Recolonization following past persecution questions the importance of persistent snow cover as a range limiting factor for wolverines
Abstract
Globally, climate is changing rapidly, which causes shifts in many species' distributions, stressing the need to understand their response to changing environmental conditions to inform conservation and management. Northern latitudes are expected to experience strongest changes in climate, with milder winters and decreasing snow cover. The wolverine (Gulo gulo) is a circumpolar, threatened carnivore distributed in northern tundra, boreal, and subboreal habitats. Previous studies have suggested that wolverine distribution and reproduction are constrained by a strong association with persistent spring snow cover. We assess this hypothesis by relating spatial distribution of 1589 reproductive events, a fitness-related proxy for female reproduction and survival, to snow cover over two decades. Wolverine distribution has increased and number of reproductive events increased 20 times in areas lacking spring snow cover during our study period, despite low monitoring effort where snow is sparse. Thus, the relationship between reproductive events and persistent spring snow cover weakened during this period. These findings show that wolverine reproductive success and hence distribution are less dependent on spring snow cover than expected. This has important implications for projections of future habitat availability, and thus distribution, of this threatened species. Our study also illustrates how past persecution, or other factors, that have restricted species distribution to remote areas can mask actual effects of environmental parameters, whose importance reveals when populations expand beyond previously restricted ranges. Overwhelming evidence shows that climate change is affecting many species and ecological processes, but forecasting potential consequences on a given species requires longitudinal data to revisit hypotheses and reassess the direction and magnitude of climate effects with new data. This is especially important for conservation-oriented management of species inhabiting dynamic systems where environmental factors and human activities interact, a common scenario for many species in different ecosystems around the globe.
1 INTRODUCTION
Mounting evidence show that climate is changing rapidly and acts on ecosystems already stressed by human activities (Brodie et al., 2013; Hansen et al., 2010). Still, our understanding of how climate change affects biodiversity remains superficial (McMahon et al., 2011). Climate change causes shifts in species distributions (Moritz et al., 2008; Pearson & Dawson, 2003), but there is a large variation in observed and predicted responses among species; while some expand, others retract (Aryal et al., 2016; Baltensperger et al., 2017; Brodie et al., 2013; Byrne et al., 2015; Ray et al., 2012), and the ecological mechanisms driving changes are often unknown. Considering ongoing changes in the environment, it is important to confront existing assumptions with new data, especially when they are relevant to contemporary conservation challenges. A better understanding of how species respond to climate change is crucial for assessing vulnerability (Moritz & Agudo, 2013). Long-term data may challenge previously established assumptions (Barsalou, 1993; Hind, 2015), and are essential to inform and update conservation and management programs (e.g., Able, 2016).
Northern latitudes are predicted to experience some of the most severe changes in climate (Flannigan et al., 2000; Williams et al., 2014). This includes milder winters, decreasing snow persistence, and depth, resulting in a higher frequency of snow-free winters (Williams et al., 2014). Milder winters and reduction in snow depth may positively affect the distribution of some species (Morellet et al., 2013; Williams et al., 2014), whereas the opposite effect is expected for snow-adapted species (Fordham et al., 2013; Ray et al., 2012; Williams et al., 2014). In boreal latitudes, like those of Scandinavia, generalist species are expanding northwards, while cold-adapted species face a range contraction in response to both a warming climate and human-caused habitat change (Elmhagen et al., 2015). Assessments of climate change effects on biodiversity distribution have often been based on empirical niche or climate-envelope models, which for many species predict large geographic displacements and widespread extinctions (Dawson et al., 2011).
Wolverines (Gulo gulo) are both physiologically and behaviorally adapted to cold climate and snow (Fisher et al., 2022; Inman et al., 2012; Liu et al., 2018; van der Veen et al., 2020), and it is suggested that successful denning (in February–May) is dependent on a persistent spring snow cover that provides protection to juveniles from weather and predators (Aronsson, 2017; Inman et al., 2012; Magoun & Copeland, 1998). In an influential study, Copeland et al. (2010) correlated spatial data on wolverine den sites and spring snow cover (April 24–May 15) in North America and Fennoscandia, and suggested that the global distribution of wolverines is intrinsically linked to the persistence of spring snow cover. Subsequently, numerous other studies have reiterated and/or predicted that an expected decline in snow cover induced by global warming would result in a contraction of wolverine distribution, due to a reduction in suitable habitat and restricted connectivity (e.g., Barsugli et al., 2020; Bonamy et al., 2020; Ellis et al., 2013; Hof et al., 2012; Manning & Garton, 2011; McKelvey et al., 2011; Peacock, 2011; Schwartz et al., 2009, 2016). In turn, the design of potential corridors for wolverines has assumed that the presence of a late-spring snowpack is crucial for the species (Dilkina et al., 2017), and the relation between snow and wolverines centers much of the discussion on the species' habitat requirements (Wolverine Science Panel, 2014). Primarily based on the hypothesis that wolverines are dependent on snow cover during spring, wolverines have repeatedly been considered for protection as a threatened species under the US Endangered Species Act on different occasions (e.g., Earthjustice, 2022; USFWS, 2013), and deteriorating snow cover has been identified as a serious threat to populations in southern Canada (COSEWIC, 2014). However, niche or climatic-envelope models, in which those assumptions are based, are most appropriate to identify exposure to climate change. However, that is only one aspect of vulnerability, which also includes sensitivity and adaptive capacity (Dawson et al., 2011). Newer studies have shown, however, that wolverines have recently expanded and reproduced outside areas of spring snow cover, both in Europe (Aronsson & Persson, 2017; Lansink et al., 2020) and North America (Jokinen et al., 2019; Webb et al., 2016).
This situation reinforces the idea that assessing impacts of climate change on biodiversity is a multifaceted issue that requires attention to several components of vulnerability (Dawson et al., 2011) and to other factors that modulate species distribution patterns, such as interspecific interactions (Wiens et al., 2009) and dispersal capacity (Diffenbaugh & Field, 2013). The latter allows for geographic shifts to follow optimal conditions and the combination of all these factors highlights the complexity to predict species responses to climate change (Moritz & Agudo, 2013).
Our aim is to revisit the proposed hypothesis that wolverine distribution and reproduction are constrained by a strong association with persistent spring snow cover. We test this by investigating the relationship between snow cover and the occurrence of wolverine reproductive events in Sweden. Using long-term data spanning a two-decade period (2000–2018), and considering the recent southward expansion of the population (Aronsson & Persson, 2017) we predict that the strength of this relationship has decreased over time. Long-term spatial distribution of reproductive events represents a fitness-related proxy (of both female survival and reproduction), which is more relevant than mere estimates of occurrence or habitat use (Gaillard et al., 2010).
2 METHODS
2.1 Study system
Intense persecution during the 1900s led to drastic decline in the Scandinavian wolverine population, and at the time of legal protection (1969 in Sweden) the remaining wolverines were restricted to refugia in northern alpine areas (Flagstad et al., 2004). Currently, the wolverine distribution largely overlaps with the reindeer husbandry area that covers the northern half of Scandinavia, where semidomestic reindeer (Rangifer tarandus) is the main prey for wolverines (Mattisson et al., 2016). Predation by wolverines and other predators causes significant economic losses for indigenous Sámi reindeer herding communities (Hobbs et al., 2012). Consequently, a conservation performance payment system has been implemented in Sweden since 1996 to mitigate depredation conflicts; reindeer herding districts are paid in relation to yearly number of wolverine reproductive events (here; field documentation of active den sites and females with offspring showing that reproduction has taken place) documented in each district within the Scandinavian monitoring program (Persson et al., 2015). The annual count of reproductive events is also the main unit for estimating the size and conservation status of the wolverine population (SEPA, 2018).
The Scandinavian wolverine monitoring program focuses on documenting reproductive events, as well as confirmed wolverine presence based on DNA from scats, visual observations, and tracks on snow. The search for reproductive events largely relies on snow tracking in late winter and spring, mostly in March–April; earlier in southern areas with poor snow cover and later in northern areas with consistent snow cover. The monitoring program follows strict protocol regulated by the Swedish Environmental Protection Agency (SEPA) and Rovdata (SEPA, 2018). Registered reproductive events are documented with coordinates in the Scandinavian database Rovbase (rovbase30.miljodirektoratet.no), and are reviewed by an independent central coordinator to validate that they fulfill stipulated criteria to be considered a documented reproductive event (SEPA, 2018). These criteria include photo documentation of females with cubs (e.g., when outside the den site), tracks of females with cubs or documentation of regular and long-term activity at den sites (Aronsson & Persson, 2017; SEPA & Rovdata, 2019).
In Sweden, wolverines are protected and only limited lethal control occurs to mitigate depredation conflicts in the reindeer husbandry area (Gervasi et al., 2019; Persson et al., 2009). In contrast, Norway implements a stricter carnivore control management with high annual harvest quotas (Gervasi et al., 2019). In 2000–2018, the mean annual harvest in Norway was 75 (33–135) wolverines of an average approximate population of 323 individuals (>1 year old), as compared to an average annual harvest of eight (0–33) wolverines from a population of an average 527 individuals in Sweden (Tovmo et al., 2018; rovbase30.miljodirektoratet.no). Consequently, there is a much stronger human influence on wolverine density and distribution in Norway compared to Sweden (Gervasi et al., 2019), where environmental factors are presumably more important. Thus, as we aim to assess the relationship between the distribution of wolverine reproductive events and an environmental parameter, snow cover, we limited our analysis to the Swedish part of the Scandinavian population.
In recent years, the wolverine population in Sweden has increased and recolonized the boreal forest landscape outside alpine areas (Figure 1). Importantly, spatial differences in both monitoring incentive (i.e., the importance of monitoring for the conservation performance payment system within the reindeer husbandry area) and availability of snow for tracking, result in geographic differences in monitoring effort and efficiency (Aronsson & Persson, 2017). Although there is a strive to reduce these differences to obtain reliable population size estimates for the entire distribution, differences need to be considered when using the monitoring data. Specifically, monitoring has been most intense in the alpine regions of the reindeer husbandry area, the refugia where wolverines persisted historical persecution and snow availability are highest. Monitoring has been less intense in the boreal region in the reindeer area due to both the recolonization pattern and lower snow availability. Finally, monitoring has been mainly opportunistic in boreal regions south of the reindeer husbandry area, due to the minimal human–wolverine conflict, low snow availability, and management focus on other carnivore species (Aronsson & Persson, 2017).
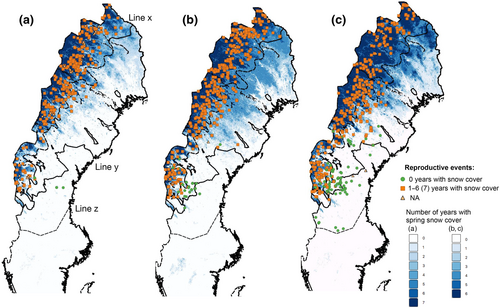
2.2 Data
In this study we used coordinates of all 1589 validated wolverine reproductive events registered in Sweden (rovbase30.miljodirektoratet.no) in 2000–2018 (mean = 83.7 reproductions per year; SD = 26.6; range = 42–125). We created annual maps of persistent snow coverage (2000–2018) for two parts of the wolverine denning period: (i) March (March 1–21), representing the critical time following parturition, when the young are small, immobile, and most vulnerable (Aronsson, 2017; Persson et al., 2003), and (ii) spring (April 24–May 15), corresponding to the period of den abandonment (Aronsson et al., 2023), which is the period that has been used to predict the importance of snow cover for wolverines (e.g., Copeland et al., 2010). For the consolidation of snow data into the snow cover maps we used the same procedure as described in Copeland et al. (2010), for each of the two periods. We downloaded moderate-resolution imaging spectroradiometer (MODIS) data for the Scandinavian peninsula using the MODIStsp package (Busetto & Ranghetti, 2016) in R (R Core Team, 2017). Based on the daily Normalized Difference Snow Index (NDSI; ranging between 0 and 100), available at a 500 × 500 m pixel resolution, we classified each pixel as “snow covered” or “bare ground” using a NDSI threshold of 40 (i.e., bare ground for NDSI <40, and snow covered for NDSI ≥40, following Rittger et al., 2013). We consolidated the 21 days to improve coverage through minimizing cloud cover and night images, and produced final annual maps of persistent snow coverage, where each pixel was given a value of 0 if it was classified as bare ground at any of the 21 days, or 1 if classified as snow covered during all days. This classification resulted in 38 maps of persistent snow cover (19 for March 1–21, hereafter “March snow coverage,” and 19 for April 24–May 15, hereafter “spring snow coverage”).
For each year, the reproductive events were classified as either snow covered (1) or bare ground (0), based on the year and 500 × 500 m pixel where it was located, for both March snow coverage (March 1–21) and spring snow coverage (April 24–May 15), respectively (Figure 2a). To avoid bias, reproductive events in pixels that did not have spring snow coverage information for the same year were excluded (n = 8; 0.5%), resulting in 1581 reproductive events with yearly snow cover information. Furthermore, we divided the study period into three separate time periods; 2000–2006, 2007–2012, and 2013–2018, and used the spring snow coverage maps for each time period to calculate the proportion of years with persistent spring snow cover for each reproductive event and year. Consequently, the proportion of years with spring snow ranged between 0 (the 500 × 500 m pixel where the reproductive event was located was never snow covered during the time period) and 1 (the pixel was snow covered in all years during the time period) (Figures 1 and 2b). Pixels that did not have spring snow coverage information for all years in each time period were excluded. As a result, 59 (3.7%) reproductive events located in pixels with incomplete snow cover information were removed; that is, 1530 reproductive events were included in the analyses (363 in 2000–2006, 617 in 2007–2012, and 550 in 2013–2018, respectively).
To consider potential sampling biases due to differences in management practices and focus (inside or outside the reindeer husbandry area), biogeographic region (alpine or boreal) and wolverine population expansion, we divided the wolverine distribution into three study regions: Alpine-Reindeer, Boreal-Reindeer, and Boreal regions (Figure 1). The reindeer husbandry region was defined by the areas that were entirely encompassed by active reindeer herding, and biogeographic regions were based on Aronsson and Persson (2017). We used the southern limit of the historical wolverine distribution in Sweden (Persson & Brøseth, 2011) as the southern limit for the Boreal region. Furthermore, we used average snow depth in April (meters) each year, to consider potential bias in monitoring efficiency, that is, for snow cover pixels, snow depth provides additional information on conditions for snow tracking (finding and following wolverine snow tracks) and travel by snow machines (main mean of transport for monitoring personnel). We used average April snow depth data from 1764 weather stations spread across the study area, obtained from www.smhi.se, and each reproductive event was assigned yearly April snow depth from the closest weather station.
2.3 Statistical analysis
To assess potential temporal change in the yearly proportion of reproductive events that were snow covered in March and in spring, we used a logistic regression with annual number of snow covered (1) and bare ground (0) reproductive events as response variables, that is, generalized linear model with a binomial distribution and a logit-link function in R (R Core Team, 2017). We used year, snow cover period, and their interaction as explanatory variables.
To assess if the distribution of wolverine reproductive events were linked to spring snow coverage (April 24–May 15), and if this relationship changed over time, we used sites with available information on proportion of years with spring snow cover (as explained above) in the Alpine-Reindeer (n = 1207 reproductive events) and Boreal-Reindeer (n = 281 reproductive events) study regions. The Boreal study region was not included in this analysis due to lack of spring snow cover (Figures 1 and 2) and low sample size (n = 44 reproductive events). A common approach to estimate the relationship between a species distribution and environmental variables is to compare the locations where the species was detected (presences) with a set of randomly selected locations (pseudo-absences) from a predefined sampling domain (e.g., Pearce & Boyce, 2006). We grouped each reproductive event (i.e., presence location) together with 50 pseudo-absence points randomly sampled from all 500 × 500 m pixels with complete spring snow cover information in the corresponding study region. We used logistic regression with presence (=1) and pseudo-absence (=0) of reproductive events as response variable to assess the relative probability of wolverine reproductions as a function of the following explanatory variables; time period (categorical), proportion of years with spring snow cover during the corresponding time period (continuous: 0–1), and April snow depth (continuous: 0–1.93 m). There was no collinearity between explanatory variables (all Pearson correlation coefficients |r| < .7). We compiled a set of candidate models representing alternative hypotheses relating the probability of reproductive events to spring snow cover (Table 1). First, reproductive events related to spring snow cover only, including a quadratic effect to allow for optimal proportion of years with spring snow cover (model 1) and allowing the effect of snow cover to vary between time periods (model 2). Second, reproductive events related to April snow depth only (model 3) and allowing the effect of April snow depth to vary between time periods (model 4). Third, reproductive events related to both spring snow cover and April snow depth (model 5), allowing for temporal difference in one (models 6–7) or both (model 8) explanatory variables. Candidate models were compared using the sample-size corrected Akaike information criterion (AICc) and AICc weights (Burnham & Anderson, 2002).
| Set | Models | Alpine-Reindeer | Boreal-Reindeer | ||
|---|---|---|---|---|---|
| ∆AICc | w i | ∆AICc | w i | ||
| 1 | PSC+PSC2 | 17.94 | 0.00 | 72.37 | 0.00 |
| 2 | PSC+PSC2+T+PSC*T+PSC2*T | 0.00 | 0.66 | 31.94 | 0.00 |
| 3 | ASD | 132.99 | 0.00 | 155.34 | 0.00 |
| 4 | ASD+T+ASD*T | 140.79 | 0.00 | 137.97 | 0.00 |
| 5 | PSC+PSC2+ASD | 19.29 | 0.00 | 43.41 | 0.00 |
| 6 | PSC+PSC2+ASD+T+PSC*T+PSC2*T | 1.79 | 0.27 | 6.40 | 0.04 |
| 7 | PSC+PSC2+ASD+T+ASD*T | 25.59 | 0.00 | 20.07 | 0.00 |
| 8 | PSC+PSC2+ASD+T+PSC*T+PSC2*T+ASD*T | 4.66 | 0.06 | 0.00 | 0.96 |
- Note: Model sets were compared using sample-size corrected AIC (AICc). For each model we show difference in AICc relative to the highest ranked model (∆AICc) and AICc weights (wi). Bold numbers highlight the highest ranked models in each study region.
- Abbreviation: AICc, corrected Akaike information criterion.
3 RESULTS
3.1 Temporal change in the distribution of wolverine reproductive events in relation to snow cover
A total of 1589 wolverine reproductive events were documented in Sweden within the national monitoring program in 2000–2018. Of these, 79% (1262) were found in the Alpine-Reindeer region, 18% (282) in the Boreal-Reindeer region, and 3% (45) in the Boreal region.
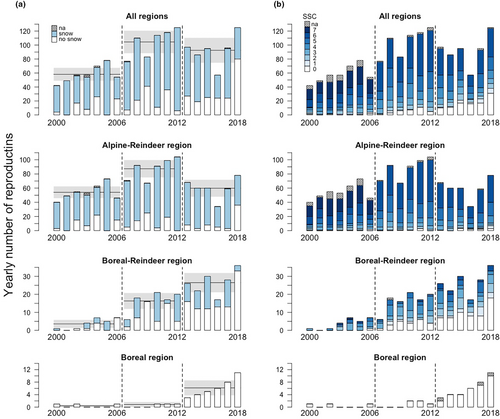
Both the annual number of wolverine reproductive events and their spatial distribution increased during the study period with an expansion south and eastwards from the alpine area; from 42 in 2000 (95% in the Alpine-Reindeer, and 2.5% each in the Boreal-Reindeer and Boreal regions) to 125 in 2018 (62%, 29%, and 9% in each region, respectively; Figures 1 and 2).
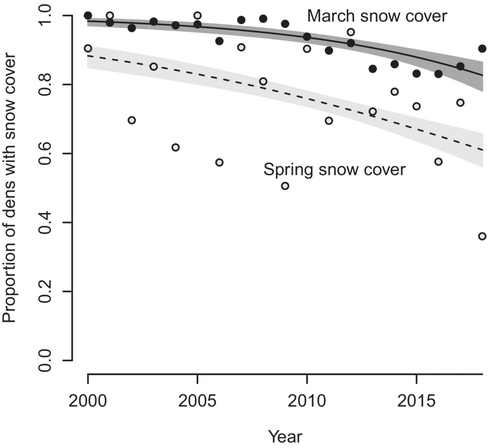
The proportion of yearly reproductive events in snow covered pixels (500 × 500 m) in both March (March 1–21) and spring (April 24–May 15), varied among years and decreased with time during the study period (Figures 2a and 3). However, the decrease was slightly smaller for spring snow coverage (logit-link estimate of the slope (βyear) for March: −0.14 ± 0.022 SE, p < .001, and the difference in slopes between March and spring: 0.053 ± 0.025 SE, p = .04). As expected, the proportion of yearly reproductive events that was located within snow covered pixels was higher in March compared to spring (logit-link intercept [β0] for March: 4.22 ± 0.32 SE, p < .001 and the difference in intercepts between March and spring: −2.07 ± 0.36 SE, p < .001; Figure 3).
The wolverine expansion into areas with less snow cover is further illustrated by the yearly number and proportion of reproductive events in areas without spring snow cover (1530 reproductive events with complete spring snow cover information) in the three time periods and study regions (Figures 1 and 2b). In 2000–2006, only 4 (1%) of all 363 reproductive events were located in areas that never had spring snow cover during the time period, compared to 33 (5%) of 617 in 2007–2012, and 102 (18%) of 550 reproductive events in 2013–2018. To account for the different number of years in the first (7 years) versus the latter periods (6 years each), the latter two could be compared to the proportion of reproductive events that was in areas snow covered ≤1 year in the first period; that is, 3% (n = 11). In the Alpine-Reindeer region, only 0%–2%, 1%, and 3% of the reproductive events were located in areas without spring snow cover in 2000–2006 (0–7 of 335), 2007–2012 (5 of 515), and 2013–2018 (11 of 357), while the corresponding percentages for the Boreal-Reindeer region were 4% (1 of 25), 25% (23 of 97), and 36% (58 of 159; Figure 2b). In the Boreal region, 100% (of 44) reproductive events were in areas without spring snow cover. Accordingly, there was a decrease in the proportion of reproductive events in areas that were snow covered in all years; 63% of all reproductive events were snow covered in ≥6 years (39% snow covered in all 7 years) in 2000–2006, 47% in 2007–2012, and 34.5% in 2013–2018.
3.2 Temporal change in the relationship between snow cover and occurrence of wolverine reproductive events
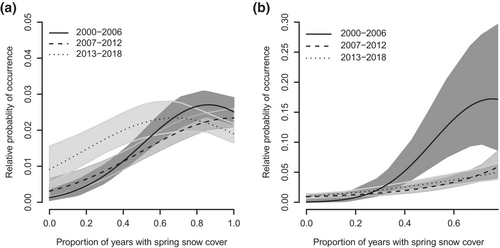
The model best explaining the probability of occurrence of wolverine reproductive events differed between the Alpine-Reindeer and Boreal-Reindeer regions (Table 1). In the Alpine-Reindeer region, the probability of occurrence of reproductive events was related to the proportion of years with spring snow cover, but the influence of spring snow cover changed between time periods. In the first and second time periods, the relative probability of occurrence of reproductive events increased with an increasing proportion of years with spring snow cover (Figure 4a; Table 2). In the last period, the relationship between reproductive events and spring snow cover was less clear, mostly due to an increase in the relative probability of occurrence of reproductive events also in areas with a low proportion of years with spring snow (Figure 4a). April snow depth was included in the second-best model for the Alpine-reindeer region (Table 1), where the relative probability of occurrence of reproductive events increased with increasing April snow depth during all time periods.
| Variable | Alpine-Reindeer | p-Value | Boreal-Reindeer | p-Value | ||
|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | |||
| Intercepta | −6.64 | 0.49 | <.001 | −8.09 | 1.17 | <.001 |
| PSCa | 7.10 | 1.46 | <.001 | 15.61 | 3.95 | <.001 |
| PSC2,a | −4.12 | 1.03 | <.001 | −10.17 | 3.46 | <.001 |
| T2b | 0.87 | 0.62 | .16 | 3.74 | 1.19 | <.001 |
| T3b | 1.96 | 0.56 | <.001 | 4.34 | 1.18 | <.001 |
| PSC*T2b | −3.03 | 1.83 | .10 | −14.23 | 4.11 | <.001 |
| PSC*T3b | −4.32 | 1.72 | .01 | −12.96 | 4.05 | <.001 |
| PSC2*T2b | 2.09 | 1.29 | .11 | 11.55 | 3.69 | <.001 |
| PSC2*T3b | 2.09 | 1.25 | .09 | 9.48 | 3.59 | .01 |
| ASDa | - | - | 1.37 | 1.19 | .25 | |
| ASD*T2b | −2.15 | 1.27 | .09 | |||
| ASD*T3b | - | - | −3.34 | 1.24 | .01 | |
- Note: The intercept corresponds to the first time period (2000–2006).
- Abbreviations: ASD, April snow depth; PSC, proportion of years with snow cover; T2, years 2007–2012; T3, years 2013–2018.
- a Period 1 is the reference.
- b This is relative to Period 1.
In the Boreal-Reindeer region, the probability of occurrence of reproductive events was related to both the proportion of years with spring snow cover and April snow depth, and the influence of both variables changed between time periods (Table 1). In the first period, the probability of occurrence of reproductive events increased with snow depth (Table 2) and spring snow cover (Figure 4b; Table 2). However, in the second and third time periods, the effect of spring snow cover almost disappeared (Table 2; Figure 4b), and the effect of snow depth was negative in the last period (Table 2).
4 DISCUSSION
The ongoing climate change requires improved understanding of its many effects on wildlife in general and on endangered species in particular to be integrated in conservation and management plans (Hansen et al., 2010). Nevertheless, there is large variation in observed and predicted responses among species (e.g., Brodie et al., 2013), and determining their vulnerability requires a deeper understanding of involved processes (Moritz & Agudo, 2013). Here we revisited the hypothesis that wolverine reproduction and distribution are dependent on persistent spring snow cover, by using two-decades fitness-related data (i.e., annual number of reproductive events) at the population level in Sweden. We documented a southward expansion of the wolverine population, from alpine areas into boreal forests, with an increasing number of reproductions in areas without spring snow cover (Figures 1 and 2). In fact, this recolonization has occurred over a notably short period; where the number of documented reproductions outside alpine areas increased from 2 to 47 in less than two decades, and we would expect this colonization to continue in the foreseeable future. This process resulted in a dampened relationship between wolverine reproductions and persistent spring snow cover across the 2000–2018 study period (Figures 3 and 4). That wolverines are expanding into areas lacking persistent spring snow cover questions the suggested importance of this factor, which predominates contemporary literature, and reinforces recent observations from both Europe and North America showing that wolverines are increasingly found outside areas with spring snow cover (Aronsson & Persson, 2017; Lansink et al., 2020; Webb et al., 2016). Still, the observed range expansion occurred subsequent to the shorter period (2000–2006) used in Copeland et al. (2010), and, in fact, the relation between wolverine reproductions and snow cover during the first period in our analyses corroborates the findings by Copeland et al. (2010), which is expected considering that 87% of their data were from Scandinavia. Our findings highlight the importance of reassessing established knowledge about environmental requirements, which is particularly critical now that ecosystems are changing rapidly. Long-term (and) fitness-related data are therefore especially needed in order to understand how species may cope with climate change, and in turn to forecast its expected effects on species and implement conservation action.
To understand and accurately predict the response of threatened species to climate change we should consider intraspecific factors (Dawson et al., 2011), as well as other biotic (e.g., food availability and interspecific interactions) and abiotic factors (e.g., past and current effects of human activities) that influence vulnerability. This is particularly important for species that warrant conservation efforts and that, at least in recent times, have been typically driven by factors other than climate, for example, carnivore populations that have been heavily impacted by past human persecution.
Two main messages arise from the range expansion of the Swedish wolverine population into areas without persistent spring snow cover. First, niche or envelope models based on a close dependency of wolverines on persistent snow cover in spring may have failed to forecast long-term effects of climate change because they estimated the exposure of the species to the conditions at a specific point in time, without including other effects that may modulate its response to environmental changes. In turn, forecasts of climate change effects, and then conservation actions, may be biased, for example, by highlighting the expected dependency on a given factor, whose actual role in drawing species distribution may be different than assumed, and thus risk overlooking other critical factors. Second, long-term human persecution that has restricted species distribution to remote or scarcely populated areas, can mask actual effects of environmental parameters, whose importance are revealed first when persecution is relaxed and populations expand naturally beyond their previously restricted ranges. Wolverine populations have recently recovered former ranges in both Scandinavia and North America subsequent to strong population reductions caused by human persecution (Aronsson & Persson, 2017; Mckelvey et al., 2014). Likewise, recolonizations by wolves (Canis lupus; Ordiz et al., 2015), lynx (Lynx lynx; Hemmingmoore et al., 2020), and brown bears (Ursus arctos; Swenson et al., 2017) in Scandinavia during the last decades support that reduced human pressure after centuries of persecution have favored recovery of the whole large carnivore guild; a pattern that applies in other areas of both Europe (Chapron et al., 2014) and North America (Bruskotter & Shelby, 2010). Thus, these large carnivore populations have likely been limited by human activities, for periods overshadowing the influence of present environmental parameters.
Wildlife monitoring efforts are typically not evenly distributed but geographically targeted to, for instance, protected areas, hotspots of management conflicts, or areas with high population densities that may facilitate to address ecological- and management-related questions (Guerrero et al., 2013; Nichols & Williams, 2006). This is the case for wolverines in Sweden, where monitoring effort has mainly focused on Alpine-Reindeer husbandry areas, and less in expansion areas where neither wolverine occurrence or conflicts were expected, and monitoring is more difficult due to poor snow conditions (Aronsson & Persson, 2017). Although, monitoring has increased in expansion areas in recent years (J. Persson, unpublished data, Milleret et al., 2022), it is still lagging behind the expansion. Consequently, it is very likely that more reproductions have gone undetected outside than inside areas with spring snow cover. Whereas missing data and sampling bias are normally a limitation for any scientific study, they paradoxically strengthen our result. That wolverines have gradually expanded south and eastwards in Sweden is a confirmed process despite a biased monitoring effort (Aronsson & Persson, 2017). A crucial component of the monitoring program is that it has focused on the counting of reproductive events, thus allowing us to depict the trend of the most important segment of the population (e.g., Andrén et al., 2002; Fernández-Gil et al., 2010; Liberg et al., 2012). Linking the wolverine expansion with this fitness-related parameter is more conclusive than if expansion was solely based on observations of single individuals or their signs. This gives reliable information about when and where a reproducing population is established. Still, observations of single individuals can be valuable for early detection of expansion patterns and understanding the development of a population, as shown in Aronsson and Persson (2017).
Although it is evident that a persistent spring snow is not limiting the distribution of wolverines in Sweden, snow is likely an important feature of wolverine habitat, as it facilitates denning, provides protection for young against predators and thermal stress, benefits food caching, and predation on ungulates (Inman et al., 2012; Sutton et al., 2017; van der Veen et al., 2020). We used MODIS imagery to infer snow cover during the study period, which might be a rough proxy to elucidate the actual snow conditions at a finer spatial scale. Dens in areas without spring snow cover may still be covered with snow during parturition and early denning (February–March, Figure 3), when temperature is generally low, and juveniles are most vulnerable. In the boreal forest without spring snow cover in central Sweden, boulders were the main structure at all (n = 49) den sites investigated in the field; all included structures that would provide cover even without snow (Makkonen, 2015; Figure 5). Similarly, wolverines in North American boreal forests den in areas with large boulders, downed trees and similar structures providing cover (Dawson et al., 2010; Jokinen et al., 2019; Scrafford, 2017). Although persistent spring snow is not limiting wolverine distribution in Sweden, its importance may vary among areas with abundance of alternative den site structures, competitors, and food resources. For example, spring snow cover may be more important in arctic Alaska where alternative den site structures are rare (Glass et al., 2022). Also, in areas with steep altitudinal gradient, such as US Rocky Mountains (Inman et al., 2013), it could be difficult to determine the importance of spring snow cover as it may covary with terrain ruggedness, vegetation, abundance of competitors, and human development.

Ongoing recolonization of Scandinavia by the whole carnivore guild allows studying interspecific interactions (e.g., Mattisson et al., 2011; Ordiz et al., 2015), which may be yet another factor behind the observed expansion of a facultative scavenger as the wolverine. For instance, wolverines benefit from scavenging lynx-killed reindeer in northern alpine areas (Mattisson et al., 2011) and wolverines recolonizing the boreal forest nowadays overlap wolf and bear ranges, which provide scavenging opportunities (e.g., Aronsson & Persson, 2017; Koskela et al., 2013; van Dijk et al., 2008; Wilmers & Post, 2006). Furthermore, the extensive annual moose (Alces alces) hunt provides large amounts of slaughter remains that wolverines scavenge (Aronsson et al., 2022). The ongoing wolverine expansion may thus be driven by a combination of the species dispersal capacity (Vangen et al., 2001), availability of areas with low human pressure, food resources provided by humans and other predators, and a combination of these. Beyond specific factors, whose clarification deserves further research, it seems clear that wolverines are less dependent on persistent snow cover than previously assumed, which is relevant to forecast the effect of climate change on the distribution of the species.
Most reproductive events are registered in March–April during monitoring, because the aim is to document occurrence of reproductions, not juvenile survival, or recruitment. However, information based on monitoring of GPS-collared females (Aronsson et al., 2022), and frequent documentation with camera traps of females with offspring in late summer, do not suggest that juvenile survival is lower in areas without spring snow cover compared to alpine areas (cf. Persson et al., 2009). In fact, data from GPS-collared females suggest that the reproductive rate is higher in the southern part of wolverine distribution compared to northern areas (Aronsson et al., 2022). This, in turn, suggests that food resources are abundant in these areas (Persson, 2005; Rauset et al., 2015). Moreover, that the annual number of documented reproductions in areas without spring snow cover is increasing further supports that reproduction and survival are relatively high, unless a constant immigration of females from other areas compensates high mortality. Indeed, an ongoing telemetry study suggests that adult survival is higher in areas outside spring snow cover than reported from core areas in the alpine region (J. Persson, unpublished data; Persson et al., 2009). Furthermore, in the central part of the area without spring snow cover (southern edge of boreal-reindeer area; Figure 2) wolverine density is among the highest in Scandinavia (Bischof et al., 2021; Milleret et al., 2022), which suggests that colonization occurs into suitable habitats for the species.
Our study shows that wolverines are less dependent on persistent snow cover than previously suggested, and highlights that further research is needed to identify other factors that, in combination with snow cover, are relevant for forecasting the effect of climate change on wolverine distribution. Understanding what environmental factors are limiting populations of species with circumpolar distributions requires studies accounting for local traits in different ecosystems. For example, ambient temperature is a climatic factor that may influence wolverines directly, through high summer temperatures influencing thermoregulation (Thiel et al., 2019), and indirectly through its influence on longevity of caches (Inman et al., 2012), and thus being something to consider when investigating factors limiting wolverine distribution (Copeland et al., 2010). Therefore, studies should include well-designed monitoring across environmental gradients at a large scale. In addition, to fully reveal mechanisms by which populations are limited, and which shape large-scale patterns, requires individual-based studies linking fitness-related parameters to variables such as snow cover and other variables that may determine habitat suitability, temperature, abundance of competitors, food availability, and human-caused disturbance. Together this could reveal spatial and temporal variation in factors limiting wolverine distribution.
Our study reinforces the importance of longitudinal data and revisiting research questions when new data are available. While overwhelming evidence shows that climate change is affecting species and ecological processes in multiple biological systems, forecasting potential consequences on a given species requires specific analyses to reveal the direction of effects and to quantify their magnitude in relation with other factors. This is particularly important for conservation-oriented management of threatened species inhabiting dynamic systems where multiple species and human activities interact. Our results have principal implications for projections of future wolverine distribution, habitat, and connectivity, and may also apply to other species. So far, projections of future wolverine habitat are largely based on the stated wolverine dependence on persistent spring snow cover, and may thus overestimate the absolute loss of wolverine habitat in the future. Importantly, this does not mean that climate change may not pose threats for this and other species. At the global scale, the wolverine population is decreasing (Abramov, 2016) and the species is classified as vulnerable or endangered in several of the countries it inhabits (Lansink et al., 2022). Furthermore, wolverines, other large carnivores, and many other species inhabiting human-dominated landscapes are influenced by multiple factors and by human activities (e.g., Ordiz et al., 2017, 2021). Disentangling the relative role of intra- and interspecific interactions and abiotic factors is crucial to properly forecast the effects of climate change on the species distribution patterns and population dynamics in a scenario of climate change. This continuous update of knowledge is essential to inform conservation-oriented management of species across their circumpolar range.
AUTHOR CONTRIBUTIONS
This study was conducted within a long-term research project led by Jens Persson and Malin Aronsson. Jens Persson and Malin Aronsson conceived the study. Jens Persson and Andrew Ladle prepared databases. Andrew Ladle, Malin Aronsson, and Henrik Andrén analyzed the data. Jens Persson, Andrés Ordiz, and Andrew Ladle led the writing of the manuscript with input from all authors.
ACKNOWLEDGMENTS
This research was funded by FORMAS (Swedish Research Council for Sustainable Development, grant number 2016-00924), Swedish Environmental Protection Agency (grant number 2020-00110), Carl Tryggers Foundation (grant number CTS: 17:359), WWF Sweden (grant number 500475), and Marie-Claire Cronstedt foundation. We thank Thomas Jung and an anonymous reviewer for constructive comments on a previous version of the manuscript. We also want to thank all the field personnel from County Administrative Boards that monitored all wolverine reproductive events, which made this study possible.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Data are available from the authors with the permission of Swedish Environmental Protection Agency. Data availably is restricted to protect locations of endangered species.




