Carbon consequences of drought differ in forests that resprout
Abstract
Prolonged drought and intense heat-related events trigger sudden forest die-off events and have now been reported from all forested continents. Such die-offs are concerning given that drought and heatwave events are forecast to increase in severity and duration as climate change progresses. Quantifying consequences to carbon dynamics and storage from die-off events are critical for determining the current and future mitigation potential of forests. We took stand measurements five times over 2+ years from affected and unaffected plots across the Northern Jarrah Forest, southwestern Australia, following an acute drought/heatwave in 2011. We found a significant loss of live standing carbon (49.3 t ha−1), and subsequently a significant increase in the dead standing carbon pool by 6 months post-die-off. Of the persisting live trees, 38% experienced partial mortality contributing to the rapid regrowth and replenishment (82%–88%) of labile carbon pools (foliage, twigs, and branches) within 26 months. Such regrowth was not substantial in terms of net carbon changes within the timeframe of the study but does reflect the resprouting resilience of this forest type. Dead carbon generated by the die-off may persist for centuries given low fragmentation and decay rates resulting in low biogenic emission rates relative to other forest types. However, future fire may threaten persistence of both dead and live pools via combustion and mortality of live tissue and impaired regrowth capacity. Resprouting forests are commonly regarded as resilient systems, however, a changing climate could see vulnerable portions of forests become carbon sources rather than carbon sinks.
1 INTRODUCTION
Globally, increased heatwave and drought events are leading to elevated rates of forest die-off events (Allen et al., 2010; Hicke et al., 2012). There have been at least 88 incidences of drought and heat related die-off events, reported from every forested continent and every major forest type (Allen, Breshears, & McDowell, 2015; Allen et al., 2010; Cobb, Ruthrof, & Breshears, 2017). These global change-type droughts (i.e., hotter droughts) are predicted to increase in frequency, duration, and severity into the future (IPCC, 2013). Die-off events may have broad repercussions, impacting the structure, function, and biodiversity of ecosystems, with some systems experiencing structural state shifts (Clark et al., 2016; Matusick, Ruthrof, Fontaine, & Hardy, 2016). For example, Martı́nez-Vilalta and Piñol (2002), reported drought-induced mortality in three pine species (Pinus pinaster, Pinus nigra, and Pinus sylvestris) on the north eastern Iberian Peninsula, and suggested that a drier climate may extirpate P. sylvestris populations in the region. In southwestern USA, Breshears et al. (2005) reported that a global change-type drought in 2002–2003 led to the die-off of the key overstorey species, Pinus edulis, across over a million hectares. Such impacts have garnered broad and intense interest and while changes in forest structure and composition have been documented, the carbon consequences of drought and heat related forest die-off events remain relatively unreported, leaving substantial uncertainty around drought/heat wave-caused emissions, and destabilization of carbon pools.
Forest carbon sinks and the maintenance of existing forest carbon stocks contribute significantly to the global carbon budget, and are an essential component of climate change mitigation strategies (Le Quéré et al., 2013). Indeed, the management of forest carbon sinks to sequester and store carbon emissions was included in the recent Paris Agreement (Grassi et al., 2017; Schleussner et al., 2016) and forms the basis of the National Determined Contributions (NDCs) of 187 countries (Grassi et al., 2017). Forests cover 30% of the earth's land mass and offset approximately 25% of the emissions from fossil fuel use, which equates to approximately 2.3 Pg C annually (Pan et al., 2011). However, forest disturbance events can have profound effects on forest carbon dynamics (Williams, Gu, MacLean, Masek, & Collatz, 2016). Such events directly emit carbon dioxide into the atmosphere (e.g., via pyrogenic emissions from wildfire), or drive large transformations in the structure of carbon pools (i.e., live to dead) in forest stands, through, for example, insect outbreaks, drought, or disease. Harvest, fire, windthrow, bark beetles, and drought collectively lead to the gross loss of approximately 200 Tg C yr−1 of live biomass annually across the conterminous USA (Williams et al., 2016). A large wildfire event in Oregon, USA, for example, was reported to have released 19 Mg C ha−1 of carbon from pyrogenic emissions (3.8 Tg C total fire emissions), which was an estimated 16 times the net annual ecosystem emissions (Campbell, Donato, Azuma, & Law, 2007). Kurz et al. (2008) reported that outbreaks of mountain pine beetles (Dendroctonus ponderosae) caused forests of Western Canada to transition from a carbon sink to a carbon source over a 20-year period; the cumulative impact of the affected regions resulted in a loss of 270 Tg of carbon. Quantifying the carbon consequences of disturbance events such as those above is imperative to understanding the stability of forest carbon storage and their feasibility as long-term carbon sinks.
Drought-induced forest mortality events alter the rate at which carbon moves through the carbon cycle (Law & Waring, 2015) by reducing the carbon sequestration potential. Given that this type of disturbance can vary in intensity and duration, so do the potential live carbon losses. The magnitude, pace, and pattern by which a system responds to drought is determined by the functional traits of the species within the stand. For example, Zeppel et al. (2015) suggest that drought stress may have a relatively minor impact on resprouting forest systems due to the lack of tree mortality compared with conifer-dominated systems, which do not resprout and require seedling-based regeneration and may experience larger and longer lasting live carbon losses. Not all drought related die-off events, however, lead to a reduction in carbon storage capacity. For example, Fauset et al. (2012) documented drought-induced structural and functional changes (including shifts from shade tolerant, evergreen, wet forest species, to deciduous, dry forest species) in tropical forests of Ghana, which led to an increase in above ground biomass (carbon) following two decades of chronic drought. Contrasting results from the boreal forest of Canada suggest that if climate change-induced drought events continue to intensify, forests could transition from a carbon sink to a carbon source as the climate warms and water deficits lead to a decline in tree growth, a reduction in net primary production and widespread increases in mortality (Ma et al., 2012). It is important to quantify the movement of carbon through forest stands, as well as changes in the volume of live and dead carbon, as these may have implications for the response of forests to future disturbance events, for example, fire, windthrow, or insect outbreak.
- quantify stand dynamics and carbon consequences of a major drought die-off event in 2011; and,
- quantify initial regrowth dynamics following the die-off event for all major above-ground biomass pools.
2 MATERIALS AND METHODS
2.1 Study area
The Northern Jarrah Forest (NJF) is located in southwestern Australia (30.8–33.5S and 115.8–117.8E) and covers an area of 1,127,600 ha (Havel, 1975). The forest ranges from an open dry sclerophyll forest in the north to a tall, closed forest in the south (Dell & Havel, 1989). Deep lateritic weathering profiles cap Archaean granite and metamorphic rocks (Gilkes, Scholz, & Dimmock, 1973). The NJF has a Mediterranean type climate, with hot dry summers and warm wet winters. Most rainfall occurs between April and October, and a seasonal drought may last between 4 and 7 months (Bates et al., 2008). There is a strong rainfall gradient across the forest, which ranges from >1,100 mm year−1 on the western edge to approximately 700 mm year-1 in the north east (Gentilli, 1989).
Southwestern Australia has experienced a significant change in climate, characterized by a reduction in rainfall (10%–15%) and increase in temperature (0.15°C per decade) since the 1970s (Bates et al., 2008). During this period of warming and drying, two extreme drought events occurred during the Australian summers of 2006–2007 and 2010–2011. The winter of 2010 was extremely dry, with rainfall 40%–50% below the annual average (BOM, 2011). In addition, the number of heatwave days in 2011 was the highest on record since 1960 (BOM, 2011). Prolonged reduction in rainfall, coupled with a heatwave, triggered significant, abrupt biotic disruptions across the region spanning both terrestrial and marine ecosystems, which included mortality as well as demographic shifts and altered species distributions (Ruthrof et al., 2018). During this event, it was estimated that approximately 16,000 ha of the NJF suffered severe canopy die-off (Brouwers, Matusick, Ruthrof, Lyons, & Hardy, 2013; Matusick et al., 2013). Areas that were severely affected were those in close proximity to granite outcrops, had soil with a lower water holding capacity compared to surrounding areas (Brouwers, Mercer et al., 2013), and were more clustered at xeric sites (Andrew, Ruthrof, Matusick, & Hardy, 2016). Following the die-off, Ruthrof et al. (2016) reported that the areas affected by drought had significantly higher amounts of fine fuels, which could elevate fire spread and intensity in subsequent fire events.
Our current study focussed on drought-affected areas that are composed of an E. marginata (Jarrah) and Corymbia calophylla (Marri) co-dominant overstorey, a midstorey composed of a mixture of Banksia grandis, Allocasuarina fraseriana, and two Persoonia species. The predominant disturbance agent in the NJF has historically been fire (Burrows, Ward, & Robinson, 1995) and the dominant overstorey species both have the ability to resprout from epicormic and lignotuberous buds.
2.2 Site selection
Following the 2011 drought-induced die-off event, 20 die-off patches were randomly selected from 236 patches identified during an aerial survey of the drought affected forest (Matusick et al., 2013). The 20 patches (Figure 1) with survey plots established spanned areas of 0.37 to 16.8 ha. These patches were delineated according to the canopy die-off, with >70% of the crown die-off considered a drought die-off patch, as outlined in Matusick et al. (2013). That is, most tree crowns were dying or recently killed on affected plots, including 74% (±3%) (mean [±SE]) of all stems that were living prior to the collapse, as opposed to only 11% (±2%) in paired control plots (Matusick et al., 2013). The 20 patches initially established were visited four times, and a subset of 12 were visited five times following the initial observations of crown die-off (3, 6, 16, and 26 months post-event). Sampling events were chosen to document the initial damage and response following the first winter rains as well as response following subsequent summer drought periods (Matusick et al., 2013, 2016).
At each of the 20 patches, three plots were randomly established within the delineated affected patch and three plots 20 m outside the drought-affected boundary in “healthy” forest (giving a total of 120 plots, 60 inside the drought-affected patches, and 60 outside). The close proximity of plots meant that topography, soil type, and fire history did not vary, thereby allowing for direct, straight-forward estimation of impact.
2.3 Plot measurements
At each of the 120 plots, a stand assessment was completed of the overstorey, understorey live vegetation and surface fuels. A plot radius of 6 m was used to sample individuals of >1 cm diameter at breast height (DBH). These individuals were identified to species and were measured for DBH, live height, and crown health class. The crown health class score, described by Worrall et al. (2008), and used by Matusick et al. (2013), Ruthrof, Matusick, and Hardy (2015), ranged from 1 to 4, with 1 signifying healthy trees, characterized by predominately green turgid foliage, 2 signifying dying trees, characterized by predominately dry and discoloured foliage, 3 signifying recently killed trees, characterized by predominately red and dead foliage, and 4 signifying long dead trees, characterized by a lack of leaves, fine twigs, and the presence of sloughing bark (Matusick et al., 2013, 2016; Ruthrof et al., 2015). This tree survey method was carried out at 0, 3, 6, 16, and 26 months postdrought die-off. Time 0 is a derived measure from the initial measurement at 3 months, given that trees impacted by the drought retained their symptomatic foliage, which was wilted, discolored, and dead (Matusick et al., 2016). At 6 and 16 months postdrought die-off, affected trees were assessed for resprouting to assess partial bole mortality. The presence, number, and height of epicormic sprouting was used as an indicator to determine individuals that had suffered partial bole mortality. After 26 months, these measurements were not repeated because vigorous resprouting made tracking individual tree sprouts unreliable (Matusick et al., 2016). No effort was made to estimate the proportion of individual stems that were dead in weakly resprouting trees. For the purposes of this study, partial mortality refers to individuals that have suffered severe canopy dieback and have epicormically resprouted on the bole.
2.4 Biomass and carbon calculations
Above ground biomass was estimated for each stem using previously published allometric equations (Grierson, Adams, & Williams, 2000; Hingston, Dimmock, & Turton, 1980). These equations relate DBH to dry weight (DW) of that individual (Table 1). Carbon content was calculated as 50% of the dry weight (Gifford, 2000).
| Species | Equation | Reference |
|---|---|---|
| Eucalyptus marginata | ln(DW) = −3.680 + 2.84 ln(DBH) | Hingston et al. (1980) |
| Corymbia calophylla | ln(DW) = −3.370 + 2.74 ln(DBH) | Hingston et al. (1980) |
| Banksia grandis | ln(DW) = −2.26 + 2.5 ln(DBH) | Grierson et al. (2000) |
| Allocasuarina fraseriana | ln(DW) = 3.57 + 2.68 ln(DBH) | Grierson et al. (2000) |
Total biomass was allocated into the tree components (foliage, twig, branch, bark, and bole) according to published proportions (Grierson et al., 2000; Hingston et al., 1980). Individual trees measured during the field assessment included both healthy and drought-affected trees, which had experienced partial mortality. Partial mortality entailed a spectrum of impacts on trees and tree components; therefore, it was necessary to quantify impacts with field-based techniques (observation of canopy impact, bole death, and resprouting extent) as well as literature-supported approaches to fractionate pools (estimation of live and dead fractions from allometric equations and similar studies; i.e., Campbell et al., 2007 Campbell et al., 2007, Gordon, Bendall, Stares, Collins, & Bradstock, 2018, Collins et al., 2019). To account for loss of foliage, fine branches, and aerial decay, each component was adjusted according to its health score (Table 2, Equation 1. Dead standing carbon biomass was calculated as the remaining biomass following the live biomass corrections (Table 1, Equation 2. It was assumed that there was no substantial fragmentation of dead material, and all dead biomass was retained in the canopy and underwent aerial decay only.
| Biomass component | Crown Mortality score | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Foliage | 1 | 0.5 | 0.15 | 0 |
| Twig | 1 | 0.6 | 0.2 | 0 |
| Branch | 1 | 0.7 | 0.3 | 0 |
| Bark | 1 | 1 | 0.75 | 0 |
| Bole | 1 | 1 | 0.75 | 0 |
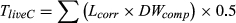 ()
()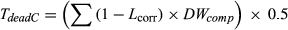 ()
()Dead carbon (TdeadC) was calculated by using the inverse of the live correction of Equation (1) to determine the weight of biomass to be allocated to the dead carbon pool (Equation 2). Once individual carbon was determined, total carbon was calculated for each plot and averaged for condition (die-off vs. control) at each site, then scaled to tonnes per hectare.
2.5 Statistical analyses
The overarching aims of this study were (1a) to quantify how stand attributes (density and basal area) varied following die-off, and (1b) to quantify the effect of drought on live and dead standing carbon stocks across multiple visits following die-off, (2) quantify initial regrowth across all above ground carbon components. Impact and early response were quantified five times over 26 months, total carbon and basal area were tested using one-way analysis of variance and change over time by pool type using a mixed model analysis. All data analyses were carried out using R (R Core Team, 2014) with the lme4 package (Bates, Maechler, Bolker, & Walker, 2014) and data visualization with ggplot2 (Wickham, 2011). In all cases, we report means and 95% confidence intervals and a lack of overlap of the mean with adjacent confidence intervals was interpreted as evidence for a statistical difference between groups, while asymmetrical overlap of means (intervals overlap one mean but not the other) was interpreted as suggestive evidence of a statistical difference between groups (Ramsey & Schafer, 2012).
Basal area was analyzed across visits to detect stand level changes to structure. Changes in basal area were tested with a one-way analysis of variance, with Tukey's multiple comparison tests used to reveal changes over time. Test assumptions (homogeneity of variance and normality of residuals) were checked visually using histograms and residual plots, and no violations were detected.
Given that the experimental design included repeated measure of plots over time and plots were nested within site, we implemented a mixed effects model structure. We assigned random effects to plot and site, and stand attributes such as density, basal area, and drought impact were fixed effects. The response variable was biomass in tonnes per hectare and was analyzed for stem density, live, and dead carbon. Model structure consisted of a two-way interaction between time since die-off (TSD) and drought impact (control vs. die-off).
Prior to analysis, the covariates were assessed for outliers and collinearity. There were only two covariates (drought impact and TSD) and no issues with collinearity were evident. Model residuals were examined graphically to ensure model assumptions were met; no violations were detected.
The same method was used to analyze differences in proportional mortality at the die-off and control plots over time (i.e., TSD). However, this model used the binomial distribution given the nature of the data.
3 RESULTS
3.1 Impact of die-off
The impact of the drought-induced die-off was evident in all stand characteristics 6 months following the event (6 months since die-off, TSD6). Six months following the drought event, live basal area in die-off plots decreased and remained significantly lower than control plots (23.40 vs. 41.19 m2 ha−1, TSD6; F8,483 = 5.29, p = 0.041, Table 3). Approximately 38% (8.97 m2 ha−-1) of the remaining live basal area in die-off plots consisted of individuals that had suffered partial mortality. Live stem density also decreased significantly at 6 months (t = 3.11, p < 0.01, Figure 2). Mean proportional stem mortality levels in die-off plots (0.39 ± 0.07) also climbed significantly 6 months following the event and remained elevated relative to control plots (0.17 ± 0.06, z = 4.55, p < 0.001, Figure 3). Within die-off plots, proportional mortality peaked at 6 months and then declined somewhat at TSD16 and TSD26 visits (p = 0.120; Figure 3) as some trees resprouted over the intervening growing season.
| Time since die-off (months) | n sites | Live | Partial mortality | Complete mortality | |||
|---|---|---|---|---|---|---|---|
| Control (CI) m2 ha−1 | Die-off (CI) m2 ha−1 | Control (CI) m2 ha−1 | Die-off (CI) m2 ha−1 | Control (CI) m2 ha−1 | Die-off (CI) m2 ha−1 | ||
| 0 | 20 | 41.46 (5.56) | 36.78 (8.73) | – | – | – | – |
| 3 | 20 | 41.46 (5.56) | 36.78 (8.73) | – | – | – | – |
| 6 | 20 | 41.19 (5.46) | 23.40 (5.58) | 3.58 (2.58) | 8.97 (3.48) | 3.20 (2.57) | 18.81 (6.46) |
| 16 | 20 | 41.19 (5.47) | 29.48 (6.40) | 0 | 8.85 (3.86) | 3.14 (2.53) | 12.55 (4.96) |
| 26 | 12 | 44.30 (8.28) | 28.47 (7.70) | – | – | – | – |

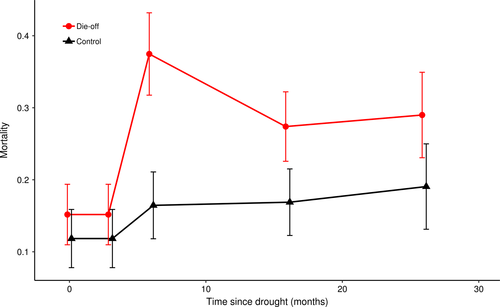
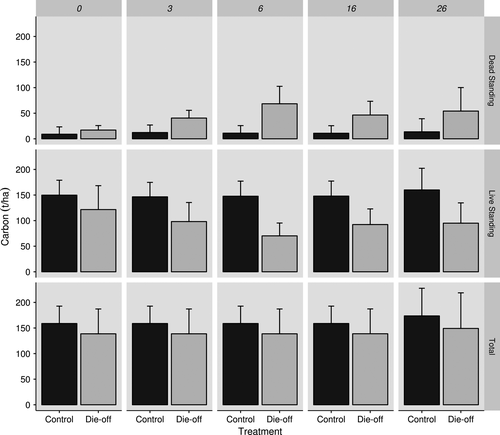
The changes found in stand characteristics 6 months following the event were mirrored in the live and dead carbon pools. Total (live + dead) carbon storage between die-off and control plots was not significantly different at any of the visits following the disturbance (F2,157 = 1.33 p = 0.27). However, the relative size and fluctuations of live and dead pools varied substantially across time and plot type (Figure 4). Three months following the disturbance, dead carbon significantly increased in the die-off plots compared to the first visit (+20.2 ± 19.33 t ha−1, t = 2.05, p < 0.05, Table 4). At 6 months following the disturbance, dead carbon increased further in the die-off plots compared to the initial visit (+49.3 ± 19.32 t ha−1, t = 5.01, p < 0.001, Table 4) and with a corresponding decrease in live carbon (−49.3 ± 25.3 t ha−1, t = −3.82, p < 0.001). Within die-off plots, the largest contributor to the decrease in live carbon of 49.3 t ha−1 (95% CI = 25.34) 6 months postdrought (t = −3.82, p < 0.001) was from bole-stored carbon (Figure 5).
| Coefficients | Response | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Live carbon (t ha−11) | Dead carbon (t ha−1) | Stem density (stems ha−1) | Proportional stem mortality | |||||||||
| Estimate (CI) | t-value | p-value | Estimate (CI) | t-value | p-value | Estimate (CI) | t-value | p-value | Odds Ratio (CI) | z-value | p-value | |
| Intercept | 150 (29.4) | 9.99 | <0.001 | 9.02 (17.7) | 1.00 | 0.32 | 1640 (369) | 8.70 | <0.001 | 0.07 (0.04) | −11.8 | <0.001 |
| Die-off | −28.2 (41.5) | −1.33 | 0.18 | 8.05 (24.3) | 0.65 | 0.51 | 453 (513) | 1.73 | 0.08 | 1.9 (1.18) | 2.62 | <0.010 |
| TSD3 | −3.28 (17.9) | −0.36 | 0.72 | 3.28 (13.7) | 0.47 | 0.76 | 0.00 (235) | 0.00 | 1.00 | 1 (0.35) | 0.00 | 1.00 |
| TSD6 | −2.07 (17.9) | −0.23 | 0.82 | 2.07 (13.7) | 0.30 | 0.78 | −97.3 (361) | −0.81 | 0.42 | 1.79 (0.57) | 4.09 | <0.001 |
| TSD16 | −1.86 (17.9) | −0.20 | 0.84 | 1.88 (13.7) | 0.27 | 0.79 | −103 (235) | −0.86 | 0.39 | 1.82 (0.58) | 4.22 | <0.001 |
| TSD26 | 7.45 (21.3) | 0.69 | 0.49 | −0.21 (16.2) | 0.02 | 0.99 | 188 (279) | 1.32 | 0.19 | 2.2 (0.81) | 5.00 | <0.001 |
| Die-off TSD3 | −20.2 (25.3) | −1.56 | 0.12 | 20.2 (19.3) | 2.05 | <0.05 | 1.47 (332) | 0.01 | 0.99 | 1 (0.46) | 0.00 | 1.00 |
| Die-off TSD6 | −49.3 (25.3) | −3.82 | <0.001 | 49.3 (19.3) | 5.01 | <0.001 | −527 (332) | −3.11 | 0.002 | 2.26 (0.94) | 4.55 | <0.001 |
| Die-off TSD16 | −27.4 (25.3) | −2.12 | <0.05 | 27.5 (19.3) | 2.79 | 0.005 | −260.8 (332) | −1.54 | 0.12 | 1.33 (0.56) | 1.56 | 0.120 |
| Die-off TSD26 | −53.4 (30.11) | −3.48 | <0.001 | 36.1 (22.9) | 3.09 | 0.002 | 1020 (395) | 5.07 | <0.001 | 1.22 (0.58) | 1.03 | 0.300 |
Note
- Model constructed from 552 observations, 20 unique sites, with 120 unique plots over 5 visits. Bold values indicate statistically significant results.
3.2 Initial regrowth
In subsequent visits following the significant decline in live carbon, early regrowth response was observed (Figure 2). Early regrowth was captured as the live stem density increased in die-off plots to a level that was significantly higher at TSD26 than TSD0 levels (t = 3.77, p < 0.001, Figure 2). Live stem density did not change across all the visits in the control plots (Figure 2) with broad overlap of 95% confidence intervals for all visits.
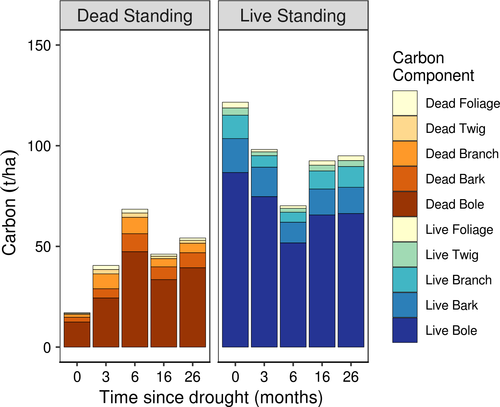
Live and dead carbon pools were relatively stable from 6 to 26 months post-die-off (Figure 4). From 6 months onwards, regrowth from resprouting trees contributed a small amount to live carbon; however, resprouting of previously “dead” individuals drove an increase in bole live carbon when comparing TSD3 and TSD6 visits (protracted epicormic resprouting above breast height led to an allocation of a proportion of the bole mass the live pool; Figure 5). Resprouting and subsequent growth led to the recovery of the more labile pools (foliage, twig, and branch) to close to predrought levels by TSD26 (Figure 6). At the 6-month visit, the labile biomass components branch, twig, and foliage were reduced to 42%, 53%, and 46% of their initial predrought mass, respectively (Figure 6). Foliage recovered to 82.4% of its original mass by the 26-month visit, while branch and twig pools recovered to 88% and 82.1% of their first visit carbon mass, respectively (Figure 6).

3.3 Discussion
This study quantifies carbon dynamics following a drought-induced forest die-off in a resprouting Australian eucalypt forest. We build on the work of other drought-induced die-off carbon studies (Fauset et al., 2012; Ma et al., 2012) by showing that although the total carbon storage did not differ over time following the die-off event, the composition of the carbon pools shifted markedly with substantial dead carbon contained in standing trees suffering complete and partial mortality. Such increases in dead carbon are in contrast to Fauset et al. (2012), who reported postdrought increases in above ground biomass (i.e., carbon) in Ghanian tropical forest due to shifts in tree species. The impacted forest underwent a structural state change, from a tall, open forest to a shorter, denser, closed forest (Matusick et al., 2016) but without changes in tree species composition (Ruthrof et al., 2015). Stem mortality occurred by 6 months post-die-off and resprouting of these trees ensued with regrowth of both basal and epicormic shoots from 6 to 26 months post-die-off. Overall, the forest transitioned from large trees to short, multi-stemmed individuals, and our study has shown that this is clearly reflected in its carbon sequestration potential.
The die-off driven carbon transformation from live to dead pools was substantial (49.3 t ha−1). Subsequent biogenic emissions and decay rates (Gunn, Ganz, & Keeton, 2012) will be contingent on a range of factors including standing vs. down, snag fragmentation rates, climatic setting, material size and wood density, and biotic agents of decay. Suspended, aerial dead wood (dead trees, portions of trees) decays more slowly (lower annual emissions) than downed wood on the forest floor in contact with the soil that experiences greater moisture content and decomposition rates (Harmon, Woodall, Fasth, Sexton, & Yatkov, 2011). The rate of snag fragmentation (recruitment of dead material from suspended to down) is undocumented for the dominant tree species in this study. However, using the knowledge available regarding the mechanisms that influence snag fragmentation rate (climate, wood density, and biotic agents), we can assume that snag fragmentation and fall rate will be more gradual than that of tropical and boreal forests. Climate plays a large role in snag decomposition rate, with decay rates decreasing from the equator toward the poles (Cooper, 1983). Warm moist environments result in faster rates of wood decay, and the Mediterranean climate (cool wet winters and warm dry summers) of southwestern Australia, therefore, do not provide optimal conditions for wood decay. In general, in a tropical forest almost all the woody material may decay within 10 years (Cooper, 1983). In contrast, the turnover time for Eucalyptus species in a temperate sclerophyll forest can range from 7 (e.g., E. regnans) to 375 years (e.g., Eucalyptus camaldulensis and Eucalyptus tereticornis) based on climate setting (Mackensen & Bauhus, 1999). The initial wood density of a species will also contribute to decay rate, with higher wood densities decaying slower than lower initial wood densities (Mackensen & Bauhus, 1999). Climatic conditions coupled with high wood density of the species in this study, E. marginata (0.67 g/cm3) and Corymbia calophylla (0.65 g/cm3), dead individuals may remain standing for decades before being added to the coarse woody debris pool and remain in the stand and ecosystem as decaying wood for a century or more in the absence of fire. This suggests that the loss of carbon (decay and subsequent heterotrophic respiration of dead wood) from the NJF may not translate to total carbon storage loss at the same rate as other forest types more commonly studied. Thus, it follows that in the absence of disturbance the total carbon storage in the die-off sites could increase as new growth counteracts the carbon lost from biogenic emissions of dead material. However, with a drying climate (Bates et al., 2008) regrowth is unlikely to grow to the extent of the individuals that died. The postdisturbance regrowth is also more likely to have a severe fire occur within it (Zylstra, 2018), further reducing carbon stored in both live and dead pools. A combination of these two factors are likely to prevent these stands from regaining or increasing carbon storage.
Fluctuations in live and dead carbon storage have implications on forest structure and future carbon sequestration capacity by potentially putting an upper limit on the amount of carbon a forest stand can fix (Ma et al., 2012). This study quantified live carbon loss and initial regrowth in die-off plots and found significant losses to live carbon occurred 6 months following the event. However, the resprouting nature of the dominant tree species in this study, E. marginata and C. calophylla, resulted in many large trees resprouting at 16 months resulting in partial mortality, which permitted rapid regrowth of the same individual and live carbon being “regained” in the system. The loss of canopy also allows plants to compete for available resources, potentially elevating regrowth, and subsequently increasing live carbon in smaller stems. These results are consistent with Zeppel et al. (2015) who reported the ability of resprouters to avoid complete mortality, through partial mortality, and withstand drought, indicating that the impact of drought stress in resprouting forest systems may be lower than in other forests dominated by nonsprouting species (i.e., conifers).
It has been suggested that even in the most rapidly growing forests, another disturbance (drought or fire) may occur before stands have recovered similar sized individuals and forest structure (Adams et al., 2009; Allen et al., 2015; Frank et al., 2015). Furthermore, postdisturbance regrowth forests have been linked to an increase the likelihood of occurrence, severity, or extent of another disturbance, particularly fire, as the structure of the regrowing forest is growing closer to the surface and more likely to be ignited (Kitzberger, Aráoz, Gowda, Mermoz, & Morales, 2012; Kitzberger et al., 2016; Zylstra, 2018). The NJF of southwestern Australia is considered a frequent fire forest, unlike forests that naturally experience infrequent standing replacing disturbances. Many of the stands that experienced drought-induced die-off are likely to be burnt by either wildfire or planned burns before they have completely recovered. Given that the drought-affected patches in this study were comprised of many younger, multiple, resprouting stems (Matusick et al., 2016), these younger/shorter individuals are more susceptible to mortality during a fire, or future drought event, as they do not have thick bark to withstand fire events (Abbott & Loneragan, 1986). Thus, the already stressed and drought affected mature individuals may not have the resilience to resprout again (Fairman, Bennett, & Nitschke, 2019; Galiano, Martínez-Vilalta, Sabaté, & Lloret, 2012), which would result in large losses in carbon storage and future sequestration potential. In a study of Quercus ilex forest in Spain, Galiano et al. (2012) suggested that progressive depletion of carbon reserves through repeated drought events may lead to a loss of resilience in resprouting species. Therefore, these systems may be at an even greater risk of future carbon loss because large trees have undergone canopy retraction (partial mortality) and resprouted epicormically, and resulting new small stems lack the thick bark required to survive even a low intensity fire. Thus, it follows that the resilience of these stands has been compromized compared with the surrounding vegetation.
The distribution of live carbon (recalcitrant soil fractions or live bole wood vs. labile live pools or dead decomposing pools) within a forest stand will influence future carbon storage and sequestration. Partial mortality of large individuals, and the increase in the number of small individuals, may temporarily buffer live carbon loss from the system (Zeppel et al., 2015). In our study, 6 months after the drought event, a reduction in live carbon was recorded, which was attributed to the movement of bole-stored carbon from the live to dead carbon pool. The slight rebound in live carbon following the initial drop can be mainly attributed to the large individuals (DBH > 30 cm) resprouting in the intervening period of 6–26 months when originally scored as dead at 6 months. This was also highlighted in the labile pools (foliage, twigs, and branches), which nearly recovered to pre-die-off levels by 26 months after the drought event. Mean foliage, twig, and branch biomass were approximately 82.5, 82.0, and 88.9% of predrought levels, respectively. Fluctuations like this highlight the significance of large trees in this forest ecosystem. Bole-stored carbon, and the transition from dead to live, plays a substantial role in the structure and carbon storage capacity of these stands. However, the rapid return of labile pools in this study highlights that partial bole mortality in resprouting forests may lead to leaf area recovery and return to predrought-carbon storage levels in a shorter time scale compared to coniferous forests.
The potential for live carbon recovery is largely dependent on the pattern of disturbance in the future. Given the fire frequency and climatic predictions for southwestern Australia that include an increase in fire risk (Pitman, Narisma, & McAneney, 2007), it is likely that subsequent disturbances will occur before the complete forest recovery. Indeed, wildfire and prescribed burning recently have impacted the sites reported in this work. Subsequent disturbances will act to substantially reduce total stand carbon storage in two ways; firstly, the drought affected patches studied here are made up of younger/shorter individuals (Matusick et al., 2016), which are more susceptible to mortality during a fire or future drought event than larger, extant individuals. Secondly, once dead trees eventually fall and are converted to coarse woody debris, they are more likely to be consumed by fire, even if it takes multiple fire events to be completely consumed (Abbott & Loneragan, 1983; Donato, Fontaine, & Campbell, 2016). Both pathways transition away from live carbon sequestration as well as an eventual reduction in dead carbon storage of die-off affected stands.
ACKNOWLEDGMENTS
This research was conducted under the Western Australian State Centre of Excellence for Climate Change Woodland and Forest Health, which is a partnership between private industry, community groups, Universities, and the Government of Western Australia. We thank Michael Pez and Geoffrey Banks, Western Australian Department of Biodiversity, Conservation, and Attractions (formerly Department of Environment, and Conservation) for their assistance in collecting spatial datasets. We also thank two anonymous reviewers for their comments and contribution to the final manuscript.




