Soil carbon and nitrogen stocks in forests along an altitudinal gradient in the eastern Himalayas and a meta-analysis of global data
Abstract
High-altitude soils potentially store a large pool of carbon (C) and nitrogen (N). The assessment of total C and N stocks in soils is vital to understanding the C and N dynamics in terrestrial ecosystems. In this study, we examined effects of altitude and forest composition on soil C and N along a transect from 317 to 3300 m a.s.l. in the eastern Himalayas. We used meta-analysis to establish the context for our results on the effects of altitude on soil C, including variation with depth. Total C and N contents of soils significantly increased with altitude, but decreased with soil depth. Carbon and N were similarly correlated with altitude and temperature, and temperature was seemingly the main driver of soil C along the altitudinal gradient. Altitude accounted for 73% of the variation in C and 47% of the variation in N stocks. Soil pH and cation exchange capacity were correlated with both soil C and N stocks. Increases in soil C and N stocks were related to forest composition, forest basal area as well as quantity of leaf litter that were in turn influenced by altitude and temperature. Concentrations of C in foliage increased by 2.1% for every 1000 m rise in altitude, while that in leaf litter increased by 2.3%.
Introduction
Carbon stocks in soil vary substantially across the globe depending on the type of forest, their location, and soil depth. In mixed forests, C stocks are highly variable, with 63–88 Mg C ha−1 reported for Picea and Abies, mixed broadleaf and Betula forests in the northeast of China (Zhu et al., 2010); 184 Mg C ha−1 (0–100 cm) for a Picea dominated forest in Poland (Galka et al., 2014); and 93–101 Mg C ha−1 (0–30 cm) for an Abies and pine–oak forest in Mexico (Ordóñez et al., 2008). Similarly, highly variable soil C stocks (0–100 cm) have been reported in a study from India, with values ranging from 34 to 411 Mg ha−1 for tropical evergreen forest, from 24 to 525 Mg ha−1 for montane temperate forest, and from 57 to 213 Mg ha−1 for tropical moist deciduous forest (Chhabra et al., 2003). These highly variable data for soil C stocks for different forest types across different geographical zones highlight the importance of site-specific measurements of parameters with predictive capacity for a good approximation of C stocks in different ecosystems around the world.
Limited data are available for the N stock for natural forests in relation to forest type or along altitudinal gradients. However, comparisons have been made in the context of land-use change effects of N stocks, for example, with afforestation programs and conversion of forest to pasture landscapes. Reported soil N stocks ranged from 3.5 to 4.9 Mg ha−1 (0–15 cm) in a mountainous forest (Finzi et al., 1998), from 1.5 to 5.0 Mg ha−1 (0–30 cm) in an Amazon tropical forest (TF; Neill et al., 1997), and 18.2 Mg ha−1 (0–100 cm) for a forest in southern Ethiopia (Demessie et al., 2011).
Although most studies considered only C, soil C and N cycles are interdependent. Nitrogen is an essential nutrient for plants and a constituent of the Rubisco enzyme responsible for photosynthesis (Raines & Lloyd, 2007). Carbon assimilated during photosynthesis is stored in plant tissues, transported to microbial symbionts in the rhizosphere, respired, or released into the soil (Pidwirny, 2012). Nitrogen introduced to soil as plant litter is a nutrient for microorganisms, which decompose the organic matter and release C to the atmosphere as CO2. Under increased CO2, net primary productivity of many ecosystem is expected to initially increase, causing N to be immobilized in biomass, depleting soil N, and thereby increasing C : N ratios in soils and slowing rates of mineralization (Adams et al., 2004). The feedback effect could ultimately limit responses to increased CO2. It is imperative that soil N stocks and C : N ratios are assessed in order to determine the C sequestration potential of soils (Finzi et al., 2006; Luo et al., 2006).
The southwestern foothills of the Bhutan Himalayas are characterized by different forest types within short distances due to a steep altitudinal gradient. Variation in altitude creates a gradient in abiotic factors such as temperature, moisture, and solar radiation, which in turn influence forest composition (Laughlin & Abella, 2007) and soil organic carbon (SOC) (Jobbagy & Jackson, 2000; Singh et al., 2011). Numerous studies have suggested that variation in abiotic factors as well as species composition contributes to variations in C density in soil and biomass (Jobbagy & Jackson, 2000; Lamlom & Savidge, 2003; Zhu et al., 2010; Martin & Thomas, 2011). As a consequence of steep altitudinal gradients in Bhutan, there are at high-altitudes climatic regimes similar to those of widely separated latitudinal zones (Beniston et al., 1997), which makes mountainous ecosystems more vulnerable to climate change, the effects of which can be more rapid and severe than at lower altitudes. Most studies report that soil C stocks increase with altitude. Such results have been reported for different regions and different forest types, including spruce, fir, and mixed hardwood forest in the USA (Garten & Hanson, 2006), mixed broad-leaved and pine forest in Nepal (Maraseni & Pandey, 2014), and mixed broad-leaved and pine forest, grasslands, agricultural and horticultural lands in the western Himalayas in India (Singh et al., 2011). In contrast, other researchers have reported decreasing soil C stocks (Kumar et al., 2013) or no relationship of soil C stock (Tewksbury & Van Miegroet, 2007; Godgift et al., 2014) with increasing altitude. We thus synthesized all relevant published literature to investigate the global relationship of soil C with altitude. In order to understand the influence of altitude and forest type on potential to sequester or release C, and to inform forest management decisions, we aimed to:
- quantify the total soil C and N stocks at different depths along an altitudinal gradient in Bhutan;
- investigate the influence of forest composition, altitude, and biomass input on the soil C and N; and
- synthesize a global relationship between soil C and altitude in different ecoregions of the world via a meta-analysis of published literature.
Material and methods
Study area
Our sampling sites are in the eastern foothills of the Himalayas, more particularly in southwestern Bhutan (Fig. 1). Soil and plant samples were taken along a transect running from the foothills at an elevation of 317 m a.s.l. (N 26° 51′, 89° 23′E) to the mid-hills, where the elevation reached to 3300 m a.s.l. (N 26° 59′, 89° 32′E). A land cover map of Bhutan (2009) and Google Earth maps were used to georeference the transect, as well as the sampling sites, prior to field studies. Based on vegetation composition, forests were zoned into five types (Table 1), following the classification proposed by Ohsawa (1987). (i) TF from 317 to 900 m a.s.l.), (ii) subtropical forest (STF) from 900 to 1870 m a.s.l., (iii) warm-temperate broadleaf forest (WTBLF) from 1870 to 2450 m a.s.l., (iv) cool-temperate broadleaf forest (CTBLF) from 2450 to 3000 m a.s.l., and (v) cold-temperate forest (CTF) from 3000 to 3300 m a.s.l. The study sites fall within two of the four Himalayan tectono-stratigraphic zones found in Bhutan. TF and STF falls under the Lesser Himalayan Formation composed of low-grade meta-sedimentary rocks, including quartzite, phyllite, and limestone (Tobgay et al., 2010; Long et al., 2011). WTBLF, CTBLF, and CTF fall under the Greater Himalayan Formation which consists of orthogneiss and meta-sedimentary rocks (Gasser, 1983; Tobgay et al., 2010).
| Altitude (m a.s.l.) | Forest | No. of Species | H′ | Density (trees ha−1) | BA (m2 ha−1) | MAT (°C) |
|---|---|---|---|---|---|---|
| 317–900 | TF | 33 | 2.96 | 313 | 17.8 | 22.9 |
| 900–1870 | STF | 54 | 3.47 | 383 | 30.1 | 15.4 |
| 1870–2450 | WTBLF | 42 | 3.21 | 433 | 41.1 | 13.3 |
| 2450–3000 | CTBLF | 47 | 3.14 | 824 | 46.3 | 10.9 |
| 3000–3300 | CTF | 10 | 1.96 | 511 | 66.6 | 5.5 |
- TF, tropical forest; STF, subtropical forest; WTBLF, warm-temperate broadleaf forest; CTBLF, cool-temperate broadleaf forest; CTF, cold-temperate forest; No. of species, number of tree species surveyed for each forest type; H′, Shannon diversity index; Density, number of trees per ha; BA, basal area of trees per ha (m2 ha−1); MAT, mean annual temperature.
Forested land has traditionally been used for grazing by migratory cattle herds. In the last few decades, portions of forests have been clear-felled in a number of localities. Clear-felled patches were avoided and not included in the study as they represented a small portion of total forest area.
The southern foothills have tropical climate with an average annual rainfall of 4600 mm and average annual temperature of 22.9 °C (2009, Department of Hydro-Met Services, Bhutan). In the mid-hills, between 2000 and 3000 m a.s.l., annual precipitation is about 3500 mm, and summer temperatures can reach as high as 29 °C while the winter temperatures can drop as low as 3 °C in December (Wangda et al., 2009).
Soil sampling and analysis
A total of 40 soil profiles, spaced at 75 m altitude interval, were dug along the transect. Prior to digging the soil profile, a 1 × 1 m plot was laid out and all leaf litter was collected and weighed and subsample was taken for further analysis. Soil samples were collected from 0 to 100 cm depth from each profile. Soil profiles were sampled and described as per guidelines of the Soil and Plant Analytical Laboratory (SPAL), Simtokha Bhutan (SPAL, 1993).
Duplicate bulk density (BD) samples were taken from each of the four depth categories, that is, 0–10 cm measured from the top of the soil after removal of the leaf litter, 10–30 cm, 30–60 cm, and 60–100 cm. Subsequently, bulk soil samples of about 1 kg were collected from each of the four depth categories. Soil samples were oven-dried at 40 °C, gently crushed by hand, weighed, and passed through a 2 mm sieve. The proportion of the >2 mm fraction which consisted mostly of pebbles was quantified by weighing and then discarded.
Soil pH and electrical conductivity were measured in a soil/water(1 : 5) suspension, after shaking for 30 min (Rayment & Higginson, 1992). Particle-size analysis was based on the pipette method, and cation exchange capacity (CEC) was measured with 1 m ammonium acetate at pH 7. BD was determined by drying a known volume of soil to constant weight at 105 °C (SPAL, 1993). For total C and N analyses, representative soil samples were finely ground (<53 μm) using a Fritsch Pulverisette 2 Mortar Grinder Mill (RETSCH GmbH, Haan, Germany). The total C and N were analyzed using a Vario MAX CNS analyzer (Elementar Analysensysteme GmbH, Hanau, Germany).
 (1)
(1)To compare our C and N stock data with other studies, a cubic spline function in Microsoft Excel was used to interpolate C and N stocks data to match with soil depths reported in other studies.
Forest inventory, biomass sampling, and analysis
Along the transect, we developed a vegetation inventory by sampling 30 × 30 m plots at 150 m altitudinal intervals. In total, 20 vegetation inventory plots were surveyed. All trees above 10 cm diameter at breast height (DBH) measured at 1.3 m from the uphill side were identified and measured for diameter and height. Diameters and heights were used to calculate bole volumes using equations developed for Bhutan (FSI, 1996). A global database (Zanne et al., 2009) was used as the source of tree density data and to convert volumes to mass for tree boles. Default values of biomass expansion factors (IPCC, 2003) were used to calculate tree biomass. Within the 30 × 30 m inventory plots, three 1 × 1 m subplots were randomly chosen to harvest the herb layer and collect the leaf litter and dead wood (<5 cm diameter).
Harvested plants were segregated into stems, branches, and foliage, and each biomass category was weighed in the field. Subsamples of each biomass categories were weighed and taken to the laboratory and oven-dried at 60 °C to constant weight. Dry weights of subsamples were used to estimate dry weight of each biomass category.
Dried biomass fractions were coarsely ground to a powder using Philips HL 1606/00 mixer grinder, and then, a representative aliquot was finely ground using Retsch MM400 Mixer Mill (RETSCH GmbH). Finely ground portion of the sample was used for C and N analyses in a Thermo Finnigan Delta V isotope ratio mass spectrometer coupled to ConfloIV and FlashHT peripherals (Thermo Fisher Scientific, Bremen, Germany). Total C and N contents for each of the biomass components of the different forest types were estimated as product of dry weight and C and N concentrations.
Statistical analysis
ibm spss statistics 21 (Armonk, NY, USA) was used to perform statistical analyses. Total soil C and N concentrations and stocks at different depth categories for the different forest types along the altitudinal gradient were compared using multivariate GLM (generalized linear model) combined with a Tukey HSD post hoc test. Relationships between total soil C and N stocks along the altitudinal gradient were assessed using a linear regression analysis.
For biomass components, a similar multivariate GLM analysis was used to compare C and N concentrations and stocks for different forest types along the altitudinal gradient. Correlations (Pearson product-moment correlation coefficients) among altitude, environmental, edaphic, allometric parameters and soil C and N concentrations and stocks were considered significant at P < 0.05.
Meta-analysis for soil C along the altitudinal gradient
We compiled data from 28 studies (Appendix 1) that investigated SOC changes with altitude in forest landscapes. Included studies originated from Europe (n = 2), Africa (n = 3), the Americas (n = 7), and Asia (n = 16). Experimental data included altitudinal ranges from sea level to 4800 m a.s.l., a MAT range from −7 to 26 °C and a MAP range from 30 to 4800 mm. The data provided 36, 15, 13, 9, and 3 observations for correlation between SOC (stocks or concentration at variable depths) and altitude, MAT, MAP, basal area (BA), and leaf litter, respectively. Meta-analysis was based on correlations. When studies reported P-values instead of correlation coefficients, P-values were converted to standard normal deviates Z and then transformed to correlation coefficients using Meta Calc (Rosenberg et al., 1999). Effect size and variance of each individual study were calculated using Meta Win v.2.1. based on reported correlation coefficient (r) and sample sizes. Individual effect sizes and variance were used to generate mean effect size and a bias-corrected 95% confidence interval (CI) by bootstrapping (4999 iterations). Correlations were considered significant if the 95% of CI did not overlap with zero. The mean effect size and CI from Fisher's z metric were reconverted to correlation coefficients using Meta Calc for interpretation of results.
Results
Influence of forest type and soil depth on carbon and nitrogen stocks in the soil
Soil C and N stocks increased significantly with altitude. For soil depths 0–30 cm and 0–100 cm, altitude explained 55% and 73%, respectively, of the variation in total C stocks. Correspondingly, for every 100 m rise in altitude, C stocks increased by 4.3 Mg C ha−1 for soil depths of 0–30 cm and 12.4 Mg C ha−1 for 0–100 cm (Fig. 2). Nitrogen stocks increased with altitude similar to C stocks. Altitude explained 35% of the variation in N stocks for soil depths 0–30 cm, and 47% for the depths 0–100 cm. For every 100 m rise in altitude, N stocks increased by 0.23 Mg ha−1 for 0–30 cm and by 0.67 Mg ha−1 for 0–100 cm (Fig. 2).
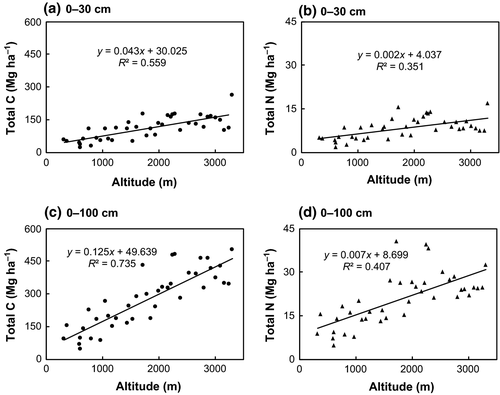
Total soil C and N stocks varied significantly with forest types and at soil depths (Table 2). Total C stock for the top 30 cm soil in TF was 49 Mg ha−1 and in STF was 91 Mg ha−1, which were significantly less than stocks in WTBLF, CTBLF, and CTF (130–187 Mg ha−1). Of the total C stock contained in 0–100 cm depth, the 0–30 cm interval constituted between 35% and 46%, while the 0–60 cm interval provided 64–74%. Deeper soils (e.g. 30–100 and 60–100 cm) contributed between 24% and 65% of the total C in 0–100 cm.
| Altitude (m a.s.l.) | Forest zones | Total: 0–100 cm | 0–10 cm | 0–30 cm | 30–100 cm | 60–100 cm | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Carbon | Mg ha−1 | Mg ha−1 | % | Mg ha−1 | % | Mg ha−1 | % | Mg ha−1 | % | |
| 317–900 | TF | 114.5 | 20.4 | 17.8 | 49.3 | 43.1 | 65.2 | 56.9 | 31.6 | 27.6 |
| 900–1870 | STF | 217.6 | 35.7 | 16.4 | 91.3 | 42.0 | 126.3 | 58.0 | 66.1 | 30.4 |
| 1870–2450 | WTBLF | 326.1 | 49.6 | 15.2 | 130.3 | 40.0 | 195.8 | 60.0 | 117.4 | 36.0 |
| 2450–3000 | CTBLF | 408.2 | 51.0 | 12.5 | 143.8 | 35.2 | 264.4 | 64.8 | 135.9 | 33.3 |
| 3000–3300 | CTF | 403.5 | 78.5 | 19.5 | 186.5 | 46.2 | 217.1 | 53.8 | 97.5 | 24.2 |
| Nitrogen | Mg ha −1 | Mg ha −1 | % | Mg ha −1 | % | Mg ha −1 | % | Mg ha −1 | % | |
| 317–900 | TF | 10.8 | 1.9 | 17.6 | 4.5 | 41.7 | 6.3 | 58.3 | 3.1 | 28.7 |
| 900–1870 | STF | 18.6 | 3.1 | 16.7 | 7.6 | 40.9 | 11.0 | 59.1 | 5.7 | 30.6 |
| 1870–2450 | WTBLF | 25.7 | 4.0 | 15.6 | 10.5 | 40.9 | 15.2 | 59.1 | 9.1 | 35.4 |
| 2450–3000 | CTBLF | 26.4 | 3.6 | 13.6 | 9.5 | 36.0 | 17.0 | 64.4 | 8.7 | 33.0 |
| 3000–3300 | CTF | 25.7 | 5.4 | 21.0 | 12.3 | 47.9 | 13.4 | 52.1 | 5.8 | 22.6 |
Similar to C stocks, total N stocks in the top 30 cm soil for TF (4.5 Mg ha−1) and STF (7.6 Mg ha−1) were significantly lower than the other three higher altitude forest types. Total N stocks in the 0–30 cm soil were 10.5 Mg ha−1 in the WTBLF, 9.5 Mg ha−1 in the CTBLF, and 12.3 Mg ha−1 in the CTF. Proportions of N stored in each soil depth category were similar to those of C stocks (Table 2).
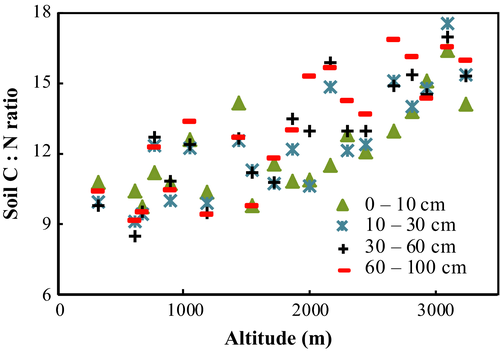
C : N ratios of soil were highly correlated with altitude (r = 0.69, P = 0.01) and increased from 9.8 for TF to 15.4 for CTF. Increases in C : N ratio with altitude were greater for deeper soils compared to surface soils. Additionally, the C : N ratios for surface soils (0–10 cm) were more variable than for deeper soils (Fig. 3).
In the present study, more C was found in shallow soils when compared to forest soils from other studies (Fig. 4). The proportion of total C (0–100 cm) found in the 0–20 cm interval ranged from 31% to 69% for the present study compared to 33–52% from other studies. Whereas the proportion of total C to 100 cm that is in the deeper soils (60–100 cm) ranged from 3.5% to 36% for the present study, which is more variable than 9.4–14.2% for other similar studies.

Influence of forest type and altitude on carbon and nitrogen concentrations in understorey live biomass, canopy dead wood, and leaf litter
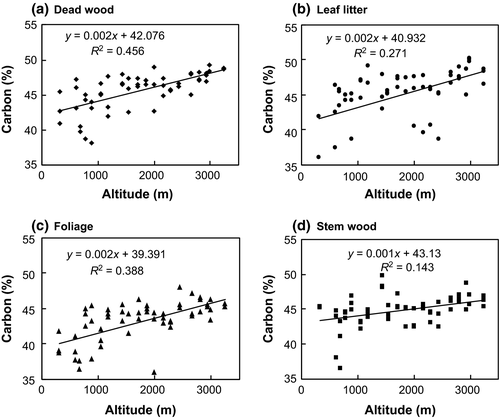
Carbon concentrations in understory foliage and stem wood, canopy dead wood, and leaf litter varied significantly with forest types (Table 3) and altitude (Fig. 5). All biomass components from CTF had the greatest C concentrations (45.4–48.6%), and TF showed the least C concentrations (39.7–43.0%). Increasing elevation resulted in increasing C concentrations in deadwood from 42% to 48.6% (corresponding to an increase of 2.0% for every 1000 m) (Fig. 5a), in leaf litter from 42% to 47.8% (corresponding to an increase of 2.3% for every 1000 m) (Fig. 5b), in understorey foliage from 42% to 45% (corresponding to an increase of 2.1% for every 1000 m) (Fig. 5c), and in stem wood from 43% to 46% (corresponding to an increase of 1.0% for every 1000 m) (Fig. 5d). Regression analysis revealed that altitude accounted for changes in C concentrations of 45% in deadwood, 27% in leaf litter, 38% in foliage, and 14% in stem wood. Additionally, total leaf litter C density (g m−2) increased significantly from low- to high-altitude forest (TF = 62.0 g m2, STF = 109.1 g m−2, WTBLF = 189.9 g m−2, CTBLF = 274.0 g m−2, and CTF = 166.2 g m−2; Table 3). There was no significant difference in the total C density with altitude for other understorey biomass components.
| Forest zone | C (%) | N (%) | C (g m−2) | N (g m−2) | Biomass (g m−2) |
|---|---|---|---|---|---|
| Foliage | |||||
| TF | 39.7a ± 3.3 | 2.9a ± 0.7 | 147.6a ± 109.1 | 9.6a ± 5.3 | 372.5a ± 273.2 |
| STF | 43.2b ± 2.2 | 2.8a ± 0.6 | 156.5a ± 69.2 | 10.6a ± 5.6 | 360.6a ± 154.5 |
| WTBLF | 43.2b ± 2.4 | 2.9a ± 0.5 | 166.9a ± 55.6 | 10.9a ± 3.4 | 388.3a ± 133.6 |
| CTBLF | 45.7b ± 1.3 | 2.6a ± 0.7 | 97.6a ± 61.7 | 6.1a ± 5.7 | 166.7b ± 124.1 |
| CTF | 45.4b ± 0.2 | 1.7a ± 0.1 | 60.6a ± 19.2 | 2.3a ± 0.6 | 133.3b ± 41.6 |
| Stem wood | |||||
| TF | 43.0a ± 2.9 | 0.8a ± 0.3 | 127.7a ± 138.8 | 1.8a ± 1.1 | 286.9a ± 303.5 |
| STF | 45.4b ± 2.0 | 0.8a ± 0.2 | 76.3ab ± 38.5 | 1.5a ± 0.9 | 161.5ab ± 86.4 |
| WTBLF | 44.2ab ± 1.2 | 0.8a ± 0.3 | 85.0ab ± 42.8 | 1.7a ± 1.1 | 190.8ab ± 94.1 |
| CTBLF | 45.8b ± 1.6 | 1.0a ± 0.3 | 36.1b ± 26.4 | 0.7a ± 0.4 | 63.2b ± 57.9 |
| CTF | 46.1ab ± 0.7 | 0.7a ± 0.2 | 44.2ab ± 25.0 | 0.8a ± 0.6 | 96.3ab ± 56.2 |
| Deadwood | |||||
| TF | 42.0a ± 4.2 | 0.9ab ± 0.2 | 108.9a ± 67.6 | 2.4a ±1.3 | 264.3a ± 172.9 |
| STF | 45.1b ± 2.5 | 1.1a ± 0.2 | 114.8a ± 77.1 | 2.9a ± 2.0 | 249.6a ± 177.4 |
| WTBLF | 46.1b ± 1.5 | 1.1a ± 0.1 | 174.8a ± 140.7 | 4.2a ± 3.4 | 378.4a ± 306.1 |
| CTBLF | 47.0b ± 1.0 | 1.0ab ± 0.2 | 117.2a ± 78.0 | 2.6a ± 2.3 | 249.6a ± 164.7 |
| CTF | 48.6b ± 0.0 | 0.6b ± 0.0 | 109.1a ± 35.8 | 1.4a ± 0.4 | 224.2a ± 73.6 |
| Leaf litter | |||||
| TF | 41.9a ± 4.8 | 1.2ac ± 0.2 | 62.0a ± 24.8 | 1.8a ± 0.8 | 148a ± 54.4 |
| STF | 44.9ab ± 3.7 | 1.7b ± 0.3 | 109.1a ± 56.8 | 4.3a ± 2.4 | 234.9a ± 123.5 |
| WTBLF | 44.8ab ± 2.8 | 1.8b ± 0.1 | 189.9b ± 96.9 | 7.8b ± 4.1 | 418.3b ± 205.2 |
| CTBLF | 46.4b ± 3.0 | 1.5ab ± 0.2 | 274.0b ± 111.3 | 9.2b ± 4.8 | 547.1b ± 222.9 |
| CTF | 47.8b ± 1.1 | 0.9c ± 0.2 | 166.2ab ± 28.4 | 3.3ab ± 1.3 | 347.6ab ± 60.9 |
- Different letters within each column indicate significant difference between the forest types (P < 0.05).
Nitrogen concentrations in branch wood (P = 0.473) and foliage (P = 0.087) did not vary significantly between the forest types. However, N concentration differed significantly among forest types for dead wood (P = 0.001) and leaf litter (P = <0.001; Table 3). In accordance with total C density, total N density in leaf litter also differed significantly among TF, STF, WTBLF, CTBLF, and CTF (density ranged from 1.8 g to 9.2 g m−2). However, total N (g m−2) in the dead wood and live biomass components did not differ among forest types.
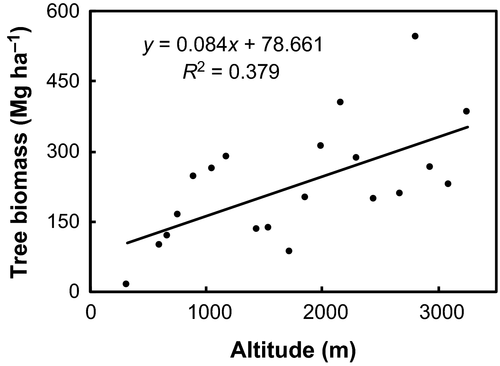
Biomass contributions from deadwood less than 5 cm in diameter did not vary among forest types. While the understorey foliage biomass for TF, STF, and WTBLF was significantly greater than the CTBLF and CTF, the biomass from the leaf litter for TF and STF was significantly less than the WTBLF and CTBLF (Table 3). For trees >10 cm DBH, the biomass increased by 8.39 Mg C ha−1 for every 100 m rise in altitude (Fig. 6).
Influence of edaphic parameters on the carbon and nitrogen stocks in soil
Soil C and N stocks were significantly (P < 0.01) correlated with altitude (Fig. 2), leaf litter (g m−2), clay (%), CEC (not for N stock), and soil C : N ratios (Fig. 7). In contrast, C and N stocks were negatively correlated soil pH. However, regression analysis of soil pH with C and N stocks were not significant. Species richness of forests were significantly correlated with C and N concentrations (P = 0.05), but not with C and N stocks. Although BA of the forest was positively correlated with altitude, it was neither significantly correlated with soil C and N stocks, nor with soil C and N concentrations (Table 4).

| C (Mg ha−1) | N (Mg ha−1) | Alt | Temp (°C) | Soil C (%) | Soil N (%) | BD | PH | EC | CEC | Clay (%) | Sand (%) | Silt (%) | Sp. richness | Basal area | Leaf litter | C/N | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C (Mg ha−1) | 1 | ||||||||||||||||
| N (Mg ha−1) | 0.72** | 1 | |||||||||||||||
| Alt (m) | 0.57** | 0.24* | 1 | ||||||||||||||
| Temp (°C) | −0.50** | −0.17 | −0.91** | 1 | |||||||||||||
| Soil C (%) | 0.32** | 0.17 | 0.62** | −0.54** | 1 | ||||||||||||
| Soil N (%) | 0.25* | 0.16 | 0.48** | −0.44** | 0.97** | 1 | |||||||||||
| BD (g cm−3) | −0.42** | −0.22 | −0.75** | 0.72** | −0.80** | −0.75** | 1 | ||||||||||
| Soil pH | −0.15 | −0.09 | −0.36** | 0.44** | −0.49** | −0.46** | 0.46** | 1 | |||||||||
| EC | −0.11 | −0.09 | 0.20 | −0.13 | 0.53** | 0.55** | −0.46** | −0.18 | 1 | ||||||||
| CEC | 0.35** | 0.15 | 0.62** | −0.52** | 0.88** | 0.83** | −0.81** | −0.39** | 0.56** | 1 | |||||||
| Clay (%) | 0.26* | 0.28* | −0.04 | 0.06 | −0.02 | −0.02 | −0.10 | −0.07 | 0.05 | 0.22* | 1 | ||||||
| Sand (%) | −0.13 | −0.12 | 0.06 | −0.03 | −0.05 | −0.06 | 0.27* | 0.07 | −0.19 | −0.30** | −0.64** | 1 | |||||
| Silt (%) | −0.01 | −0.05 | −0.05 | 0.00 | 0.08 | 0.09 | −0.28** | −0.04 | 0.21 | 0.24* | 0.14 | −0.85** | 1 | ||||
| Sp. richness | 0.18 | 0.10 | 0.33** | −0.22 | 0.24* | 0.26* | −0.31** | 0.05 | 0.20 | 0.22* | −0.13 | 0.12 | −0.07 | 1 | |||
| Basal area (m2 ha−1) | 0.20 | 0.00 | 0.69** | −0.71** | 0.30** | 0.20 | −0.60** | −0.24* | 0.20 | 0.35** | −0.13 | −0.02 | 0.11 | 0.22* | 1 | ||
| Leaf litter (g m−2) | 0.47** | 0.20 | 0.73** | −0.66** | 0.56** | 0.47** | −0.51** | −0.30** | 0.19 | 0.57** | −0.04 | 0.07 | −0.05 | 0.55** | 0.37** | 1 | |
| C/N ratio | 0.53** | 0.23** | 0.77** | −0.68** | 0.34** | 0.15 | −0.55** | −0.31** | −0.06 | 0.37** | −0.02 | 0.02 | −0.01 | 0.14 | 0.54** | 0.63** | 1 |
- *Correlation coefficients are significant at 0.05 and **correlation coefficients are significant at 0.01 level.
Meta-analysis of studies on soil carbon trend along altitudinal gradients
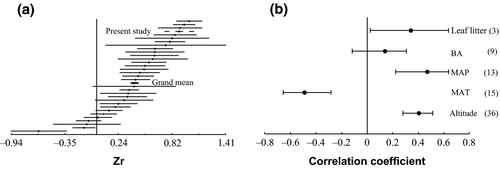
The present study and a majority of previous studies show positive correlations of soil C with altitude. A few exceptions showed no relation or negative correlation (Fig. 8a). Overall results of meta-analysis highlight a positive correlation of SOC with altitude (r = 0.38, CI = 0.27–0.49, Fig. 8b). When altitude was binned into two categories of high (>1500 m a.s.l.) and low (<1500 m a.s.l.), the correlation coefficient did not differ significantly. SOC was negatively correlated with MAT (r = −0.49, CI = −0.66 to −0.28) and positively correlated with MAP (r = 0.47, CI = 0.22–0.64). When MAT and MAP were binned into high and low classes, meta-analysis suggested no significant change in the strength of correlation between SOC and MAT or MAP. While forest BA was not related to SOC, quantities of leaf litter (kg m−2) on the forest floor were positively correlated with SOC (r = 0.35, CI = 0.01–0.63) (Fig. 8b), as found in the present study.
Discussion
Soil carbon and nitrogen trends with altitude
Soil C and N stocks and concentrations were significantly correlated with altitude, as well as temperature and edaphic parameters, which are in turn strongly determined by altitude. Correlation coefficients of altitude with C and N stocks were less than for C and N concentrations, mainly due to variation in BD and its effect on calculated C and N stocks. BD decreased with increasing altitude. Weak correlations of C and N stocks with clay content were not repeated for sand and silt contents. Increases in soil C and N stocks with altitude were mainly influenced by increased C and N input to soils from forest canopies, and the effect of temperature, rather than by soil texture per se. CEC was also highly correlated with both C and N concentrations and stocks, and CEC was generally high in soils with high organic content (Parfitt et al., 1995), suggesting that increases in C and N with altitude are due to increased organic matter content. Increases with altitude in BA, forest density (Table 1) and leaf litter (Table 3) and tree biomass (Fig. 6) supports this conclusion.
Increasing soil C and N stocks with altitude (and with decreasing temperature) is in agreement with meta-analysis wherein soil C increased with altitude (r = 0.40, CI = 0.29–0.50). Dieleman et al. (2013) compared SOC stocks for TF along an altitudinal gradient for 10 studies and, similar to our results, found a positive correlation (r = 0.43, P < 0.001). Altitude and temperature showed very similar correlation coefficients, pointing to temperature being the main driver for the C and N cycles and soil chemistry along the altitudinal gradient. In the global data set, temperature was the most important environmental parameter after precipitation (Fig. 8b), which was not available for the current study. Microbial decomposition rates decrease as altitude increases and temperatures fall. Accordingly, SOC is typically less in STF compared to temperate forest (Yang et al., 2007). Besides the effect of temperature on microbial activity, total microbial biomass, composition, and activity are influenced by soil pH. For two similar TFs in the Peruvian Amazon, the less acidic soil had a greater diversity of soil bacteria (Fierer & Jackson, 2006). In our study, slower rates of organic matter decomposition at higher altitudes were accompanied by increasing soil acidity, and increased stocks of C and N (Table 2) (see also Garten & Hanson, 2006).
Soil C : N ratio also increased with altitude. The dominant influence of temperature on soil respiration (Miller, 2000; Hopkins et al., 2012) ensures that at lower elevations, where temperatures are greater, losses of C to the atmosphere are maximized relative to those of N. At higher altitude with lower temperatures, organic matter decomposition slows and more C is retained relative to N. In our study, deeper soils (30–60 and 60–100 cm) at lower altitudes had lower C : N ratios than the surface soils, suggesting a greater degree of decomposition and older SOM in deeper soils. With increasing altitude, the C : N ratios of deeper soils tend to be higher than the surface soils (0–10 and 30–60 cm), probably due to decreased decomposition – arguably, organic matter in deeper soils at higher elevations represents a far reduced role of microbial turnover in the chemical nature of stored organic matter. Additionally to microbial processes, dissolved organic carbon (DOC) may have contributed to the increase in C : N ratio with altitude and depth. SOC is a major source of DOC (Ying et al., 2013) and C : N ratios in DOC were greater than in SOC (Neff et al., 2000). DOC can contribute as much as 25% of the total C in the soil (Neff & Asner, 2001). Conifer forests are characterized by a greater flux of DOC into the soil than deciduous forest (Hope et al. (1994). As high-altitude CTBLF and CTF were dominated by conifers, the greater C : N ratios at higher altitudes and in deeper soil horizons may have been influenced by DOC percolation, especially relative to the slower rates of C input and incorporation into the soil from biomass decomposition (Kalbitz & Kaiser, 2008).
Total C and N stocks of prime forests in the southern foothills of the Himalayas in Bhutan are among the highest reported in the world, especially for mountainous regions. Even though C and N are preferentially accumulated at shallower depths, deeper soils (60–100 cm) store substantial amounts of C and N stocks (from 24% to 36% and 22% to 35%, respectively, of the total C and N contents) and need to be considered for an accurate estimation of the C and N stocks in forest soils. A study in southwestern Australia has reported C stock in soils from 0 to 5 m depth to vary between 47% and 77% of the total C stock to bed rock (Harper & Tibbett, 2013). They also found organic C even at depths of 38, which suggests that there can be substantial underestimations of C and N stocks when deeper soils are ignored. In contrast, the greater C and N stocks in shallower soils at high altitude were mostly contained in organic matter due to slower decomposition rate (i.e. high CEC and C : N ratios) which is a good indication of the vulnerability of these stocks to increasing temperatures under climate change scenarios.
Biomass carbon concentration with altitude
Carbon concentration in understorey biomass components along the altitudinal gradient varied from 39.7% in the foliage of the TF to 48.6% in the dead wood of the CTF. Accurate estimation of C stocks and C sink potential are crucial for C accounting, and different methodological approaches may over or underestimate C stocks, for example, estimating standing dead tree biomass (Woodall et al., 2012) and tree allometry (Chave et al., 2005). Generally, biomass C concentration is considered to be 50% of the plant dry mass (IPCC, 2003; Epa, 2011, Keith et al., 2014); however, this simplified generalization is not a good approximation for all species and ecosystems and can lead to an erroneous estimation of C stock in forest ecosystems. In order to improve the modeling of C stock in Australian forests (FullCAM, National Inventory Report 2010), the C concentrations of different biomass components were taken into account (Gifford, 2000). Biomass C concentration may vary by as much as 10% even within same tree species because of age and tree components (Fu et al., 2013). Carbon concentration has also been shown to vary between species as well as between plant organs (Lamlom & Savidge, 2003; Thomas & Malczewski, 2007; Martin & Thomas, 2011; Fu et al., 2013). In the present study, most live biomass samples contained less than 47% C, and for all biomass samples, concentrations were <50%. Carbon concentrations differed among the various components of understorey plants and dead biomass as well as with altitude (Fig. 5). Similarly, tropical species have been previously noted to have reduced concentrations of C than temperate species (Thomas & Martin, 2012). The lower C concentrations in TF biomass could lead to an overestimation of C stocks in the tropics compared to high-altitude forests, if a general biomass C concentration of 50% of the dry mass is used. Even small stems (<10 cm) have a significant impact on C accounting (Preece et al., 2012). More detailed mapping of C concentrations in different forest components of various forest types will improve global C stock estimation of terrestrial forest ecosystems. Furthermore, studies conducted in regions with low MAT (<8 °C) have reported an increasing trend of soil C with increasing temperature (Liski & Westman, 1997; Callesen et al., 2003). This implies that there is a threshold temperature wherein with increasing altitude and decreasing temperature the soil C stocks will start to decrease, and can be further explored along targeted altitudinal gradients.
Acknowledgements
Sonam would like to thank the Australian Government for supporting this study through the Endeavour postgraduate scholarship award. We would like to thank the Ministry of Agriculture and Forest in Bhutan for kindly providing the necessary field and logistic assistance during the entire field work. We would like to acknowledge Floris Van Ogtrop for his valuable inputs in the experimental design and Lori Watson for her help in getting the import permit to bring soil and plant samples to Australia.
Notes
Appendix 1: Data sources used in the meta-analysis that provided correlation data on SOC with altitude, MAT, MAP, BA, and forest floor leaf litter
| Grouping | Number of studies | Authors |
|---|---|---|
| Altitude | 28 | Alexander et al., 1993, Raich et al., 1997, Garten et al., 1999, Lemenih & Itanna, 2004, Dai & Huang, 2006, Garten & Hanson, 2006, Tewksbury & Van Miegroet, 2007, Li et al., 2010, 2012, Zhu et al., 2010, Zimmermann et al., 2010, Razakamanarivo et al., 2011, Singh et al., 2011, Zhang et al., 2011, Chuai et al., 2012, Liu et al., 2012, Charan et al., 2013, Dieleman et al., 2013, Kumar et al., 2013, Ali et al., 2014, Campos et al., 2014, Du et al., 2014, Godgift et al., 2014, Longbottom et al., 2014, Maraseni & Pandey, 2014, Prietzel & Christophel, 2014, Shelukindo et al., 2014 |
| MAT | 10 | Garten et al., 1999, Lemenih & Itanna, 2004, Dai & Huang, 2006, Li et al., 2010, Singh et al., 2011, Zhang et al., 2011, Liu et al., 2012, Campos et al., 2014, Du et al., 2014, Prietzel & Christophel, 2014 |
| MAP | 8 | Lemenih & Itanna, 2004, Dai & Huang, 2006, Singh et al., 2011, Liu et al., 2012, Campos et al., 2014, Du et al., 2014, Longbottom et al., 2014, Prietzel & Christophel, 2014 |
| BA | 7 | Raich et al., 1997, Garten & Hanson, 2006, Tewksbury & Van Miegroet, 2007, Li et al., 2010, Zhu et al., 2010, Zhang et al., 2011 |
| Leaf litter | 3 | Li et al., 2010, Zhang et al., 2011, Galka et al., 2014 |
Appendix 2: Soil properties at different soil depths (mean ± SD) for forest types in Bhutan
| Forest zone | 0–10 cm | 10–30 cm | 30–60 cm | 30–100 cm |
|---|---|---|---|---|
| Soil pH (1 : 5 H2O) | ||||
| TF | 5.46a ± 0.7 | 5.43a ± 0.7 | 5.58a ± 0.7 | 5.83a ± 0.7 |
| STF | 4.85b ± 0.4 | 4.96b ± 0.4 | 5.15b ± 0.4 | 5.34b ± 0.5 |
| WTBLF | 4.71b ± 0.2 | 4.92b ± 0.2 | 4.97b ± 0.2 | 5.24b ± 0.3 |
| CTBLF | 4.66b ± 0.3 | 4.74b ± 0.2 | 4.91b ± 0.1 | 5.09b ± 0.1 |
| CTF | 4.88ab ± 0.4 | 4.81ab ± 0.2 | 4.88b ± 0.1 | 5.13ab ± 0.1 |
| Electrical conductivity (1 : 5; µS cm−1) | ||||
| TF | 42a ± 21 | 28a ± 12 | 25a ± 14 | 27a ± 19.0 |
| STF | 52a ± 26 | 36a ± 16 | 35a ± 27 | 21a ± 6.0 |
| WTBLF | 64a ± 30 | 31a ± 11 | 23a ± 5.0 | 20a ± 7.0 |
| CTBLF | 64a ± 39 | 60b ± 32 | 43a ± 29 | 29a ± 8.0 |
| CTF | 67a ± 15 | 54ab ± 5.0 | 36a ± 18 | 16a ± 0.1 |
| Sand (%) | ||||
| TF | 49.4a ± 7.2 | 51.4a ± 8.4 | 51.8a ± 9.7 | 53.6ab ± 11.8 |
| STF | 39.3a ± 12.8 | 36.5b ± 11.0 | 40.4a ± 9.3 | 43.1a ± 11.9 |
| WTBLF | 51.6a ± 17.1 | 53.1a ± 16.5 | 52.8a ± 21.8 | 57.8b ± 18.8 |
| CTBLF | 46.9a ± 18.7 | 41.8ab ± 15.9 | 42.3a ± 15.7 | 43.5ab ± 17.8 |
| CTF | 74.3* | 72.2* | 80.4* | 87.3* |
| Silt (%) | ||||
| TF | 30.9ab± 6.7 | 29.0ac ± 6.1 | 27.5ac ± 5.9 | 24.3a ± 5.7 |
| STF | 39.3a ± 8.1 | 40.9bc ± 6.7 | 37.6bc ± 8.5 | 38.3b ± 12.0 |
| WTBLF | 28.8b ± 8.2 | 24.7a ± 4.7 | 24.2a ± 7.1 | 22.4a ± 5.1 |
| CTBLF | 34.8ab ± 12.1 | 36.7c ± 16.8 | 33.4c ± 8.6 | 29.4a ± 7.6 |
| CTF | 12.1* | 11.8* | 7.8* | 3.1* |
| Clay (%) | ||||
| TF | 19.6a ± 5.5 | 19.5a ± 5.5 | 20.6a ± 7.1 | 22.0a ± 8.3 |
| STF | 21.2a ± 6.7 | 22.5a ± 8.9 | 21.9a ± 8.9 | 18.5a ± 6.4 |
| WTBLF | 19.4a ± 9.7 | 22.0a ± 11.9 | 22.8a ± 15.0 | 19.7a ± 13.9 |
| CTBLF | 18.2a ± 9.7 | 21.4a ± 9.8 | 24.6a ± 10.6 | 26.9a ± 11.2 |
| CTF | 12.8* | 15.8* | 11.7* | 9.5* |
| Cation exchange capacity (cmolc kg−1) | ||||
| TF | 11.13a ± 7.0 | 8.69a ± 4.5 | 6.7a ± 2.3 | 8.53a ± 5.4 |
| STF | 18.87ab ± 9.6 | 14.98a ± 7.2 | 11.6ab ± 6.7 | 8.73a ± 6.4 |
| WTBLF | 23.81bc ± 7.8 | 17.52ac ± 9.5 | 16.4b ± 7.4 | 12.89a ± 6.5 |
| CTBLF | 31.33c ± 7.4 | 30.55b ± 8.1 | 26.6c ± 4.8 | 24.45b ± 6.1 |
| CTF | 33.23bc ± 2.8 | 34.16bc ± 9.6 | 21.1bc ± 7.8 | 15.13ab ± 2.4 |
| Bulk density (g cm−3) | ||||
| TF | 1.32a ± 0.2 | 1.32a ± 0.2 | 1.42a ± 0.2 | 1.47a ± 0.3 |
| STF | 0.91b ± 0.2 | 0.98b ± 0.2 | 1.08b ± 0.2 | 1.07b ± 0.2 |
| WTBLF | 0.70c ± 0.1 | 0.89b ± 0.2 | 0.92b ± 0.2 | 0.98b ± 0.2 |
| CTBLF | 0.56c ± 0.2 | 0.62c ± 0.2 | 0.68c ± 0.1 | 0.72c ± 0.1 |
| CTF | 0.52c ± 0.1 | 0.52c ± 0.1 | 0.82bc ± 0.2 | 0.86bc ± 0.1 |
Appendix 3: Total carbon and nitrogen stocks in Mg ha−1 (mean ± SD) at various depths in soils from different forest zones in Bhutan
| Altitude (m a.s.l.) | Forest zones | 0–10 cm | 10–30 cm | 30–60 cm | 60–100 cm |
|---|---|---|---|---|---|
| Total C (Mg ha−1) | |||||
| 317–900 | TF | 20.4a ± 11.0 | 28.9a ± 16.5 | 33.5a ± 19.2 | 31.6a ± 18.6 |
| 900–1870 | STF | 35.7ab ± 12.6 | 55.5ab ± 27.8 | 60.1ac ± 30.6 | 66.1a ± 47.4 |
| 1870–2450 | WTBLF | 49.6b ± 13.0 | 80.6bc ± 22.7 | 78.4ac ± 21.6 | 117.4b ± 44.0 |
| 2450–3000 | CTBLF | 51.0bc ± 5.8 | 92.7c ± 24.2 | 128.5b ± 42.2 | 135.9b ± 32.2 |
| 3000–3300 | CTF | 78.5c ± 46.3 | 107.9bc ± 59.9 | 119.5bc ± 58.2 | 97.5ab ± 23.9 |
| Total N (Mg ha−1) | |||||
| 317–900 | TF | 1.9a ± 0.9 | 2.5a ± 1.2 | 3.1a ± 1.5 | 3.1a ± 1.8 |
| 900–1870 | STF | 3.1b ± 1.0 | 4.5b ± 2.5 | 5.3a ± 3.1 | 5.7a ± 4.3 |
| 1870–2450 | WTBLF | 4.3cd ± 1.2 | 6.2b ± 1.8 | 6.1ab ± 1.8 | 9.1b ± 3.9 |
| 2450–3000 | CTBLF | 3.6bc ± 0.8 | 5.8b ± 1.4 | 8.3b ± 2.1 | 8.7b ± 2.7 |
| 3000–3300 | CTF | 5.4d ± 3.1 | 6.8b ± 3.4 | 7.6ab ± 4.1 | 5.8ab ± 1.2 |




