and macrofauna groups have relatively diverse diets, potentially providing a buffer when some sources of food are scarce (Potapov et al., 2022). Similarly, reductions in precipitation can reduce litter input, reducing the quantity of food for some detritivore mesofauna, and increase the carbon content of litter (Deng et al., 2021), reducing decomposition rates, resulting in a shortage of readily available nutrients for litter feeding fauna.
Under increased precipitation, the hump-shaped relationship between size and abundance appears to be reversed. This implies that mesofauna is more sensitive to increases in water resources than either micro- or macrofauna. Mesofauna appear to be more easily affected by seasonal changes than macrofauna due to their smaller body size and shorter life cycles (Wu & Wang, 2019), thus explaining their increase in abundance with increased precipitation. Mesofauna may also be less affected by predation under wetter conditions (Aupic-Samain, Baldy, et al., 2021). Meanwhile, for microfauna such as nematodes, increased precipitation may reduce the overall fungal biomass, reducing populations of fungivorous nematodes (Liu et al., 2020). At the same time, some fungal groups, such as saprotrophic fungi, on which mesofauna such as Collembola preferentially feed, are expected to increase under wetter conditions (Sanders et al., 2024).
The effect of body size seen in our meta-analysis represents an advance in our understanding of the responses of soil biota to changes in precipitation associated with climate change. However, the mechanisms that regulate this response to changes in water availability are currently unclear and further research could substantially improve our ability to predict the future impact of climate change on the resilience of soils and their functioning in the face of climate change. One such potential impact is that the loss of mesofauna could cause a reduction in the rate of litter decomposition (Song et al., 2020) resulting in a reduction in the incorporation of organic matter into soils and a reduction in the complexity of soil structure. Equally, such a reduction in soil mesofauna could lead to increases in the abundance of taxa belonging to other size groups that also feed on litter (e.g. earthworms) resulting in a change in the structure of soil food webs, which may potentially buffer the impacts of precipitation changes on soil functioning. However, this replacement could entail major changes in the physical structure of the soil, since earthworms are ecosystem engineers which can alter soil porosity (Flores et al., 2021).
Although our explanations for the observed patterns are grounded in theory and empirical evidence from the literature, new experiments and observations are needed to test them. In particular, within broad taxonomic groups, there are large differences in key traits that determine their response to precipitation change. For example, Collembola, vary in body size, permeability of cuticle and occupancy of different soil layers, all of which influence how species respond to drought conditions (Ferrín et al., 2023). For Nematodes, body size and trophic group are linked with fungivores and bacterivores being relatively small, and omnivores and predators being relatively large (Sechi et al., 2018). Given these variations, we urge researchers to identify soil and litter fauna to a higher taxonomic resolution, thus allowing for more nuanced interpretations of how body size and other functional traits impact responses to precipitation change.
4.3 Study biases and recommendations for future research
Our study identified a need for changes in studies of precipitation change impacts on forest soil and litter fauna. The proposed changes may be difficult to implement, and we acknowledge that decisions about study practicalities are the result of a mixture of factors such as socioeconomics (Llorente-Culebras et al., 2023) and the obsession with academic productivity (Fischer et al., 2012). First, linked to our finding that many experiments use precipitation regime alterations that are much more extreme than projected future changes, we advocate for researchers to clearly distinguish between experiments which aim to simulate changes in mean annual precipitation and those that aim to simulate extreme events such as droughts and extreme rainfall (Korell et al., 2020). Second, this study shows that the scale of experimental manipulations in many studies may be too small to capture changes in more mobile macrofauna taxa, and so larger-scale studies are needed that allow for a wider range of organisms and processes to be studied (Hanson & Walker, 2020). Third, we found strong geographic biases, with few studies found outside temperate and Mediterranean forest biomes, and thus suggest the greater need for studies outside these regions.
While there is a need for changes in how primary studies are conducted, the same is true for syntheses relating to soil fauna. Our study represents one of most methodologically robust meta-analyses to date in soil ecology, collating more studies on the impacts of precipitation changes than previous similar meta-analyses (Blankinship et al., 2011; Peng et al., 2022), despite our narrower focus on precipitation changes in forests, and thus providing greater statistical power than previous efforts. We encourage more researchers to strive for more robust evidence syntheses and familiarise themselves with existing guidance for evidence synthesis in ecology (Collaboration for Environmental Evidence, 2018; Haddaway et al., 2018; Figure S1). In our study, we used the log response ratio as an effect size metric, due to differences between studies in the units of abundance. Because the log response ratio measures proportionate change in biodiversity relative to a control or baseline value, there is a loss of information that can render meta-analyses vulnerable to possible inferential errors when baselines vary across studies (Spake et al., 2023).
In addition, existing meta-analyses on the impacts of global change on soil fauna (This study; Beaumelle et al., 2023; Blankinship et al., 2011; Bristol et al., 2023; Goncharov et al., 2023; Peng et al., 2022; Phillips et al., 2023) use biodiversity metrics related to abundance and alpha diversity, meaning we know little about impacts on more complex aspects of biodiversity such as community composition and functional diversity. We advocate for researchers to collate and use raw data from field studies to allow for more nuanced ‘full data’ analyses which can avoid issues associated with the use of effect sizes (Spake et al., 2023) and that are becoming the gold standard in other fields, such as medicine (Culina et al., 2018; Spake et al., 2022). Finally, we recognise that we were unable to explicitly examine the impact of study scale (e.g. grain, extent) on observed changes in soil fauna biodiversity as a result of precipitation changes. However, given the general importance of scale for observations in ecology (Spake et al., 2021) and the findings that precipitation change experiments have scale-dependent effects on other taxa (Korell et al., 2021), we encourage researchers to address this topic in future syntheses.
We acknowledge that our meta-analysis focussed solely on the impacts of precipitation changes and ignored the potential impacts of other drivers that could have synergistic impacts on soil and litter fauna. In the real world, ecosystems are typically affected by more than one of global change factor (e.g. temperature increases and nitrogen deposition) at any given time (Bowler et al., 2020). Importantly, interactions between different global change factors can cause unpredictable changes in soil biodiversity and functioning (Eisenhauer et al., 2012; Rillig et al., 2019). In the case of precipitation reductions, there is a clear synergy with temperature increases as these can lead to increased evapotranspiration and further reductions in soil moisture. Such conditions could further stress soil fauna sensitive to changes in moisture by limiting their diets (Sanders et al., 2024) or directly causing desiccation. However, the recent meta-analysis by Peng et al. (2022) suggests there are currently few primary studies that have investigated this synergy, especially in forests. We urge researchers to prioritise experiments investigating the impacts of multiple global change factors to inform more realistic predictions of future change.
5 CONCLUSION
Overall, our results suggest that forest soil and litter fauna abundance is sensitive to changes in precipitation, and that for mesofauna this impact depends on the magnitude of precipitation change. Meanwhile, alpha diversity appeared to be relatively insensitive, with little evidence that changes were related to the magnitude of precipitation change. Given soil mesofauna affect soil functions, such as litter decomposition, changes in the abundance of this group may result in changes in the soil physical structure, soil nutrients and soil carbon. In turn, changes in mesofauna abundance will also alter the trophic structure of belowground food webs. Our results provide new insights into belowground biodiversity change in forests that can inform more realistic soil models in the future (Deckmyn et al., 2020; Flores et al., 2021). In addition, we call on global change researchers to conduct more realistic studies of changes in mean annual precipitation, droughts and extreme precipitation in future, in line with representative concentration pathways (RCPs; van Vuuren et al., 2011) as well as larger scale experiments to capture impacts on soil fauna more fully.
AUTHOR CONTRIBUTIONS
Charlotte Biryol: Data curation; writing – review and editing. Claire Menival: Data curation; visualization; writing – review and editing. Bertrand Guenet: Conceptualization; funding acquisition; writing – review and editing. Jan C. Axmacher: Conceptualization; methodology; supervision; writing – review and editing. Jorge Curiel Yuste: Conceptualization; funding acquisition; investigation; project administration; resources; supervision; validation; writing – original draft; writing – review and editing. Leonora Fisher: Data curation; formal analysis; investigation; methodology; visualization; writing – original draft; writing – review and editing. Leticia Pérez-Izquierdo: Data curation; writing – review and editing. Mathieu Santonja: Conceptualization; funding acquisition; writing – review and editing. Philip A. Martin: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; supervision; validation; visualization; writing – original draft; writing – review and editing. Rebecca Spake: Conceptualization; methodology; visualization; writing – review and editing. Sebastiaan Luyssaert: Conceptualization; funding acquisition; writing – review and editing. Stefano Manzoni: Conceptualization; writing – review and editing.
ACKNOWLEDGEMENTS
Philip Martin, Leticia Pérez-Izquierdo, Charlotte Biryol, Bertrand Guenet, Sebastiaan Luyssaert, Stefano Manzoni, Claire Menival, Mathieu Santonja and Jorge Curiel Yuste were funded by the grant Holistic management practices, modelling and monitoring for European forest soils—HoliSoils (EU Horizon 2020 grant agreement no. 101000289). JCY was also funded by the coordinated project ATLANTIS (PID2020-113244GB-C21), the Basque Government through the BERC 2022-2025 programme, and the Spanish Ministry of Science and Innovation through the BC3 María de Maeztu excellence accreditation (MDM-2017-0714). We would also like to thank two anonymous referees for help in improving this article.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data and code that support the findings of this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.10987485.




