The importance of benthic–pelagic coupling for marine ecosystem functioning in a changing world
Abstract
Benthic–pelagic coupling is manifested as the exchange of energy, mass, or nutrients between benthic and pelagic habitats. It plays a prominent role in aquatic ecosystems, and it is crucial to functions from nutrient cycling to energy transfer in food webs. Coastal and estuarine ecosystem structure and function are strongly affected by anthropogenic pressures; however, there are large gaps in our understanding of the responses of inorganic nutrient and organic matter fluxes between benthic habitats and the water column. We illustrate the varied nature of physical and biological benthic–pelagic coupling processes and their potential sensitivity to three anthropogenic pressures – climate change, nutrient loading, and fishing – using the Baltic Sea as a case study and summarize current knowledge on the exchange of inorganic nutrients and organic material between habitats. Traditionally measured benthic–pelagic coupling processes (e.g., nutrient exchange and sedimentation of organic material) are to some extent quantifiable, but the magnitude and variability of biological processes are rarely assessed, preventing quantitative comparisons. Changing oxygen conditions will continue to have widespread effects on the processes that govern inorganic and organic matter exchange among habitats while climate change and nutrient load reductions may have large effects on organic matter sedimentation. Many biological processes (predation, bioturbation) are expected to be sensitive to anthropogenic drivers, but the outcomes for ecosystem function are largely unknown. We emphasize how improved empirical and experimental understanding of benthic–pelagic coupling processes and their variability are necessary to inform models that can quantify the feedbacks among processes and ecosystem responses to a changing world.
Introduction
Coastal and estuarine ecosystems are hot spots of environmental variability, biogeochemical transformations, and biological interactions, where dynamic exchanges of energy, mass, and nutrients occur between benthic and pelagic habitats via diverse pathways. Consequently, they are among the world's most productive ecosystems (Nixon, 1988; Berger et al., 1989; Costanza et al., 1995) that provide important ecosystem services, such as food provision and water filtration (Agardy et al., 2005; Granek et al., 2010). These transitional ecosystems between land and sea are often densely populated and experience multiple anthropogenic pressures including climate change, nutrient loading, and fishing (Lotze et al., 2006; Halpern et al., 2008; Cloern et al., 2016).
The implementation of effective management strategies that mitigate or adapt to human-driven changes in these ecosystems requires a better understanding of how anthropogenic pressures can cause changes in ecosystem structure and function. Essential ecosystem functions, such as production and energy transfer in food webs, biogeochemical cycling, and provisioning of fish nursery areas (Granek et al., 2010; Seitz et al., 2014), are supported by multiple and interacting benthic–pelagic coupling processes (e.g., Chauvand et al., 2000). We define benthic–pelagic coupling as those processes which connect the bottom substrate and the water column habitats through the exchange of mass, energy, and nutrients. However, the compartmentalization of these ecosystems into their benthic and pelagic components in empirical studies and models often limits our understanding of the scope and strength of interactions between these habitats, their role in maintaining ecosystem function, and their sensitivity to future change.
The traditional view of benthic–pelagic coupling has focused on the deposition of nonliving organic material to benthic habitats (Hargrave, 1973; Suess, 1980; Smetacek, 1985; Graf, 1992), bioresuspension (Graf & Rosenberg, 1997), and the release of inorganic nutrients from the sediments (Raffaelli et al., 2003). These fluxes have been quantified in a variety of ecosystems (e.g., Duineveld et al., 2000; Smith et al., 2006), including the seasonal variation and spatial heterogeneity of these fluxes. Substantial limitations remain, however, in our quantitative predictive capacity of flux occurrence and magnitude and in our ability to generalize among ecosystems. Efforts are increasing to describe and understand the diversity of processes that couple benthic and pelagic habitats, especially those mediated by living organisms (Marcus & Boero, 1998; Schindler & Scheuerell, 2002; Raffaelli et al., 2003; Baustian et al., 2014). These include pelagic predation on benthic fauna, ontogenetic shifts in habitat use, reproductive (life-cycle) fluxes, diel and seasonal migrations, nutrient-cycling effects of benthic bioturbation and bioirrigation, and filter-feeding by benthic organisms. For many of these processes, however, the limited knowledge of their rates and importance impedes our ability to do quantitative syntheses.
Anthropogenic pressures regulate benthic–pelagic coupling directly and indirectly through their effects on the physical (e.g., salinity, oxygen, temperature) and biological (e.g., species, communities, functional traits) components of ecosystems. In coastal and estuarine ecosystems, climate change, nutrient loading, and fishing have been shown to have direct effects on benthic–pelagic coupling with clear consequences for ecosystem function. For example, increased water temperatures in Narragansett Bay (USA) have caused shifts in the timing and a decrease in the magnitude of phytoplankton blooms. This has decreased the deposition of organic material to the benthos and ultimately reduced inorganic nutrient release from the sediment (Fulweiler & Nixon, 2009; Nixon et al., 2009). Additionally, the loss of oyster reefs in Chesapeake Bay (USA) initiated by overfishing resulted in a decline of water filtration capacity by nearly 200-fold in the last century leading to increased phytoplankton production and declines in water clarity and quality (Kemp et al., 2005). In contrast, the successful establishment of an invasive filter-feeding clam in San Francisco Bay (USA) has resulted in an increased flow of energy into the benthic habitat while depriving pelagic pathways of phytoplankton production (Cloern & Jassby, 2012). Importantly, and despite the above examples, it is still more common to investigate the response of a specific species or community to anthropogenic pressures than to investigate the effects of anthropogenic pressures on processes that couple benthic and pelagic habitats. This strongly limits our ability to assess ecosystem resilience, that is, the ability of an ecosystem to retain its structure and function when exposed to pressures. Advancing the knowledge of how habitat coupling processes respond to anthropogenic pressures will significantly improve our ability to predict ecosystem responses to environmental change and to implement the appropriate management actions to maintain or reach healthy ecosystems.
We use the Baltic Sea as a case study to illustrate how benthic–pelagic coupling shapes coastal and estuarine ecosystems and to evaluate the sensitivity of coupling processes to three anthropogenic pressures: climate change, nutrient loading, and fishing. The high-latitude position of the Baltic Sea (associated with higher rates of warming, for example, Belkin, 2009; Rutgersson et al., 2014) and its large catchment area populated with over 85 million people expose this ecosystem to multiple regional and global anthropogenic pressures that are expected to continue to impact its overall function and health (Elmgren et al., 2015). We examine two categories of benthic–pelagic coupling processes, those that control inorganic nutrient fluxes and those that control organic material fluxes. Within these two categories, we identify key physical and biological processes and review their potential responses to the three anthropogenic pressures listed above. We also identify knowledge gaps and conclude with recommendations about how to address them in coastal and estuarine ecosystems worldwide through observational, experimental, and modeling approaches.
The Baltic Sea
The Baltic Sea is one of the largest brackish water bodies in the world with a geographically stable salinity gradient (surface salinity 1–25; Fig. 1a; Table 1) providing comparisons of benthic–pelagic coupling across the entire salinity range from marine to almost-freshwater conditions. Temperature and ice cover also show a north (colder/longer) to south (warmer/shorter) latitudinal gradient (Leppäranta & Myrberg, 2009; Table 1; Fig. 1b) as well as strong seasonal dynamics. The Baltic Sea is relatively shallow with an average depth of 54 m. Mixing and resuspension continue to occur at water depths greater than the photic zone (max. depth ~20 m), but a semipermanent halocline at ~70 m prevents full water column mixing in the Baltic Proper and Gulf of Finland (Fig. 1b). Deep-water oxygen conditions vary by basin (Table 1), but large areas of the central Baltic Sea, as well as the Gulf of Finland, are permanently hypoxic (Carstensen et al., 2014). North–south abiotic gradients are associated with gradients in biological diversity (species richness increases with increasing salinity, Table 1) and phenology.
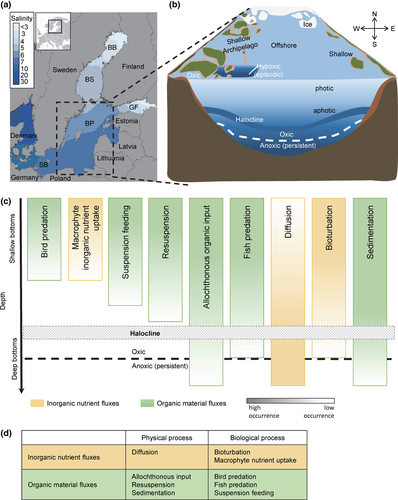
| Depth | Bothnian Bay | Bothnian Sea | Gulf of Finland | Baltic Proper | Southern/Western Baltic | Data source | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shallow (68 m) | Deep (108 m) | Shallow (104 m) | Deep (125 m) | Shallow (47 m) | Deep (70 m) | Shallow (63 m) | Deep (81 m) | Shallow (47 m) | Deep (90 m) | ||
| Station Name | CVI | BO3 | F18 | SR5 | GOF2 | GOF6 | EGB1 | LF2 | BY2 | BY5 | |
| Abiotic characteristics | |||||||||||
| Salinity | 3.2 | 3.8 | 6.1 | 6.5 | 7.2 | 9.0 | 7.8 | 9.4 | 15.6 | 15.9 | Norkko et al. (2015) |
| Oxygen (ml l−1) | 8.5 | 8.6 | 5.2 | 5.2 | 3.4 | 0.9 | 4.9 | 0.0 | 5.2 | 0.0 | Norkko et al. (2015) |
| Temperature (°C) mean (min – max) | 3.7 (−0.4 to 21.2) | 6.0 (−0.4 to 21.4) | 4.0 (−0.4 to 21.0) | 6.5 (−0.4 to 21.4) | 5.7 (−0.5 to 22.9) | 7.4 (−0.5 to 24.4) | 6.2 (−0.6 to 23.2) | 8.3 (−0.7 to 24.1) | 9.4 (−1.0 to 22.7) | 9.5 (−1.0 to 23.9) | Kotta et al. (2014) |
| Ice period (days) mean (min – max) | 23.5 (16.8–35.2) | 30.3 (17.8–43.4) | 9.4 (4.3–15.9) | 13.1 (8.8–18.5) | 17.6 (6.4–50.1) | 28.7 (10.6–54.5) | 2.6 (0.1–8.7) | 4.2 (0.3–8.2) | 14.4 (7.3–24.8) | 11.2 (2.9–30.3) | Armstrong & Knowles (2010) |
| % Surface area of shallow waters | 29 | 11 | 13 | 15 | 69 | Based on Helcom data portal GIS layers | |||||
| Biological characteristics | |||||||||||
| Mean regional macrofauna species diversity (1 mm sieve) | 2.1 | 3.3 | 5.3 | 5.3 | 8.8–14.6 | Villnäs & Norkko (2011) | |||||
| Number of benthic invertebrate species | 132 | 147 | 482 | 53–164 | 300–1028 | HELCOM (2012) | |||||
| % invasive benthic invertebrate species | 9 | 8 | 4 | 10–30 | 2–8 | HELCOM (2012), Baltic Sea Alien Species Database (2010) | |||||
Air temperature in the Baltic Sea region has increased more rapidly than the global average since the 1870s (BACC II Author Team, 2015), ice season length and ice thickness have declined (Merkouriadi & Leppäranta, 2014), and, since the 1980s, the Baltic Sea is the world's fastest warming large marine ecosystem (net sea surface temperature change of 1.35 °C (1982–2006), Belkin, 2009). The Baltic Sea has been highly impacted by eutrophication throughout the 20th century (Andersen et al., 2015), although the decrease in external nutrient loads since 1980 (1990–2006 decline of 45% total phosphorus, 28% total nitrogen (not normalized for river flow); HELCOM, 2011), has led to local improvements in coastal zones (Elmgren et al., 2015). Fishing pressure along the coast varies in space and time, but is generally moderate. Both recreational and commercial fishery sectors mainly target the same predatory and (often) benthivorous fish species. There are two dominant offshore fisheries: the commercial cod fishery, which is concentrated in the southern and more saline areas, and the mixed fishery for sprat and herring (ICES, 2014).
Future projections of anthropogenic pressures
With continued climate change, the Baltic Sea is projected to become more strongly stratified (Hordoir & Meier, 2011) but with dampened north–south gradients in temperature and salinity (BACC II Author Team, 2015). Climate change projections suggest a continued warming, with summer surface water temperature increasing from 2 °C (south) to 4 °C (north) by the end of this century (BACC II Author Team, 2015). Projections for future salinity are uncertain because Baltic Sea salinity responds both to precipitation in the catchment area (runoff) and saltwater inflows from the North Sea. However, most studies project declines in both surface and bottom salinities with the largest declines in surface salinity in the more saline (south and west) regions due to both increased runoff and decreasing inflows (BACC II Author Team, 2015).
External nutrient loads have been a major cause of Baltic Sea eutrophication, but the recovery of the ecosystem is governed by internal nutrient recycling (Vahtera et al., 2007). With adherence to the Baltic Sea Action Plan, an international agreement that includes nutrient load reduction targets (HELCOM Ministerial Meeting, 2007), reduction in nitrogen and phosphorus (target reduction from 1997 to 2003 levels is 18.3% of total nitrogen and 42% of total phosphorus) would eventually result in decreased eutrophication under present climate conditions. However, climate change scenarios indicate that increased precipitation and runoff in combination with changes in water column stratification may offset the effects of reduced nutrient input (Meier et al., 2012).
Fishing pressure has the greatest potential for quick adaptation to changes in ecosystem state due to its short response time. The internationally managed commercial fisheries are subject to annual management decisions, while there is less regulation of recreational and small-scale coastal fisheries. Socioeconomic drivers have a strong influence on fishery management decisions, and long-term projections of future changes in fishing pressure are therefore highly uncertain (Lade et al., 2015).
Sensitivity of benthic–pelagic coupling to anthropogenic pressures
A wide range of benthic–pelagic coupling processes control the flow of inorganic nutrients and organic material in the Baltic Sea (Fig. 1c). In the following section, we discuss key physical and biological processes (e.g., diffusion, sedimentation, predation) in view of their current and projected responses to anthropogenic pressures (Fig. 1d). A subset of these processes has been measured in multiple Baltic Sea basins, which we summarize in Table 2.
| Depth | Bothnian Bay | Bothnian Sea | Gulf of Finland | Baltic Proper | Southern/Western Baltic | Data source | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shallow (68 m) | Deep (108 m) | Shallow (104 m) | Deep (125 m) | Shallow (47 m) | Deep (70 m) | Shallow (63 m) | Deep (81 m) | Shallow (47 m) | Deep (90 m) | ||
| Station Name | CVI | BO3 | F18 | SR5 | GOF2 | GOF6 | EGB1 | LF2 | BY2 | BY5 | |
| Inorganic nutrient fluxes | |||||||||||
| NO3- (μmol m−2 day−1) | 151 ± 70 | 217 ± 69 | −43 ± 141 | −205 ± 83 | −626 ± 229 | −1125 ± 99 | 111 ± 56 | 26 ± 3 | 200 ± 34 | −811 ± 290 | Norkko et al. (2015) |
| NO2- (μmol m−2 day−1) | 1 ± 10 | 8 ± 41 | −12 ± 20 | −14 ± 32 | −16 ± 5 | 54 ± 21 | −6 ± 17 | −7 ± 6 | 9 ± 17 | −1 ± 26 | Norkko et al. (2015) |
| NH4+ (μmol m−2 day−1) | 32 ± 18 | −10 ± 39 | 195 ± 87 | −68 ± 87 | 890 ± 916 | 4554 ± 881 | 133 ± 70 | 3005 ± 1130 | −12 ± 153 | 1522 ± 514 | Norkko et al. (2015) |
| PO43- (μmol m−2 day−1) | 2 ± 24 | 33 ± 10 | 124 ± 113 | 38 ± 16 | 297 ± 395 | 2795 ± 1066 | −263 ± 58 | 457 ± 236 | 38 ± 25 | 361 ± 220 | Norkko et al. (2015) |
| Bioturbation depth (aRPD, cm) | 9.6 | 9.3 | 13.1 | 10.9 | 7.5 | 0.0 | 7.3 | 0.0 | 8.5 | 1.1 | Norkko et al. (2015) |
| Organic material fluxes | |||||||||||
| Pigments top 1 cm of sediment (μg cm−3) | |||||||||||
| Total pigments | 0.97 ± 0.16 | 7.84 ± 5.26 | 22.4 ± 6.31 | 9.55 ± 4.06 | 5.98 ± 3.06 | Josefson et al. (2012) | |||||
| Chlorophyll a | 0.04 ± 0.02 | 3.41 ± 3.86 | 8.92 ± 3.49 | 2.52 ± 1.77 | 0.81 ± 0.38 | Josefson et al. (2012) | |||||
| Sediment accumulation rate (g dry wt m−2 yr−1) | 290.00 | 910.00 | 5935.00 | 1160.00 | 970.00 | 1261.00 | NA | 680.00 | 400.00 | NA | Josefson et al. (2012) |
| Sedimentation of organic matter (g C m−2 yr−1) | 20* | NA | NA | 198.4† | 9.4–34‡ | 38§ | NA | 6.13¶–50.1** | 50–65†† | NA |
aElmgren (1984),† Lehtonen & Andersin (1998), §,¶Heiskanen & Tallberg (1999), **Gustafsson et al. (2013), ††Smetacek (1980) |
| % benthos in diet, 20–30 cm cod‡‡ | NA | NA | NA | NA | NA | NA | 80–95 (except 45 in 1976–79)¶¶ | 60–95*** | 80.80 | NA | ¶¶Uzars (1994), *** Weber & Damm (1991) |
| % benthos in diet, >30 cm cod‡‡ | NA | NA | NA | NA | NA | NA | 50–95¶¶ | 10–65*** | 25.20 | NA | ¶¶Uzars (1994), ***Weber & Damm (1991) |
| % benthos in herring diet§§ | NA | NA | NA | NA | NA | NA | ~5 benthos, 30–55 mysids | NA | NA | NA | Tomczak et al. (2012) |
- *Estimated from annual primary production assuming export efficiency (e-ratio). Depth not specified; †Sediment trap at 80 m (max depth 125 m); ‡Sediment trap at 15 m (max depth 38 m) and sediment trap at 20 m (max depth 36 m); §Sediment trap at 20 m (max depth 50 m); ¶Sediment trap at 140 m (max depth c. 200 m); **Sediment trap at 40 m (max depth 459 m); ††Sediment trap at 15 or 18 m (max depth 20 m); ‡‡May not reflect current pelagic prey availability, size class >30 cm may include sizes consuming large quantities of benthic prey and the largest individuals that eat primarily fish; §§Annual average, not depth specific, modeling result.
Inorganic nutrient exchange
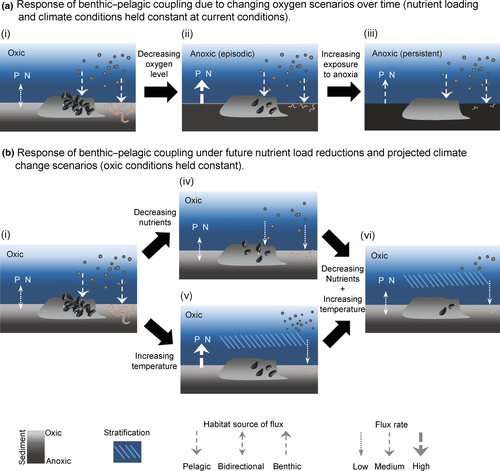
Oxygen is the overriding environmental regulator of inorganic nutrient fluxes across the sediment–water interface (Conley et al., 2009; Carstensen et al., 2014; Norkko et al., 2015) because it determines the extent to which diffusion and bioturbation govern these fluxes. Oxygen also determines flux rates and directionality while responding to climate and nutrient conditions (Fig. 2a). The focus here is on fluxes of nitrogen and phosphorus, elements that are of particular importance for biological production in aquatic ecosystems (e.g., Baltic Sea estimates of flux rates in Table 2).
Physical processes
Diffusive exchange
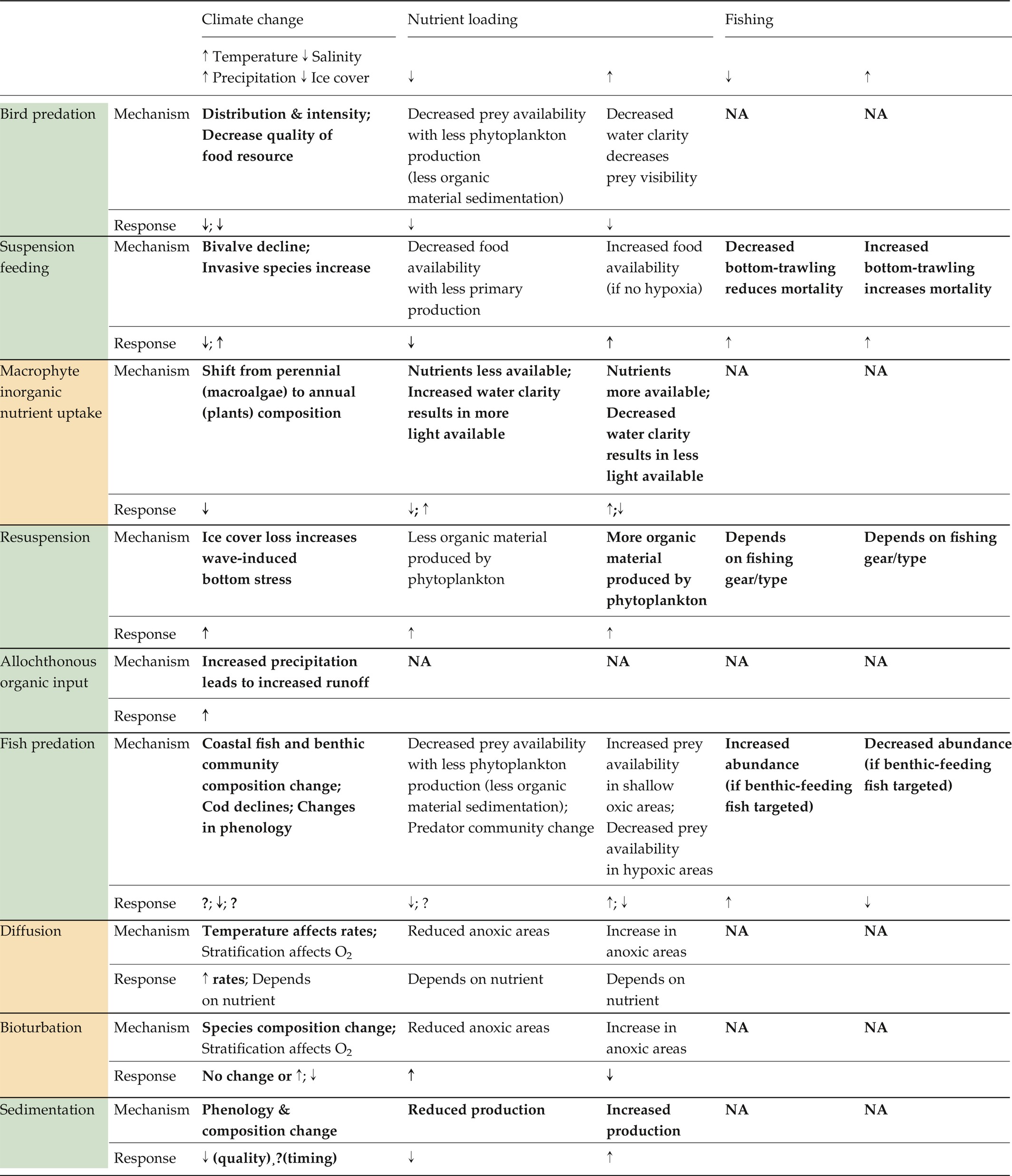
The direction of nutrient fluxes varies with oxygen conditions, from a balanced nitrogen and phosphorus exchange in oxic conditions, to a slow sedimentary efflux during permanent anoxia (Fig. 2a). In the deep basins of the central Baltic Sea, exchange processes are dominated by slow, molecular diffusion (Figs 1c and 2a). These basins have been almost permanently anoxic since the 1990s, due to high external inputs of nutrients, increased sedimentation, and semipermanent water stagnation (Hansson & Andersson, 2014; Vahtera et al., 2007; Fig. 2a). The projected strengthening of stratification, due to climate change, will decrease mixing and increase the extent of anoxia in deep waters of the Baltic Sea (Meier et al., 2011; Table 3). This could expand the hypoxic/anoxic area to currently oxygenated benthic sediments triggering stronger fluxes of inorganic nutrients (especially phosphorus) from benthic habitats to the water column (Eilola et al., 2014; Fig. 2a; Table 3). Warmer temperatures could also exacerbate fluxes of inorganic nutrients as rates of organic material degradation processes (i.e., aerobic respiration and denitrification) are temperature sensitive (Bonaglia et al., 2014a; Table 3). If external nutrient load reductions are achieved according to the Baltic Sea Action Plan (HELCOM Ministerial Meeting, 2007), the resulting decrease in the areal extent of anoxia could lower the release of inorganic nitrogen and phosphorus from the sediment (e.g., reduce internal recycling, Bonaglia et al., 2013, 2014a; Viktorsson et al., 2013).
Biological processes
Bioturbation
Meio- and macrofauna inhabiting benthic habitats have a direct effect on inorganic nutrient fluxes between the sediment and the water column. In the oxygenated areas of the Baltic Sea (Carstensen et al., 2014), they enhance inorganic nutrient fluxes by advective fluid flow and bioturbation (Aller & Aller, 1992; Elmgren, 1978; Figs 1c and 2a). The presence of meiofauna can double nutrient fluxes while macrofauna can enhance nutrient fluxes by a factor of 2 to 10 because of enhanced physical exchange and physiological factors (Aller & Aller, 1992; Nascimento et al., 2012; Bonaglia et al., 2014b). Morphological traits of macrofauna, such as size, may influence the nutrient flux more than species richness, community composition, or abundance, as larger and older individuals have a disproportionately large effect on oxygen and nutrient fluxes (Norkko et al., 2013). Overall, the net direction of the inorganic nutrient flux due to bioturbation can vary substantially because of organism geometry, density, or bioturbation mode. For example, surface-mixing amphipods such as M. affinis stimulate denitrification rates (Karlson et al., 2005, 2007a) while deep-burrowing, bioirrigating polychaetes have minimal effect on this process (Kristensen et al., 2011; Bonaglia et al., 2013). In addition, bioturbation effects are not uniform across nutrients; for example, bioturbation by deep-burrowing polychaetes has been shown to strongly enhance sediment phosphorus retention (Norkko et al., 2012), while on the other hand increasing the fluxes of dissolved nitrogen to the water column (Bonaglia et al., 2013; Ekeroth et al., 2016).
Current and projected abiotic conditions of the Baltic Sea suggest an ongoing reduction of macrofaunal abundance due to more common hypoxic events in shallow coastal areas (Conley et al., 2011). Macrofaunal abundance decreases will consequently lower the enhancement effects of bioturbation on inorganic nutrient flux (Cederwall & Elmgren, 1990; Karlson et al., 2002; Villnäs et al., 2012; Fig. 2a). In addition, the importance of bioturbation also declines with decreasing salinity, mirroring the decline of native macrobenthic species abundance and diversity (Bonsdorff, 2006; Kautsky & Kautsky, 2000; Table 1). Projected decreased salinity and increased temperature, in conjunction with increased hypoxia, could further reduce native benthic fauna bioturbation capacity (Fig. 2b).
Invasive species can provide new functional traits to communities, and this may enhance the resilience of bioturbation capacity in the Baltic Sea. For example, the three invasive species of the polychaete genus Marenzelleria burrow and irrigate deeper than most native species and have a broad tolerance to salinity, oxygen, and even sulfidic conditions (Maximov et al., 2015). Since the mid-1980s, they have spread throughout the Baltic Sea to become a dominant member of the benthic macrofaunal community (Kauppi et al., 2015). Marenzelleria bioturbation can enhance phosphorus retention and ammonium regeneration in sediments (Norkko et al., 2012; Bonaglia et al., 2013). However, a recent mesocosm study suggests that these effects on inorganic nutrient fluxes are species specific and might be different for the three different Marenzelleria species (Renz & Forster, 2014).
Macrophyte inorganic nutrient uptake
Aquatic macrophytes, microphytobenthos, macroalgae, and their epibionts take up inorganic nutrients from the water column and are important in shallow coastal zones where light is sufficient to sustain benthic primary production (Fig. 1c). As the Baltic Sea becomes fresher, a transition from macroalgal-dominated coastal ecosystems toward nonvegetated areas or habitats dominated by vascular plants is expected (Kotta et al., 2014). The loss of perennial macroalgae (Kotta & Möller, 2014) would reduce the uptake of pelagic nutrients by benthic primary producers, resulting in increased phytoplankton production (Smith et al., 2006) and potentially pelagic fish yields (Kotta et al., 2004).
Organic material fluxes
Phytoplankton production fuels benthic secondary production through sedimentation of organic matter. In turn, the benthic organisms provide additional food sources for pelagic fish and birds, supporting and stabilizing pelagic dynamics (Rooney & McCann, 2012). The physical and biological processes transferring organic material between benthic and pelagic habitats have variable responses to changes in anthropogenic pressures and exhibit complex feedbacks among each other.
Physical-dominated processes
Sedimentation
Phytoplankton production is the largest source of particulate organic material sinking to the benthos at the basin scale (Fig. 1c; Table 2). Sedimentation is temporally and spatially variable and regulated by climate and nutrient conditions, which can be seen in the decrease of phytoplankton production from south to north (Table 2; Bonsdorff & Pearson, 1999) due to the shorter productive season and lower inorganic nutrient concentrations at higher latitudes. Reduction in nutrient loading would in the long run decrease the pelagic to benthic organic material flux by reducing phytoplankton production and sedimentation in offshore regions (Fig. 2b; Table 3). In these regions, benthic primary production is limited because the bottom depth is greater than the euphotic zone. Thus, the benthos is dependent on the sinking organic material and offshore benthic productivity may eventually be significantly affected by decreased nutrient loads.
The projections of further climate-induced winter ice cover declines and increased temperatures (BACC II Author Team, 2015) may alter the timing, duration, and quantity of organic material transfer to the benthos in all basins of the Baltic Sea (Table 3). For example, the initiation of the spring bloom has shifted earlier in the central Baltic Sea during the past 20 years (Kahru et al., 2015) and models have projected earlier blooms in the future due to decreased ice cover (Eilola et al., 2013). High-latitude regions of the Baltic Sea (Bothnian Sea, Bothnian Bay), however, will continue to be light-limited by long winters and higher concentrations of humic substances than other Baltic Sea basins. The consequences of these changes in phytoplankton phenology for benthic communities are largely unknown as they are also affected by the ability of both pelagic (zooplankton) and benthic consumers to adjust to such shifts.
There is clear spatial and temporal variation of organic material sedimentation already affecting resource availability to benthic consumers. This is due to strong seasonality, spatial variation in seasonality, smaller-scale oceanographic processes, and a coastal-to-offshore gradient in phytoplankton productivity. Across the Baltic Sea, the spring bloom accounts for the largest flux of matter from the pelagic habitat to benthic communities. The late summer blooms (and autumn in the south-central regions) provide a secondary input to the benthos, albeit less regular in occurrence and magnitude than during spring (Gustafsson et al., 2013) and of lower nutritional quality (Nascimento et al., 2009). Phytoplankton production also decreases from the open sea to the coast, as water transparency decreases with increasing sediment resuspension and dissolved and particulate organic material input from land (Olafsson & Elmgren, 1997; Tallberg & Heiskanen, 1998; Gustafsson et al., 2013). Substantial recovery from eutrophication would not only reduce phytoplankton production (as mentioned above) but also increase water transparency, favoring benthic primary production in shallow regions. In turn, community dominance could change from phytoplankton to macroalgae and seagrasses (Riemann et al., 2015) as well as benthic microalgae production and this benthic production would result in feedbacks to inorganic nutrient cycling (see Macrophyte inorganic nutrient uptake).
Resuspension
Resuspension of sedimentary material commonly occurs in the Baltic Sea due to the shallow average depth of the water column (Fig. 1c). Sinking particles due to resuspension account for >50% of the total sinking material in shallow (<50 m) coastal areas (Blomqvist & Larsson, 1994; Heiskanen, 1998), and this source often dominates the diet of benthic suspension feeders, as opposed to the traditional view that phytoplankton are their primary food source (Lauringson et al., 2014). In deeper waters, mixing is prevented by the permanent halocline at ~70 m depth and resuspension is low. Despite no active resuspension in deep water, deep benthic habitats receive resuspended materials through advection offshore of organic material resuspended in shallow waters (c.f. Eilola et al., 2013).
Resuspension is sensitive to both projected climate change and the use of bottom trawl gear (Table 3). For example, reduced ice cover during spring has already increased wave-induced bottom stress (BACC II Author Team, 2015) and a potential consequence is increased resuspension of organic material during spring (Eilola et al., 2013). Furthermore, bottom-trawling increases resuspension and can cause long-term impacts on nutrient fluxes (Olsgard et al., 2008), as well as on benthic fauna abundance, biomass, and community structure (Rumohr & Krost, 1991; Hinz et al., 2009). Trawling has a large spatial footprint (Korpinen et al., 2013), but any fishing-related effects in the future on resuspension will depend on future gear use (e.g., extent of trawl use) and fishing intensity.
Allochthonous organic material inputs
Organic material from terrestrial sources and riverine primary and secondary production contributes substantially to organic material deposition in the nearshore environment (Tallberg & Heiskanen, 1998; Malmqvist et al., 2001) (Fig. 1c). High levels of colored dissolved organic material can, however, also reduce phytoplankton production by decreasing light availability (Wikner & Andersson, 2012), thus dampening the autochthonous pelagic flux of high-quality organic material to the benthos. River flow and precipitation events control allochthonous inputs and lead to strong seasonal patterns, but these dynamics differ across the Baltic Sea region (Reader et al., 2014).
The extent of the benthos response to either increased allochthonous organic matter inputs or to indirect effects of dampened phytoplankton production due to projected increase in precipitation and earlier peak river discharge (BACC II Author Team, 2015) is still unclear. While increased freshwater runoff may increase the deposition of organic material in nearshore and coastal sediments (Table 3), this would lower the quality of the food available to the marine food web because terrestrial organic material typically has lower nitrogen content compared to autochthonous sources (Grebmeier et al., 1988). In salinity-transition zones, increased flocculation of dissolved organic material occurring with increased freshwater runoff would also introduce low-nitrogen-content organic material due to the higher carbon:nitrogen ratio in dissolved vs. particulate organic material (c.f. Asmala et al., 2013; Tamelander & Heiskanen, 2004).
Biological processes
A wide array of biological processes that are inherently linked to species-specific life-history traits and phenology contribute to shaping the exchange between benthic and pelagic habitats (Baustian et al., 2014). These processes are spatially and temporally highly variable and sensitive to human pressures. The Baltic Sea is a relatively species-poor ecosystem (Elmgren & Hill, 1997; Bonsdorff & Pearson, 1999; Villnäs & Norkko, 2011), but its food webs are sufficiently complex to highlight the challenge of evaluating the sensitivity of biologically mediated benthic–pelagic coupling to anthropogenic pressures (Yletyinen et al., 2016). Disentangling the relative effects of different pressures on species-mediated energy transfer between benthic and pelagic habitats is challenging – especially due to limited understanding of the factors regulating the timing and magnitude of trophic interactions. We focus on two trophic processes (suspension feeding and predation) to describe our current understanding of their role in benthic–pelagic coupling. While processes such as diel migrations and reproductive (life-cycle) fluxes (Marcus & Boero, 1998; Baustian et al., 2014) may result in large exchange of organic material, these processes are poorly quantified from the perspective of energy transfer between Baltic Sea benthic and pelagic habitats (but see Katajisto et al., 1998) and are not discussed here.
Suspension feeding
Suspension feeding by benthic macrofauna in the Baltic Sea, especially by bivalves (Elmgren, 1984), transfers organic materials from the pelagic zone to the benthos. In addition to secondary production (somatic growth), the deposition of feces from benthic consumers constitutes a significant organic input to the sediment which is locally important, particularly in areas shallower than 30 m dominated by blue mussels (Kautsky & Evans, 1987; Fig. 1c).
The filtering function of benthic macrofauna decreases sharply when moving toward the less saline northern basins (Elmgren, 1984). This results from the decreasing diversity and biomass of suspension feeders with decreasing salinity in both soft-bottom (Bonsdorff & Pearson, 1999) and hard-bottom areas (blue mussels, Westerbom et al., 2008). Projected changes in nutrients and salinity could have negative effects on the distribution and productivity of mussels (Kotta et al., 2015) and diminish their role in benthic–pelagic exchange (Fig. 2b; Table 3). For example, decreased nutrient loading by humans would lessen the sedimentation of organic material and reduce mussel stock growth (Riemann et al., 2015; Fig. 2b). The persistence of suspension-feeding traits in benthic communities may be supported by invasive species despite decreasing salinity (Table 3). Very dense populations of the invasive mussel Dreissena polymorpha now occur in the low-saline regions of the Baltic Sea and perform the same suspension-feeding function as marine-origin bivalves (Lauringson et al., 2007). Alternatively, the loss of filter-feeding functions from benthic communities may also occur due to invasive species. The predatory round goby (Negobius melanostomus), for example, can decimate local populations of suspension-feeding mussels (Ojaveer & Kotta, 2015).
Fish predation
Most fish species in the Baltic Sea feed on benthic invertebrates during at least part of their life cycle (Casini et al., 2004; Hüssy et al., 1997; Snickars et al., 2015; see Table 2 for cod and herring), yet the patterns and relative importance of benthic–pelagic coupling by fish predation are often poorly understood or quantified. This is because predation strength depends on population abundances, which vary considerably over time and space (e.g., stickleback, Bergström et al., 2015; herring, Casini et al., 2011), and the spatial and temporal dynamics of predation also depend upon fish life histories (spawning and feeding migrations, ontogenic diet shifts). The relative importance of pelagic and benthic prey sources will also depend on prey availability, and therefore, changes in prey composition and biomass may alter trophic coupling pathways.
Coastal benthic invertebrate and fish communities in the Baltic Sea have already experienced substantial changes in species composition, abundance, and biomass since the early 1970s (Olsson et al., 2013; Weigel et al., 2015), increasingly due to climate impacts (Snickars et al., 2015). There has been a decrease in coastal abundances of fish species of marine origin that prefer colder waters (i.e., herring, cod and sculpins), and an increase in freshwater species and those favored by warmer waters (i.e., perch and cyprinid fishes, Olsson et al., 2012), with concurrent changes in their benthic invertebrate prey (Olsson et al., 2013; Weigel et al., 2015). With increased warming and decreasing salinity of the Baltic Sea, the future coastal fish communities are expected to mainly be comprised of benthic-feeding fish species of freshwater origin. Despite the changing composition of invertebrate macrozoobenthos and fish communities, it is unknown whether this will also alter the magnitude of predation on the benthos. Future oxygen conditions will also govern predator–prey relationships, and hypoxic vs. anoxic conditions could have different effects. Hypoxia is more likely to result in species composition shifts in the benthic community while anoxia results in dead zones with no prey (Karlson et al., 2002; Villnäs et al., 2012).
The Eastern Baltic cod, a commercially important fish species, preys mainly on benthic invertebrates during juvenile life stages (Hüssy et al., 1997; Table 2) and pelagic fish prey as adults in addition to larger benthic invertebrates. Cod populations, and therefore their predation pressure on the benthos, are sensitive to both climate and fisheries management. Under reduced salinity and continued spread of hypoxic and anoxic waters, some model projections show continued decline of the Eastern Baltic cod stock despite reductions in fishing mortality (Lindegren et al., 2010; Gårdmark et al., 2013). Alternatively, under favorable environmental conditions, the cod population size may increase substantially if management decisions and the actual exploitation adhere to current fishery management plans (Niiranen et al., 2013). However, scenario projections vary greatly across different models depending on which species’ interactions each model accounts for (Gårdmark et al., 2013). Modeling studies demonstrate the importance of ontogenetic shifts from benthic to pelagic predation by cod for feedbacks between the structure and dynamics of fish communities and their prey (van Leeuwen et al., 2013, 2014), thereby determining the extent of ontogenetic benthic–pelagic coupling. These feedbacks increase the difficulty of quantifying current and future benthic–pelagic coupling through cod predation.
The consequences of changing cod predation for benthic–pelagic coupling will vary spatially. In the southwestern Baltic Sea, greater taxonomical and functional diversity in the benthos and fish community (Törnroos et al., 2015; Pécuchet et al., 2016) may uphold benthic–pelagic coupling despite reduced cod predation due to compensatory increases in functionally similar gadoid and flatfish species (Lindegren et al., 2012; Sparrevohn et al., 2013). In contrast, the less saline southeastern Baltic Sea has fewer benthic-feeding fish species, mainly flounder and gobies besides cod (Ojaveer & Kotta, 2015), which may not compensate for decreased cod predation.
Bird predation
The Baltic Sea is a favored habitat for benthivorous sea ducks (Skov et al., 2011), which consume large quantities of bivalves (Nilsson, 1980; Stempniewicz & Meissner, 1999). Bivalves can also be an important prey for populations of generalist bird species (e.g., gulls Garthe & Scherp, 2003). Bird predation on benthos takes place mainly in the shallow and transition zones (Bonsdorff et al., 1990), as greater depth limits the accessibility of benthic resources to diving birds (down to 25 m, Skov et al., 2011; Fig. 1c). Predation magnitude is mainly determined by bird abundance, so trends in breeding success and survival may affect the strength of coupling over time while seabird migration patterns and phenology lead to strong seasonal variation. Some benthivorous species remain in the Baltic Sea year-round, but migrate within the region, while others only overwinter there. The spatial dynamics and intensity of coupling varies during the overwintering period, as birds gather in contracted areas during arrival/departure but then disperse throughout shallow coastal waters and offshore banks (Skov et al., 2011).
Climate change is expected to influence waterbird phenology and distribution (Guillemain et al., 2013; Lehikoinen et al., 2013), increasing the duration and intensity of benthic predation in the northeastern Baltic Sea while decreasing their presence in the southern and western part of the area (Table 3). Decreasing salinity is likely to shift the occurrence, size, and densities of mussel beds (as discussed above, Kotta et al., 2015; Fig. 2b) in turn affecting the availability and quality of benthic prey and bird consumers. In addition, exposure to increasing temperatures can reduce the meat-to-shell ratio in overwintering mussels which decreases their quality as food for birds (Waldeck & Larsson, 2013). Bottom-up factors may also limit bird predation on the benthos as a negative response of mussel growth to decreased nutrient loading reduces bird prey availability (Laursen & Møller, 2014).
Summary and outlook
Our review highlights the importance of an integrated, whole-system perspective for understanding how estuarine and coastal ecosystems will respond to anthropogenic drivers through their effects on benthic–pelagic coupling. We identify key processes that define the type and level of interdependency between benthic and pelagic habitats in coastal and estuarine environments. Based on our Baltic Sea example, the most significant processes can be divided into three groups: nutrient release from sediments, sedimentation, and biological processes, which include pelagic consumer predation on benthic fauna and the response of community function to changes in composition. These processes all respond to widespread human impacts on the environment (climate change, nutrient loading, and fishing) and are not independent of one another. Historical and ongoing changes of the Baltic Sea ecosystem contribute to our general understanding of many of the world's coastal and estuarine ecosystems facing increasing pressures from these impacts (Cloern et al., 2016). Our review focused on the most likely effects on benthic–pelagic coupling processes from projected anthropogenic pressures, and below we highlight the role of oxygen, interactive effects of climate change and nutrient load reductions, and key uncertainties for biological processes. We then provide our recommendations on how we can improve our quantification of benthic–pelagic coupling processes in any ecosystem such that the feedbacks among processes can be better understood.
Oxygen concentration is a main driver of inorganic nutrient and organic material exchange between benthic and pelagic habitats particularly affecting nutrient release and biological communities (Table 3). The extent of low-oxygen areas in the Baltic Sea is controlled by water exchange, climate, and eutrophication. Oxygen directly regulates the flux of inorganic nutrients and the potential for biological activity to contribute to bidirectional inorganic nutrient fluxes (Norkko et al., 2015). There is great uncertainty related to the nature and magnitude of inorganic nutrient cycling in the future due to the complexity of internal feedbacks that may contribute to maintaining the Baltic Sea in a state of hypoxia/anoxia, despite major nutrient load reduction. Oxygen availability also governs the spatial and temporal dynamics of the biological interactions that lead to organic material exchange. During hypoxia or anoxia, the flow of organic material from the benthos to pelagic consumers decreases. Given the widespread increase in reports of hypoxia in coastal ecosystems since the 1960s (Diaz & Rosenberg, 2008; Conley et al., 2011), changing oxygen conditions will affect benthic–pelagic coupling globally.
Climate change impacts, in combination with management actions to reduce nutrient loading, suggest that organic fluxes from pelagic primary producers to benthic habitats (sedimentation) will decrease in the future due to shifts in phytoplankton composition, phenology, and physiology. This combined response may also be expected in other systems with similar management goals to reduce eutrophication. Although organic matter sedimentation is likely to decline, the transfer of this organic matter between habitats through biological pathways remains uncertain in the Baltic Sea and responses are likely system specific, based upon the unique properties of pelagic and benthic communities. The interdependency of these processes results in a large degree of uncertainty in the ultimate, systemwide effects. Moreover, sinking of particulate material is rarely covered by monitoring programs and improving this knowledge base will improve our ability to draw conclusions on its response to environmental change.
Biologically mediated couplings (bioturbation, suspension feeding, and predation) respond to the interactive effects of anthropogenic pressures acting through multiple pathways (Table 3), and their sensitivity depends on the functional traits in the community. The continued increase in species invasions globally (Hulme, 2009; Walther et al., 2009) will influence the biological processes of habitat coupling in multiple ways sustaining, increasing, or reducing current coupling (e.g., Norkko et al., 2012) as well as potentially introducing new coupling pathways (e.g., as seen in San Francisco Bay, Cloern & Jassby, 2012). Overall, biological processes coupling habitats have greater unpredictability in their responses and are more difficult to quantify than other processes.
Recommendations
An important step forward is the quantification of inorganic nutrients and organic material exchange between the two habitats, which will improve our understanding and the predictability of these processes. Quantifying these fluxes and their sensitivity to anthropogenic pressures at different spatial and temporal scales (e.g., Fig. 2) requires the following: (1) coherent spatiotemporal measurements of rates across ecosystem components; (2) experimental studies that explicitly evaluate the benthic–pelagic coupling to multiple pressures and linkages among processes; and (3) ecosystem models incorporating benthic–pelagic coupling processes.
Coherent spatiotemporal measurements
A coordinated and comprehensive monitoring of ecosystems across benthic and pelagic habitats is needed to fill our knowledge gaps. The temporal and spatial scatter of observations, methodological differences between studies, and regional bias of observations (e.g., specific species or habitats) complicate the assessment of specific fluxes or comparisons among processes. Strong seasonal dynamics likely shape many benthic–pelagic coupling processes, but, overall, these dynamics are poorly captured by current monitoring. First, measuring processes in a common currency using a standardized methodology (sampling frequencies, incubation periods) is essential. Second, depending on the process under consideration, there are often observations of either biomass or rates but both are necessary for a more integrative understanding of benthic–pelagic coupling in ecosystems. For example, sedimentation rates of organic material are measured (Table 2) but benthic biomass, not secondary production rates, is typically monitored. Improving our observational extent and consistency will allow us to track the relative responses of the coupling processes to anthropogenic stress and evaluate ecosystem change.
Experimental studies
In addition, experimental studies specifically targeting the interactive effects of various pressures on benthic–pelagic processes would enhance our mechanistic understanding. For example, building upon experiments that quantify species density (Karlson et al., 2007b) or functional group (Michaud et al., 2006; Bonaglia et al., 2014b) effects on sediment–water solute exchange and carbon mineralization would help to evaluate the consequences of projected changes in temperature and oxygen. While mechanistic laboratory studies are important for exploring specific processes, the emphasis should be put on resolving our real-world understanding of how particular processes and pressures may be modulated by environmental drivers. Hence, embedding experimental work along environmental gradients may be particularly powerful for resolving the context dependency of patterns in benthic–pelagic coupling (Snelgrove et al., 2014; Norkko et al., 2015). Designing experiments to be valid at the seascape level would also ensure that results are applicable to ecosystem modeling efforts.
Ecosystem models
Ecosystem models are needed to explore the sensitivity of ecosystem structure and function to projected future anthropogenic pressures. Models allow the exploration of complex feedback loops between biological and physical processes that are challenging to measure (e.g., as described in Fig. 2), as well as the sensitivity of benthic–pelagic coupling processes to synergistic changes in anthropogenic pressures. The development of ecosystem models including hydrology, biogeochemical cycles, and some components of biological system (e.g., phytoplankton groups) is highly advanced for the Baltic Sea (e.g., BALTSEM model, Savchuck et al., 2012). The BALTSEM model already provides inputs to benthic trait models for assessing eutrophication effects (Timmermann et al., 2012) and to food web models (e.g., Niiranen et al., 2012). Integrating feedbacks from biological responses to the abiotic dynamics and vice versa in these types of models would allow for an assessment that ranks the most important feedbacks and sensitivities, providing grounds to evaluate the consequences of multiple pressures throughout complex ecosystems now and in the future. The parameterization and validation of these coupled models require both the coherent spatiotemporal measurements and experimental approaches discussed above.
Outlook
Common management goals for many of the world's coastal–estuarine ecosystems are to improve their ecological status and to protect or enhance their provision of ecosystem services. However, management advice and ecological targets are often based upon the current compartmentalization of benthic and pelagic habitats. Management indicators, such as used for the European Water Framework Directive (WFD; Directive 2000/60/EC) and Marine Strategy Framework Directive (MSFD; EU Directive 2008/56/EC), often describe the status of pelagic or benthic habitats separately, and there are few attempts to combine indicators across habitats (Dimitriou et al., 2015) despite this being the overarching goal in some of these directives (e.g., the MSFD).
Human activities alter important benthic–pelagic linkages and disrupt the flow of ecosystem services in coastal and estuarine ecosystems. In many coastal–estuarine systems, eutrophication and climate change continue to affect the physical and biological processes that cycle nutrients between benthic and pelagic habitats. Simultaneously, food web dynamics are responsive to direct physical habitat changes, predator–prey feedbacks, and fishing. Consequently, understanding the interdependency between benthic and pelagic communities in specific ecosystems, such as the Baltic Sea, can be instrumental for projecting their future trajectories, status, and contribution to ecosystem services. To maintain the function of coastal and estuarine ecosystems and to safeguard the services they deliver under future anthropogenic change, we need to ensure that the inorganic and organic exchange between pelagic and benthic habitats is understood, monitored, modeled, and included in management frameworks.
Acknowledgements
This synthesis originated with a Benthic–Pelagic Coupling workshop held in Stockholm in May 2015, and we thank those attendees for their initial contributions: R. Elmgren, O. Hjerne, M. Viitasalo, L. Viktorsson, and J. Warzocha. We also thank R. Elmgren for thoughtful and detailed comments on the manuscript. Stockholm University's Baltic Ecosystem-Based Management (BEAM) program provided funding for J.R. Griffiths and T. Blenckner as well as for the workshops. Research presented in this article contributes to the BONUS BIO-C3, COCOA, Baltic-App, and INSPIRE projects and was supported by BONUS (Art 185), funded jointly by the EU, the Academy of Finland, the Estonian Research Council, and the Swedish Research Council FORMAS. It also contributes to the Nordforsk-funded project Green Growth Based on Marine Resources: Ecological and SocioEconomic Constraints (GreenMAR) and to the Nordic Centre for Research on Marine Ecosystems and Resources under Climate Change (NorMER). EB and MCN are funded by the Åbo Akademi University Endowment.




