Multiple stressor effects on coral reef ecosystems
Abstract
Global climate change has profound implications on species distributions and ecosystem functioning. In the coastal zone, ecological responses may be driven by various biogeochemical and physical environmental factors. Synergistic interactions can occur when the combined effects of stressors exceed their individual effects. The Red Sea, characterized by strong gradients in temperature, salinity, and nutrients along the latitudinal axis provides a unique opportunity to study ecological responses over a range of these environmental variables. Using multiple linear regression models integrating in situ, satellite and oceanographic data, we investigated the response of coral reef taxa to local stressors and recent climate variability. Taxa and functional groups responded to a combination of climate (temperature, salinity, air-sea heat fluxes, irradiance, wind speed), fishing pressure and biogeochemical (chlorophyll a and nutrients - phosphate, nitrate, nitrite) factors. The regression model for each species showed interactive effects of climate, fishing pressure and nutrient variables. The nature of the effects (antagonistic or synergistic) was dependent on the species and stressor pair. Variables consistently associated with the highest number of synergistic interactions included heat flux terms, temperature, and wind speed followed by fishing pressure. Hard corals and coralline algae abundance were sensitive to changing environmental conditions where synergistic interactions decreased their percentage cover. These synergistic interactions suggest that the negative effects of fishing pressure and eutrophication may exacerbate the impact of climate change on corals. A high number of interactions were also recorded for algae, however for this group, synergistic interactions increased algal abundance. This study is unique in applying regression analysis to multiple environmental variables simultaneously to understand stressor interactions in the field. The observed responses have important implications for understanding climate change impacts on marine ecosystems and whether managing local stressors, such as nutrient enrichment and fishing activities, may help mitigate global drivers of change.
1 INTRODUCTION
Anthropogenically induced global climate change has profound implications for marine ecosystems (Hewitt, Ellis, & Thrush, 2016). While ‘natural’ cycles are the result of seasonal variability, there is evidence that ‘anthropogenic’ factors may increase the ecological effects associated with such variability (Hauck, 2018). Regardless, the marine environment is changing rapidly (Doney et al., 2012; Hoegh-Guldberg & Bruno, 2010) with unprecedented alterations in seawater temperature throughout much of the globe occurring in the last decade (Philippart et al., 2011). Changes also include rising sea level, increased storm intensity and frequency, more variable precipitation, loss of ice cover in polar regions and ocean acidification (IPCC, 2007). Although marine species and ecosystems have responded to variations in their environment throughout evolutionary history, a primary concern is the rapid rate of change currently observed (Root et al., 2003). Seminal reviews in the early 1990s (Fields, Graham, Rosenblatt, & Somero, 1993; Lubchenco, Navarrete, Tissot, & Castilla, 1993) identified that the distribution and abundance of species would shift according to their thermal tolerance and ability to adapt with a primary research focus on the effects of rising temperatures. However, more recently the role of ocean chemistry, UV radiation and hydrodynamic changes have also been identified as important factors, not just temperature effects alone (Harley et al., 2006) creating the potential for multiple stressor effects.
Despite this, many marine studies still focus on temperature predicting changes in distributional boundaries of species and replacement of cold-water taxa by others with warm-water affinities (Hewitt et al., 2016). Increasingly there has also been a call for a better understanding of the effect of multiple stressors on biodiversity and associated ecosystem services (Ban, Graham, & Connolly, 2014). Much of this concern over multiple stressor interactions has developed due to the potential for their combined effects to exceed their individual effects, referred to as synergism (Folt, Chen, Moore, & Burnaford, 1999). When the combined effect of stressors is less than the sum of their individual effects this is considered an antagonistic interaction. Two review articles provide evidence for a high number of synergistic and antagonistic effects in marine ecosystems (Crain, Kroeker, & Halpern, 2008; Darling & Cote, 2008). Importantly, increasing evidence reveals the existence of ecological surprises where the behavior of a natural system sometimes drastically deviates from expectations and in many cases, synergistic effects may have played a role in these ecological surprises (Lindenmayer, Likens, Krebs, & Hobbs, 2010).
Effective coastal management requires an understanding of how multiple stressors interact and coral reefs are a particularly good example of the interplay between global and local stressors (Ban et al., 2014). Coral reefs belong to some of the world's most stressed ecosystems (Carpenter et al., 2008; Hoegh-Guldberg & Bruno, 2010; Hughes et al., 2003; Walther et al., 2002), therefore understanding and managing multiple stressor interactions is particularly urgent (Ban et al., 2014). Coral reefs are in serious decline with an estimated 30% already severely damaged, while 60% of reefs have been predicted to be lost by the year 2030 (Wilkinson, 2000). Direct and indirect effects of overfishing and pollution from agriculture and land development have been identified as the major drivers of widespread changes in reef ecosystems over the past two centuries (Hughes, 1994; Hughes et al., 2003; Jackson et al., 2001; Pandolfi et al., 2003), causing regime shifts from a dominance of scleractinian corals to turf and macroalgal species (Hughes, 1994; McClanahan, Polunin, & Done, 2002). However, these changes are now compounded by the more recent superimposed impacts of global climate change (Hughes et al., 2003, 2018).
Coral reefs are also among the most biologically diverse and socioeconomically valuable biomes and identifying synergisms between stressors would allow prioritization of management to mitigate the most severe interactions. This study therefore aims to determine which stressors impact important coral reef taxa and to detect the nature of these interactions (i.e. antagonistic, additive or synergistic) signifying priorities for coastal management. Previous studies have concluded that our ability to mitigate global change is negligible compared to our ability to manage local stressors (Gurney, Melbourne-Thomas, Geronimo, Alino, & Johnson, 2013), for example, controlling nutrient discharges or managing fishing pressure to increase coral reef resilience. By identifying local stressors and those that exert the largest or most frequent synergistic effects, it may be possible to maximize the effectiveness of management actions (Ban et al., 2014). In this paper we subsequently use the term ‘driver’ of ecosystem change rather than ‘stressor’ for nutrient and climate related variables. This terminology is adopted because whilst eutrophication can occur at high levels of nutrient enrichment and would be considered a ‘stressor’, at background levels nutrients are essential for the health of coral reefs.
This research was conducted in the Red Sea where coral reefs thrive in one of the warmest and most saline environments of any extensive coral reef system in the world. The Red Sea is characterized by strong gradients in temperature, salinity, and nutrients along the latitudinal axis which provides a unique opportunity to study ecological responses over a wide range of these environmental variables. For example the Red Sea currently experiences higher temperature conditions in the South with up to over 33°C recorded during summer and high saline conditions in the Northern Red Sea up to 42 psu. Consequently, the Red Sea is considered a contemporary example for understanding how reefs may fare under predicted scenarios of global climate change (Berumen et al., 2013). By modelling along gradients, such as sea surface temperature, the model provides information on whether the species increases in abundance, decreases or shows a uni-modal response to elevated temperature. These trends are indicative of whether species are sensitive or tolerant to the physical variable being modelled. This study, therefore, assesses how coral reef ecosystems in the Red Sea are influenced by gradients in large-scale environmental and oceanographic variables using regression-based techniques and investigates whether critical benthic components including hard corals are affected by multiple drivers. By modelling along the strong natural gradients of temperature, salinity and nutrient regimes in the Red Sea, which at the upper ranges are already above IPCC global predictions for other ocean systems, this research may provide novel climate change insights.
2 METHODS
2.1 Quantitative surveys of coral reefs
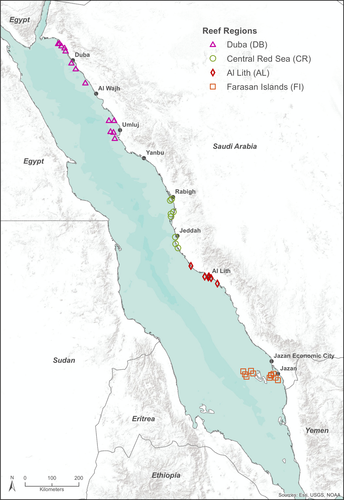
In 2014 and 2015 a total of 40 coral reef locations from the Red Sea were surveyed using an adapted photo quadrat belt-transect method (Figure 1). At each site, three 20 m by 5 m transects followed two depth horizons at 3–5 m and 8–10 m. Along each transect, a photo (1 m2) was taken every 2 m with a Canon G16 digital camera (16 megapixel). Benthic groups in each photo were identified and quantified using the software Coral Point Count with Excel extensions (CPCe; Kohler & Gill, 2006) for 48 points randomly distributed per 1 m2 frame. On each substrate image the features underlying the points were user-identified based on 90 benthic taxonomic groups. Counted points were averaged for each site and depth to calculate percentage cover of each of the main taxonomic groups including hard corals, soft corals, coralline algae, turf algae and macroalgae.
2.2 In situ data
At all reef stations Conductivity Temperature Depth vertical casts were collected for conductivity, depth, temperature and dissolved oxygen measurements and at the Al Lith stations chlorophyll measurements. The ratio of the water conductivity to that of a standard solution was used to calculate psu. Surface water samples for chlorophyll a and nutrient quantification (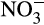 , NO2,
, NO2, 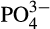 , SiO2) were taken at each reef. Approximately 50 ml water was filtered through 0.2 μm Supor PES membrane disc filters to collect nutrient samples that were frozen at −20°C for later analysis. Nutrient samples were analyzed using a Continuous Flow Analyzer (SEAL AutoAnalyser 3 with XY2/3 Sampler; EPA, 1983). Detection limits were 0.083 μM SiO2, 0.002 μM NO2, 0.011
, SiO2) were taken at each reef. Approximately 50 ml water was filtered through 0.2 μm Supor PES membrane disc filters to collect nutrient samples that were frozen at −20°C for later analysis. Nutrient samples were analyzed using a Continuous Flow Analyzer (SEAL AutoAnalyser 3 with XY2/3 Sampler; EPA, 1983). Detection limits were 0.083 μM SiO2, 0.002 μM NO2, 0.011 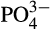 μM and 0.032 μM
μM and 0.032 μM 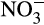 . For the chlorophyll a analysis 0.5–3 L of water were filtered through GF/F filters. The filters were then wrapped in aluminum foil and refrigerated in −80°C in the laboratory until later analysis. Chlorophyll a was extracted using 90% acetone (EPA, 1983) and the fluorescence was measured with a Trilogy fluorometer (Turner Designs) and calibrated using known concentration standards.
. For the chlorophyll a analysis 0.5–3 L of water were filtered through GF/F filters. The filters were then wrapped in aluminum foil and refrigerated in −80°C in the laboratory until later analysis. Chlorophyll a was extracted using 90% acetone (EPA, 1983) and the fluorescence was measured with a Trilogy fluorometer (Turner Designs) and calibrated using known concentration standards.
2.3 Modeled environmental data
Air sea variables were examined based on a high-resolution (5 km) long term downscaled version of the ERA-interim atmospheric reanalysis initially available at 0.75° × 0.75°. Data from this model included air-sea heat and freshwater fluxes and incoming solar radiation. Advanced Research WRF model version 3.9.1 developed by NCEP/NCAR was used to generate the Arabian Peninsula regional reanalysis. The model simulations were performed over 37 years starting from 1980 to 2017 by assimilating all available observations (Yesubabu, Hari Prasad, Langodan, Srinivas, & Hoteit, 2016). This resulting regional reanalysis has been used to investigate various climatic characteristics over the Red Sea (Langodan et al., 2017), as well as for estimations of wave and wind energy for the Red Sea region (Langodan, Yesubabu, & Hoteit, 2016). Wind speed, air temperature, surface freshwater fluxes (evaporation), irradiance (solar radiation) and turbulent components of heat flux (latent and sensible) time series were extracted to examine their potential influence on the local ecosystem.
Wave information in terms of significant wave height, period and speed, were provided by model simulations (Langodan et al., 2016) using the third generation WAVEWATCH III model. The simulations were forced by the same downscaled atmospheric reanalysis. The analyzed fields, including wind speed, period and direction, were provided on a regular grid with 0.05° resolution in space, daily integrated.
2.4 Remote sensing data
Mapped photosynthetically active radiation (PAR) Level-3 data, acquired from the Moderate Resolution Imaging Spectroradiometer sensor onboard the Aqua (NASA EOS PM-1) satellite were downloaded from http://oceancolor.gsfc.nasa.gov/cgi/l3. Data were acquired at a daily temporal resolution for the period spanning 2013–2015 on a spatial resolution of ~4 km × 4 km. PAR is defined as the spectral range of solar radiation between 400 and 700 nm and typically represents the range of wavelengths that can be utilized for photosynthesis.
2.5 Fishing index
A fishing index was derived from Rowlands et al. (2012). The index utilized data from the Saudi Ministry of Agriculture fishery statistics, fishing effort that combined properties of the fishing fleet derived from satellite imagery (notably industrial vs. traditional boat and port density information), depth and a distance from port decay model. The metric is a spatial representation of potential fishing effort and is bounded between 0 and 1. A value of 0 represents no fishing effort while a value of 1 represents the highest fishing potential in the Red Sea.
2.6 Data analysis
2.6.1 Identifying key drivers of change
Environmental variables that accounted for a range of natural and anthropogenic drivers were generated (Table 1). A total number of 24 (11 in situ and 13 modeled) variables were available. In order to avoid overfitting, due to the relatively low number of observations, a typical approach is to reduce the dimension of the system for the statistical analysis, by removing variables that are highly correlated. Examination of the correlation structure (Figure S1) reveals clustered groups of strongly correlated variables. A reasonable outcome of the correlation analysis is that the identified groups consist of variables representing similar or related physical and/or biochemical mechanisms. For example, wind speed, wave direction and wave height, were highly correlated, as expected, since wind speed largely characterizes the wave dynamics and their variability. A simple approach for reducing the number of variables by removing the highest correlated variables was initially examined, however, this method excluded entire groups and, therefore, did not account for the different mechanisms represented in the initial set of variables. A more suitable approach was then considered, by removing the highest correlated variables ranked in each representative group of each physical/biochemical forcing. Therefore, variables were excluded by correlation rank, while retaining at least one of these variables in each group. Uncorrelated variables were, in most cases, included in the model (e.g. chlorophyll, 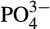 , oxygen, temperature, fishing index). Consequently, this approach allowed the number of variables to be reduced, while retaining the bulk of physical and biological information included in the original set of analyzed variables. Ιt should also be noted that the “excluded” variables may still be relevant and were considered in the analysis of our results. For example, wind speed was selected as representative of the variables characterizing the wave dynamics, which include the wave height and wave period. Similarly, latent heat flux was selected as most representative of air-sea exchanges for the Red Sea.
, oxygen, temperature, fishing index). Consequently, this approach allowed the number of variables to be reduced, while retaining the bulk of physical and biological information included in the original set of analyzed variables. Ιt should also be noted that the “excluded” variables may still be relevant and were considered in the analysis of our results. For example, wind speed was selected as representative of the variables characterizing the wave dynamics, which include the wave height and wave period. Similarly, latent heat flux was selected as most representative of air-sea exchanges for the Red Sea.
| In situ data | Units | Modeled data | Units |
|---|---|---|---|
| PO4 | μM | Wave period | s |
| SiO2 | μM | Wave height | m |
| NO3 | μM | Wind speed | m/s |
| NO2 | μM | Latent heat flux | W/m2 |
| NOx | μM | Evaporation | W/m2 |
| Chlorophyll a | μg/L | Remotely sensed chlorophyll a | mg/m3 |
| Temperature | °C | Rainfall | mm |
| Salinity | psu | PAR | W/m2 |
| Oxygen | mg/L | Specific humidity | kg/kg |
| Distance to shore | km | Fishing pressure | Index (0–1) |
| Latitude longitude | DMS |
Note
- Variables in bold were retained in the final model as important in explaining the percent cover of the main reef benthic communities surveyed.
- Abbreviations: NO2, nitrite; NO3, nitrate; NOx, nitrite/nitrate; PAR, photosynthetically active radiation; PO4, phosphate; SiO2, silicate.
One environmental variable, distance to shore, had a skewed distribution and was therefore log-transformed. However this variable was not carried forward in the model. Once the final set of environmental variables was determined, each environmental variable was standardised (so that the mean was 0 and the SD was 1). The final set of variables is presented in Table 1. While we have selected one variable that represents a group of highly correlated parameters, it should be noted that the “excluded” variables are still relevant. For example while wind speed was carried forward in the model, it was highly correlated with wave height and wave period. Hence wind speed also characterises the variables representative of wave dynamics. Similarly for latent and sensible heat flux, while they were highly correlated, latent heat flux was the dominant variable between the two and therefore carried forward in the model. Irradiance, evaporation and latent heat fluxes were overall selected as most representative of air-sea exchanges for the Red Sea. For nutrients, we also conducted a separate model run replacing nutrient values that fell below the limit of detection with the detection value, which was not found to influence the model performance.
2.7 Modeling taxa and taxonomic group responses to drivers of change
In community ecology, environmental variables should not be considered in isolation as they influence and often co-vary with each other. We used multivariate linear regression to model the in situ, modeled variables and in situ-modeled interactions that contribute to the coral species distribution model. Quantile-quantile and residuals versus fitted plots were used to check for homogeneity and normality. Model selection in regression methods is often performed through sequential testing using a stepwise algorithm. One of the aims of model selection is a trade-off between model complexity and accuracy. The selection of the best model from exhaustive models is computationally challenging as the number of models grows exponentially with the number of factors.
Prior to analysis, taxa with a small number of occurrences (≤10 sites) were removed; leaving a total of 68 taxa. The benthic percentage coverage data were square-root arcsine transformed. We also conducted a separate model run based on logistic regression which was not found to influence the model performance. Some variables were not relevant in explaining the benthic taxa (e.g. rainfall and currents) and therefore they were dropped from the analysis. The stepwise regression builds the model from a set of candidate predictor variables by bringing in and removing predictors that meet the criteria for entry or removal until a stable set of variables is attained. The model selection is based on Akaike's Information Criterion (AIC), which is calculated as AIC = 2k – 2log LL, where k is the number of parameters estimated by the model and log LL is the log likelihood for the given the model. The model selection with AIC attempts to select the model that best explains the data (highest likelihood), while still not fitting too many parameters. Comparison of the AIC values for a set of candidate models may simply indicate if a superior model exists within the given candidate models. AIC compares candidate models to a reference model. If the best candidate model is superior it becomes the reference model for the next step. This process is repeated until the increase in log-likelihood is no longer improved. The stepwise regression involves starting with the most complex model (all main effects and interactions terms), and sequentially removing or adding the terms in an effort to lower the AIC until no further variables can be deleted or added without a loss of fit. Finally, this algorithm determines the most informative environmental predictors explaining the benthic taxa distributions along the latitudinal gradient. Results reported are based on a screening-criteria of p < .05 for environmental variables. Effect sizes were also reported following Cohen (1988) as the proportion of Y variance accounted for by the source in question relative to the proportion of error. It should be noted that the results from this research would benefit from validation with comparable data from other regions.
The multivariate regression analysis was also performed on taxonomic groups as a way to investigate potential shifts from important hard coral dominated systems to nuisance macroalgae or turf algae groups. Taxonomic groups included hard corals, soft corals, coralline algae, macroalgae and turf algae.
2.8 Multiple driver interactions
We are also interested in the potential for multiple stressor effects to occur. We therefore used multiple regression models to determine the relationships between the abundance of individual taxa or taxonomic groups to the identified key drivers including climate related (temperature, salinity, air-sea heat exchanges, evaporation, irradiance), fishing pressure and biogeochemical variables (chlorophyll a, phosphate, nitrate, nitrite, silicate and dissolved oxygen). Multiple stressor effects were identified through interaction terms. These models are only intended to be used to explore the prevalence and nature of interactions, not to make predictions about taxa abundances. Taxa and functional data were used and initial models included all variables and their pairwise interaction terms. All analyses were conducted using R software.
The final regression models were interpreted to identify the presence of additive and multiplicative effects (as defined by Thrush, Hewitt, Hickey, & Kelly, 2008). A screening criteria was applied whereby variables with p > .03 parameter estimates were ignored. Additive effects (i.e. when the combined effect of two stressors is equal to the sum of the individual effects) occurred when the effect of one explanatory variable was independent of the others in the model (e.g. expressed as + or − in the equation: y = a + b1x − b2x2). Multiplicative effects were identified by the presence of terms (e.g. b3x1x2) in the final model. Multiplicative effects were further interpreted as either synergistic (i.e. when the combined effect of two stressors is greater than the sum of the individual effects and both stressors operate in the same direction), or antagonistic (i.e. when the combined effect of two stressors is less than the sum of the individual effects) based on whether the sign of the parameter estimate for the multiplicative effects indicated that it worked to increase or decrease the effect of strongest variable identified by the model.
2.9 Literature review
Based on an adapted protocol by Ban et al. (2014), we searched for all studies that reported the effect of two (or more) stressors on tropical coral reefs or scleractinian coral organisms in a natural setting (no laboratory or mesocosm studies were included). Stressors selected for this study were previously identified in a meta-analysis (Ban et al., 2014) and comprised: Ocean acidification; Crown-of-thorns starfish outbreaks, Eutrophication; Fishing pressure; Temperature; Irradiance; Pollution; Storms; Sedimentation; Ultraviolet radiation; and Sea level rise. Studies were searched using the ‘Topic’ search feature on ISI Web of Knowledge. All studies up to June 2018 were included. Studies returned from the search engine were manually examined for their applicability. The number and type of stressor was identified, and the response variable was categorized by its organizational level (i.e., response on an organism, community or ecosystem level). Reviews and modeling studies without a field-based component were excluded.
3 RESULTS
3.1 Physical and biogeochemical gradients
The considered physical and biochemical environmental parameters varied along the latitudinal axis of the Red Sea. The stations located in the northern part of the Red Sea were characterized by higher salinity, evaporation rates and heat exchange with the atmosphere while those located in the southern Red Sea by higher temperatures, chlorophyll a, irradiance and nutrient levels (Figure 2). Water temperature and remotely sensed chlorophyll a levels ranged from 26.6°C and 0.22 mg/m3 respectively in the north with higher average temperatures of 29.5°C and chlorophyll a levels of 0.97 mg/m3 in the southern Red Sea (Figure 2). Irradiance (PAR) also showed similar latitudinal trends ranging from 45.08 W/m2 in the north to higher irradiance levels of 47.5 W/m2 in the south. Conversely, salinity, and turbulence components of heat fluxes were consistently higher in the north, ranging from an average of 40.02 and 15.9 W/m2 respectively in the north to 38.9 and 9.3 W/m2 in the south (Figure 2). A few variables had higher values in the central region, notably wind speed, while fishing index was regionally specific with higher fishing pressure occurring in the Central Region and the South.
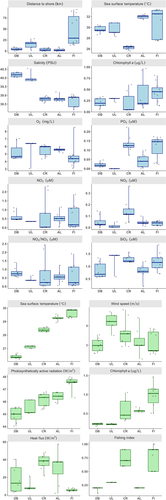
3.2 Taxa
Regression models were successfully developed for 63 taxa where the R2 values ranged from 69% to 98%. All 63 taxa displayed differences in percent cover as a function of the main modeled environmental variables. Variables that were consistently retained in the model included oceanographic and climate related variables (temperature, salinity, latent heat flux, wind speed, irradiance) fishing pressure and biogeochemical measures (chlorophyll a, phosphate, nitrate and nitrite, oxygen, photosynthetically active radiance; Table 1). The nature of the variables identified was taxa dependent (Tables 2 and 3). However temperature, latent and sensible heat fluxes, solar radiation (irradiance), salinity, PAR, and fishing index were important environmental variables associated with negative regression coefficients for hard corals (Table 2). Other environmental variables that explained changes in the percent cover for hard coral taxa included wind speed, chlorophyll a, nitrate and nitrite (NOx), and phosphate (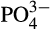 ) concentrations, where some of these variables consistently interacted. For example, wind speed was often identified as an important variable with an increase in wind speed associated with an increase in the percent cover of hard corals. Mutual interactions were evident and could be due to coupled feedbacks. Consistent positive associations with wind speed and a negative relation with water temperature were observed for the following hard corals: Acanthastrea, Dipsastrea, Distichopora, Favites, Galaxea, Platygyra, Pavona and Porites encrusting (see Table S1). Consistent positive associations with wind speed and associated positive relation with nutrient levels (NOx or chlorophyll a) were observed for Pavona, Acanthastrea, Favities, Galaxea and Playtgyra. Only three taxa of corals, Acropora, Fungia and Seriatopora, were found to decrease with increasing wind speed. These taxa have branching or plate like growth forms and may therefore be more susceptible to damage from increasing wave dynamics. Taxa from the hard coral group with the highest number of environmental variables and the highest adjusted R2 values included Acanthastrea, Turbinaria, Galaxea, Montipora, Acropora, Hydrophora, Pavona, Pachyseris and Porites columnar (Table 2). For soft corals, including the taxa Lobophyton, Lithophyton, Rhytisma and Xeniidae, important variables that were related with decreased percent cover for two or more soft coral taxa included temperature, salinity, oxygen and fishing index. Other variables related with the percent cover of soft corals included
) concentrations, where some of these variables consistently interacted. For example, wind speed was often identified as an important variable with an increase in wind speed associated with an increase in the percent cover of hard corals. Mutual interactions were evident and could be due to coupled feedbacks. Consistent positive associations with wind speed and a negative relation with water temperature were observed for the following hard corals: Acanthastrea, Dipsastrea, Distichopora, Favites, Galaxea, Platygyra, Pavona and Porites encrusting (see Table S1). Consistent positive associations with wind speed and associated positive relation with nutrient levels (NOx or chlorophyll a) were observed for Pavona, Acanthastrea, Favities, Galaxea and Playtgyra. Only three taxa of corals, Acropora, Fungia and Seriatopora, were found to decrease with increasing wind speed. These taxa have branching or plate like growth forms and may therefore be more susceptible to damage from increasing wave dynamics. Taxa from the hard coral group with the highest number of environmental variables and the highest adjusted R2 values included Acanthastrea, Turbinaria, Galaxea, Montipora, Acropora, Hydrophora, Pavona, Pachyseris and Porites columnar (Table 2). For soft corals, including the taxa Lobophyton, Lithophyton, Rhytisma and Xeniidae, important variables that were related with decreased percent cover for two or more soft coral taxa included temperature, salinity, oxygen and fishing index. Other variables related with the percent cover of soft corals included 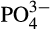 and PAR (Table 3). Macroalgae taxa (Dictyota, Halimeda, Turbinaria, macroalgae assemblage) increased in response to nutrients (NOx), PAR and oxygen levels (Table 3). Other important variables related to heat fluxes, fishing index and wind speed however the nature of the effects were taxon specific. For both Dictyota and the brown algae Turbinaria lower wind speed was associated with higher percent cover of these two algae.
and PAR (Table 3). Macroalgae taxa (Dictyota, Halimeda, Turbinaria, macroalgae assemblage) increased in response to nutrients (NOx), PAR and oxygen levels (Table 3). Other important variables related to heat fluxes, fishing index and wind speed however the nature of the effects were taxon specific. For both Dictyota and the brown algae Turbinaria lower wind speed was associated with higher percent cover of these two algae.
| Unadjusted R2 | Adjusted R2 | Effect size | Number of variables | Variables that negatively affected percent cover | |
|---|---|---|---|---|---|
| Hard corals | |||||
| Acanthastrea | 0.92 | 0.74 | 0.11 | 5 | Salinity, temperature, PAR |
| Acropora branching | 0.89 | 0.69 | 0.08 | 4 | Fishing index, salinity |
| Astreopora | 0.94 | 0.79 | 0.15 | 6 | Temperature, wind speed, PAR, PO4 |
| Coscinarea | 0.84 | 0.56 | 0.05 | 4 | Salinity, PO4, NOx |
| Cyphastrea | 0.83 | 0.59 | 0.04 | 3 | Chlorophyll a |
| Dipsastrea | 0.80 | 0.41 | 0.04 | 2 | Salinity |
| Echinopora | 0.84 | 0.72 | 0.05 | 4 | Fishing index |
| Favites | 0.93 | 0.83 | 0.13 | 8 | Fishing index, salinity, temperature |
| Fungia | 0.81 | 0.55 | 0.04 | 4 | Salinity, wind speed, PAR |
| Galaxea | 0.92 | 0.76 | 0.11 | 6 | Fishing index, salinity, temperature |
| Goniastrea | 0.81 | 0.45 | 0.04 | 1 | PO4 |
| Goniopora | 0.82 | 0.52 | 0.04 | 2 | PO4 |
| Hydnophora | 0.69 | 0.26 | 0.02 | 1 | PO4 |
| Leptastrea | 0.89 | 0.72 | 0.08 | 5 | PO4, oxygen |
| Lopophyllia | 0.88 | 0.61 | 0.07 | 4 | Fishing index, salinity, temperature |
| Leptoseris | 0.90 | 0.64 | 0.09 | 1 | |
| Millepora | 0.96 | 0.84 | 0.24 | 1 | |
| Montipora | 0.89 | 0.72 | 0.08 | 6 | Fishing index, latent heat flux |
| Pavona | 0.96 | 0.88 | 0.24 | 6 | Fishing index, salinity, temperature |
| Pachyseris | 0.96 | 0.88 | 0.24 | 6 | Fishing index, salinity, temperature |
| Pocillopora | 0.92 | 0.77 | 0.11 | 1 | NOx |
| Porites Branching | 0.83 | 0.65 | 0.04 | 6 | Fishing index, salinity, temperature, latent heat flux |
| Porites Columnar | 0.96 | 0.90 | 0.24 | 1 | PAR |
| Porites Encrusting | 0.96 | 0.87 | 0.24 | 4 | Fishing index, temperature, PAR |
| Porites Massive | 0.87 | 0.63 | 0.06 | 4 | Fishing index, temperature |
| Seriatopora | 0.90 | 0.76 | 0.09 | 4 | NOx, wind speed |
| Stylophora | 0.80 | 0.49 | 0.04 | 3 | Salinity |
| Symphyllia | 0.77 | 0.34 | 0.03 | 4 | PO4, salinity |
| Turbinaria | 0.89 | 0.71 | 0.08 | 5 | NOx, wind speed |
| Soft corals | |||||
| Lithophyton | 0.92 | 0.61 | 0.11 | 6 | Fishing index, salinity, temperature, oxygen, PAR |
| Lobophyton | 0.88 | 0.61 | 0.07 | 4 | Fishing index, salinity, temperature |
| Rhytisma | 0.98 | 0.91 | 0.49 | 4 | Chlorophyll a, temperature, oxygen, latent heat flux |
| Xeniidae | 0.94 | 0.78 | 0.15 | 2 | PO4, oxygen |
| R 2 | Adjusted R2 | Effect size | Number of variables | Variables that positively affected percent cover | |
|---|---|---|---|---|---|
| Algae | |||||
| Halimeda | 0.93 | 0.69 | 0.13 | 5 | Latent heat flux, wind speed, PAR |
| Macroalgae assemblage | 0.93 | 0.75 | 0.13 | 5 | Wind speed, latent heat flux, NOx |
| Dictyota | 0.91 | 0.76 | 0.10 | 4 | Oxygen |
| Turbinaria | 0.98 | 0.93 | 0.49 | 7 | NOx, oxygen |
3.3 Taxonomic groups
The dominance of hard corals were negatively affected by increasing fishing pressure, temperature and salinity. Notably, higher fishing pressure, salinity and elevated temperature were associated with reduced hard coral cover. Irradiance was also associated with reduced coral cover. Conversely, chlorophyll a, oxygen levels and wind speed were positively associated with increases in hard corals (Table 4).
| Variable | Hard coral p-value | Soft coral p-value | Corraline algae p-value | Macroalgae p-value | Turf algae p-value |
|---|---|---|---|---|---|
| Fishing index | <.001 (−) | .07 | .55 | .96 | .63 |
| PO4 | * | <.001 (−) | .15 | .21 | .11 |
| NOx | .21 | .96 | .68 | .01 (+) | .99 |
| Chlorophyll a | <.001 (+) | .26 | .20 | .22 | .81 |
| Salinity | <.001 (−) | .05 (−) | .15 | .83 | * |
| Temperature | .04 (−) | .88 | .04 (−) | <.001 (−) | .33 |
| Oxygen | .01 (+) | <.001 (−) | .28 | .58 | .30 |
| Latent heat flux | .84 | .03 (+) | .41 | <.001 (−) | .55 |
| Windspeed | <.001 (+) | .05 | .69 | .01 (−) | .10 |
| PAR | .07 | .14 | .76 | .02 (+) | .37 |
Note
- Where * not selected in final model and brackets indicate whether there was a negative (−) or positive (+) association provided for criterion of p < .05 (in bold).
Coralline algae was negatively related with increasing temperature while soft corals were negatively related with salinity, oxygen and 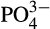 . In general, the central regions supported the highest abundances of sensitive calcifying taxa (hard corals and coralline algae; Figure 3).
. In general, the central regions supported the highest abundances of sensitive calcifying taxa (hard corals and coralline algae; Figure 3).
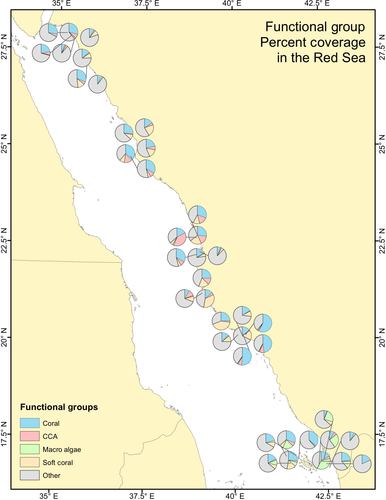
The central areas under the period surveyed and modeled did not experience the extremes of high salinity and evaporation rates present in the north or conversely high water temperatures or fishing pressure present in the southern Red Sea. Variables that influenced macroalgae dominance were climate related including wind speed, irradiance, temperature and atmospheric heat exchanges as well as NOx (Tables 4 and 5).
| Hard corals | Coralline algae | Soft corals | Macroalgae | Turf algae | Total | |
|---|---|---|---|---|---|---|
| Total | 5 | 10 | 0 | 4 | 4 | |
| Latent heat flux | 1 | 1 | 2 | 1 | 5 | |
| Temperature | 1 | 2 | 1 | 1 | 5 | |
| Wind speed | 1 | 3 | 4 | |||
| Fish index | 1 | 1 | 2 | |||
| Salinity | 1 | 1 | 2 | |||
| PAR | 1 | 1 | 2 | |||
| Chlorophyll a | 1 | 1 | ||||
| PO4 | 1 | 1 | ||||
| Oxygen | 1 | 1 |
Increasing wind speed and heat flux were negatively associated with algal cover. Hence decreased wind wave dynamics and associated lower heat loss rates from coastal waters was associated with an increased percentage cover of nuisance macroalgae. Nutrients (NOx) had a positive relation with increasing macroalgae cover. Of interest increased fishing pressure also had a positive effect on the percent cover of macroalgae however the effect size was low. Hard corals and coralline algae were susceptible taxonomic groups to changing environmental conditions. When assessing multiple stressor interactions, the highest number of synergistic interactions between stressor pairs that reduced abundance were recorded for coralline algae (Table 5). Negative synergistic interactions in corals were most pronounced when climate variables, nutrient and fishing pressure interacted. Further, the heat exchanges with the atmosphere, as expressed here by the latent heat fluxes, as well as temperature had the highest number of multiple stressor interaction pairs for sensitive corals. Heat fluxes, wind speed and temperature, followed by fishing pressure, salinity, and irradiance levels were found to be important stressors exhibiting high numbers of stressor pair interactions across all taxonomic groups modeled (Table 5). Chlorophyll a, phosphate and oxygen were also important but with fewer interactions. For macroalgae and turf algae, however, stressor combinations increased the percent cover.
4 DISCUSSION
This study documents latitudinal gradients in the dominance of functionally important coral reef communities in the Red Sea. Individual taxa and taxonomic groups responded to a combination of climate, fishing pressure and biogeochemical variables, where synergistic interactions reduced the abundance of sensitive hard coral taxa. Many mechanisms have been associated with climate change impacts on corals. For example, changes in ocean chemistry due to higher carbon dioxide may weaken coral skeletons (Kleypas et al., 1999) while increased storm frequency leads to shorter timeframes for recovery between recurrences. However the most pressing impact of climate change has been identified as coral bleaching events that are strongly associated with elevated temperatures, where stressed corals expel their pigmented zooxanthellae (Harvell et al., 2002; Hoegh-Guldberg, 1999). In this field study, the top three variables responsible for decreases in the percentage cover of hard corals were those normally associated with heat stress, namely temperature and air-sea heat exchanges as expressed by latent heat flux and wind speed. In general our results also highlight the role of climate, water quality and fishing pressure as factors influencing coral reef ecosystems (Hughes et al., 2003). Notably climate related factors had the highest number of synergistic interactions followed by fishing and then water quality variables. In general the variables identified for hard coral taxa reflect the importance of air-sea interactions that shape the local oceanographic environment and define their variability as well as local stressor impacts from overfishing and nutrient levels. The role of wave dynamics in supporting hard coral dominance has not been widely reported in the literature and therefore represents an area for future research efforts in other coral dominated systems.
4.1 Taxonomic group drivers
Increased wind speed was consistently associated with decreases in water temperature and an increase in the dominance of hard coral taxa. Increased wind speed is naturally related to increased heat losses to the atmosphere that generally lead to lower temperatures and increased vertical mixing. Furthermore, increased wind-wave action can also redistribute nutrients in the water column. Accordingly, consistent positive relations with wind speed and nutrient levels were also observed for hard coral taxa. Mixing of the water column due to increased wind speed can in turn influence temperature, nutrient and oxygen levels in the water column.
Important variables also associated with synergistic interactions for corals included both fishing pressure and nutrients. Our results are consistent with other published studies that highlight the role of climate, fishing pressure and water quality interactions in effecting coral reef resilience and health (Hughes et al., 2003). The role of fishing is not surprising and has been widely reported in the literature as having impacts on coral reef health. Overfishing, particularly of herbivorous fish such as parrotfish and surgeonfish, effect the dynamics of reef populations (Hughes, 1994; Jackson et al., 2001; Pandolfi et al., 2003). Herbivores regulate and influence the competitive interaction between corals and macroalgae, notably when they are not present, macroalgae can overgrow corals. Reduced herbivory from overfishing (Jackson et al., 2001) and excess nutrients (D'Angelo & Wiedenmann, 2014) have therefore been shown to impair the resilience of corals and prevent their recovery following acute-disturbance events resulting in phase shifts to algae dominated systems (Hughes, 1994; Jackson et al., 2001; Pandolfi et al., 2003).
Modeling responses of individual taxa to changing environmental conditions can provide insights into sensitive and tolerant taxa. Some of the most sensitive corals to changing environmental conditions included branching corals such as Acropora, Stylophora, Seriatopora and some Porites taxa. In general, branching corals have been found to be more prone to bleaching than massive and encrusting corals (Marshall & Baird, 2000) due to a lower tolerance to thermal stress (Loya et al., 2001). At the taxa level, higher nitrate and nitrite concentrations were associated with increases in the dominance of many hard coral taxa. While tropical reef-building corals commonly thrive in nutrient-poor environments, these results suggest that corals in the Red Sea may still be nitrogen limited. Recent studies also indicate that the capacity to control nitrogen cycling may stabilize coral holobiont functioning under both oligotrophic and eutrophic conditions (reviewed in Rädecker, Pogoreutz, Voolstra, Wiedenmann, & Wild, 2015), limiting the impacts of excess nitrogen as previously thought. In addition, the direct effects are not necessarily caused by the nutrient presence/absence itself but by skewed nitrogen to phosphorus ratios (Rosset, Wiedenmann, Reed, & D'Angelo, 2017; Wiedenmann et al., 2012). Likewise, phosphorous was identified as an important environmental variable for hard corals in this study. The nature of the response to changing phosphorous levels was taxa specific with both increases and decreases observed. Hence, the present study strengthens findings that indicate the necessity of a fine balance between nitrogen and phosphorous as stabilizers for corals to resist temperature and light induced stress (Ezzat, Maguer, Grover, & Ferrier-Pagès, 2016).
Coralline algae were also found to be sensitive to changing environmental conditions with the highest number of synergistic interactions, generally exhibiting similar patterns to hard coral along the environmental gradients modeled. For example, both hard corals and coralline algae displayed the lowest percent cover in the northern sites, which were characterised by high rates of evaporation and salinity, similarly lower percent cover at the southern sites, which were characterised by higher water temperatures and nutrients. Higher temperature was a key variable associated with decreases in the percentage cover of coralline algae, when modeled alone and also when investigating synergistic interaction terms. Laboratory-based research has provided evidence for increased necrosis of coralline algae under experimental conditions of increased temperature regimes (Martin & Gattuso, 2009). Within coral reef ecosystems, coralline algae function as frame-builders and encrusting or binding organisms that stabilize reef accretion, prevent bioerosion and induce settlement of coral larvae (Littler & Littler, 2013). Coral reefs with exposed coralline algae maintain higher biodiversity than those covered by fleshy algae (Belliveau & Paul, 2002) and a drop in coralline algae abundance has been shown to hinder coral recruitment (Weiss & Martindale, 2017). Coralline algae are therefore essential to sustaining healthy coral reef ecosystems. Because of the similar physiological traits of coralline algae and coral reefs, that is, slow growing, calcifying organisms that need limited nutrients, both groups showed similar trends along the gradients modeled in this study.
Soft corals were less susceptible to environmental change where nutrients, salinity and oxygen were often associated with changes in the percentage cover of soft corals. When modeled at the taxonomic level there were no synergistic interactions recorded. Soft corals are fast growing and versatile species capable of switching their feeding modes from autotrophic to heterotrophic. They are not reef builders and hence energy that would be used in calcification processes can be used directly for growth. In general, they have been reported as being more adaptable to environmental changes and more widely distributed than hard corals. A recent study further reported that the loss of hard corals on reefs can ultimately result in shifts to macroalgae and soft coral dominated communities (Thurber, Payet, Thurber, & Correa, 2017). This study provides further evidence that soft corals were more resilient to changes in climate and nutrients than sensitive calcifying hard corals.
Increases in fast growing and opportunistic macroalgae and turf algae were associated with increased nutrients, irradiance and temperature with Dictyota dominance and abundance exhibiting particularly notable increases. Macroalgae were also affected by changes in wind speed and heat fluxes. The relationship with latent heat flux and wind speed was negative for macroalage, both suggesting a higher macroalage abundance with reduced heat loss to the atmosphere, while similarly they were negatively affected by increased wind/wave dynamics. Air-sea heat exchanges, represented by their dominant part, the evaporative heat fluxes, was the co-stressor involved in the highest number of multiple stressor pair interactions for macroalgae. Elevated fishing pressure, and hence removal of herbivorous fish, had an overall positive affect on the percent abundance of nuisance macroaglae.
Competition is an important process in determining the structure and composition of benthic communities on coral reefs (McCook, Jompa, & Diaz-Pulido, 2001) especially during “regime shifts” when reefs dominated by reef-building corals can switch to being dominated by macroalgae (Littler & Littler, 1984). In general macroalgae are considered to be competing with corals for space under conditions of increased nutrient availability or reduced herbivory. In a major review of coral macroalgae interactions, McCook et al. (2001) concluded that competition between corals and algae is widespread on coral reefs, and that replacement of corals by algae often indicates coral mortality due to external disturbance, rather than competition overgrowth. Although once established, algal cover can lead to competitive inhibition of coral recruitment (Dixson, Abrego, & Hay, 2014; McCook et al., 2001). Hence, understanding the external disturbance mechanisms that result in these phase shifts is important for reef management. In this paper, climate variables were the external disturbances associated with shifts to a higher dominance of macroalgae in the Red Sea.
Latitudinal gradients in species richness and community diversity have previously been described in the Red Sea (Sheppard & Sheppard, 1991). However more recent studies have reported a weaker variability in coral reef communities with functional consistency along latitudinal gradients suggesting a general homogenization of the coral reef communities in response to anthropogenic stressors (Riegl, Bruckner, Rowlands, Purkis, & Renaud, 2012). Our research is consistent with these studies where the dominant benthic taxa were present across the majority of sites surveyed. However, despite this homogenization of the coral reef community composition in recent decades we were still able to distinguish changes in the percentage cover of the dominant benthic taxa along the strong environmental gradients present providing important insight into the role of multiple drivers in shaping reef health and resilience.
4.2 Multiple stressors
Despite over a decade of research into multiple stressor effects, conducted across a variety of ecosystems in both field and laboratory settings, prediction of when and where synergistic effects may occur remains difficult (Ban et al., 2014; Crain et al., 2008; Folt et al., 1999). Previous studies have reviewed the evidence for synergistic and antagonistic effects (Crain et al., 2008; Darling & Cote, 2008). These studies conclude that the overall interactive effect across the majority of studies was synergistic. However, the interaction type varied greatly in relation to response level, trophic level and the specific stressor pair (Crain et al., 2008). For corals, a recent review concluded that the majority of multiple stressor interactions have deleterious consequences from organismal to ecosystem levels (Ban et al., 2014).
While laboratory experiments are often only able to manipulate two (and less often three) stressors, field-based studies aid in understanding the role of multiple drivers by modelling combinations of variables ranging from local to global factors. Only recently, have responses of coral reefs to multiple anthropogenic drivers been modeled (Hughes et al., 2017; van de Leemput, Hughes, van Nes, & Scheffer, 2016). Identified drivers include temperature, irradiance, acidification, storm events, nutrient pollution and fishing pressure with evidence that synergistic human impacts can reduce resilience and cause unexpected ecological collapse, even when individual drivers or stressors remain at levels that are considered to be low. However, the number of studies that have collected field data and modelled more than two or three stressors are still very limited (Figure 4). We conducted a comprehensive literature review which identified 106 studies that examined the interaction of at least two stressors. Our review identified that 86% of all studies only assessed the effects of one or two stressors. Further, only 14 studies examined the effects of three stressors, while only a single study assessed the effects of four stressors at a time. Therefore, this work is unique in applying regression analysis to multiple environmental variables simultaneously to understand multiple stressor interactions in the field.
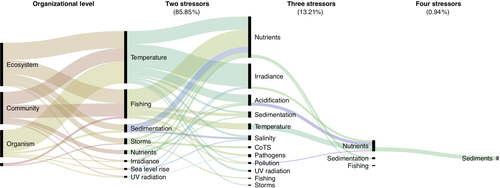
This study demonstrates synergistic multiple stressor interactions between a combination of stressor pairs for important benthic coral reef communities. Similar to previous reviews (Ban et al., 2014; Crain et al., 2008), the nature of the interaction effects in this study was stressor and taxa specific, whereby for sensitive hard corals and coralline algae climate, fishing pressure and nutrient interactions acted synergistically to decrease their prevalence. Conversely, synergistic interactions increased macroalgae abundance (Figure 5). In their literature review Crain et al. noted that the addition of a third stressor changed the interactive effects significantly, doubling the number of synergistic interactions. Most of the studies reviewed were conducted in laboratories, where stressor effects can be carefully isolated. Their conclusions suggest that synergies may be common in nature where more than two stressors generally coexist (Ellis et al., 2017). While laboratory studies are important as they provide the mechanistic understanding of how and why interactions occur, direct transfer to the field can be confounded by the complexity of communities existing in nature, differing temporal and spatial scales of studies, and the presence of additional stressors that could alter the direction and intensity of laboratory observed interactions (e.g. hydrodynamics, temperature variations; Ellis et al., 2017). Crain et al. concluded that stressor effects can be reinforced when other pressures or stressors are present in nature. We would concur with these conclusions as we also documented a high number of synergistic interactions indicating that interaction type varied by stressor pair and response level, in this case taxa or taxonomic group. As the findings from field and laboratory studies converge regarding the role of multiple drivers and their impact on coral reefs, management actions and policy can more effectively be prioritized.
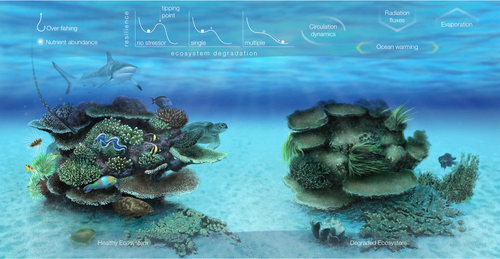
5 CONCLUSION
Coral reefs are among the most biologically diverse and socioeconomically valuable coastal habitats providing important services including the provisioning of food, coastal protection, spawning and nursery habitats for commercially important species and recreational opportunities. Hence understanding how specific coral taxonomic groups are affected by environmental factors is of critical importance to understand and manage reef ecosystems (Mumby & Steneck, 2008). In general, three prominent drivers of reef decline have been synthesized from the existing literature which include increased disturbance associated with climate change, pollution and fishing. This study provides evidence for impacts on hard corals facing temperature conditions already above IPCC predictions for other world ocean systems in the Red Sea and identifies a combination of global (climate) and local drivers that influenced the coral reef communities. A greater focus on identifying and, thereby, reducing drivers of change to avoid crossing thresholds that lead to environmental degradation has been identified as an important management tool (Hughes et al., 2017). This study recommends seeking methods to reduce impacts from known local drivers such as improving coastal water quality through waste-water treatment facilities or utilizing innovations or modifications in fishing gear. Understanding thresholds and feedbacks at a range of scales (Hughes et al., 2017) and managing drivers of change will be integral to addressing the escalating problems that confront the sustainability of coral reefs. Finally, the climate related variables identified for hard coral taxa in this research (notably waves, latent heat flux and temperature) reflect the importance of air-sea interactions not widely reported in the literature representing an area for future research efforts in other coral dominated systems.
ACKNOWLEDGEMENTS
This research has been developed using funding from the Saudi Aramco KAUST Marine Environmental Observations (SAKMEO) research program. We would like to thank Ute Langner and Michael Campbell for producing Figures 1 and 3 respectively. Figure 5 was created by Ivan Gromicho, Scientific Illustrator at King Abdullah University of Science and Technology (KAUST). We also wish to thank Benjamin Bowes, Benjamin Taylor, CMOR and the crew of the R.V. Thuwal for their assistance in collecting the field samples.
CONFLICT OF INTEREST
All authors declare that they have no potential competing financial interests.
AUTHOR CONTRIBUTIONS
Conceived and designed the experiments: S.C., B.J., I.H., J.E., H.A.. Collected the data: H.A., S.C., J.C., B.K., D.C.. Conceived and designed the statistical and modelling analysis: J.E., I.H., T.J., J.H., G.K., D.H.. Analyzed the data: T.J., J.E. Conducted literature review: F.R. Wrote the paper: J.E. All authors reviewed the final paper.




