Pathogen exposure disrupts an organism's ability to cope with thermal stress
Abstract
As a result of global climate change, species are experiencing an escalation in the severity and regularity of extreme thermal events. With patterns of disease distribution and transmission predicted to undergo considerable shifts in the coming years, the interplay between temperature and pathogen exposure will likely determine the capacity of a population to persist under the dual threat of global change and infectious disease. In this study, we investigated how exposure to a pathogen affects an individual's ability to cope with extreme temperatures. Using experimental infections of Daphnia magna with its obligate bacterial pathogen Pasteuria ramosa, we measured upper thermal limits of multiple host and pathogen genotype combinations across the dynamic process of infection and under various forms (static and ramping) of thermal stress. We find that pathogens substantially limit the thermal tolerance of their host, with the reduction in upper thermal limits on par with the breadth of variation seen across similar species entire geographical ranges. The precise magnitude of any reduction, however, was specific to the host and pathogen genotype combination. In addition, as thermal ramping rate slowed, upper thermal limits of both healthy and infected individuals were reduced. Our results suggest that the capacity of a population to evolve new thermal limits, when also faced with the threat of infection, will depend not only on a host's genetic variability in warmer environments, but also on the frequency of host and pathogen genotypes. We suggest that pathogen-induced alterations of host thermal performance should be taken into account when assessing the resilience of any population and its potential for adaptation to global change.
1 INTRODUCTION
Many species are currently experiencing a period of unprecedented environmental change in response to shifting global temperature patterns (Stocker, Qin, & Plattner, 2013). Not only are average temperatures on the rise, but an increase in environmental variability is predicted to escalate extreme climatic events, including heat waves (e.g. Meehl & Tebaldi, 2004). Yet, the risks of global change for populations extend beyond increased thermal stress alone. Global change is also predicted to result in a dramatic shift in the transmission and distribution of infectious diseases (Altizer, Ostfeld, Johnson, Kutz, & Harvell, 2013; Rodó et al., 2013). For example, disease vectors such as Zika virus carrying mosquitoes are predicted to experience large range shifts as our planet warms, leading to a spread in the transmission of socio-economically important infectious diseases (Tesla et al., 2018). The combination of increasing temperatures and more prevalent outbreaks of disease has the potential to place populations closer to the brink of extinction than previously thought (Cohen, Civitello, Venesky, McMahon, & Rohr, 2018).
Missing from the connection between infectious disease and global change is the capacity of pathogen exposure to disrupt a host's ability to cope with extreme environments (Greenspan et al., 2017). Assessing thermal limits has been a mainstay of global change biology (Bennett et al., 2018; Hoffmann, Chown, & Clusella-Trullas, 2012; Klockmann, Günter, & Fischer, 2017; Sunday, Bates, & Dulvy, 2011), but rarely applied to the study of host–pathogen interactions. While emerging research has shown how temperature can influence the timing and size of epidemics (Auld & Brand, 2017b; Gehman, Hall, & Byers, 2018; Rohr et al., 2011; Shocket, Vergara, et al., 2018), pathogen transmission and virulence (Mordecai et al., 2017; Tesla et al., 2018), and host susceptibility to infection (Cohen et al., 2017; Garbutt, Scholefield, Vale, & Little, 2014), the thermal limits of a host and the impact of pathogen exposure are rarely considered in unison (but see Greenspan et al., 2017). By causing stress and disrupting homeostasis, a pathogen should be expected to reduce the upper thermal limits of its host upon infection. But the extent to which this impairment occurs, and indeed if at all, could depend on the severity and duration of infection, the genotype of the host and pathogens involved, and thermal tolerance of the pathogen itself; factors that have yet to be considered in the light of host–pathogen interactions and thermal ecology.
Integrating an understanding of infectious disease into the study of thermal performance begins by following the approaches common to thermal ecology (Chown, Jumbam, Sørensen, & Terblanche, 2009; Jørgensen, Malte, & Overgaard, 2019; Kingsolver & Buckley, 2017; Rezende, Castañeda, & Santos, 2014; Sgrò et al., 2010). Thermal stress can take many forms, from short exposure in extreme environments, to slow incremental increases in temperature towards stressful levels (Kellermann, van Heerwaarden, & Sgrò, 2017). Methodologies for measuring thermal limits are thus varied, including both static lethal heat exposure and gradual temperature ramps that begin at ambient temperatures (e.g. Sgrò et al., 2010). Importantly, investigations into the thermal limits of ectotherms have shown that the rate at which temperature increases towards lethal levels can have dramatic impacts on individual responses (Chown et al., 2009; van Heerwaarden, Malmberg, & Sgrò, 2016; Sgrò et al., 2010; Terblanche, Deere, Clusella-Trullas, Janion, & Chown, 2007; Terblanche et al., 2011). Some populations are able to buffer against thermal stress through physiological plastic responses when exposed to longer periods of incrementally increased thermal stress (Chown et al., 2009; Rezende, Tejedo, & Santos, 2010; Rohr et al., 2018). While other populations appear to suffer from longer and slower exposures to sublethal thermal stress, as a consequence of accumulating physiological damage, resulting in reduced thermal limits (Chown et al., 2009; Terblanche et al., 2007). How infectious disease interacts with different forms of thermal stress to impact host survival is still unknown.
In this study, we use the crustacean Daphnia magna and its bacterial pathogen Pasteuria ramosa to characterize how infectious disease impacts on an organism's upper thermal limits. Daphnia have been shown to be locally adapted to a wide range of environmental conditions, including both their thermal environment (Declerck, Cousyn, & De Meester, 2001; Geerts et al., 2015; Yampolsky, Schaer, & Ebert, 2014), and local pathogens, in particular P. ramosa (Carius, Little, & Ebert, 2001; Decaestecker et al., 2007; Ebert, Zschokke-Rohringer, & Carius, 1998). Infection with P. ramosa is associated with reduced lifespan, castration and gigantism (Clerc, Ebert, & Hall, 2015; Ebert, Carius, Little, & Decaestecker, 2004; Hall & Ebert, 2012), and both host and pathogen fitness characteristics are known to be mediated by a combination of host genotype, pathogen genotype, and importantly, environmental factors (Auld & Brand, 2017a; Hall, Vettiger, & Ebert, 2012; Michel, Ebert, & Hall, 2016; Mitchell, Rogers, Little, & Read, 2005). Temperature, in particular, has been implicated in affecting both the evolution and spread of infectious disease. Increases in average temperature have been shown to modify interactions between host and pathogen genotypes (Allen & Little, 2011; Vale & Little, 2009; Vale, Stjernman, & Little, 2008; Vale, Wilson, Best, Boots, & Little, 2011), as well as increase epidemic sizes in seminatural populations (Auld & Brand, 2017b). However, how infection affects the upper thermal limits of Daphnia or other model systems of global change, and the result this has on thermal adaptation, population persistence and disease dynamics remains unexplored.
To address this issue, we assayed the upper thermal limits of multiple host and pathogen genotype combinations as part of a standard cross-infection experiment. P. ramosa genotypes were chosen that cover the spread of virulence-transmission trade-offs from high to low rates of pathogen reproduction and subsequent host harm (Clerc et al., 2015; Hall & Mideo, 2018). In a series of experiments, we first explored the impact of infection on upper thermal limits under a variety of static and ramping temperatures. We then explored how thermal limits vary across the course of infection as the damage caused by the pathogen accumulates. With this approach, we aimed to answer important questions regarding the influence of disease on thermal limits, including whether they: (a) vary with thermal ramping rates and the type of thermal stress an organism encounters; (b) depend on the prevalence or intensity of a given infection; and (c) are specific to host and pathogen genotypic combinations. By answering these questions, we shed light upon how important biotic interactions affect species’ ability to survive thermal stress, and the implications this could have for the dynamics of infectious disease and the persistence of populations under scenarios of global change.
2 MATERIALS AND METHODS
2.1 Host and pathogen
Daphnia magna Straus is a cyclically parthenogenic crustacean that occurs naturally in brackish and freshwater environments, ranging from shallow pools to lakes across the northern hemisphere (Ebert, 2005). P. ramosa Metchnikoff is a common Gram-positive bacterial pathogen of D. magna found across the Northern Hemisphere (Ebert et al., 2016). During the course of infection, P. ramosa supresses reproduction of its host, causes pathogen-induced gigantism and severely reduces host lifespan, before releasing millions of spores into the environment at host death (Clerc et al., 2015; Ebert et al., 2016; Hall & Ebert, 2012). Thus, transmission is exclusively horizontal in this system and depends on a balance between the virulence of the pathogen and its production of mature transmission spores (see Hall & Mideo, 2018).
In this study, we utilized Daphnia of two distinct genotypes (genotype ID: BE-OMZ-M10 and HU-HO-2, hereafter M10 and HO2) originally collected from sites in Belgium and Hungary respectively. We also used three P. ramosa genotypes (genotype ID: C1, C14 and C20), each of which was originally isolated through single cell infections, and therefore represent true single genotype lines (Luijckx, Ben-Ami, Mouton, Pasquier, & Ebert, 2011). Each bacterial genotype is known to vary in its virulence and transmission potential (Clerc et al., 2015; Hall & Mideo, 2018), and all are readily able to infect both Daphnia genotypes used in this study.
Prior to all experiments, female Daphnia were taken from stock cultures and kept in isolation in 70 ml jars filled with 50 ml of Artificial Daphnia Medium (ADaM; Klüttgen, Dülmer, Engels, & Ratte, 1994; modified via Ebert et al., 1998) for three generations before setting up experimental blocks. Daphnia were changed into fresh ADaM twice weekly and fed daily with algae (Scenedesmus sp.), with food levels increased from 0.5 million cells per animal per day at birth to 5 million cells per animal per day from day 8 onwards to meet the growing needs of the animals. All animals were maintained under standard conditions (20°C, 16L:8D), and repositioned regularly to minimize any positional effects within the incubator.
2.2 Experiment 1: Thermal limits under different forms of temperature stress
2.2.1 Experimental animals and infection
All experimental animals were taken from clutches three and four of the standardized mothers. A total of 960 females were set up in a full factorial design, with 30 individuals per treatment (2 hosts × [3 pathogens + uninfected controls] × 4 thermal limit assay methods), and approximately equal numbers per treatment assigned to one of three blocks. We infected Daphnia with 20,000 P. ramosa spores over 2 days (i.e. 40,000 total), starting 3 days after birth. During the infection period, Daphnia were kept in 70 ml jars filled with 20 ml of ADaM to promote infection and then transferred into new jars with 50 ml of ADaM after 3 days. Following the infection period, all animals were maintained in isolation as described above until their respective thermal limits assays. With the exception of exposure to bacterial spores, uninfected control animals were treated identically.
2.2.2 Thermal limit assays
Four distinct thermal limit assays were conducted to test how temperature ramping rate affected thermal limits of infected and uninfected Daphnia. These included a 37°C static heat shock assay (as in Yampolsky et al., 2014) and three temperature ramping assays which exposed Daphnia to ramping rates of either 0.06, 0.04 or 0.02°C/min starting at an ambient 20°C. These temperature assays were chosen to capture a range of possible temperatures documented in aquatic environments similar to those in which Daphnia regularly inhabit (Jacobs, Heusinkveld, Kraai, & Paaijmans, 2008; Paaijmans et al., 2008). In small ponds or rock pools, for example, water temperatures closely track that of the air, with maxima ranging from 32°C in Northern Europe to over 40°C in the tropics (Jocque, Vanschoenwinkel, & Brendonck, 2010), and ramping rates of 0.04°C/min concordant with pools warming by as much as 15°C following the overnight lows (Ganning, 1971). Thirty Daphnia from each treatment were used per assay. Forty-eight individuals could be measured in a single assay run; so assays were conducted over 5 days spanning 2 weeks, with each experimental block being used for one to two assay days. All Daphnia were between 29 and 31 days post-infection (dpi) at the time of the assays.
For each assay, Daphnia were placed in individual 5 ml glass fly vials covered by a mesh and immersed in a constantly agitated water bath filled with ADaM set to the desired temperature (see Sgrò et al., 2010, for similar methodology using Drosophila). The mesh allowed for circulation of ADaM between the vials and tank to minimize oxygen depletion within the vials over the course of the assays. For each ramping assay, the temperature of the water baths was increased appropriately every 10 min, at which time Daphnia were checked for activity. As the baths approached stressful temperatures, they were observed constantly until all Daphnia were immobile. For all assays, time until knockdown, starting from when they were first placed in the bath, was recorded for each individual when there was no visible movement including filtering from the animal (Yampolsky et al., 2014).
2.3 Experiment 2: Thermal limits across stages of infection
2.3.1 Experimental animals and infection
All experimental animals were taken from clutches three and four of standardized mothers as described above. A total of 2,400 females were assigned to treatments in a full factorial design (2 hosts × [3 pathogens + uninfected controls] × 5 ages × 2 experimental assays). Infection protocol and animal husbandry was carried out as in Experiment 1 described above.
2.3.2 Thermal limit assays
In order to capture how the dynamics of infection impact upon thermal limits, assays were conducted on individuals from all treatments at 10, 20, 30, 40 or 50 dpi. These times were chosen in order to capture the range of the within-host disease dynamics seen in this system (Clerc et al., 2015; Hall & Mideo, 2018). Approximately 30 individuals per treatment were used for each assay method at each dpi. Based on the results of the previous experiment, two experimental assays were conducted to measure upper thermal limits of the Daphnia, including a static 37°C heat shock and a 0.06°C/min temperature ramp assay. At each dpi time point, we conducted five runs of each assay method over two consecutive days. Knockdown times were measured as described for Experiment 1 above. After each assay, all animals were measured for body size using a stereo microscope and then frozen individually in 500 μl of water for later bacterial spore counting.
2.4 Bacterial spore counts
Bacterial spore counts were conducted using an Accuri C6 flow cytometer (BD Bioscience, San Jose, CA). Infected Daphnia individuals were thawed and crushed in 500 μl of water before 10 μl of each sample was pipetted into 190 μl of 5 mM EDTA in a round-bottomed PPE 96-well plate. Gates based on fluorescence (via the 670 LP filter) and side scatter pulse area (cell granularity) were used to identify mature spores based on their distinct size, morphology and fluorescence, compared to immature spores, algae or animal debris. Each sample was counted twice and counts were averaged. A total of 12 Daphnia were counted per run (32 wells including 8 ‘blank’ wells containing only EDTA).
2.5 Statistical analysis
All analyses were performed in R (v. 3.3.3; R Development Core Team, www.R-project.com). For both experiments, knockdown times from ramping assays were converted into critical thermal maxima (CTmax) for further analysis, while knockdown times from the static heat shock assays were retained in minutes.
For experiment 1, we investigated the effect of infection on thermal limits by fitting linear mixed effect models (nlme package: Pinheiro, Bates, DebRoy, & Sarkar, 2018) separately for the static heat shock assay and the temperature ramp assays. For the heat shock assay, host and pathogen genotype, and their interaction were treated as fixed effects, and assay run was treated as a random effect (to account for variation between assay replicates). For the ramping assays, host genotype, pathogen genotype and ramping rate were treated as (interacting) fixed effects, and assay run was treated as a random effect. The significance of fixed effects was then tested using analysis of variance (ANOVA Type III, car package: Fox & Weisberg, 2018). These data were subsequently split by host genotype, and linear models conducted separately for each assay type to more directly explore the effects of pathogen genotypes and infection on thermal limits. Individuals that died at low temperatures in ramping assays (quantified as being more than three standard deviations away from the mean) were removed from these data as they caused heavy left skew. Removal of these individuals (3, 5 and 1 from 0.06, 0.04 and 0.02°C/min ramps, respectively) did not qualitatively affect the results.
For experiment 2, we again used linear mixed effect models to analyse thermal limits under both static heat shock and ramping assays. Host genotype, pathogen genotype and dpi were treated as fixed effects (including all higher order interactions), and assay run was treated as a random effect. To account for heteroscedasticity in residual variance in these models, residual variance was allowed to vary independently at the levels of both host genotype and dpi using the ‘varIdent’ function (nlme package: Pinheiro et al., 2018; but see Zuur, Ieno, Walker, Saveliev, & Smith, 2009). The significance of the fixed effects was tested using analysis of variance (ANOVA Type III).
Finally, we explored how any reduction in thermal limits caused by a pathogen might change over the course of infection. To do this, we calculated the relative change in thermal limits for each infected individual by subtracting their trait value by the corresponding control group mean (i.e. age and genotype matched). Using linear models, simultaneous changes in within-host spore load, relative host body size (calculated by subtracting the mean body size of control animals) and the increasing age of the animal following infection were modelled, allowing predictions of linear partial effects; for example, how a reduction in thermal limits relative to controls might vary with increasing spore loads after controlling for the influence of infection age and relative body size. To do so, we first excluded animals below 20 dpi as bacterial spore counts are unreliable before this time (Clerc et al., 2015; Ebert et al., 2016) and further removed individuals with CTmax below 36°C. We then fit a full model for each assay type that allowed the three symptoms of infection to covary by both host and pathogen genotype. Data were then split by host genotype, and linear models were constructed that allowed symptoms of infection to vary at the level of pathogen genotype, permitting a more direct test of pathogen-specific effects across host genotypes.
3 RESULTS
3.1 Thermal limits under different forms of temperature stress
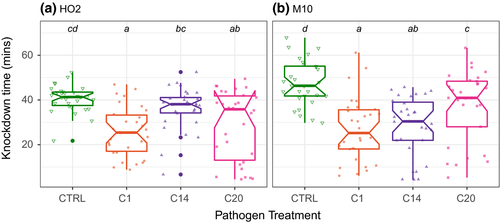
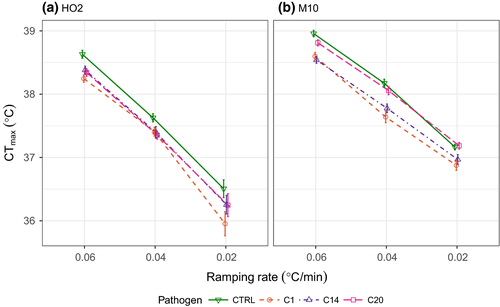
We first considered how infection affected upper thermal limits under different forms of thermal stress, using four different heating rates including both ramped and static heat shock assays. Across all thermal assays, we found that infection by the pathogen generally reduced the upper thermal limits of its host, with the results broadly consistent across all types of thermal stress for each host genotype (Figures 1 and 2; Table 1). Pathogen C1, for example, consistently resulted in the greatest reduction in a host's capacity to cope with thermal stress, leading to a 21 min or more reduction in knockdown times under static heat stress and up to 0.5°C lower CTmax values compared to the uninfected individuals of each heating ramp (see Table S1). However, the precise magnitude of any reduction in a host's upper thermal limits varied with both the host and pathogen genotype (Table 1), as well as the type of thermal stress they encountered (Figure 2).
| Term | χ 2 | df | p-value |
|---|---|---|---|
| (a) Static heat shock | |||
| Host | 2.609 | 1 | 0.106 |
| Pathogen | 87.649 | 3 | <0.001 |
| Host × pathogen | 22.957 | 3 | <0.001 |
| (b) Ramping assays | |||
| Host | 228.500 | 1 | <0.001 |
| Pathogen | 68.485 | 3 | <0.001 |
| Ramp rate | 1,974.400 | 2 | <0.001 |
| Host × pathogen | 9.464 | 3 | 0.024 |
| Host × ramp rate | 32.377 | 2 | <0.001 |
| Pathogen × ramp rate | 2.971 | 6 | 0.812 |
| Host × pathogen × ramp rate | 6.485 | 6 | 0.371 |
- Significant p-values (α = 0.05) are in bold.
The results from the static heat shock assays provide the most striking example of how the reduction in upper thermal limits caused by any pathogen depends on the genotype of the host (Figure 1a,b). On average, host genotype M10 saw the greatest reduction in upper thermal limits upon infection (17 min in M10 across pathogen genotypes compared with 10 min in HO2), with a clear hierarchy in the severity of the loss caused by each pathogen genotype, beginning with genotype C1 and ending with C20 (Figure 1b; Table S1). For host genotype HO2, the lower knockdown times of the control animals (40 min compared with 47 min in M10 controls) contributed to an overall reduced pathogen impact, and we also saw a change in the rank order of pathogen effects (Figure 1a; Table S1).
We observed similar patterns of thermal limit reduction for infected animals across ramping rates (Figure 2; Table S1), but with a clear reduction in the overall thermal limits as the ramping rates slowed (e.g. CTmax of uninfected HO2 dropped from 39 to 36°C across the ramping rates). The extent to which thermal limits declined was determined by an interaction with host genotype, but not pathogen genotype (Table 1b). This suggests that the two hosts responded differently (but still detrimentally) to the extended length of time spent at sublethal temperatures, while pathogen exposure further degraded thermal limits in a mostly additive way across ramping rates (Figure 2; Table 1b). The impact of infection on host thermal limits was therefore generally consistent across ramping rates (Figure 2), although with a decrease in effect sizes for some host and pathogen combinations at the slowest ramping rate (Table S1).
3.2 Thermal limits across stages of infection
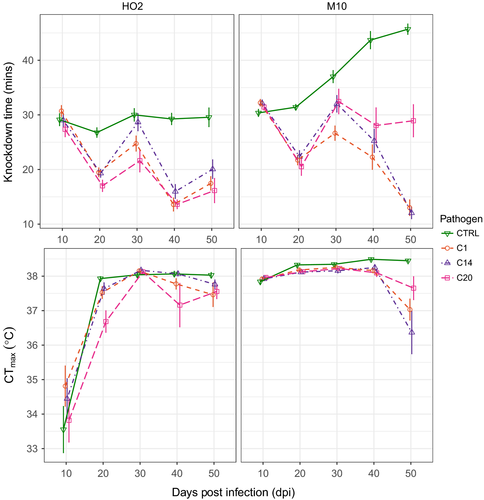
Following infection, a pathogen continues to replicate within a host, potentially reducing thermal limits even further due to the accumulation of host damage or by changes in host morphology and physiology. Using the static heat shock and 0.06°C/min temperature ramp assays, we investigated how a host's thermal limits change over the course of infection. Overall, we found that differences in knockdown times and CTmax values between control and infected animals progressively diverged with the time since infection (Figure 3). This change, however, was specific to both the host and pathogen involved, as indicated by the significant three-way interactions between host genotype, infection treatments and dpi (Table 2). In the static heat shock assays, for example, infection resulted in a far greater reduction in thermal performance for host genotype M10 (knockdown times up to 35 min less at 50 dpi) compared to genotype HO2 (15 min less at 50 dpi), in part driven because the upper thermal limits of M10 animals, when uninfected, improves as the animals’ age (Figure 3). Under the 0.06°C/min heating ramp, thermal performance again continued to diverge between control and infected animals over time, leading to a 0.5°C (Host HO2) to 2°C (Host M10) reduction in thermal limits by 50 dpi (Figure 3). Host HO2 appears particularly intolerant to extended exposure to thermal stress at early developmental stages (Figure 3).
| Term | χ 2 | df | p-value |
|---|---|---|---|
| Static heat shock | |||
| Host | 146.842 | 1 | <0.001 |
| Pathogen | 385.585 | 3 | <0.001 |
| Age | 56.241 | 4 | <0.001 |
| Host × pathogen | 56.957 | 3 | <0.001 |
| Host × age | 45.638 | 4 | <0.001 |
| Pathogen × age | 298.083 | 12 | <0.001 |
| Host × pathogen × age | 71.354 | 12 | <0.001 |
| Ramp | |||
| Host | 132.371 | 1 | <0.001 |
| Pathogen | 9.702 | 3 | 0.021 |
| Age | 133.555 | 4 | <0.001 |
| Host × pathogen | 14.570 | 3 | 0.002 |
| Host × age | 195.017 | 4 | <0.001 |
| Pathogen × age | 50.924 | 12 | <0.001 |
| Host × pathogen × age | 30.989 | 12 | 0.002 |
- Significant p-values (α = 0.05) are in bold.
We next partitioned the effects of three changes in disease symptoms that occur as an infection progresses—changes in body size, pathogen spore load and age of infection—on the reduction in a host's thermal limits that is caused by a pathogen (i.e. relative to the control animals; Figures 4 and 5). Overall, the best fitting model for both the static heat shock and 0.06°C/min heating ramp was one that allowed the relationship between relative thermal limits and the symptoms of disease to vary by both host and pathogen genotypes (Table S2). Thus, while the reduction in the thermal limits of a host are generally at their lowest under a high infection intensity (i.e. increased spore loads), small relative body size and old age of infection, both the strength of these relationships, and in some cases, the direction, depend on an interaction between host and pathogen genotypes (Figures 4 and 5).
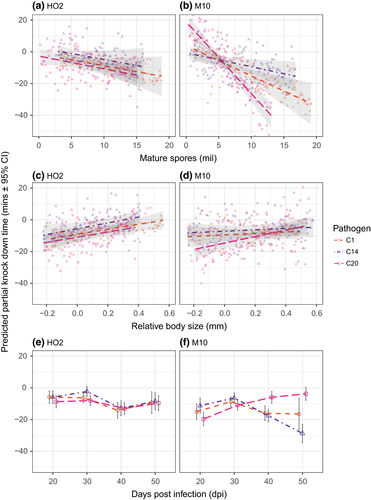
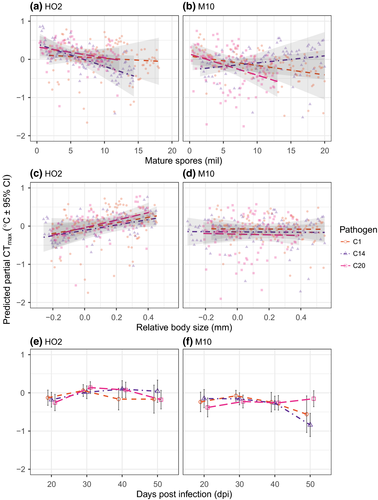
In host HO2, for example, the relationships between the symptoms of disease and the reduction in thermal limits due to infection were uniform across pathogen genotypes, in both the ramp and heat shock assays (Figures 4 and 5; Table 3). Conversely, in host M10, pathogen differences defined the relationship between symptoms of infection and the reduction in thermal limits across assay types (Figures 4 and 5; Table 3). Pathogen-specific responses were most evident in the effects of spore load and infection age. The effect of relative body size, however, was largely consistent across pathogen genotypes, but did switch from being positive (static heat shock) to having no detectable effect (temperature ramp) across assay types.
| Term | SS | df | F-value | p-value |
|---|---|---|---|---|
| Static heat shock | ||||
| HO2 | ||||
| Pathogen | 7.1 | 2 | 0.078 | 0.925 |
| Spore load | 503.2 | 1 | 11.054 | <0.001 |
| Body size | 988.0 | 1 | 21.703 | <0.001 |
| Age | 1,624.3 | 3 | 11.894 | <0.001 |
| Pathogen × spore load | 0.8 | 2 | 0.009 | 0.991 |
| Pathogen × body size | 4.4 | 2 | 0.049 | 0.952 |
| Pathogen × age | 207.1 | 6 | 0.758 | 0.603 |
| M10 | ||||
| Pathogen | 2,363.6 | 2 | 15.172 | <0.001 |
| Spore load | 4,820.6 | 1 | 61.888 | <0.001 |
| Body size | 380.7 | 1 | 4.888 | 0.028 |
| Age | 2,418.0 | 3 | 10.347 | <0.001 |
| Pathogen × spore load | 2,231.0 | 2 | 14.321 | <0.001 |
| Pathogen × body size | 246.7 | 2 | 1.583 | 0.207 |
| Pathogen × age | 2,604.9 | 6 | 5.574 | <0.001 |
| Ramp | ||||
| HO2 | ||||
| Pathogen | 0.287 | 2 | 0.821 | 0.441 |
| Spore load | 0.729 | 1 | 4.173 | 0.042 |
| Body size | 1.879 | 1 | 10.750 | 0.001 |
| Age | 2.374 | 3 | 4.527 | 0.004 |
| Pathogen × spore load | 0.260 | 2 | 0.743 | 0.476 |
| Pathogen × body size | 0.053 | 2 | 0.152 | 0.859 |
| Pathogen × age | 0.842 | 6 | 0.803 | 0.569 |
| M10 | ||||
| Pathogen | 1.052 | 2 | 3.909 | 0.021 |
| Spore load | 0.358 | 1 | 2.660 | 0.104 |
| Body size | 0.007 | 1 | 0.049 | 0.826 |
| Age | 2.524 | 3 | 6.252 | <0.001 |
| Pathogen × spore load | 0.874 | 2 | 3.248 | 0.040 |
| Pathogen × body size | 0.003 | 2 | 0.013 | 0.987 |
| Pathogen × age | 2.476 | 6 | 3.068 | 0.006 |
- Models were run separately for each assay method and host genotype. Relative measures of upper thermal limits and body size were calculated by subtracting the corresponding control group means. Significant p-values (α = 0.05) are in bold.
4 DISCUSSION
Despite growing interest in predicting how populations will respond to amplified environmental pressures caused by global change (e.g. Ockendon et al., 2014; Sinclair et al., 2016), our understanding of how co-occurring stressors will interact to shape the responses of species and populations remains poor (see Schäfer & Piggott, 2018). With both thermal environments and the geographical distributions of pathogens predicted to undergo considerable shifts in the coming years (Gehman et al., 2018; Tesla et al., 2018; Zhan, Ericson, & Burdon, 2018), the interplay between temperature and pathogen exposure will likely determine both the capacity of a population to cope with global change and the spread of infectious disease (Gehman et al., 2018; Greenspan et al., 2017; Shapiro, Whitehead, & Thomas, 2017; Shocket, Vergara, et al., 2018; Sternberg & Thomas, 2014). Few studies, however, have examined the impact of infection on a host's capacity to cope with thermal stress (but see Greenspan et al., 2017, and references therein). Here, we demonstrate how thermal limits are profoundly sensitive to infection and its severity, the intensity of thermal stress, and the type of pathogen genotype that is encountered.
Our results reveal how the reduction in thermal limits caused by pathogen exposure will have substantial consequences for host persistence and adaptation under global change. We found that infected individuals experienced significantly reduced thermal limits (Figures 1 and 2), and that the progression of disease (i.e. increases in pathogen load and host damage accumulation) contributed to the further degradation in an individual's response to extreme temperatures (Figure 3). Individuals most at risk under heat stress had a higher intensity of infection (i.e. spore loads), relatively smaller body sizes and older ages of infection (Figures 4 and 5). In all cases, infection had the capacity to alter host thermal limits at an equivalent magnitude to the natural variation seen across species entire geographical ranges (Sgrò et al., 2010; see Geerts, De Meester, & Stoks, 2014; Yampolsky et al., 2014 for Daphnia example), or even the phylogeny of related species (Janion-Scheepers et al., 2018; Kellermann et al., 2012). Across the native range of Drosophila in Australia, for example, thermal limits show a latitudinal cline from tropical to temperate populations, with knockdown times varying by 3 min along this cline and differences in CTmax ranging from 0.5°C to 1.1°C, depending on the type of assay (Sgrò et al., 2010). Although seemingly small, changes in thermal limits of this magnitude are all that is required for many Drosophila species to reduce range losses under global change (Bush et al., 2016). We observed that the magnitude of a pathogens impact could easily dwarf these observations, with infection reducing knockdown times by 20 min or more (and up to 35 min as disease symptoms progress) and CTmax by 0.5–2°C.
In adapting the framework that has previously been used to explore thermal limits across many taxa (Chown et al., 2009; Sgrò et al., 2010; Terblanche et al., 2007), we have highlighted the importance of considering different forms of thermal stress in the context of disease. Previous studies looking at the effect of disease on host thermal limits have focused on static temperature treatments (Gehman et al., 2018) or a single ramping rate (Greenspan et al., 2017). Our results show that more acute thermal stress resulted in the largest differences between healthy and infected individuals (both static and fast ramps, Figures 1 and 2). As ramping rates slowed, however, both upper thermal limits generally and the magnitude of the difference between infected and uninfected animals gradually declined (e.g. Figure 2), most likely because prolonged exposure to thermal stress caused an accumulation of physiological damage (Jørgensen et al., 2019; Rezende et al., 2014; Rohr et al., 2018). Both observations are consistent with previous work investigating the effects of slow rates of temperature change on survival of ectotherms (Kingsolver & Buckley, 2017; Rezende et al., 2010). We suggest that the type of thermal change, which will depend on both a population's environment and the severity of climate events (Rezende et al., 2014; Vinagre, Leal, Mendonça, & Flores, 2015), will define the risk of extinction for populations that face both pathogens and thermal stress, with the impact of infection greatest under more acute thermal conditions.
Adding to the complexity of understanding an organism's thermal performance in the face of both global change and pathogen infection is that any response we observed depended heavily on the specific host and pathogen genotypes involved. In particular, the host (M10) that was most able to cope with thermal stress when uninfected (higher CTmax and knockdown time, particularly at older ages) was less able to limit the damage caused by a pathogen to their thermal limits when infected (i.e. tolerance to infection. Medzhitov, Schneider, & Soares, 2012; Råberg, Graham, & Read, 2009; Rohr, Raffel, & Hall, 2010). Such patterns even gave rise to the scenario where a healthy individual of one host genotype may have a thermal limit equal to, or even lower, than an infected individual of a different host genotype (e.g. Figure 3). Some host genotypes, therefore, may be better able to compensate for the detrimental impacts of infection on thermal limits (higher tolerance to infection), while being considerably less tolerant to stressful temperatures when healthy (lower tolerance to thermal stress). This suggests that for populations exposed to the co-occurrence of infection and thermal stress, a trade-off might possibly arise between how well hosts balance tolerance to one stressor or another.
We also found that genetic interactions between host and pathogen genotypes defined the outcome of both static and ramped thermal assays. There were rank order shifts across host genotypes in the extent to which pathogen genotypes reduced thermal limits, as well as how the symptoms of disease associated with a pathogen genotype manifested in a further reduction of a host's thermal limits. As a consequence, the evolution of a hosts’ thermal limits will no longer be driven simply by host performance and genetic variability in warmer environments (sensu Hoffmann & Sgrò, 2011), but also by the frequency of host and pathogen genotypes occurring in a population and how these genotypes interact. While similar genetic interactions have been well documented for many, if not most, disease-related traits (Hall, Bento, & Ebert, 2017; Lambrechts, 2010), the potential for them to mediate a host's thermal limits has yet to be incorporated into studies of host and pathogen thermal ecology (e.g. Gehman et al., 2018).
Studies on Daphnia have increasingly been used to highlight the complexity of the interaction between infectious disease and global change. Increases in average temperature have been shown to result in an escalation in the size of epidemics (Shocket, Strauss, et al., 2018), and a reduction in host genetic diversity as the most susceptible genotypes are purged from the population (Auld & Brand, 2017b). Independently, work on local adaptation has shown that populations of Daphnia vary in their thermal limits, and that this variation correlates with the native environmental temperatures of those populations (Yampolsky et al., 2014). Here, we connect these aspects of thermal biology by characterizing the impact of infection on the thermal limits of the host itself. Our results suggest that epidemics that emerge under elevated temperatures will result in infected animals being far less able to cope with increased thermal stress. The risk of death in warmer waters is thus twofold—driven by both increased proliferation and virulence of pathogens as temperatures rise (Vale et al., 2008) and the reduction in a hosts thermal limits that coincides with infection.
In conclusion, our results emphasize how an understanding of multiple co-occurring stressors, including those directly related to thermal stress, will be vital for predicting the response of a population to global change (Carilli, Norris, Black, Walsh, & McField, 2010; Nõges et al., 2016). Not only will the joint thermal performance of hosts and pathogens determine whether pathogen transmission will increase under scenarios of global change (Caminade et al., 2017; Cohen et al., 2018), or instead peak at intermediate temperatures (Shocket, Ryan, & Mordecai, 2018), but as we have shown here, so too will the capacity of pathogens to fundamentally alter the environmental tolerances of their hosts. These effects appear at minimum equal to both the scale of variation in thermal limits that species exhibit over large geographical ranges (Sgrò et al., 2010), and the scale of temperature change that is predicted to have significant impacts on disease dynamics in natural populations (Gehman et al., 2018; Tesla et al., 2018). Our results suggest that host populations exposed to disease will be less able to cope with extreme climatic events and that predictions of populations’ resilience and adaptation to global change may be overestimated if they fail to consider the complex effects of disease on host thermal limits.
ACKNOWLEDGEMENTS
We thank F. Cockerell, S. Gipson, L. Heffernan, T. Laidlaw, L. Nørgaard and J. di Valentine for assistance with laboratory work, and Monash University and the Australian Research Council for financial support.
AUTHORS' CONTRIBUTIONS
TEH, MDH and CMS conceived and designed all experiments. TEH performed the experiments and data analyses. All authors contributed to writing the manuscript and approved the final version.
Open Research
DATA ACCESSIBILITY
Datasets supporting this article are available via a figshare repository at https://doi.org/10.26180/5ceb7e7939b25.




