Denitrification as a major regional nitrogen sink in subtropical forest catchments: Evidence from multi-site dual nitrate isotopes
Funding information
PhD Scholarship from China Scholarship Council (CSC), Norwegian Research Council Project 209696/E10, Grant from National Natural Science Foundation of China, Grant/Award Number: 41603082); Hundred-Overseas Talents Introduction Plan of Colleges and Universities in Guangxi
Abstract
Increasing nitrogen (N) deposition in subtropical forests in south China causes N saturation, associated with significant nitrate (NO3−) leaching. Strong N attenuation may occur in groundwater discharge zones hydrologically connected to well-drained hillslopes, as has been shown for the subtropical headwater catchment “TieShanPing”, where dual NO3− isotopes indicated that groundwater discharge zones act as an important N sink and hotspot for denitrification. Here, we present a regional study reporting inorganic N fluxes over two years together with dual NO3− isotope signatures obtained in two summer campaigns from seven forested catchments in China, representing a gradient in climate and atmospheric N input. In all catchments, fluxes of dissolved inorganic N indicated efficient conversion of NH4+ to NO3− on well-drained hillslopes, and subsequent interflow of NO3− over the argic B-horizons to groundwater discharge zones. Depletion of 15N- and 18O–NO3− on hillslopes suggested nitrification as the main source of NO3−. In all catchments, except one of the northern sites, which had low N deposition rates, NO3− attenuation by denitrification occurred in groundwater discharge zones, as indicated by simultaneous 15N and 18O enrichment in residual NO3−. By contrast to the southern sites, the northern catchments lack continuous and well-developed groundwater discharge zones, explaining less efficient N removal. Using a model based on 15NO3− signatures, we estimated denitrification fluxes from 2.4 to 21.7 kg N ha−1 year−1 for the southern sites, accounting for more than half of the observed N removal. Across the southern catchments, estimated denitrification scaled proportionally with N deposition. Together, this indicates that N removal by denitrification is an important component of the N budget of southern Chinese forests and that natural NO3− attenuation may increase with increasing N input, thus partly counteracting further aggravation of N contamination of surface waters in the region.
1 INTRODUCTION
Anthropogenic changes to the nitrogen (N) cycle affect the environment both regionally and globally (Galloway et al., 2003; Vitousek et al., 1997) with profound effects on ecosystem functions (Aber et al., 1998; Jaworski, Howarth, & Hetling, 1997), climatic feedbacks (Bernal, Hedin, Likens, Gerber, & Buso, 2012; Shi, Cui, Ju, Cai, & Zhu, 2015; Zaehle, Ciais, Friend, & Prieur, 2011) and human health (Townsend et al., 2003). Reactive N input into the biosphere has doubled in the last century (Cui, Shi, Groffman, Schlesinger, & Zhu, 2013; Gu et al., 2012), and China's relative contribution is growing (Liu et al., 2013). Currently, forests in South China receive up to 60 kg N ha−1 year−1 from the atmosphere (Xu et al., 2015), leading to N saturation with significant nitrate (NO3−) leaching from well-drained soils (Huang, Kang, Mulder, Zhang, & Duan, 2015). Yet, at the catchment scale, large proportions of dissolved inorganic N appear to be attenuated in water-saturated soils just before entering the streams (Duan et al., 2016; Larssen, Duan, & Mulder, 2011).
Biogeochemical N sinks in forest ecosystems include assimilation by plants and microbial biomass, and NO3− removal by denitrification (Yanai et al., 2013). In the acidic soils of South China, with low availability of phosphorus, N uptake by standing biomass is limited due to slow tree growth (Li et al., 2014; Wang et al., 2007), while net N assimilation into by soil microbes is restricted by low carbon availability (Chen & Mulder, 2007a; MacDonald et al., 2002). Several N mass balance studies have hypothesized that denitrification in near-stream soils acts as a significant N sink on the catchment level (Chen, Mulder, Wang, Zhao, & Xiang, 2004; Larssen et al., 2011; Zhu et al., 2013). Recently, this hypothesis was strengthened by stable isotope studies (Fang et al., 2015). However, the importance of denitrification at the catchment scale is under debate, owing the scarcity of spatially resolved data for soil denitrification (Duncan, Groffman, & Band, 2013).
Previous studies in temperate forests have documented significant, but transient denitrification activities in shallow water-saturated soils (Duncan, Band, Groffman, & Bernhardt, 2015; Duncan et al., 2013; Rose, Sebestyen, Elliott, & Koba, 2014; Wexler, Goodale, McGuire, Bailey, & Groffman, 2014), where NO3− is derived from soil N cycling rather than from atmospheric sources (Rose, Elliott, & Adams, 2015; Rose et al., 2014; Sabo, Nelson, & Eshleman, 2015). Others have emphasized the importance of coupled nitrification–denitrification, i.e. the simultaneous production and consumption of NO3− in shallow, fluctuating groundwater bodies (Duncan et al., 2015, 2013; Griffiths et al., 2016). These scenarios differ fundamentally from observations in South China, where large amounts of NO3− are produced in well-drained top soils on hillslopes and removed in groundwater discharge zones (Larssen et al., 2011). In a previous study in SW China, we observed a robust multi-year 15N and 18O pattern in nitrate (NO3−) along a hydrological flow path in a headwater catchment, suggesting that large amounts of NO3− are produced by nitrification on hillslopes and transported by interflow over argic horizons of common Acrisols to wet soils in groundwater discharge zones, where they are denitrified (Yu, Zhu, Mulder, & Dörsch, 2016). Similar geomorphological and soil-related patterns of N turnover, transport and removal have been recently reported in temperate and subtropical systems (Anderson, Groffman, & Walter, 2015; Griffiths et al., 2016).
Denitrification in soil is difficult to measure directly (Kulkarni, Burgin, Groffman, & Yavitt, 2014), making dual isotopic signatures of NO3− an attractive tool for integrating temporal and spatial variability of N turnover (Griffiths et al., 2016; Kendall, Elliott, & Wankel, 2007; Schwarz, Oelmann, & Wilcke, 2011; Wexler et al., 2014). Biological transformations fractionate stable isotopes (Fry, 2007; Kendall et al., 2007) and the resulting changes in 15N and 18O signatures of residual NO3− can be used to partition NO3− sources and sinks as well as to elucidate underlying biological processes. For instance, NO3− produced through nitrification is often 15N-depleted compared to NH4+, even though the fractionation effect is small (Kendall et al., 2007). Nitrification-derived NO3− has usually distinctively smaller δ18O than NO3− from precipitation, and this could be used to distinguish soil NO3− from microbial and atmospheric sources (Barnes, Raymond, & Casciotti, 2008; Rose et al., 2015), as NO3− produced from NH4+ in soils by nitrification incorporates O from both 18O-depleted atmospheric O2 and 18O-enriched H2O (Kendall et al., 2007). Microbial denitrification results in enrichment of both 15N and 18O in residual NO3− with a ratio between 1:1 and 2:1. The latter is routinely used to identify denitrification in riparian and aquatic systems (Kendall et al., 2007; Osaka et al., 2010; Wexler et al., 2014).
Several studies have applied 15N natural abundance models to estimate ecosystem denitrification fluxes (Fang et al., 2015; Houlton & Bai, 2009; Soper et al., 2017). Assuming steady state for ecosystem N cycling, Houlton and Bai (2009) attributed 15N enrichment in the terrestrial biosphere to solely gaseous (denitrification) and hydrological (leaching) losses. Based on distinct isotopic effects by denitrification and leaching, they estimated the contribution of denitrification to total N loss and from this the denitrification N flux. Recently, Fang et al. (2015) presented a more complex isotopic model based on transformation rates and δ15N signatures of soil NO3− pools. This model follows the dynamic turnover of NO3− and thus provides more realistic estimates of denitrification. When quantifying isotopic effects of N cycling processes, both models depend on 15N fractionation factors (ε) as reported in the literature. In contrast to the small ε for leaching and biological N uptake, the isotopic effect of denitrification varies over a wide range from 5‰ to 30‰, with a global average of 16‰ (Granger, Sigman, Lehmann, & Tortell, 2008; Houlton & Bai, 2009; Knöller, Vogt, Haupt, Feisthauer, & Richnow, 2011). Under field conditions, the apparent 15N enrichment is usually smaller, due to complete consumption of NO3− by denitrification or mixing with NO3− produced by other processes (Mariotti, Landreau, & Simon, 1988). Thus, apparent 15N isotopic fractionation effects by riparian and groundwater denitrification have been reported to be around 5‰ (Bottcher, Strebel, Voerkelius, & Schmidt, 1990; Mariotti et al., 1988; Osaka et al., 2010; Søvik & Mørkved, 2008; Spalding, Exner, Martin, & Snow, 1993).
On the global scale, terrestrial denitrification fluxes largely depend on water balance and N load (Bouwman et al., 2013; Seitzinger, Harrison, & Bohlke, 2006). Chinese forests naturally cover a wide range of climate conditions and N deposition rates (Du, Jiang, Fang, & Vries, 2014; Fang & Yoda, 1990), which makes them highly suitable for studying ecosystem-level denitrification. In the present study, we monitored N fluxes in seven Chinese forest catchments for two years. The five southern sites are similar in geomorphology, but differ in N deposition rates. We hypothesized that denitrification in soils of the groundwater discharge zone in warm-humid subtropical forests in South China is a significant N sink, thus contributing to the observed N attenuation at the catchment scale. For comparison, we included two temperate northern sites which strongly differ in climatic and hydrological conditions from the southern. To evaluate the role of denitrification in the different catchments, we conducted sampling campaigns in two rainy summer seasons and analysed dual NO3− isotopes along the hydrological flow paths, comprising topographically distinct landscape elements like hillslopes and groundwater discharge zones. In addition, we use the N deposition gradient across these sites to assess the question how increased atmospheric N deposition affects the N-sink function of Chinese forested catchments.
2 MATERIALS AND METHODS
2.1 Study sites
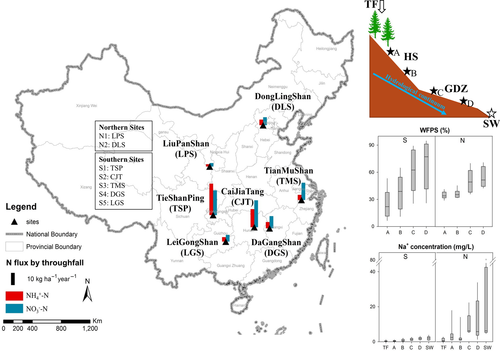
Data were collected from five catchments in South China and two catchments in North China (Figure 1). The southern and northern catchments are named sites S1 to S5 and sites N1 to N2, respectively. Details about site location, mean annual temperature and precipitation, vegetation and soil characteristics are given in Table 1. The five southern sites are subtropical and have a monsoonal climate with annual precipitation ranging from 1,028 to 1,591 mm. The two northern sites are in the temperate zone, also affected by monsoonal rains, but have smaller annual precipitation (612–676 mm). At all studied sites, precipitation occurs mostly in summer. The pH of soil O/A horizons (excluding undecomposed litter) at southern sites (4.2–6.3) tends to be lower than at northern sites (7.0 and 6.1 for N1 and N2, respectively). The seven sites represent a gradient of inorganic N flux in throughfall ranging from 5.0 to 48.6 kg N ha−1 year−1 (observed during 2013 and 2014; Table 2).
| Site name | S1 (TSP) | S2 (CJT) | S3 (TMS) | S4 (DGS) | S5 (LGS) | N1 (LPS) | N2 (DLS) |
|---|---|---|---|---|---|---|---|
| Location | Chongqing | Hunan | Zhejiang | Jiangxi | Guizhou | Ningxia | Beijing |
| Longitude | 106°41′ | 112°22′ | 119°26′ | 114°34′ | 108°11′ | 106°20′ | 115°26′ |
| Latitude | 29°38′ | 27°50′ | 30°19′ | 27°35′ | 26°23′ | 35°15′ | 39°58′ |
| Mean annual temperature (oC) | 18.2 | 17.5 | 11.9 | 15.8 | 15.7 | 5.8 | 4.8 |
| Mean annual precipitation (mm) | 1028 | 1250 | 1581 | 1591 | 1120 | 676 | 612 |
| Annual runoff (mm)a | 172 | 303 | 414 | 486 | 335 | 68 | 61 |
| Vegetation | Massone pine-dominated, coniferous-broad leaf mixed forest | Massone pine dominated, coniferous-broad leaf mixed forest | Broad-leaf forest | Evergreen broad-leaf forest | Pinus armandii dominated, coniferous-broad leaf mixed forest | Mixed deciduous broad-leaf and coniferous forest | Mixed, secondary deciduous broad-leaf and coniferous forest |
| Soil type | Loamy yellow mountain soil (Haplic Acrisol) | Yellow mountain soil (Haplic Acrisol) | Yellow soils (Cambisol) | Red and yellow soils (Alisol) | Yellow mountain soil (Alisol and Acrisol) | Gray cinnamon soil (Luvisol) | Cinnamon soil (Luvisol) |
| Soil pHb | 4.2 | 4.8 | 6.3 | 4.9 | 4.4 | 7.0 | 6.1 |
| Soil C/N ratioc | 14.0 | 14.2 | 13.2 | 14.4 | 12.0 | 10.4 | 11.5 |
- a Runoff for the northern sites was estimated by multiplying annual precipitation with runoff coefficient (see Materials and Methods for details).
- b Soil pHH2O of the O/A horizon (0-5 cm).
- c Soil C/N ratio of the O/A horizon (0-5 cm).
| Site no. | N deposition by thoughfall | N export via stream water | N removalb | Nitrification | Denitrification−15NO3− model | Denitrification−15N-soil model | 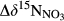 (‰)f (‰)f |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NH4+-N | NO3−-N | TIN | NH4+-N | NO3−-N | TIN | ||||||
| S1 | 27.2 | 21.5 | 48.6 | 0.03 | 9.5 | 9.5 | 39.1 | 108 | 18.3 (9.3)e | 10.1 (1.7) | 11.9 |
| S2 | 15.6 | 23.2 | 38.8 | 0.03 | 2.7 | 2.7 | 36.1 | 116 | 21.7 (10.5) | 4.8 (1.1) | 22.2 |
| S3 | 4.4 | 14.9 | 19.4 | 0.01 | 4.8 | 4.8 | 14.6 | 130 | 14.1 (11.4) | 4.7 (0.8) | 13.7 |
| S4c | 4.7 | 11.9 | 16.6 | 0.02 | 5.7 | 5.7 | 10.9 | 65 | 3.8 (4.0) | 2.4 (0.3) | 6.4 |
| S5 | 3.9 | 5.9 | 9.8 | 0.02 | 1.9 | 1.9 | 7.8 | 59 | 2.4 (5.5) | 1.9 (0.3) | 6.9 |
| N1d | 1.8 | 3.2 | 5.0 | 0.25 | 0.2 | 0.5 | 4.6 | 56 | –g | – | −1.2 |
| N2 d | 4.8 | 6.8 | 11.6 | 0.12 | 6.6 | 6.8 | 4.9 | – | – | – | – |
- a Annual fluxes are averages for 2012–2014. Nitrification fluxes are estimated based on 18O–NO3− method. Denitrification fluxes are estimated with the 15NO3− and the 15N-soil models, respectively (see Materials and Methods for details).
- b N removal refers to the difference between N deposition and N export by stream (input–output)
- c Discharge during November to April was estimated based on precipitation. Therefore, N export during this period was also estimated.
- d Annual runoff was calculated from the estimated annual discharge and annual average inorganic N concentrations.
- e Values in the brackets indicate uncertainty of model results (1 SD).
- f
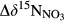 represents overall 15N enrichment in NO3− along the catchment (
represents overall 15N enrichment in NO3− along the catchment (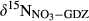 –
–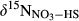 ; see Materials and Methods for details).
; see Materials and Methods for details).
- g Data not available.
All catchments are characterized by well-drained hillslopes (HS) and hydrologically connected, terraced valley bottoms acting as groundwater discharge zones (GDZ). Soil moisture expressed as water filled pore space (WFPS) increases markedly from HS to GDZ (Figure 1). Sodium (Na+) is a virtually inert cation in forest soils, and its concentration increased gradually due to evapotranspiration along the water flow path (Figure 1), indicating hydrological continuity from plots A to D at all sites (Osaka et al., 2010). Thus, we could identify hydrological flow paths from throughfall (TF) to hillslope (plots A and B), groundwater discharge zone (plots C and D), and finally to stream water (SW) in both southern and northern catchments (Figure 1). The northern catchments are significantly drier than the southern catchments, as indicated by significantly smaller annual runoff volumes (Table 1) and lower values for soil WFPS (Figure 1), resulting in less developed and discontinuous groundwater discharge zones.
2.2 Sampling design
In each of the seven catchments, soil and soil water samples were collected along the hydrological flow path, from two permanent sampling plots (A and B) on a hillslope and two plots (C and D) in a groundwater discharge zone. Additional water samples were collected at stream outlets (SW; Figure 1). Plots A and B were situated at the top and foot of the hillslope, respectively, while plots C and D represent the elevational gradient along the groundwater discharge zone. Samples of throughfall, soil water and stream water were collected bi-weekly at all sites from August 2012 to August 2014. Throughfall was sampled in triplicate in 3 L PET (polyethylene) bottles, equipped with PET funnels with nylon gauze to exclude canopy litter. Throughfall samples were pooled to monthly samples prior to analysis, and total volumes were recorded. Soil water was sampled at each plot in triplicate from the surface soil (0–5 cm) with macrorhizon soil moisture samplers (Rhizosphere Research Products, The Netherlands). In parallel with soil water collection, we measured soil volumetric moisture content with a hand-held TDR probe (Hydraprobe; Stevens Water Monitoring Systems, USA) at 10-cm soil depths. Soil WFPS was calculated using average bulk densities for soils at 0–10 cm. Stream water was collected above V-notch weirs at the outlet of each catchment. At the southern sites, the weirs were equipped with WL705 ultrasonic water level sensors (Global Water, Xylem Inc., College Station, TX, USA) measuring water discharge rates at 5-min intervals. Surface soils (0–3 cm, O/A horizon) were collected in triplicate using a garden spade, while excluding the litter (Oi) layer. Water samples were kept frozen at −20°C until analysis, whereas soil samples were stored at +4°C.
For stable isotope analysis, we conducted sampling campaigns in summers, when most of precipitation and runoff occur. We sampled throughfall, soil water and stream water twice at all sites (only throughfall for site S4) in July and August 2014. As shown by Yu et al. (2016), N turnover associated with hydrological transport are most active in warm and humid summers, resulting in pronounced NO3− isotopic signatures. Due to drought at site N2, no soil pore water could be sampled in the summer months at A, B and C plots. Additional samples of surface soil (0–3 cm excluding Oi), soil water and stream water (at the outlet) were collected during a sampling campaign in July 2015 at all southern sites except S1. During this campaign, little water was sampled at A, B and C plots of site S4, but pooling of triplicate samples at each of the plots B and C, produced enough sample for analyzing NO3− isotope. For site S5, soil samples were obtained from a depth of 0–10 cm. During the 2015 sampling campaign, all soil and water samples from a single site were collected on the same day. Previously published isotope data from site S1 (Yu et al., 2016), obtained in 2013 using the same methods, are included for comparison.
2.3 Chemical analyses
The concentrations of NH4+ and NO3− in the water samples were analysed by ion chromatography (DX-500, DIONEX) at the Research Center of Eco-Environmental Sciences, Chinese Academy of Science (CAS), Beijing. Inorganic N concentrations of water samples collected for isotope analysis were determined spectrophotometrically by flow injection analysis (FIA star 5020, Tecator, Sweden) at the Norwegian University of Life Sciences. Air-dried soil samples were sieved (2 mm) and milled before analysis of total organic carbon (C) and N by LECO-EA (TruSpec®CHN, USA). The soil pH was measured in suspensions of 10 g dry weight soil in 50 ml DI water, using an Orion SA720 electrode pH-meter.
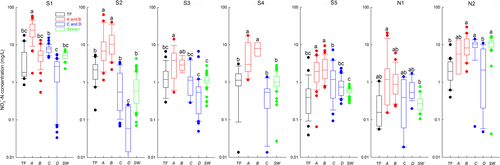
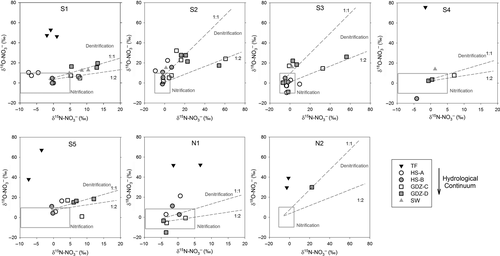
Natural abundance of 15N and 18O in NO3− (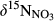 and
and  ) were analyzed using a modified denitrifier method converting NO3− to N2O (Yu et al., 2016; Zhu et al., 2018). Briefly, tryptic soya broth (TSB) was pretreated with Paracoccus denitrificans (ATCC 17741) to remove background NO3−, amended with NH4Cl, autoclaved and filtered, before inoculation with an overnight culture of Pseudomonas aureofaciens (ATCC 13985), a denitrifier lacking N2O reductase. After 6–8 hr aerobic growth, 2 ml aliquots were transferred aseptically to Helium-washed 120 ml vials. Sample volumes were adjusted to ~100 nmol NO3− before injecting them into the vials for conversion to N2O at room temperature. 1 ml of a 1M NaOH solution was added to stop the conversion and to trap excess CO2. Analysis of δ15N and δ18O of N2O was done directly from the headspace using purge-and-trap isotope ratio mass spectrometry (PreCon-GC-IRMS, Thermo Finnigan MAT, Bremen, Germany) at the Norwegian University of Life Sciences. International standards (IAEA N3, USGS 32 and 34) were included in each batch for calibration and correction (Zhu et al., 2018). Overall repeatability of our measurements was 0.1‰–0.3‰ for δ15N and 0.2‰–0.7‰ for δ18O.
) were analyzed using a modified denitrifier method converting NO3− to N2O (Yu et al., 2016; Zhu et al., 2018). Briefly, tryptic soya broth (TSB) was pretreated with Paracoccus denitrificans (ATCC 17741) to remove background NO3−, amended with NH4Cl, autoclaved and filtered, before inoculation with an overnight culture of Pseudomonas aureofaciens (ATCC 13985), a denitrifier lacking N2O reductase. After 6–8 hr aerobic growth, 2 ml aliquots were transferred aseptically to Helium-washed 120 ml vials. Sample volumes were adjusted to ~100 nmol NO3− before injecting them into the vials for conversion to N2O at room temperature. 1 ml of a 1M NaOH solution was added to stop the conversion and to trap excess CO2. Analysis of δ15N and δ18O of N2O was done directly from the headspace using purge-and-trap isotope ratio mass spectrometry (PreCon-GC-IRMS, Thermo Finnigan MAT, Bremen, Germany) at the Norwegian University of Life Sciences. International standards (IAEA N3, USGS 32 and 34) were included in each batch for calibration and correction (Zhu et al., 2018). Overall repeatability of our measurements was 0.1‰–0.3‰ for δ15N and 0.2‰–0.7‰ for δ18O.
The δ15N of bulk soil (δ15Nsoil) was measured by EA-Conflow-IRMS (Thermo Finnigan MAT, Bremen, Germany). Before analysis, air-dried samples were milled and weighed in tin capsules (8*5 mm, Elemental Microanalysis). IAEA standards (N1 and N3) as well as house standards (lab-mixed forest soils) were included in each batch for calibration and drift correction. The analytical precision was 0.2‰.
2.4 Partitioning nitrification and denitrification with box ranges of dual NO3− isotopes
Soil NH4+, the substrate for nitrification, may derive from atmospheric deposition or mineralization of soil organic N. 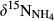 in throughfall measured previously at sites S1, S2 and S5 were in the range of −5‰ to −3.5‰ (Yu, Li, Zhang, & Wang, 2013; Yu et al., 2016). In this study, we determined δ15N-bulk of soil, varying between −4‰ and +5‰. Considering the small isotopic fractionation effect by nitrification (Mariotti et al., 1981), we assigned a range of −10‰ to +5‰ for
in throughfall measured previously at sites S1, S2 and S5 were in the range of −5‰ to −3.5‰ (Yu, Li, Zhang, & Wang, 2013; Yu et al., 2016). In this study, we determined δ15N-bulk of soil, varying between −4‰ and +5‰. Considering the small isotopic fractionation effect by nitrification (Mariotti et al., 1981), we assigned a range of −10‰ to +5‰ for 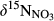 to indicate nitrification-derived NO3− in soils, similar to which has been proposed by Kendall et al. (2007). During nitrification, NO3− obtains two O atoms from soil water and one from O2. The δ18O of NO3− derived from nitrification could therefore be estimated with empirical ranges of δ18O for ambient water (−25‰ to +4‰) and ambient O2 (+23.5‰), yielding a range of −10‰ to +10‰ (Buchwald & Casciotti, 2010; Kendall et al., 2007).
to indicate nitrification-derived NO3− in soils, similar to which has been proposed by Kendall et al. (2007). During nitrification, NO3− obtains two O atoms from soil water and one from O2. The δ18O of NO3− derived from nitrification could therefore be estimated with empirical ranges of δ18O for ambient water (−25‰ to +4‰) and ambient O2 (+23.5‰), yielding a range of −10‰ to +10‰ (Buchwald & Casciotti, 2010; Kendall et al., 2007).
Denitrification enriches both 15N and 18O in residual NO3−, with a theoretical ratio of 1:1 (Fry, 2007). However, field observations, e.g. studies in natural waters, often find that denitrification enriches 15N and 18O in ratios between 1:1 and 2:1 (Kendall et al., 2007; Wexler et al., 2014). This is due to O exchange between the denitrification intermediate nitrite (NO2-) and H2O or interfering reactions that produce NO3−. In this study, we adopted enrichment ratios between 1:1 and 2:1 for 15N: 18O, to identify denitrification.
2.5 Rayleigh enrichment factor
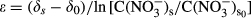 ()
()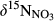 from hillslopes to groundwater discharge zones.
from hillslopes to groundwater discharge zones.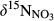 along the hydrological continuum in the catchments, we defined
along the hydrological continuum in the catchments, we defined 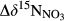 as
as
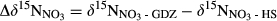 ()
()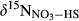 and
and 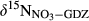 refer to concentration-weighted averages of
refer to concentration-weighted averages of 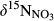 in soil water from hillslopes and groundwater discharge zones, respectively.
in soil water from hillslopes and groundwater discharge zones, respectively.2.6 Catchment N mass balances
We used total inorganic N (TIN) flux via throughfall to represent total N influx. The annual N efflux was calculated by summing up monthly N fluxes through stream runoff (multiplying average TIN concentration with monthly discharge). For site S4, where discharge data were only available from May to October, we estimated catchment discharge for November to April based on monthly rain distribution. For the northern sites, we lack discharge measurements and annual N efflux was estimated by multiplying the annual average inorganic N concentration at stream outlet with estimated annual discharge. To estimate annual discharge (Table 1), we adopted runoff coefficients (0.1; the ratio of stream discharge to throughfall) given by Wang et al. (2011). A runoff coefficient of 0.1 appears realistic, as annual evapotranspiration in North China is commonly above 500 mm (Xu & Yang, 2010). Catchment N removal was calculated by subtracting N flux in the stream from N flux in throughfall.
2.7 Model estimation of denitrification flux: 15NO3− model
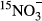 (Fang et al., 2015). The model is parameterized with the observed changes of
(Fang et al., 2015). The model is parameterized with the observed changes of 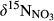 and estimated fluxes of other NO3− source and sink processes:
and estimated fluxes of other NO3− source and sink processes:
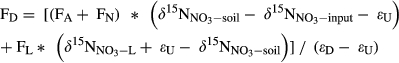 ()
()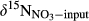 refers to the mixed δ15N signature of nitrification-produced and atmospherically deposited NO3−;
refers to the mixed δ15N signature of nitrification-produced and atmospherically deposited NO3−; 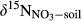 refers to δ15N signature of whole-soil NO3−;
refers to δ15N signature of whole-soil NO3−; 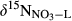 refers to δ15N signature of leached NO3−; εD and εU refer to isotopic enrichment effects (‰) by denitrification and biological NO3− uptake, respectively.
refers to δ15N signature of leached NO3−; εD and εU refer to isotopic enrichment effects (‰) by denitrification and biological NO3− uptake, respectively.This model was applied only to southern site, for which sufficient data were available. FA was given by NO3− flux in throughfall. FN was estimated by partitioning atmosphere-derived NO3− and soil-produced NO3− by a two-end member mixing approach based on 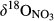 in throughfall and in hillslope soil water (Barnes et al., 2008).
in throughfall and in hillslope soil water (Barnes et al., 2008). 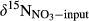 and
and 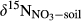 were determined as concentration-weighted averages of
were determined as concentration-weighted averages of 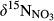 on hillslopes (plots A and B) and across whole catchments (plots A to D), respectively. We did not capture changes of
on hillslopes (plots A and B) and across whole catchments (plots A to D), respectively. We did not capture changes of 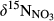 with soil depths, but previous studies of
with soil depths, but previous studies of 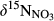 in southern Chinese forest catchments demonstrated the vertical transport and transformation of NO3− is negligible because of limited infiltration in the argic B horizon (Sørbotten, Stolte, Wang, & Mulder, 2017; Yu et al., 2016). Concentration-weighted averages of
in southern Chinese forest catchments demonstrated the vertical transport and transformation of NO3− is negligible because of limited infiltration in the argic B horizon (Sørbotten, Stolte, Wang, & Mulder, 2017; Yu et al., 2016). Concentration-weighted averages of 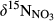 in stream water were taken as indicative for
in stream water were taken as indicative for 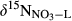 . Ranges for εD and εU were assigned from values reported in the literature, 16‰–20‰ for denitrification (Granger et al., 2008; Houlton & Bai, 2009) and 0‰–4‰ for uptake (Evans, 2001; Granger, Sigman, Rohde, Maldonado, & Tortell, 2010). For more details on model parameterization, see SI - Estimation of denitrification flux by 15NO3− model.
. Ranges for εD and εU were assigned from values reported in the literature, 16‰–20‰ for denitrification (Granger et al., 2008; Houlton & Bai, 2009) and 0‰–4‰ for uptake (Evans, 2001; Granger, Sigman, Rohde, Maldonado, & Tortell, 2010). For more details on model parameterization, see SI - Estimation of denitrification flux by 15NO3− model.
2.8 Model estimation of denitrification flux: 15N-bulk soil model
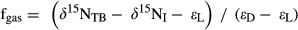 ()
()where fgas is the fraction of gaseous N loss to total N loss (leaching +gas emission), δ15NTB and δ15NI are the 15N isotopic ratios of total biospheric and atmospheric N, respectively, and εL and εD the isotopic enrichment effects (expressed as ‰) for N leaching and denitrification, respectively.
δ15NTB was approximated with δ15N of bulk soils down to 50 cm depth. Since δ15N was only measured in surface soils, we applied a correction factor of +2.5‰ to account for the typical 15N enrichment with soil depths (Fang et al., 2015; Hobbie & Ouimette, 2009). For site S5 where soils were sampled at 0–10 cm, a smaller correction factor of +1.5‰ was applied. For isotopic fractionation effects, we used ranges of 0‰–0.8‰ for εL and 16‰–20‰ for εD according to previous literature data (Houlton & Bai, 2009). For further details concerning model parameterization, see SI - Estimation of denitrification flux by 15N-bulk soil model.
2.9 Statistics
Statistical analyses were performed with Minitab 16.2.2 (Minitab Inc., State College, PA, USA). All data was tested for normality before any statistical analysis. If not normally distributed, data were normalized by logarithmic or minus reciprocal transformations. One-way ANOVA with post hoc Tukey test was used to test differences in NH4+ and NO3− concentrations, as well as in 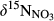 ,
, 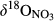 and δ15NSoil among different sampling plots and sites at a significance level of p < 0.05, unless specified otherwise. Model uncertainty adhering to the different estimates of catchment denitrification fluxes due to variable input parameters was accounted for by a Monte Carlo simulation approach (for details, see SI sections for two model estimations).
and δ15NSoil among different sampling plots and sites at a significance level of p < 0.05, unless specified otherwise. Model uncertainty adhering to the different estimates of catchment denitrification fluxes due to variable input parameters was accounted for by a Monte Carlo simulation approach (for details, see SI sections for two model estimations).
3 RESULTS
3.1 Soil chemical properties and mineral N concentrations
At the five southern sites, soil C and N contents and C/N ratios generally decreased from hillslopes (A and B plots) to groundwater discharge zones (C and D plots), while no clear trend was found at the two northern sites (Table S1). Despite similar C/N ratios, S4 and the northern sites had lower soil C and N contents than the southern sites. Soil pH increased from hillslopes to groundwater discharge zones, at all sites except N2. Site S1 had the lowest soil pH.
On hillslopes, soil water NH4+ concentrations were generally <0.1 mg N L−1 (except for N1, Figure S1), which was significantly smaller than in throughfall, while soil water NO3− concentrations were significantly larger than in throughfall (Figure 2). Along the hydrological continua from hillslopes to groundwater discharge zones and streams, NH4+ concentrations in soil water remained small, while NO3− declined (Figure 2). Only at sites S2 and N2, NO3− concentrations in the streams exceeded those in the soil of the groundwater discharge zones. Across all catchments, sites S1 and S2 had the highest NO3− concentrations on the hillslope, whereas site N1 had the lowest NO3− concentrations, both for hillslopes and groundwater discharge zones (Figure 2).
The samples collected during the summer campaign for isotope analysis showed similar spatial patterns of inorganic N concentrations (Figure S2), as the long-term data (Figure 2 and Figure S1). However, at site S4, NH4+ and NO3− concentrations were unexpectedly high in the groundwater discharge zone compared to those on the hillslope (Figure S2), possibly due to strong evaporation in the groundwater discharge zone, similar to what Yu et al. (2016) found during a drought period at S1 in 2013. However, these elevated N concentrations play little role for the annual NO3− flux, as water transport was small.
To account for concentration changes due to evapotranspiration, we normalized NO3− concentrations against Na+ concentrations along the flow path. The spatial pattern of the NO3−/Na+ ratios showed a sharp increase from throughfall to hillslopes and a subsequent pronounced decrease from hillslopes to groundwater discharge zones (Figure S3b), confirming that the observed change of NO3− concentration was due to net production and consumption and not due to evapotranspiration.
3.2 Isotopic composition of soil water NO3− and bulk soil along water flow paths
Mean 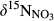 in soil water was ≤0‰ on hillslope, which is smaller than in throughfall, at all sites except for plot A at S5 (Figure 3 and Figure S4). The negative
in soil water was ≤0‰ on hillslope, which is smaller than in throughfall, at all sites except for plot A at S5 (Figure 3 and Figure S4). The negative 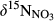 signatures on hillslopes were associated with low NH4+ and high NO3− concentrations (relative to those in throughfall; Figure 2, Figures S1 and S2). The
signatures on hillslopes were associated with low NH4+ and high NO3− concentrations (relative to those in throughfall; Figure 2, Figures S1 and S2). The 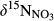 signals increased significantly from hillslopes to groundwater discharge zones at the five southern sites (Figure 3 and Figure S4a), while NO3− concentrations decreased significantly (Figure 2 and Figure S2b). The
signals increased significantly from hillslopes to groundwater discharge zones at the five southern sites (Figure 3 and Figure S4a), while NO3− concentrations decreased significantly (Figure 2 and Figure S2b). The 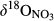 values in throughfall were distinctively higher than in soil water (Figure 3 and Figure S4b). Similar to
values in throughfall were distinctively higher than in soil water (Figure 3 and Figure S4b). Similar to 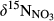 ,
, 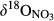 increased from hillslopes to groundwater discharge zones at the five southern sites.
increased from hillslopes to groundwater discharge zones at the five southern sites. 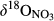 in stream runoff was in the range of 15‰ to 20‰, not significantly different from that in the groundwater discharge zone.
in stream runoff was in the range of 15‰ to 20‰, not significantly different from that in the groundwater discharge zone.
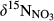 and
and 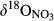 along the hydrological continua of the seven catchments: from throughfall (TF) to hillslope (HS-A and HS-B), groundwater discharge zone (GDZ-C and GDZ-D), and then to stream water (SW). The grey boxes indicate the ranges of dual isotopic compositions for nitrification, while the 1:1 and 1:2 lines indicate the typical relationship between
along the hydrological continua of the seven catchments: from throughfall (TF) to hillslope (HS-A and HS-B), groundwater discharge zone (GDZ-C and GDZ-D), and then to stream water (SW). The grey boxes indicate the ranges of dual isotopic compositions for nitrification, while the 1:1 and 1:2 lines indicate the typical relationship between 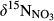 and
and  of residual NO3− undergoing denitrification (Kendall et al., 2007). Data for S1 were collected in summer 2013 and are from Yu et al. (2016). Data for the other southern sites were collected in summers 2014 and 2015, while those for northern sites were collected in summer 2014. Site codes are as in Figure 1.
of residual NO3− undergoing denitrification (Kendall et al., 2007). Data for S1 were collected in summer 2013 and are from Yu et al. (2016). Data for the other southern sites were collected in summers 2014 and 2015, while those for northern sites were collected in summer 2014. Site codes are as in Figure 1.At site N1, both 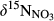 and
and 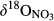 decreased gradually from throughfall to soil water on the hillslope and further to the groundwater discharge zone (Figure 3 and Figure S4). At site N2, with only a single observation at plot D,
decreased gradually from throughfall to soil water on the hillslope and further to the groundwater discharge zone (Figure 3 and Figure S4). At site N2, with only a single observation at plot D, 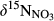 and
and 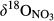 were high compared to observations in ground water discharge zones of other sites. This strong 15N enrichment in NO3− occurred simultaneously with a sharp decrease in NO3− concentration (Figure S2b).
were high compared to observations in ground water discharge zones of other sites. This strong 15N enrichment in NO3− occurred simultaneously with a sharp decrease in NO3− concentration (Figure S2b).
Rayleigh fractionation factors for denitrification were calculated for southern sites except S4 (Figure 4), yielding a range from −6.24‰ to −2.97‰. 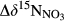 , representing the overall difference in
, representing the overall difference in 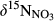 between hillslopes and groundwater discharge zones, was positive in all southern sites and negative in the northern site N1 (Table 2). In the southern sites,
between hillslopes and groundwater discharge zones, was positive in all southern sites and negative in the northern site N1 (Table 2). In the southern sites, 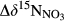 varied in a range from 6.9 to 22.2‰, with site S2 having the highest value.
varied in a range from 6.9 to 22.2‰, with site S2 having the highest value.
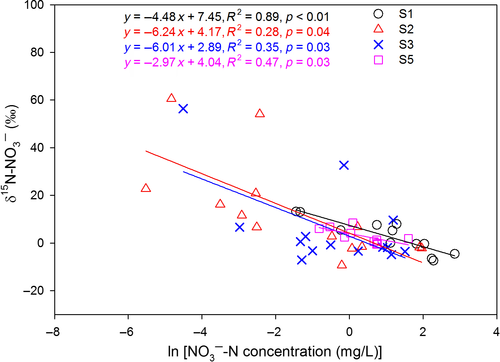
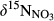 and NO3− concentrations (logarithmic) in soil water (from plots A–D) at southern sites except S4 which had limited data. Data for S1 site were collected in summer 2013 and from Yu et al. (2016). Data for the other southern sites were collected in summers 2014 and 2015. Site codes are as in Figure 1. The slopes obtained by linear regression denote the apparent “Rayleigh fractionation factors” of denitrification.
and NO3− concentrations (logarithmic) in soil water (from plots A–D) at southern sites except S4 which had limited data. Data for S1 site were collected in summer 2013 and from Yu et al. (2016). Data for the other southern sites were collected in summers 2014 and 2015. Site codes are as in Figure 1. The slopes obtained by linear regression denote the apparent “Rayleigh fractionation factors” of denitrification.At the southern sites (except S5), bulk soil δ15N values ranged from −3‰ to +3‰, gradually increasing from hillslopes to groundwater discharge zones (Figure S5). At site S5, bulk soil δ15N was relatively constant along the hydrological continuum (4‰ to 5.5‰). Across the southern sites, bulk soil δ15N was negatively correlated with the soil C/N ratio (Figure S6).
3.3 Catchment-scale N mass balance
TIN deposition (throughfall) in the studied forests varied from 5.0 to 48.6 kg N ha−1 year−1 (Table 2), covering the typical range of N deposition rates in Chinese forests (Du et al., 2014). At sites S1 and S2, about half of the N was deposited as NH4+. At the other sites, inorganic N fluxes in throughfall were dominated by NO3−. Compared with earlier throughfall data for the period 2001–2004 (Chen & Mulder, 2007b), this suggests that the relative importance of NO3− in N deposition has increased significantly during the last 10 years (Huang et al., 2015).
Inorganic N export by stream water was dominated by NO3− at all sites except N1, where TIN runoff was very small (Table 2). Although increased N deposition caused increased annual stream N export, this relationship was weak and stream runoff only accounted for a small fraction of the observed N loss, similar to what has been reported previously for southern Chinese catchments (Figure 5a) (Larssen et al., 2011; Yu et al., 2017). Catchment N removal rates, computed as the differences between N deposition and stream N export, scaled positively with N input (Table 2). For the southern sites, more than 65% of annual N input was removed within their catchments, with S2 removing most (93%). The remainder was exported from the catchments, dissolved in stream water. The northern site N1 removed most of its N input while site N2 was poorest in attenuating N runoff (42%).
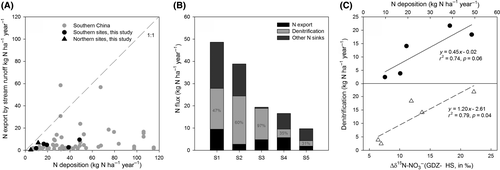
adopted from
a regional observation in South China (Yu et al., 2017). (b) Stacked bars showing N fluxes of stream export, denitrification and other N sinks at five southern sites. Denitrification fluxes are estimated with the 15NO3− model. The sum of the stacked fluxes for each site is equivalent to total N input. N removal refers to the difference between N deposition and N export by stream (input–output), and thus is the sum of denitrification and other N sinks. The percentages noted in the denitrification bars refer to the contribution of denitrification to total N removal at each site. Site codes are as in Figure 1. (c) Upper: Relationship between N deposition and denitrification fluxes (estimated with 15NO3− model). Lower: Relationship between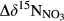 and denitrification fluxes (estimated with 15NO3− model).
and denitrification fluxes (estimated with 15NO3− model). 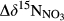 represents overall 15N enrichment in NO3− from the hillslope to the groundwater discharge zone at each site (
represents overall 15N enrichment in NO3− from the hillslope to the groundwater discharge zone at each site (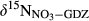 –
–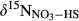 ; see Materials and Methods for more details).
; see Materials and Methods for more details).Whole-catchment denitrification fluxes for the five southern sites estimated by the 15NO3− model ranged from 2.4 to 21.7 kg N ha−1 year−1 (Table 2). These fluxes accounted for 31% to 97% of the total N sinks (Figure 5b) and were positively related with both N deposition and removal rates. Gross nitrification rates estimated for parameterizing the 15NO3− model ranged from 59 to 130 kg N ha−1 year−1 (Table 2). High gross nitrification rates were found at sites with high N deposition rates. Denitrification fluxes estimated with the 15N-bulk soil model were generally lower (1.9 to 10.1 kg N ha−1 year−1, Table 2).
4 DISCUSSION
Soils on hillslopes seemed to efficiently nitrify NH4+ (Figure 2 and Figure S1), as indicated by the low 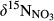 and
and 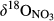 values observed during summers (Figure S4). Especially, the significantly smaller
values observed during summers (Figure S4). Especially, the significantly smaller 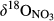 in soil water than in throughfall suggests that NO3− in soil water mostly originated from nitrification and not from atmospheric deposition (Curtis, Evans, Goodale, & Heaton, 2011; Rose et al., 2015). Dual NO3− isotopic signatures on hillslopes generally fell into a box range of −10‰ to +5‰ and −10‰ to +10‰ for
in soil water than in throughfall suggests that NO3− in soil water mostly originated from nitrification and not from atmospheric deposition (Curtis, Evans, Goodale, & Heaton, 2011; Rose et al., 2015). Dual NO3− isotopic signatures on hillslopes generally fell into a box range of −10‰ to +5‰ and −10‰ to +10‰ for 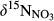 and
and 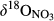 , respectively (Figure 3), which has previously been used to identify nitrification-derived NO3− in soils (Kendall et al., 2007; Wexler et al., 2014).
, respectively (Figure 3), which has previously been used to identify nitrification-derived NO3− in soils (Kendall et al., 2007; Wexler et al., 2014).
At all southern sites, the decrease in NO3− concentrations from hillslopes to groundwater discharge zones (Figure 2) was associated with a significant increase of both 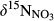 and
and 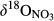 (Figure 3). Given the negligible isotopic fractionation by assimilation (Mariotti, Germon, & Leclerc, 1982), this suggests that denitrification in near-stream groundwater discharge zones acts as a significant N sink, similar to what has been reported for temperate forest ecosystems (Osaka et al., 2010) and agroecosystems (Billy, Billen, Sebilo, Birgand, & Tournebize, 2010). A regression of
(Figure 3). Given the negligible isotopic fractionation by assimilation (Mariotti, Germon, & Leclerc, 1982), this suggests that denitrification in near-stream groundwater discharge zones acts as a significant N sink, similar to what has been reported for temperate forest ecosystems (Osaka et al., 2010) and agroecosystems (Billy, Billen, Sebilo, Birgand, & Tournebize, 2010). A regression of 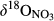 against
against 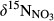 values for each site showed slopes between 0.5 and 1 (Figure 3), which are well within the range considered diagnostic for denitrification (Kendall et al., 2007; Lehmann, Reichert, Bernasconi, Barbieri, & McKenzie, 2003; Mayer et al., 2002; Wexler et al., 2014). The apparent isotopic effects of denitrification determined with Rayleigh equations generally agreed within the southern sites (not assessed for S4), with an average absolute 15N enrichment effect of 5‰ (Figure 4), which is similar to the values reported for groundwater denitrification (Bottcher et al., 1990; Mariotti et al., 1988; Spalding et al., 1993). Spatial patterns of dual NO3− isotopic signatures found in the southern catchments were largely in line with our previous long-term study at S1 (Yu et al., 2016), suggesting efficient N turnover and NO3− removal in hydrologically connected landscapes. Our finding of similar patterns at four additional subtropical catchments in South China confirms that denitrification in near-stream groundwater discharge zones is quantitatively important and a widespread phenomenon in monsoonal, subtropical forest catchments.
values for each site showed slopes between 0.5 and 1 (Figure 3), which are well within the range considered diagnostic for denitrification (Kendall et al., 2007; Lehmann, Reichert, Bernasconi, Barbieri, & McKenzie, 2003; Mayer et al., 2002; Wexler et al., 2014). The apparent isotopic effects of denitrification determined with Rayleigh equations generally agreed within the southern sites (not assessed for S4), with an average absolute 15N enrichment effect of 5‰ (Figure 4), which is similar to the values reported for groundwater denitrification (Bottcher et al., 1990; Mariotti et al., 1988; Spalding et al., 1993). Spatial patterns of dual NO3− isotopic signatures found in the southern catchments were largely in line with our previous long-term study at S1 (Yu et al., 2016), suggesting efficient N turnover and NO3− removal in hydrologically connected landscapes. Our finding of similar patterns at four additional subtropical catchments in South China confirms that denitrification in near-stream groundwater discharge zones is quantitatively important and a widespread phenomenon in monsoonal, subtropical forest catchments.
Unlike the southern sites, the northern site N1, having the lowest N deposition rates of all catchments, showed a more continuous decrease of 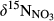 and
and 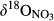 along the flow path (Figure 3 and Figure S4), suggesting that denitrification is less important for N retention in this catchment. In addition, dual NO3− isotopic signals indicated that nitrification occurred in the groundwater discharge zone, particularly during dry periods. Besides the smallest N deposition rates (Table 2), N1 has least NO3− leaching from hillslope soils (Figure 2) and lacks significant changes in NO3− concentration along the flow path. This indicates that N1 is N-limited, with N largely being assimilated by plants and immobilized in soils, similar to what is commonly seen in N-limited temperate forest ecosystems in Northeastern America (Goodale, 2017; Nadelhoffer, Colman, Currie, Magill, & Aber, 2004).
along the flow path (Figure 3 and Figure S4), suggesting that denitrification is less important for N retention in this catchment. In addition, dual NO3− isotopic signals indicated that nitrification occurred in the groundwater discharge zone, particularly during dry periods. Besides the smallest N deposition rates (Table 2), N1 has least NO3− leaching from hillslope soils (Figure 2) and lacks significant changes in NO3− concentration along the flow path. This indicates that N1 is N-limited, with N largely being assimilated by plants and immobilized in soils, similar to what is commonly seen in N-limited temperate forest ecosystems in Northeastern America (Goodale, 2017; Nadelhoffer, Colman, Currie, Magill, & Aber, 2004).
Even though high  (23.4‰) and
(23.4‰) and 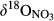 (30.0‰) were found in pooled soil water samples from the groundwater discharge zone at site N2 (Figure 3), indicating strong denitrification, NO3− export via stream water was high relative to its input (Table 2). This suggests that the N2 catchment has an overall weak N sink strength. As already indicated the northern sites have drier conditions, both in terms of annual runoff volume and in soil moisture contents of the groundwater discharge zone (Table 1; Figure 1). Thus, we hypothesize that the groundwater discharge zones at the northern sites are less developed and consequently less effective in removing NO3− through denitrification. This is supported by end-member-mixing analyses (Figure S7 and Table S2), which showed that stream runoff at N2 receives more than 50% of its NO3−-rich water from the hillslope, compared with significantly smaller contributions (<20%) at the southern sites.
(30.0‰) were found in pooled soil water samples from the groundwater discharge zone at site N2 (Figure 3), indicating strong denitrification, NO3− export via stream water was high relative to its input (Table 2). This suggests that the N2 catchment has an overall weak N sink strength. As already indicated the northern sites have drier conditions, both in terms of annual runoff volume and in soil moisture contents of the groundwater discharge zone (Table 1; Figure 1). Thus, we hypothesize that the groundwater discharge zones at the northern sites are less developed and consequently less effective in removing NO3− through denitrification. This is supported by end-member-mixing analyses (Figure S7 and Table S2), which showed that stream runoff at N2 receives more than 50% of its NO3−-rich water from the hillslope, compared with significantly smaller contributions (<20%) at the southern sites.
Although the southern sites showed a common pattern of NO3− attenuation (Figure 2; Table 2), the relationship between stream N export and atmospheric N deposition could be further elucidated by catchment hydrology. At the sites with largest precipitation and runoff (S3 and S4), we observed highest N export by stream relative to N deposition (19% and 22%; Table S2), indicating that NO3−-rich hillslope water by-passes the groundwater discharge zone during heavy precipitation events, and directly feeds into the stream (Rose et al., 2014). These findings lead to the conclusion that the efficiency of catchments to remove NO3− depends on the extent to which groundwater discharge zones act as a conduit for water as it passes from hillslope to stream.
Distinct, hydrologically connected landscape elements with prevailing oxidative or reductive conditions appear to be crucial for N retention observed at the catchment scale. Argic horizons with restricted vertical hydraulic conductivity are widespread in Acrisols of subtropical China and favor the transport of NO3− produced on hillslopes by lateral “interflow” to groundwater discharge zones in which NO3− is denitrified. By contrast, direct seepage of NO3− on the hillslopes to deeper groundwater plays a minor role (Sørbotten et al., 2017). Topographical control on denitrification is well-known (Anderson et al., 2015; Duncan et al., 2013), but seems to be favored by the high degree of hydrological connectivity in southern Chinese forest catchments. This is also supported by increasing bulk soil-δ15N and decreasing C/N ratios along the topographical gradient of our southern sites (Figures S5 and S6). As 15N enrichment in soils is often related to losses of 15N-depleted N (e.g. as NO3− and N2; Amundson et al., 2003; Billings & Richter, 2006), the observed 15N enrichment in groundwater discharge zones likely reflects long-term denitrification activity, as indicated by isotopic enrichment in NO3− (Figure 3).
Annual N mass balances for the seven catchments indicated that up to 93% of N input via throughfall was removed without being exported by the stream (Table 2). An exception was the northern site N2, which exported more than half of the N received by throughfall to the stream. Possible N sinks comprise N uptake by the biosphere and removal as gaseous N by denitrification (Yanai et al., 2013). Given the excessive amounts of NO3− leached from well-drained hillslope soils but moderate N export by the streams (Figure 2; Table 2), gaseous N losses from the water-saturated soils appear to be important for the N budget of southern Chinese forests (Van Breemen et al., 2002; Sudduth, Perakis, & Bernhardt, 2013). We therefore estimated catchment denitrification fluxes based on two alternative models, using either 15NO3− (Fang et al., 2015) or 15N-bulk soil (Houlton & Bai, 2009). The estimated fluxes varied from 1.9 to 21.7 kg N ha−1 year−1 (Table 2). Although the two isotopic approaches differed fundamentally in their parameterization, the estimated denitrification fluxes showed a consistent pattern across the five sites, which correlated positively with N deposition rates. Comparing the denitrification rates estimated by the two models, the 15N-bulk soil approach yielded 22% to 78% lower estimates than the 15NO3− model. This has also been found in other studies (Fang et al., 2015; Soper et al., 2017), and attributed to the fact that 15N signature of bulk soil carries only diluted 15N effects of denitrification especially on large spatial scales and does not account for the isotopic effect during complete NO3− consumption by denitrification (Houlton & Bai, 2009). Thus, the very low denitrification fluxes estimated by the 15N-bulk soil model for site S2 reflects the moderate 15N enrichment of bulk soil in its groundwater discharge zone (Figure S5), while it showed the highest 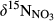 in soil water (Table 2 and Figure S4) among all sites during summer. This calls for more systematic studies into the
in soil water (Table 2 and Figure S4) among all sites during summer. This calls for more systematic studies into the 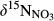 in forest catchments to scrutinize temporal patterns, as has been done for site S1 (Yu et al., 2016).
in forest catchments to scrutinize temporal patterns, as has been done for site S1 (Yu et al., 2016).
Denitrification estimates by isotopic model approaches are fraught by uncertainties adhering to isotopic fractionation effects for N loss processes (Soper et al., 2017). While isotopic effects for N uptake and leaching show little variation (Evans, 2001; Granger et al., 2010), isotopic effects of denitrification itself vary over large ranges. Isotopic effects have been described ranging from 5‰ to 30‰ for pure culture (Granger et al., 2008; Mariotti et al., 1981) and 6‰ to 65‰ for natural soils (Houlton & Bai, 2009; Wang et al., 2018). The apparent average isotopic effect of denitrification determined with our field data was ~5‰ (Figure 4), which is at the low end of the global reported range. However, this value likely underestimates the actual isotopic effect of in situ denitrification due to complete NO3− consumption by denitrification or mixing of NO3− from other sources (Fang et al., 2015). Therefore, we adopted a range of 16‰ to 20‰ for both isotopic models. This range is close to the global average reported for denitrification, and has been used in previous model studies (Fang et al., 2015; Houlton & Bai, 2009). Model uncertainties given in Table 2 show that the 15NO3− model is more sensitive to variations in isotopic effects than the 15N-bulk soil model.
Global isotopic models indicate that fluxes of N gas emission from tropical forests are in the range of 6 to 26 kg N ha−1 year−1 (Bai, Houlton, & Wang, 2012). Site-specific model estimates based on field isotopic measurements reported smaller denitrification fluxes, ranging from 1 to 7.5 kg N ha−1 year−1 (Bai & Houlton, 2009; Soper et al., 2017; Weintraub, Cole, Schmitt, & All, 2016), which may be attributed to a conservative N cycle in these forests with low N deposition (Soper et al., 2017). By contrast, based on the 15NO3− model, Fang et al. (2015) estimated denitrification fluxes of 5.6 to 30.1 kg N ha−1 year−1 for a few tropical forests in southern China and a few temperate forests in Japan. Having similar inorganic N deposition rates as our sites (Table 1), their estimated denitrification fluxes were close to or larger than TIN input, while our estimates based on the 15NO3− model accounted for 23%–73% of the annual input (Table 2). The larger N loss (gaseous and leaching) than N input may indicate that these forest ecosystems are not at steady state, i.e. that they lose N from organic pools in soil and plant biomass over time which is leached or denitrified. Another reason for the relatively smaller N removal in our study may be that our 15NO3− model was based on the isotopic difference between NO3− found along the lateral water flow path (concentration-weighted whole-soil NO3−) and NO3− on hillslopes (input), while Fang et al. (2015) considered the isotopic difference between NO3− in stream water and that of whole soil profiles (Equation 3). Hence, our estimates may miss denitrification in the hyporheic zone and are strictly speaking only valid for NO3− removal along the hydrological flow path from the hillslope to the groundwater discharge zone. We substantiated N removal by denitrification by plotting the overall change of 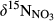 along the hydrological flow path in each southern catchment (
along the hydrological flow path in each southern catchment (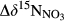 , Table 2) against estimated denitrification fluxes, and found a significant positive correlation (Figure 5c). This validates the idea that
, Table 2) against estimated denitrification fluxes, and found a significant positive correlation (Figure 5c). This validates the idea that 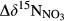 obtained along hydrological flow paths may serve as a proxy for the denitrification N sink strength of hydrologically connected landscapes.
obtained along hydrological flow paths may serve as a proxy for the denitrification N sink strength of hydrologically connected landscapes.
Our study provides evidence for significant denitrification in subtropical forests on a regional scale. N mass balances, process identification by isotopic box ranges and model estimates of nitrification and denitrification largely agreed and support our hypothesis that denitrification in groundwater discharge zones acts as a strong N sink. The estimated denitrification fluxes help us to close the N budget for subtropical Chinese forest catchments, which have long been reported to be strong N sinks (Figure 5a; Chen & Mulder, 2007a; Larssen et al., 2011). Denitrification estimated by the 15NO3− model accounted for 31% to 97% of total N removal (Figure 5b), illustrating that denitrification may indeed account for a large fraction of the missing N. In addition, denitrification scaled proportionally (slope = 0.45) with N deposition (Figure 5c), implying that subtropical Chinese forests may play an important role in attenuating the ever-increasing atmospheric N loads in this region (Liu et al., 2013) before entering into water courses.
ACKNOWLEDGEMENT
Longfei Yu thanks the China Scholarship Council (CSC) for supporting his PhD study. Support from the Norwegian Research Council to project 209696/E10 ‘Forest in South China: an important sink for reactive nitrogen and a regional hotspot for N2O?' is gratefully acknowledged. Jing Zhu received additional grants from the National Natural Science Foundation of China (Grant No. 41603082) and “The Hundred-Overseas Talents Introduction Plan of Colleges and Universities in Guangxi”. We thank Wang Yanhui, Zhang Yi, Cui Juan, Wang Bing, Xiao Jinsong, Qin Pufeng, Jiang Hong, Zou Mingquan for their help during data collection.




