Urban ponds as an aquatic biodiversity resource in modified landscapes
Abstract
Urbanization is a global process contributing to the loss and fragmentation of natural habitats. Many studies have focused on the biological response of terrestrial taxa and habitats to urbanization. However, little is known regarding the consequences of urbanization on freshwater habitats, especially small lentic systems. In this study, we examined aquatic macro-invertebrate diversity (family and species level) and variation in community composition between 240 urban and 782 nonurban ponds distributed across the United Kingdom. Contrary to predictions, urban ponds supported similar numbers of invertebrate species and families compared to nonurban ponds. Similar gamma diversity was found between the two groups at both family and species taxonomic levels. The biological communities of urban ponds were markedly different to those of nonurban ponds, and the variability in urban pond community composition was greater than that in nonurban ponds, contrary to previous work showing homogenization of communities in urban areas. Positive spatial autocorrelation was recorded for urban and nonurban ponds at 0–50 km (distance between pond study sites) and negative spatial autocorrelation was observed at 100–150 km and was stronger in urban ponds in both cases. Ponds do not follow the same ecological patterns as terrestrial and lotic habitats (reduced taxonomic richness) in urban environments; in contrast, they support high taxonomic richness and contribute significantly to regional faunal diversity. Individual cities are complex structural mosaics which evolve over long periods of time and are managed in diverse ways. This facilitates the development of a wide range of environmental conditions and habitat niches in urban ponds which can promote greater heterogeneity between pond communities at larger scales. Ponds provide an opportunity for managers and environmental regulators to conserve and enhance freshwater biodiversity in urbanized landscapes whilst also facilitating key ecosystem services including storm water storage and water treatment.
Introduction
Land-use change has been predicted to be the greatest driver of biodiversity change in the 21st century (Sala et al., 2000). The conversion of natural landscapes to urban areas represents a common land-use transition and is a significant process contributing to the loss of freshwater habitats and the degradation of those that remain, placing considerable pressure on native flora and fauna (McKinney, 2002). The fragmentation of natural habitats and development of uniform landscapes in urban areas has been demonstrated to cause the biotic homogenization of flora and fauna through the decline and exclusion of native species by land-use modification (and associated anthropogenic pressures) and the establishment and spread of non-native invasive species through habitat disturbance and human introductions (McKinney, 2006; Grimm et al., 2008; Shochat et al., 2010). Previous research has demonstrated that high levels of urbanization reduce macro-invertebrate and macrophyte species richness (e.g. in urban streams, Roy et al., 2003; Walsh et al., 2005) to the point where urban environments are viewed as ‘ecological deserts’, although at moderate levels of urbanization greater diversity has been recorded for plant communities (McKinney, 2008). In recent decades, significant improvements to the physical, chemical and ecological quality of urban freshwater ecosystems have been made in economically developed nations reflecting the decline in industrial developments, improved waste water treatment, and more effective environmental legislation (e.g. The Water Framework Directive in Europe; EC, 2000 and The Water Act 2007 in Australia; Commonwealth of Australia, 2007). Although there have been significant improvements to the quality of many urban aquatic habitats, the number of water bodies in urban areas has declined over the past century (Wood et al., 2003; Vaughan & Ormerod, 2012; Thornhill, 2013). Commercial and residential developments are expanding in urban areas to keep pace with population growth (66% of the global population are predicted to live in urban areas by 2050; United Nations, 2014) at the expense of urban green spaces (Dallimer et al., 2011). Such losses of green/blue space are likely to place significant pressure on remaining urban freshwaters to support native flora and fauna and may lead to substantial shifts in the diversity and composition of species in urban areas (Fitzhugh & Richter, 2004; McKinney, 2006).
Ponds are ubiquitous habitat features in both urban and nonurban landscapes. In nonurban landscapes, ponds have been demonstrated to support greater regional diversity of flora and fauna compared to rivers and lakes (Davies et al., 2008). This biodiversity value may result from spatial and temporal diversity in pond environmental variables (Hassall et al., 2011, 2012), which create a highly heterogeneous ‘pondscape’ of habitats that provide a diverse array of ecological niches. Ponds have been acknowledged as providing important network connectivity across landscapes, acting as ‘stepping stones’ that facilitate dispersal (Pereira et al., 2011). Within urban areas, ponds provide a diverse array of habitats and occur in a wide range of forms including garden ponds (Hill & Wood, 2014), sustainable urban drainage systems (SUDS; Briers, 2014; Hassall & Anderson, 2015), industrial, ornamental and park ponds (Gledhill et al., 2008; Hill et al., 2015), recreation and angling ponds (Wood et al., 2001), and nature reserve ponds (Hassall, 2014) which typically display heterogeneous physicochemical conditions (Hill et al., 2015). Urban ponds are almost always of anthropogenic origin and often demonstrate different environmental characteristics to nonurban (seminatural/agricultural) ponds; urban ponds commonly have concrete margins, a synthetic base, reduced vegetation cover, lower connectivity to other waterbodies and are subject to run off from residential and industrial developments which can greatly increase the concentration of contaminants (Hassall, 2014). Whilst the definition of a ‘pond’ vs. a ‘lake’ is still very much debated, a general rule is that ponds are standing water bodies <2 ha in size. Urban waterbodies are frequently much smaller (closer to 1–5 m2 for garden ponds) but show a large variation in size (>10 ha for park lakes). For a discussion of the definitions of ponds and lakes, we refer the reader elsewhere (Hassall, 2014; Appendix 1 in Biggs et al., 2005). Despite the considerable anthropogenic pressures on urban ponds, recent studies have demonstrated that ponds located within an urban matrix can provide important habitats for a wide range of taxa including macro-invertebrates (Hassall, 2014; Goertzen & Suhling, 2015; Hill et al., 2015) and amphibians (Hamer et al., 2012). In addition, many support comparable diversity to surrounding nonurban ponds (Hassall & Anderson, 2015) and also provide a wide range of ecosystems services in urban areas to offset the negative impacts of urbanization (Hassall, 2014). However, these patterns are inconsistent, and other studies have reported a lower diversity of macro-invertebrate and floral taxa in urban ponds reflecting the greater isolation of pond habitats (Hitchings & Beebee, 1997) and management practices designed for purposes other than biodiversity (e.g. emergent vegetation removal, Noble & Hassall, 2015).
Whilst there has been increasing research interest in the biodiversity and ecosystem services of urban ponds across Europe (Hassall, 2014; Jeanmougin et al., 2014; Goertzen & Suhling, 2015), the question remains as to whether urban ponds can provide similar levels of biodiversity to that recorded in ponds in the wider landscape. Few studies have compared urban pond faunal communities with nonurban pond communities (see Hassall & Anderson, 2015), and no known studies have examined urban pond macro-invertebrate diversity at a national scale. Furthermore, there are a series of ecological patterns within cities (e.g. reduced taxonomic diversity, biotic homogenization, increase in non-native and invasive taxa) that have been described in terrestrial systems (particularly birds, butterflies and plants: McKinney, 2008), but these have not been tested in aquatic ecosystems. This study provides a comparative analysis of environmental characteristics and macro-invertebrate communities contained within >1000 UK ponds, including ponds located in a number of cities and towns across the UK and nonurban ponds that cover a wide range of nonurban habitats including nature reserves, agricultural land (pasture and crop), meadows, woodland and other wetlands. We test the following hypotheses: (i) urban ponds support lower macro-invertebrate richness and diversity (family and species level) than nonurban ponds, as would be predicted from the greater anthropogenic stressors in urban areas; (ii) urban macro-invertebrate communities would be more homogeneous than nonurban communities at a family and species scale, due to the greater similarity of urban habitats as has been reported for terrestrial taxa; and (iii) urban pond communities demonstrate stronger spatial structuring at smaller scales than nonurban communities, through reduced connectivity, dispersal and gene flow.
Materials and methods
Data management
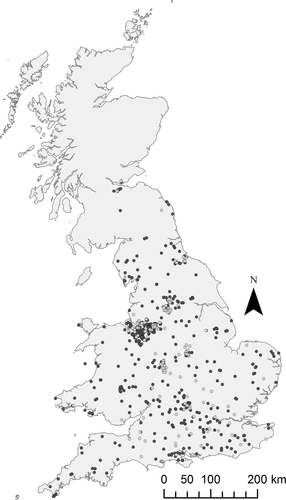
The United Kingdom covers a total area of 242 495 km2 and has a population of approximately 64.6 million inhabitants. Over 6.8% of the UK land mass is classified as urban and approximately 80% of the population resides in urban areas (defined as areas >20 ha containing >20 000 people, UKNEA, 2011). Aquatic macro-invertebrate community data from 230 urban and 607 nonurban ponds and environmental data from 240 urban ponds and 782 nonurban ponds in the United Kingdom were collated from 12 previous studies (Table 1). The spatial distribution of the studied urban and nonurban ponds is displayed in Fig. 1.
| Reference number | Geographical scale | Aquatic macro-invertebrate sampling methodology | Taxonomic resolution | Taxa included | Reference |
|---|---|---|---|---|---|
| 1 | UK wide n = 152 | Individual ponds sampled for 3 min in spring, summer and autumn using a sweep sample technique. Sampling time was divided between the mesohabitats recorded in each pond. | Species, except for Oligochaeta, Diptera and small bivalves | Aquatic macro-invertebrates (water mites, zooplankton and other micro-arthropods were not included) | Biggs et al., (1998) |
| 2 | Dunfermline, Fife, Scotland n = 14 | Individual ponds were sampled annually between 2007 and 2011 in the summer following the methods of the National Pond Survey. | Species, except for Oligochaeta, Ostracoda and Diptera | Aquatic macro-invertebrates | Briers, (2014) |
| 3 | Leicestershire, United Kingdom n = 41 | Individual ponds were sampled over spring, summer and autumn seasons. Sampling time was proportional to surface area, up to a maximum of three minutes. Sampling time designated to each pond was divided between the mesohabitats recorded. | Species, except for Diptera, Oligochaeta, Hydrachnidiae and Collembola | Aquatic macro-invertebrates (zooplankton and other micro-arthropods were not included) | Hill et al., (2015) |
| 4 | West Yorkshire, United Kingdom n = 36 | Individual ponds were sampled during the summer and autumn, following the guidelines of the National Pond Survey. In addition, soft benthic samples were taken using an Eckman Grab. | Species, except Ostracoda, Copepoda and Diptera | Aquatic macro-invertebrates | Wood et al., (2001) |
| 5 | Bradford, United Kingdom n = 21 | Individual ponds were sampled for 3 min in the summer. Sampling time was divided between the mesohabitats present. | Family level | Aquatic macro-invertebrates (presence of fish and amphibians noted) | Noble & Hassall, (2015) |
| 6 | Birmingham, United Kingdom n = 30 | Individual ponds were sampled for 3 min in the spring and summer, following the guidelines of the National Pond Survey. | Species, except Diptera, Sphaeriidae and Oligochaeta | Aquatic macro-invertebrates | Thornhill, (2013) |
| 7 | Halton, United Kingdom n = 37 | Individual ponds were sampled twice per year (summer and autumn) for 2 years. Samples were taken from all available mesohabitats using a standard pond net until no new species were recorded. | Species | Aquatic macro-invertebrates, Aquatic macrophytes, Amphibians | Gledhill et al., (2008) |
| 8 | North West England n = 425 | Samples were taken from all available mesohabitats using a standard pond net until no new species were recorded. Logs and debris was lifted to look for macro-invertebrates located beneath. | Species except Diptera, and Oligochaeta which were not examined. | Aquatic macro-invertebrates, Aquatic macrophytes, Amphibians | Pond life Project, (2000) |
| 9 | Leeds, United Kingdom n = 11 | Individual ponds were sampled for 3 min in the summer. Sampling time was divided between the mesohabitats present. | Family level | Aquatic macro-invertebrates | D. Moyers & C. Hassall unpub. |
| 10 | UK wide n = 169 | Individual ponds were sampled for 3 min in spring, summer and autumn using a sweep sample technique. Sampling time was divided between the mesohabitats recorded in each pond. | Species, except for Oligochaeta, Diptera and small bivalves | Aquatic macro-invertebrates (water mites, zooplankton and other micro-arthropods were not included) | FHT Realizing Our Potential Award data set unpub. |
| 11 | UK wide n = 76 | Individual ponds sampled for 3 min in spring, summer and autumn using a sweep sample technique. Sampling time was divided between the mesohabitats recorded in each pond. | Species, except for Oligochaeta, Diptera and small bivalves | Aquatic macro-invertebrates (water mites, zooplankton and other micro-arthropods were not included) | FHT Temporary Ponds data set unpub. |
| 12 | Leeds, United Kingdom n = 10 | Individual ponds were sampled for 3 min in the summer. Sampling time was divided between the mesohabitats present. | Family level | Aquatic macro-invertebrates | N. Barber & C. Hassall unpub. |
Data collection methodologies employed by the majority of contributing studies (Table 1) broadly followed the standardized guidelines of the National Pond Survey (Biggs et al., 1998) including a 3-min sweep sample divided between the mesohabitats present (studies 1, 2, 3, 4, 5, 6, 9, 10, 11 and 12; Table 1). The other studies also sampled for aquatic macro-invertebrate taxa in all available mesohabitats, but sampling was undertaken until no new species were recorded (studies 7 and 8). The majority of studies were sampled across two or three seasons (studies 1, 3, 4, 6, 7, 10 and 11; Table 1) although five studies were only sampled during the summer months (studies 2, 5, 8, 9 and 12; Table 1). Environmental data recorded from pond sites varied between studies, but always included a common core of variables that were used in the comparative analysis: pond area, pH, percentage coverage of emergent macrophytes, percentage pond shading and altitude. Ponds were categorized as urban or nonurban based on whether they were located within developed land-use areas (DLUAs) – a landscape designation used by the UK-based Ordnance Survey to delineate urban and nonurban sites. We provide a comparison between our binary categorization and two other measures of ‘urbanness’ (proportion of urban land use in a 1 km buffer and distance from urban land-use areas in the Appendix S1). We acknowledge that the definition of an urban pond is complex. Indeed, a previous attempt to define a typology of urban ponds concluded that these sites comprise a diverse array of different habitat types (Hassall, 2014). However, the intention with this study is to evaluate the aquatic biodiversity in urban areas and to establish whether those urban sites are deserving of protection, value and enhancement. Hence, rather than attempting to define the precise characteristics of an ‘urban pond’, we are focusing on the much more tractable issue of ‘ponds in urban areas’. Similarly, the definition of a ‘nonurban pond’ for our purposes simply includes ponds outside of urban areas. Our nonurban pond data set is concentrated in agricultural landscapes which in the United Kingdom are typically characterized by low tree cover and low surrounding botanical diversity, along with high inputs of nutrients and agricultural effluents. These ponds are likely to be subject to ‘benign neglect’ (i.e. limited management), but this will vary across the ponds in the study. Urban ponds in this study encompass a broad spectrum of urban areas, from their location in densely populated cities (e.g. Birmingham: population >1 million) to smaller towns (e.g. Loughborough: estimated population of 60 000). The urban ponds chosen for investigation included ponds in domestic gardens, industrial ponds (old mill ponds), ornamental ponds located in urban parks and drainage ponds (e.g. sustainable urban drainage systems/stormwater retention ponds; see Hassall, 2014). The issue of the representative nature of UK cities compared to cities elsewhere (in Europe or the wider world) is less clear for ponds, as there has been limited study of these habitats using standardized methods (see Hassall, 2014, for a discussion and a range of biodiversity studies). It is likely that the range of urbanized areas incorporated in our study covers the range of different urban landscapes that are found in European cities, from millennia-old cities with an evolving land-use pattern (e.g. London), to centuries-old industrial towns (e.g. Leeds, Manchester), to 20th century towns which have been designed and built de novo (e.g. Milton Keynes).
The faunal data set was converted into a presence–absence matrix to ensure data provided by the 12 constituent studies were comparable and that any sampling bias was reduced. Abundance data may yield additional insights into variation in biomass and evenness among ponds, and we might expect greater biomass and evenness in nonurban sites where stressors are reduced and nutrient supply is greater. However, our primary goal within this study was to investigate variation in taxonomic richness across the pond types. Two key methodological differences exist in the 12 studies. First, although most of the corresponding studies identified the majority of macro-invertebrate taxa to species level, each study also identified selected taxa (e.g. Diptera, Oligochaeta, Copepoda and Ostracoda) at higher taxonomic levels (Table 1). The influence of a higher taxonomic resolution of identification for aquatic macro-invertebrates has been examined, primarily within lotic habitats (Monk et al., 2012; Heino, 2014). However, identification of macro-invertebrate taxa at family level has been shown to be appropriate to examine alpha, beta and gamma diversity in lentic systems (Le Viol et al., 2009; Mueller et al., 2013; Hassall & Anderson, 2015; Vilmi et al., 2016) and is the resolution used by a range of environmental monitoring indices (e.g. biological monitoring working party [BMWP] and predictive system for multimetrics [PSYM] scores; Environment Agency and Ponds Conservation Trust, 2002) and legislation (e.g. The Water Framework Directive; EC, 2000) across Europe. However, to assess the sensitivity of results to taxonomic resolution, we performed all analyses at two taxonomic levels: first, to incorporate as many sites as possible and to ensure faunal data was comparable across all studies, aquatic macro-invertebrate data were reclassified to family level and analysis was undertaken at this higher taxonomic resolution. Second, statistical analysis was also undertaken on a subset of urban (207 ponds) and nonurban ponds (578 ponds) where species-level data were available.
The second methodological variation was in the amount of sampling effort applied to the sites: sampling effort was limited to 3 min in 10 of the studies (following standard UK sampling protocols), but two studies used exhaustive sampling until no more species were found. A preliminary analysis showed that, in fact, the sites sampled for 3 min found more taxa (average of 14.7 ± 0.4 SE families, n = 392 sites; average of 30.0 ± 0.9 species, n = 340) than sites sampled exhaustively (average of 13.6 ± 0.3 SE families, n = 518 sites; average of 26.8 ± 0.6 species, n = 518). However, this lower number of species in exhaustive samples is likely to result from those sites occurring in the north of England where the regional species pool may be smaller. As a result, we find no evidence of bias between the exhaustive and time-limited samples. Finally, to provide the strongest possible test of the biodiversity value of urban ponds, urban pond communities (at a family and species level) were compared to a subset of the nonurban ponds with degraded sites excluded (leaving n = 571 nonurban ponds with family level data and 542 with species-level data).
Statistical analysis
Differences in environmental characteristics (pond area, percentage coverage of emergent macrophytes, pH, percentage pond shading and altitude) and aquatic macro-invertebrate communities at a family and species level between urban and nonurban ponds were examined. All analyses were carried out in the r environment (R Development Core Team, 2013). Prior to statistical analysis, the data were screened to remove any missing values. Estimated gamma diversity was calculated using Chao2 estimator in the vegan package in r (Oksanen et al., 2016). Mann–Whitney U-tests were used to test for differences in alpha diversity (family and species richness) between urban and nonurban ponds. To account for the fact that there were different numbers of urban and nonurban sites, taxon accumulation curves were constructed by randomized resampling of sites without replacement using the specaccum function in vegan with 1000 permutations per sample size. From these curves, the mean number of families and species in each simulated group of sites and the standard error were calculated. Variability between urban and nonurban ponds in the environmental variables was tested using Mann–Whitney U-tests. Differences between environmental variables and faunal community composition in urban and nonurban ponds were visualized using Non-Metric Multidimensional Scaling (NMDS) with the metaMDS function in the vegan package and were examined statistically using a ‘permutational analysis of variance’ (permanova). Bray–Curtis dissimilarity was used to analyse the macro-invertebrate data and Euclidean distance used for the environmental data. Homogeneity of multivariate dispersions between the environmental data and macro-invertebrate communities from urban and nonurban ponds was calculated using the betadisper function in vegan and compared using anova. To identify indicator taxa of ephemeral and perennial ponds, Indicator Value analysis (IndVal: Dufrêne & Legendre, 1997) was undertaken. To test the spatial patterns of community structure in urban and nonurban ponds, a Mantel correlogram was constructed between the aquatic macro-invertebrate distance matrix (Euclidean) and the geographical distance for urban and nonurban ponds using the mantel.correlog function in the vegan package in r. Breaks among distance classes in the Mantel correlogram were defined in 50-km intervals. The Mantel correlogram enables the identification of changes in the strength of correlation between faunal distance matrices and geographical distance matrices at different spatial scales (Rangel et al., 2010).
The relationship between macro-invertebrate assemblages and environmental variables (pH, percentage coverage of emergent macrophytes, percentage pond shading, altitude, location within urban area and pond area) was examined using redundancy analysis (RDA) in the vegan package. A stepwise selection procedure (forward and backward selection) was employed to select the best model and environmental variables that significantly (P < 0.05) explained the variance in pond macro-invertebrate assemblages using the ordistep function in vegan, which uses permutation-based significance tests (999 permutations).
Results
Urban and nonurban pond environmental characteristics
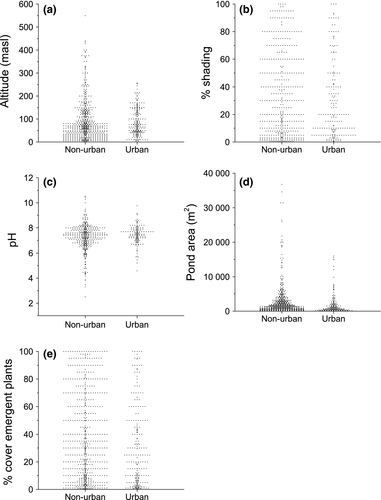
Comparisons between specific environmental variables in urban and nonurban ponds that are thought to influence diversity and composition showed that altitude (W = 108179.5 P < 0.01; Fig. 2a) and pond shading (W = 92965.5 P < 0.01; Fig. 2b) were significantly higher for urban ponds (mean altitude: 85.9 ± 3.7 masl; mean shading 22.89 ± 1.84%) than nonurban ponds (mean altitude: 78.2 ± 2.8 masl; mean shading 19.61 ± 0.95%), but the absolute differences between the pond types are small enough that they may be biologically insignificant. pH was significantly higher for urban ponds (mean 7.44 ± 0.06 SE) compared to nonurban ponds (7.37 ± 0.16; W = 37024 P < 0.05; Fig. 2c) although in both pond types pH was close to neutral. Nonurban ponds demonstrated a greater variability in pH compared to urban ponds. A total of 13% of nonurban ponds (66 ponds) recorded a pH <6.5, whilst only 4% of urban ponds (10 urban ponds) recorded a pH <6.5. In addition, pond area was on average 43% larger in nonurban ponds (2207 ± 139 m2) compared to urban ponds (1546 ± 171 m2; W = 75154.5 P < 0.01; Fig. 2d). Emergent macrophyte coverage was significantly higher in nonurban ponds (33.10 ± 1.08%) compared to urban ponds (27.77 ± 1.87%; W = 81695 P < 0.01; Fig. 2e) although the mean difference was <5%.
Aquatic macro-invertebrate diversity
Family level gamma diversity was similar between urban (observed 96 families, Fig. 3a) and nonurban ponds (observed 103 families, Fig. 3b), and the Chao2 estimator produced results taking into account sample size that were not statistically different across the two pond types (urban: 108.2, 95% CI: 91.4–125.0 families; nonurban: 107.5, 95% CI: 99.7–115.3 families). At an alpha scale, urban ponds (median richness = 13, range = 2–44) supported significantly greater macro-invertebrate family richness compared to nonurban ponds (median richness = 12, range = 2–38; W = 20430.5 P < 0.01) although median richness values were very similar between the pond types. Species-level gamma diversity was lower in urban (observed 403 species) than nonurban sites (observed 473 species), but the Chao2 estimator showed that there was no significant difference after controlling for the number of sites (urban: 496.6, 95% CI: 445.6–547.7 species; nonurban: 572.9, 95% CI: 520.2–625.7 species). No significant difference in alpha diversity between macro-invertebrate species was recorded between urban (median: 28) and nonurban ponds (median 26; W = 17310 P = 0.507).
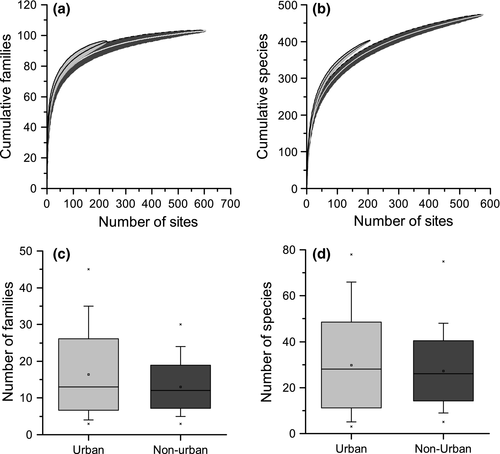
Urban ponds demonstrated a greater variability in alpha diversity among individual ponds at a family and species level (Fig. 3c, d). A total of 25 urban ponds (11% of total urban pond number) supported >25 macro-invertebrate families, whilst only 9 nonurban ponds (1.5% of total nonurban pond number) supported macro-invertebrate communities with >25 families. In addition, the greatest number of invertebrate families recorded was from an urban pond (46 taxa) and five of the six ponds with the greatest macro-invertebrate family richness were located in urban environments. Only two families of macro-invertebrates were statistically associated with nonurban ponds (one family of Plecoptera and one family of Ephemeroptera), whilst 20 families were identified as indicator taxa for urban ponds, including seven families of Diptera. Strongest associations for families are presented in Table 2 (see Table S10 for the full list of statistically significant family indicator values and Table S11 for significant indicator values of macro-invertebrate species).
| Nonurban ponds | Stat | Urban ponds | Stat |
|---|---|---|---|
| Nemouridae** | 0.34 | Chironomidae** | 0.72 |
| Heptageniidae* | 0.20 | Oligochaeta** | 0.69 |
| Crangonyctidae** | 0.63 | ||
| Sphaeriidae** | 0.51 | ||
| Certaopogonidae** | 0.48 | ||
| Dixidae** | 0.46 |
- *P < 0.05, **P < 0.01.
When nonurban ponds designated as degraded were removed and the macro-invertebrate diversity in the remaining ponds was compared to urban ponds, alpha diversity was significantly greater in urban ponds (median: 13; W = 18057 P < 0.01) than the higher quality nonurban ponds (median: 12) at a family level, although mean and median richness values were similar between the pond types (see Appendix S2). There was no significant difference in alpha diversity (W = 14653.5 P = 0.358) at the species level between urban ponds (median: 28) and higher quality nonurban ponds (median: 25). Estimated gamma diversity for higher quality nonurban ponds at a family (98.7) and species scale (575.1) was marginally higher compared to gamma diversity when all nonurban ponds were considered.
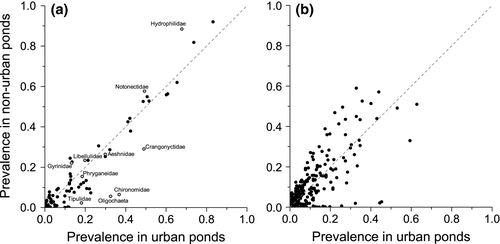
Chironomidae, Tipulidae, Crangonyctidae and Oligochaeta had a greater frequency of occurrence in urban ponds, whilst Gyrinidae, Hydrophilidae and Notonectidae displayed a greater occurrence in nonurban ponds (Fig. 4; for complete data see Tables S8 and S9 for family and species-level prevalence, respectively). Macro-invertebrate families that score highly within biological monitoring surveys of ponds and other waterbodies (e.g. PSYM and BMWP) such as Phryganeidae, Leptoceridae, Libellulidae and Aeshnidae occurred at similar frequencies in the urban and nonurban ponds (Fig. 4). Crangonyctidae were present in 49.0% of urban ponds and only 29.0% of nonurban ponds. All specimens of this family from the species-level data set were the North American invasive Crangonyx pseudogracilis. A similar pattern is also seen in the species-level data set with the invasive New Zealand mud snail, Potamopyrgus antipodarum, being found in 21.3% of urban ponds and 9.5% of nonurban ponds.
Community heterogeneity
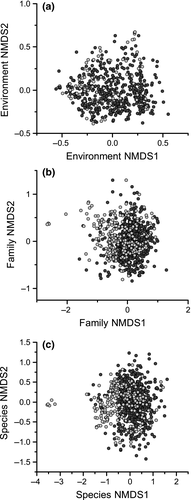
Multivariate dispersion for environmental characteristics was significantly lower in nonurban ponds (median distance: 1116) than urban ponds (median distance: 1978; F = 5.774 P < 0.05, Fig. 5a). permanova showed that there was a small but significant difference between environmental characteristics (R2 = 0.03 P < 0.001) and faunal communities at a family (R2 = 0.09 P < 0.001) and species level (R2 = 0.03 P < 0.001). A relatively clear distinction between aquatic macro-invertebrate community composition in urban and nonurban ponds was observed at the family and species level within the NMDS ordination (Fig. 5b, c). Among faunal communities, multivariate dispersion was significantly higher at the family (median distance – urban: 0.451, nonurban: 0.406; F = 27.584 P < 0.01) and species scale (median distance – urban: 0.579, nonurban: 0.550; F = 17.626 P < 0.01) for urban ponds compared to nonurban ponds.
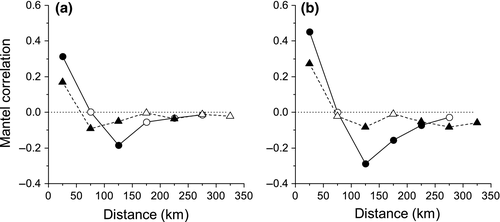
There was significant positive spatial autocorrelation for urban (r = 0.31 P < 0.01) and nonurban ponds (r = 0.17 P < 0.01) at the family level for the smallest distance class (0–50 km), indicating that those ponds in close geographical proximity have similar macro-invertebrate community compositions (Fig. 6a). At middle distance classes (distance class three: 100–150 km), urban and nonurban ponds demonstrated a significant negative Mantel spatial autocorrelation, although this effect was weak for nonurban ponds (urban: r = −0.18 P < 0.01, nonurban: r = −0.05 P < 0.01) (Fig. 6a). At larger distances, spatial autocorrelation declined in strength for urban and nonurban ponds. The same analyses carried out on species-level data showed similar spatial patterns, but with stronger positive correlation at shorter distances (0–50 km, urban: r = 0.45, P < 0.01; nonurban: r = 0.27, P < 0.01) and stronger negative correlation at middle distances (100–150 km, urban: r = −0.29, P < 0.01; nonurban: r = −0.08, P < 0.01; Fig. 6b).
Macro-invertebrate – environment relationships
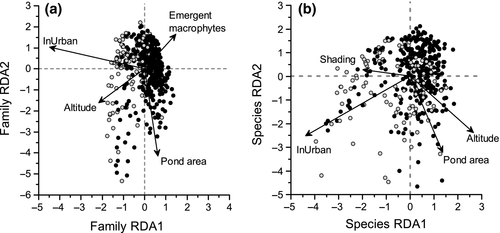
Redundancy analysis (RDA) of the pond macro-invertebrate family community data and environmental parameters highlighted clear differences between urban and nonurban ponds (Fig. 7a). The RDA axes were highly significant (F = 3.06 P < 0.001, adjusted R2 = 0.02), explaining 3.8% of the variation in family assemblage on all constrained axes (see Table S4). Stepwise selection of environmental parameters identified four significant physicochemical variables correlated with the first two RDA axes: altitude, emergent macrophytes (all P < 0.05), surface area and location within urban area (both P < 0.01) (Fig. 7a). RDA indicated that urban and nonurban pond invertebrate communities were separated on the first and second axes along gradients associated with pond surface area and emergent macrophyte cover/their location within the urban landscape (Fig. 7a). Nonurban ponds were characterized by a greater pond area and emergent macrophyte cover, whilst urban ponds were associated with smaller surface areas and less emergent macrophytes (Fig. 7). RDA of pond macro-invertebrate species community data showed similar patterns: urban and nonurban ponds were strongly separated along the first RDA axis, with significant effects of urbanization, pond area, altitude and shading on community structure (Fig. 7b). However, in both RDA analyses, the explanatory power of the models was very low (see Table S4).
Discussion
Urban freshwater diversity
This is the first study to provide a large-scale, intercity approach to test the biological response of entire pond macro-invertebrate communities to urbanization. The results provide a contrast with previous work on terrestrial and lotic habitats which has shown greater fragmentation, reduction in habitat quality (e.g. pollution/contaminant build up), alterations to biogeochemical cycles, higher air surface temperatures, increased disturbance frequencies, proliferation of non-native taxa, biotic homogenization and an overall decline in biological richness in urban areas (e.g. McKinney, 2002, 2006; Grimm et al., 2008). The ecological consequences of urbanization for ponds do not appear to follow the same patterns identified elsewhere for terrestrial habitats.
Urban ponds and nonurban ponds support similar alpha diversity of aquatic macro-invertebrates at a family and species level (reject hypothesis 1) and estimated gamma diversity was similar at a family level, although nonurban ponds recorded higher estimated gamma diversity at a species scale. These findings are consistent with a recent study of terrestrial invertebrates that showed comparable levels of diversity of particular indicator groups inhabiting birch trees (Betula pendula) between urban and agricultural areas (Turrini & Knop, 2015). However, an analysis of the same data set showed a homogenization of arboreal invertebrates within urban areas (Knop, 2016), consistent with other terrestrial ecosystem studies (McKinney, 2008) but not with our data for freshwater macro-invertebrates. The lack of agreement in ecological patterns between ponds (which, in this study, show similar patterns of diversity across urban boundaries) and lotic/terrestrial habitats (which tend to show reduced faunal richness with increasing urbanization) in cities may reflect the ability of pond communities to recover relatively quickly from temporary anthropogenic disturbance (Thornhill, 2013). This resilience is supported by the high dispersal abilities of many semi-aquatic invertebrates (Goertzen & Suhling, 2015). Despite commonly occurring in clusters, ponds are discrete habitats with small catchment areas (Davies et al., 2008) and disturbance in one pond or its catchment has little impact on others in the network cluster, whilst a single disturbance event in, for example, a river system would impact an entire reach (Thornhill, 2013). Aside from rare taxa, there were few families that showed a different prevalence between urban and nonurban ponds, including indicator taxa with high BMWP scores (indicative of high water quality). However, there was also a higher prevalence of Oligochaeta and Chironomidae in urban ponds which is consistent with historical disturbance and subsequent recolonization by disturbance tolerant taxa, and higher prevalence of the invasive C. pseudogracilis and P. antipodarum in urban ponds supports previous findings that urban ecosystems favour the establishment of invasive species (Shochat et al., 2010).
We propose two potential explanations, which are not mutually exclusive, for the similarity between urban and nonurban pond biodiversity. First, it has been estimated that 80% of ponds in the wider UK landscape are in a degraded state (Williams et al., 2010). Hence, nonurban ponds and urban ponds may be suffering from external pressures and mismanagement leading to the similar alpha diversities recorded. With both pond types in degraded states the biodiversity value of urban ponds must be treated with caution, as their richness is compared to similar degraded nonurban ponds. However, our secondary analysis demonstrated that urban ponds still show comparable biodiversity to higher quality, nondegraded nonurban ponds. Research examining the diversity of high-quality urban and nonurban ponds is required to fully quantify the biodiversity value of urban ponds. Second, intensive management in cities may actually promote biodiversity. Whilst many ponds in nonurban areas (e.g. agricultural land) are left unmanaged, neglected, and at late successional stages (Hassall et al., 2012; Sayer et al., 2012), ponds in urban areas are often managed (primarily for purposes other than biodiversity) and a wide range of successional stages are maintained. Furthermore, in many cases, local residents (e.g. pond warden schemes) monitor and manage large numbers of urban ponds for the benefit of ecological communities, improving their habitat/water quality and promoting high biological richness (Boothby, 1997; Hill et al., 2015). Results from the present study show that urban areas have the potential to become reservoirs of freshwater biodiversity rather than ‘ecological deserts’, which incorporate a wide range of aquatic habitats including ponds, canals, urban reservoirs and wetlands (Hassall & Anderson, 2015). However, it should be noted that diversity was highly variable in this study at both the family and species level of taxonomic resolution and previous research has demonstrated that some urban ponds can be of low ecological quality if anthropogenic stressors such as eutrophication are allowed to persist (Noble & Hassall, 2015).
Urban ponds were also characterized by contrasting values of some environmental parameters to nonurban ponds. As expected, urban ponds were smaller than nonurban ponds reflecting the high level of competition and the economic value of urban land. Lower emergent macrophyte coverage was recorded in urban ponds compared to nonurban ponds which reflects their primary function for flood water storage/water treatment and the management practices undertaken to achieve this (Le Viol et al., 2009). Reduced emergent macrophyte cover in urban areas may also be the result of public perceptions of pond attractiveness (clean, open water and surrounding vegetation mown; Nassauer, 2004) which pond amenity managers aim to replicate, or other management practices for amenity purposes such as angling or boating (Wood et al., 2001). Urban ponds were significantly more shaded than nonurban ponds, which is most likely the result of urban ponds location within high density, built environments providing significant additional artificial shading to that provided by trees. In addition, reduced shading of nonurban ponds may be because many nonurban ponds were located in landscapes typically free of shading (trees) including wetland meadows and the low numbers of trees in British agricultural landscapes where many nonurban ponds are situated (however, high levels of pond shading from trees have been recorded in some UK agricultural areas: Sayer et al., 2012).
Community heterogeneity
Small but significant differences in faunal communities (family and species) were observed between urban and nonurban ponds in this study (reject hypothesis 2). Differences (albeit subtle) in community composition found in the present study contrast with the findings of Hassall & Anderson (2015) and Le Viol et al. (2009) and suggest that at greater spatial scales urban ponds contribute as much to the regional biodiversity pool as nonurban ponds. The higher community dissimilarity among urban ponds may reflect the different levels of disturbance and diverse management practices (reflecting their primary function, e.g. flood alleviation, biodiversity, amenity), as well as general pond characteristics such as small catchments which result in highly heterogeneous environmental conditions (greater environmental multivariate distances than nonurban ponds) even in ponds that are in close proximity (Davies et al., 2008).
Significant positive spatial autocorrelation at the smallest distance class and significant negative spatial autocorrelation at medium distances suggest that (i) ponds within individual cities have similar communities which reflect similar city-region environmental characteristics; and (ii) ponds at greater spatial distances from one another in different cities have increasingly dissimilar communities reflecting the high variability in environmental (Heino & Alahuhta, 2015) and historical factors (Baselga, 2008; Heino & Alahuhta, 2015) among cities. Spatial patterns of management may influence geographical variation in community structure to a greater extent than landscape connectivity, making it difficult to evaluate our third hypothesis. However, we demonstrate stronger spatial structuring of urban communities at finer spatial scales, which would be expected under lower connectivity. Greater connectivity in nonurban landscapes enhances species movement leading to weaker spatial structuring at finer spatial scales in nonurban ponds. Hence, our observations support our third hypothesis, but further work is needed to evaluate the consequences of spatial patterns for management. Historically, urban environments were highly degraded (physically, chemically and biologically), but significant improvements to urban freshwater quality have been achieved in recent decades despite urban sprawl and intensification (Vaughan & Ormerod, 2012). Therefore, it is possible that cities are still being recolonized by aquatic taxa from different regional species pools using different dispersal routes, creating a dynamic pattern of communities.
Conservation implications
Urban ponds support relatively high alpha and gamma diversity comparable to nonurban ponds. A lack of monitoring of urban freshwaters (particularly ponds that are excluded from the EU Water Framework Directive) may be hiding considerably more diversity such that urban planners fail to identify high biodiversity sites (Hassall, 2014). There is a need for a concerted, comparative, empirical approach to freshwater management that incorporates biodiversity as well as other ecosystem services alongside social and political considerations. Fundamental to the conservation of ponds is an integrated landscape approach that recognizes the need for networks of ponds (Boothby, 1997). Hence, the prioritization of ponds for conservation will need to take into account their location relative to other sites, requiring a complementary approach that creates new habitats, improves degraded habitats, and conserves those habitats that have already achieved good quality. Changes in the management of ponds more generally has led to change in the environmental conditions within and around these habitats, such as the reduction in riparian tree management around agricultural ponds which has consequences for light, oxygen and temperature (Sayer et al., 2013). Urban ponds are well suited to biodiversity enhancement as many are sites of high diversity (Hassall, 2014) and even small changes to current management strategies in urban freshwaters (e.g. the planting of native macrophytes in amenity ponds; Hill et al., 2015) are likely to significantly augment biodiversity in urban landscapes. Cities are highly complex, multifunctional landscapes designed primarily for anthropogenic use yet they still support considerable aquatic diversity and represent scientifically and ecologically important habitats.
Acknowledgements
The authors would like to thank the various organizations who provided resources for the data sets included in this study: the EU Life Program funded the PondLife Project. RB would like to thank the Carnegie Trust for the Universities of Scotland. MH would like to acknowledge Leicestershire County Council and the private land owners that granted access to their land. CH is grateful for support from a Marie Curie International Incoming Fellowship within the 7th European Community Framework Programme. DG would like to thank Halton Borough Council for support and access to pond sites, and IT is grateful for the support from the Natural Environment Research Council and The James Hutton Institute.




