Isotopic evidence for the occurrence of biological nitrification and nitrogen deposition processing in forest canopies
Abstract
This study examines the role of tree canopies in processing atmospheric nitrogen (Ndep) for four forests in the United Kingdom subjected to different Ndep: Scots pine and beech stands under high Ndep (HN, 13–19 kg N ha−1 yr−1), compared to Scots pine and beech stands under low Ndep (LN, 9 kg N ha−1 yr−1). Changes of NO3-N and NH4-N concentrations in rainfall (RF) and throughfall (TF) together with a quadruple isotope approach, which combines δ18O, Δ17O and δ15N in NO3− and δ15N in NH4+, were used to assess N transformations by the canopies. Generally, HN sites showed higher NH4-N and NO3-N concentrations in RF compared to the LN sites. Similar values of δ15N-NO3− and δ18O in RF suggested similar source of atmospheric NO3− (i.e. local traffic), while more positive values for δ15N-NH4+ at HN compared to LN likely reflected the contribution of dry NHx deposition from intensive local farming. The isotopic signatures of the N-forms changed after interacting with tree canopies. Indeed, 15N-enriched NH4+ in TF compared to RF at all sites suggested that canopies played an important role in buffering dry Ndep also at the low Ndep site. Using two independent methods, based on δ18O and Δ17O, we quantified for the first time the proportion of NO3− in TF, which derived from nitrification occurring in tree canopies at the HN site. Specifically, for Scots pine, all the considered isotope approaches detected biological nitrification. By contrast for the beech, only using the mixing model with Δ17O, we were able to depict the occurrence of nitrification within canopies. Our study suggests that tree canopies play an active role in the N cycling within forest ecosystems. Processing of Ndep within canopies should not be neglected and needs further exploration, with the combination of multiple isotope tracers, with particular reference to Δ17O.
Introduction
Forest canopies play a significant role in regulating carbon and water exchanges with the atmosphere, with profound effects on climate (Schulze, 2006; Canadell et al., 2007; Bonan, 2008). However, the contribution of tree canopies in altering the chemical composition of precipitation and, consequently, the nutrient cycling within a forest has been less investigated. In particular, it is unclear whether the deposition of reactive nitrogen species (Ndep) to canopies is retained, re-emitted and/or altered by chemical or biological reactions and what portion and chemical form of deposited N eventually reaches the soil as washed out N compounds. Interception of Ndep by forest canopies contributes to the cycling of N in the terrestrial biosphere, thereby affecting plant health, community structure and biodiversity, nutrient cycling, greenhouse gas balance, soil pH and water quality (Lindberg et al., 1986; Vitousek et al., 1997; Cape & Percy, 1998; Pitcairn et al., 1998; Rennenberg & Gessler, 1999; Prescott, 2002; Galloway et al., 2004; Pitman et al., 2010; Vanguelova et al., 2011).
Understanding the interactions taking place between atmospheric N and forest canopies, under different environmental conditions and Ndep levels, for various forest types (e.g. conifer vs. broadleaf forests) and tree species remains complex. Systematic monitoring of the main N chemical species (i.e. NH4+, NO3−, dissolved organic N) in rainfall (RF) and throughfall (TF) has now been carried out for almost two decades in a network of experimental European forests (i.e. level II network of ICP plots http://icp-forests.net/). While these measurements quantify the atmospheric N inputs to forests and soils, they have not been sufficient to allow assessing in-canopy processes that may be affecting changes in N compounds.
Forests are particularly efficient at scavenging pollutants via dry and occult deposition due to their aerodynamically rough canopies (Fowler et al., 1989). As a consequence, the total N speciation and N concentrations in RF differ from those in TF. Fluxes of N in TF reflect a mixture of wet, occult (fog/cloud) and dry deposition, which may also be chemically or biologically modified during canopy exchange and uptake. Commonly, TF has a higher N compound concentration compared with RF, particularly in areas subjected to high N input from the atmosphere, which provide indication of dry Ndep inputs (Lovett & Lindberg, 1993; Lovett, 1994; Tietema & Beier, 1995; Lovett et al., 2000; Vanguelova et al., 2010; Fang et al., 2011). Occult deposition can also be marked in areas where seasonal fogs and N pollution sources coincide. This has resulted in very large N inputs (25–45 kg ha−1 yr−1) in some areas such as the most highly exposed forests of the Los Angeles air basin (Bytnerowicz & Fenn, 1996). Using a labelled N approach, foliar uptake of aqueous N was recently proved to occur in beech and birch, with NH4+ more readily taken up than NO3− (Wuyts et al., 2015). Ammonia is readily absorbed directly onto foliage (see the review by Pearson & Stewart, 1993), and TF-N fluxes are enhanced in forests that are near NH3 sources such as agricultural and farming areas (Vanguelova & Pitman, 2009). Moreover, in very low Ndep areas (e.g. total Ndep of 2–3 kg ha−1 yr−1), such as in Finland, tree canopies tend to retain much of the N they capture by dry deposition due to uptake by epiphytic lichens, microbial immobilization within the canopy, N absorption into foliage and assimilation by leaves and stems (Mustajärvi et al., 2008). A recent study conducted in Italian forests reported an apparent canopy consumption of N for sites at low Ndep, that is <4 to 6 kg N ha−1 yr−1 (Ferretti et al., 2014). Similarly, in a study conducted in three National Parks in Washington State (USA) subjected to low Ndep, up to 90% of the atmospheric N, mostly in the form of NO3-N, was found to have been consumed by the forest canopies (Fenn et al., 2013).
The stable nitrogen isotope composition (δ15N) of wet Ndep has helped to characterize the sources of atmospheric N (Heaton, 1987; Freyer, 1991; Kendall et al., 2007 and references therein) and its transformations when interacting with the biosphere, as assessed through measurements of δ15N in plants and soil (Ammann et al., 1999; Nadelhoffer et al., 1999; Saurer et al., 2004; Guerrieri et al., 2009, 2011; Savard et al., 2009). In addition, observations have been made of changes in the δ15N of NO3− in TF that suggested the occurrence of nitrification processes (i.e. from NH4+ to NO3−) in the canopy of Norway spruce of Central Europe (Sah & Brumme, 2003) and of a montane rainforest in Ecuador (Schwarz et al., 2011). Teuber et al. (2007) found evidence that autotrophic nitrifiers were present in the needles of a spruce forest exposed to high levels of Ndep (but not in needles of tree canopies exposed to low levels of Ndep), and proposed that canopy N transformations may partly be bacterial. However, a broad range of processes can lead to similar alterations of TF isotopic composition, so distinguishing between various processes using a single-isotope approach is challenging.
The application of the dual isotope approach, that is the combined measurement of δ15N and δ18O in NO3−, in bulk precipitation and stream water has provided another important step towards a better understanding of the importance of Ndep and of its cycling in forests. For example, δ18O can help assess whether the NO3− in the soil solution derives from atmospheric N or from nitrification processes. This is possible because of the large difference between the isotopic signature of the atmospherically derived NO3− (between 20 and 80‰) and the signature for the NO3− derived from nitrification (between −10 and +10‰, Kendall, 1998; Burns & Kendall, 2002).
An even more powerful approach has been proposed by Michalski et al. (2002, 2003) and Costa et al. (2011) based on the measurements of δ17O, together with δ18O, to characterize the sources of NO3−. Mass-dependent isotope fractionation leads to a consistent relationship between δ17O and δ18O, that is δ17O ≈ 0.52 × δ18O (Matsuhisa et al., 1978; Miller, 2002; Young et al., 2002). However, in the case of ozone-mediated nitrate formation in the atmosphere, mass-independent oxygen isotope compositions are observed (Michalski et al., 2002). This ‘excess’ of 17O is quantified by Δ17O = δ17O − 0.52 × δ18O. This means that ozone-derived NO3− has a Δ17O > 0, while mass-dependent nitrification produces NO3− with Δ17O = 0. These new tools offer the possibility to test some of the hypotheses previously proposed in the literature, in particular to determine the relative contribution of occult dry deposition and of bacteria-mediated nitrification in tree canopies to the chemical composition of canopy TF and the N input to the soil.
This study investigated whether N transformations occurred within the tree canopies of four different forests in the United Kingdom subjected to different levels of Ndep. The NO3-N, NH4-N and dissolved organic nitrogen (DON) concentrations in RF and TF were used to assess the role of canopy in filtering, retaining and processing atmospheric N. Furthermore, we used δ15N-δ18O and Δ17O in NO3− and δ15N in NH4+, to assess whether and how atmospheric N is processed within the canopy. In particular, we tested the following hypotheses: (1) in forests with low to intermediate levels of Ndep (i.e. about 10 kg ha−1 yr−1), no differences exist between RF and TF for either ion concentrations or their isotopic signature, regardless of the tree species. In cases when most of the atmospheric N is retained in the canopies, the isotopic signatures of NO3− and NH4+ in TF should still reflect that of atmospheric N in RF, as a result of low canopy processing and canopy uptake. (2) At high Ndep sites, exceeding critical N loads (i.e. 20–30 kg ha−1 yr−1), significant differences exist between RF and TF for both NH4-N and NO3-N concentrations and their isotopic signature, as a result of isotope fractionations during N processing within the canopy and enhanced by the high input of wet and dry Ndep. For the first time, we used two independent approaches, based on Δ17O and δ18O in NO3− to determine the occurrence of bacterial nitrification from NH4+ to NO3− in forest canopies at high Ndep levels.
Materials and methods
Site description and sampling
Two Scots pine (Pinus sylvestris L) and two beech (Fagus sylvatica L.) stands were studied. The pine stands were within the UK Forest Monitoring network (http://www.forestry.gov.uk/fr/INFD-67MEVC; Vanguelova et al., 2010), which is part of the ICP European Forest Network. The two beech stands are part of long-term experiments on monitoring of the effects of Ndep on forest and soil biochemical cycling in the United Kingdom (Vanguelova & Pitman, 2009, 2011). Two forests, one for each tree species, were situated at Alice Holt and Rogate (6 km apart) in South East England and the remaining two at Thetford (<8 km apart), east England. They were chosen on the basis of similarity in stand (age, density and management history), climate and soil conditions, but at contrasting levels of ambient Ndep (Table 1). In particular, the pine and beech stands at Thetford are subjected to higher background levels of Ndep (13 and 19 kg N ha−1 yr−1, respectively) compared to forest stands at Alice Holt and Rogate (9–10 kg N ha−1 yr−1) (Table 1). Thetford in East Anglia is known to be among the areas with highest atmospheric N inputs in the United Kingdom (Vanguelova et al., 2010; RoTAP, 2012), mostly in the reduced form, coming mainly from the intensive livestock farms (in particular pigs and chickens). Therefore, the two forest stands in Thetford will be referred to as HN (high nitrogen) and the forests in Rogate and Alice Holt as LN (low nitrogen) sites. Rainfall (RF) and throughfall (TF) sampling and analysis have been carried at the sites over a number of years by means of two bulk RF collectors and ten TF collectors per site. Sampling and analytical procedures followed the level II protocols described in detail in the ICP Forests (2010) manual. In this study, only samples collected biweekly during the 2011 growing season, from June until November, were considered.
| Site | Location | Forest stand | Stand age (years) | Soil type (WRB, 2006) | Precipitation (mm yr−1) | T (°C) | NH4+/NO3− Dry dep. (kg ha−1 yr−1) | NH4+/NO3− Wet dep. (kg ha−1 yr−1) | Tot Ndep Dry/Wet (kg ha−1 yr−1) | Tot Ndep (kg ha−1 yr−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| LN | Alice Holt | Beech | 70 | Cambisol | 800 | 11.6 | 2.7/0.2 | 3.7/3.2 | 2.9/6.9 | 9.8 |
| Rogate | Scots pine | 60 | Cambisol | 800 | 11.6 | 4.1/0.6 | 3.1/2.9 | 4.8/5.9 | 10.7 | |
| HN | Thetford | Beech | 70 | Arenosol | 600 | 11.3 | 4.9/4.6 | 7.5/2.7 | 9.5/10.2 | 19.7 |
| Scots pine | 45 | 3.2/1.8 | 5/3.3 | 5.0/8.4 | 13.4 |
Chemical and isotope analyses of water samples
After collection, RF and TF water samples were filtered through a 0.45-μm membrane filter and then analysed for NH4-N, colorimetrically, and total N by Carbon analyser (Shimadzu 5000, Osaka, Japan; Dionex UK Ltd, Surrey, UK) and for NO3-N by ion chromatography (Dionex DX-500; Dionex UK Ltd). DON was calculated from the difference between measured total and inorganic nitrogen forms. Chemical analyses were carried out on water samples collected from each of the RF and TF collectors. The RF and TF elemental fluxes were calculated using measured water volumes at the sites and measured elemental concentrations. Dry Ndep values were estimated as the difference between TF and RF for each of the N forms according to European ICP forest monitoring manual, which assumed zero canopy exchange (ICP Forests, 2010) (Tables 1 and 2). To check this assumption, we compared values measured at our sites with the 5 × 5 km grid modelled Ndep data set for the United Kingdom, as used in the RoTAP (2012) review. The estimate included wet and dry NHx-N (NH4, NH3) and NOy-N (NO2, NO3, HNO3) deposition, modelled with FRAME upon 2005 emissions data (RoTAP, 2012 – chapter 4).
| Site | Location | Forest stand | NH4-N/NO3-N RF (kg ha−1) | NH4-N/NO3-N TF (kg ha−1) | DON RF (kg ha−1) | DON TF (kg ha−1) |
|---|---|---|---|---|---|---|
| LN | Alice Holt | Beech | 1.54/1.52 | 1.05/1.26 | 1.00 | 1.68 |
| Rogate | Scots pine | 1.31/1.41 | 1.08/0.94 | 0.91 | 1.69 | |
| HN | Thetford | Beech | 3.14/0.97 | 9.96/2.12 | 1.15 | 2.48 |
| Scots pine | 1.92/1.42 | 3.67/3.83 | 0.65 | 1.19 |
A subsample of the water analysed for ion concentrations was used for stable isotope measurements. Based on measured concentrations, we worked out the volume of water needed to obtain NH4-N and NO3-N concentrations >0.5 mg. For this reason, we combined water collected from June until August and then from September until November and we considered (on average between the two time windows considered) 1.5 l for RF and 1 l for TF in the case of forests at HN, while 2 l for RF and 3–4 l for TF in the case of forests at the LN. Pooling was also necessary for RF water samples collected at the two LN and the two HN sites because not enough volume of water was available for each of the two forests at the LN. We assumed that pooling RF water samples within each level of Ndep was not likely to have an impact on the characterization of the isotopic signature of the atmospheric N, due to similar atmospheric N input and source. Indeed, no significant differences were found in the amount of NO3-N and NH4-N in RF at either of the two sites, except at Thetford where the NH4-N was significantly (P < 0.05) higher in the beech relative to the pine stand at the HN site. This was likely the result of the beech site being located only a few 100 m away from a chicken farm that generates NH3 concentrations as high as ~73 μg m3 (Vanguelova & Pitman, 2009, 2011).
Each RF and TF sample was composited as described above and then passed through cation and anion exchange resins. Ammonium from the cation resin was eluted with hydrochloric acid and converted to ammonium sulphate on a quartz filter paper using an alkaline diffusion method (Heaton, 2001). Nitrate from the anion resin was eluted with hydrobromic acid and processed to silver nitrate (Chang et al., 1999; Heaton et al., 2004). The 15N/14N ratios of the ammonium sulphate and the silver nitrate were analysed by combustion in a Flash EA online to a Delta Plus XL mass spectrometer (ThermoFinnigan, Bremen, Germany), with δ15N values vs. air (atmospheric N2) calculated by comparison with standards calibrated against IAEA N 1 and N 2 assuming these had values of +0.4 and +20.3‰, respectively. 18O/16O ratios of the silver nitrate were analysed by thermal conversion to CO gas at 1400°C in a TC-EA online to a Delta Plus XL mass spectrometer (ThermoFinnigan, Bremen, Germany), with δ18O values calculated vs. SMOW by comparison with IAEA-NO3 assuming it had a value of +25.6‰. Analytical precisions (1 SD) were typically <0.3‰ for δ15N and <0.6‰ for δ18O. Finally, a subsample of the composite RF and TF water as described above was used for δ17O measurements by Delta V Plus ratio mass spectrometer. The NO3− was converted to O2 and N2 using the denitrifier method (Casciotti et al., 2002; Kaiser et al., 2007). Analytical precisions (1 SD) for Δ17O were <1.0‰ based on replicate analysis of the reference material USGS35.
Statistical analyses
Concentrations of NH4-N and NO3-N were log-transformed to account for non-normality and variance heterogeneity, as assessed through Shapiro and Levene test, respectively. Independent sample t-tests were employed to test for differences between deposition levels (e.g. HN and LN) and water samples (i.e. RF and TF) for NH4-N and NO3-N, while, within each water sample, differences between concentrations of different compounds were tested through paired-samples t-tests (t). The nonparametric Wilcoxon test (W) was employed when log-transformed data did not conform to a normal distribution. Given the small sample size available for the isotopic data (i.e. n = 2 for RF and n = 4 for TF per level of Ndep, as a result of pooling the water samples collected from June until August and then September until November), we calculated the difference in isotopic fractionation between TF and RF without separating beech and pine stands and used a t-test to verify the significance of the difference between LN and HN stands. The level of significance of all statistical tests was set as P ≤ 0.05. R project statistical computing (vers. 3.0.2; R Core Team, 2014) was used for all the analyses.
Mass balance calculations based on Δ17O and δ18O
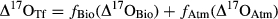 (1)
(1)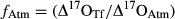 (2)
(2)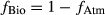 (3)
(3)
The assumption of similar Δ17O values for wet and dry Ndep stems from the fact that Δ17O in atmospherically derived nitrate is mostly determined by photochemical oxidation of NOx by tropospheric ozone (Michalski et al., 2011), not the phase (gaseous, solid, or liquid) into which it is partitioned. Measurements of aerosol nitrate and rain NO3− collected during the same season do not have significant differences in Δ17O values (Michalski et al., 2011; Riha, 2013). Hence it is not related to specific point emission sources and it is not affected by mass-dependent isotope fractionations, which, in fact play a significant role in the case of the other two isotope ratios, that is 18O/16O and particularly 15N/14N.
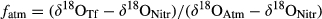 (4)
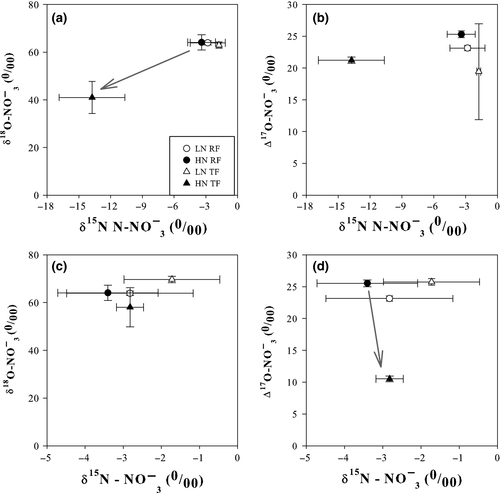
(4)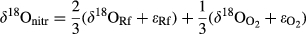 (5)
(5)Assuming negligible the isotope fractionation during water (ɛRf) and O2 ( ) incorporation (Mayer et al., 2001), δ18O of NO3− from nitrification was obtained from δ18O of atmospheric O2 (δ18OAtm = 23.9‰, Barkan & Luz, 2005) and the oxygen isotopic signature of the RF. We have assumed this latter to have values of about −5.5‰ (for June–August) and −8.5‰ (for September–November), based on the weighted mean δ18O values for June–August 2011 and September–November 2011 rainfall at a site near Oxford in the United Kingdom (W.G. Darling, personal communication).
) incorporation (Mayer et al., 2001), δ18O of NO3− from nitrification was obtained from δ18O of atmospheric O2 (δ18OAtm = 23.9‰, Barkan & Luz, 2005) and the oxygen isotopic signature of the RF. We have assumed this latter to have values of about −5.5‰ (for June–August) and −8.5‰ (for September–November), based on the weighted mean δ18O values for June–August 2011 and September–November 2011 rainfall at a site near Oxford in the United Kingdom (W.G. Darling, personal communication).
Results
Concentrations of NH4-N, NO3-N and DON in RF and TF
The concentration of N compounds varied between LN and HN sites and between RF and TF. At the two LN forests the concentrations of ions in RF were not significantly different (Fig. 1a, b) and the RF and TF had similar NH4-N and NO3-N concentrations (Scots pine: t = 1.78, 7.97 and P = 0.11, 0.56, respectively; beech: W = 163, 125 and P = 0.73, 0.48, respectively). In contrast, at the HN forests, the NH4-N and NO3-N concentrations were significantly higher in TF compared to RF, for both Scots pine (t = 6.42, 6.26, respectively; all P < 0.001) and beech (W = 265, 250, respectively; all P < 0.001) (Fig. 1a, b). Ion concentrations in both RF and TF were significantly higher at the HN than at LN sites, with the exception of RF in the beech stands, which had similar NO3-N concentrations. Concentrations of DON in both RF and TF were significantly (RF: W = 32, P < 0.05; TF: W = 34, P < 0.01) higher for the beech stand (Fig. 1c) at the HN compared to LN site. By contrast, Scots pine (Fig. 1c) subjected to different atmospheric N loads from the atmosphere showed similar values of DON concentrations in both RF and TF. However, DON concentrations in RF did not show a significant difference when comparing beech and Scots pine stand at the LN site, while concentrations were slightly higher (W = 35, P = 0.05) at the beech compared to the Scots pine stand at the HN site. Concentrations of DON in TF were similar at the two LN forests, while they were higher (W = 33; P < 0.05) at the beech than at the Scots pine stand at the HN site (Fig. 1c).
Extrapolation of our seasonal measurements over time and model validation of estimated dry Ndep fluxes
The mean of total N fluxes during the 6 months we considered in this study (i.e. June–November 2011) is reported in Table 2. TF-N fluxes were higher than RF fluxes at the two forests at the HN, with particular reference to the NH4-N at the beech site. By contrast, at the LN site, RF N fluxes were higher than TF-N fluxes for both species. An independent estimate of the dry Ndep at our sites can be obtained using the modelling approach outlined in RoTAP (2012). Figure S1 (in Supporting Information) shows a comparison of the measured wet N and estimated dry N fluxes (i.e. as a difference between TF and RF fluxes) at the two level of Ndep and shown in Table 1, with the fluxes of wet and dry Ndep obtained from the 5 × 5 km grid UK map, based on modelled Ndep with FRAME upon 2005 N emission data (RoTAP, 2012). Interestingly, a reasonably good agreement was found between the on-site measurements and the modelled values of wet Ndep. However, the fluxes of dry Ndep predicted using the RoTAP modelling approach were much higher than those estimated as the difference between TF and RF fluxes at our sites (Fig. S1).
Values of δ 15N-NH4
Values of δ15N-NH4+ in RF (Fig. 2a) ranged from positive at the HN site (+1.49 ± 3.5‰) to very negative at the LN site (−9.14 ± 0.2‰). Due to the limited number of RF measurements (i.e. n = 2 per level of Ndep), statistical analyses of isotope data were performed per level of Ndep, combining data for both tree species and focussing on the differences between RF and TF. However, TF values measured separately for beech and Scots pine are presented in Fig. 2a, to show the species-specific changes in the isotope compositions in N compounds collected below the canopies. More positive values were measured for δ15N-NH4+ in TF compared to RF at both HN (t = −2.85, P < 0.05) and LN (t = −15.16, P < 0.001) sites. The TF–RF difference for δ15N in NH4+ was much higher (t = −2.65, P < 0.05) at the LN compared to the HN site (Fig. 2b).

Values of δ 15N, δ 18O and Δ 17O-NO3
The δ15N in NO3− of RF (Fig. 3a) showed similar negative values at the HN (−3.4 ± 1.4‰) and LN sites (−2.8 ± 1.7‰). Albeit lower, the δ15N-NO3− values in TF at the HN site (diff = −4.9‰ ± 3.4) were only slightly different (t = −1.72, P = 0.06) compared to the LN sites (diff = +1.1 ± 0.54‰) (Fig. 3d). Despite differences between RF and TF for δ15N in NO3− not being significant within each level of Ndep, δ15N in NO3− showed more negative values in TF than in RF at the HN site at the Scots pine stand (Fig. 3a).
The δ18O in NO3− of RF showed similar values at the two different levels of Ndep, that is LN = 63.9 ± 0.88‰ and HN = 64.1 ± 3.2‰ (Fig. 3b). Within each level of Ndep, δ18O values did not significantly differ between RF and TF. However, similarly to δ15N, we observed lower δ18O in TF compared to RF in the case of the Scots pine at the HN. A significant contrast (t = −2.34, P < 0.05) was found in the difference between the δ18O values of NO3− in TF compared with RF across levels of Ndep (Fig. 3e), with lower δ18O-NO3− values at HN than at LN site.
Δ17O values measured in RF at our sites ranged from 23.14 (±0.58)‰ at the LN site to 25.53 (±0.76)‰ at the HN site. A significant difference was found in the Δ17O of NO3− in the TF vs. RF at the HN sites (W = 16, P < 0.05), but not at the LN sites. Within individual species, it is worth pointing out that beech showed lower Δ17O values than Scots pine (Fig. 3c). When we considered the difference between RF and TF, Δ17O values in NO3− had lower values on average at the HN sites (t = −1.86, P = 0.05) than at LN sites (Fig. 3f), but the difference was not significant.
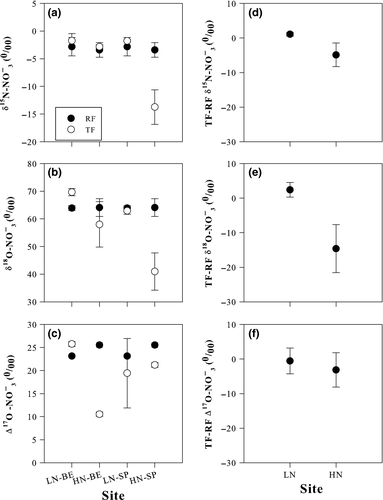
Combined plots for the three isotopic species of NO3− at the Scots pine and beech sites are given in Fig. 4 as trajectories of change from RF to TF values, to emphasize the consequences of canopy processing for the three tracers, with particular references to forests at HN levels. For Scots pine (Fig. 4a, b), only in the case of HN sites did δ15N, δ18O and to a less extent Δ17O values in TF diverge from those measured in RF. For beech (Fig. 4c, d), distinct changes in δ18O vs. δ15N were not observed and, only in the case of HN site, did Δ17O become substantially lower from RF to TF.
Assessing the source of NO3− in the TF at the sites with high atmospheric N loads
Two mixing models, partitioning fluxes based on either Δ17O or δ18O, were used to estimate the relative contributions of atmospheric vs. nitrification-derived NO3− collected underneath tree canopies. Using the two-end-member mixing model with the Δ17O [Eqns 2 and 3 in the 2] values measured in TF and RF (Table S1), the fractions of NO3− in TF coming from nitrification (fbio) ranged from 0.17 for the Scots pine up to 0.59 for the beech (i.e. 17–59%) at the two HN sites (Fig. 5a). Most of the NO3− collected in the TF at the Scots pine stand derived from the atmosphere (mean of fAtm = 0.83 ± 0.002), with only a minor contribution from nitrification (mean of fBio = 0.17 ± 0.002). By contrast, biologically derived NO3− seemed to be the dominant fraction of the NO3− in TF of the beech stand (fBio = 0.59 ± 0.03), at least for the time period considered in this study (Fig. 5a).

When using the mixing model based on δ18O partitioning [Eqns 4 and 5 in the 2], a higher fraction of NO3− in TF was estimated to derive from the atmosphere (Scots pine: fAtm = 0.62 ± 0.07; beech: fAtm = 0.90 ± 0.09) than from nitrification (Scots pine: fBio = 0.38 ± 0.07; beech: fBio = 0.10 ± 0.09) (Fig. 5b). The two approaches were more consistent for the Scots pine, while they did lead to opposite results in the case of beech. Averaging across the two methods, the proportion of the biologically derived nitrification was 27% for Scots pine (range of 17–38%) and 34% for beech (range of 10–59%).
Discussion
Four forests (two Scots pine and two beech stands) subjected to contrasting levels of Ndep in the United Kingdom were selected to assess whether and how tree canopies altered Ndep and its isotopic signature in TF. To our knowledge, this is the first study that combined measurements of NO3-N and NH4-N fluxes together with their relative isotope signatures, that is δ15N in NO3− and NH4+ and δ18O and, specifically, Δ17O in NO3− to determine the role of canopy processing of atmospherically derived Ndep. In the following sections, we discussed changes in TF fluxes at the HN and LN sites and how stable isotopes helped assessing the different processes taking place on tree canopies exposed to different atmospheric N loads.
Atmospheric N and its isotopic signatures at the contrasting Ndep levels
Both beech and Scots pine forests at HN sites were subjected to air masses with high NH3-N/NH4-N concentrations and had higher NH4-N deposition relative to the LN sites. The HN beech site, which is right next to an intensive chicken farm, is trapping the farm's NH4/NH3 emissions along a very distinct 200-m-long N gradient where concentrations decrease to levels similar to those in the nearby Scots pine stand (Vanguelova & Pitman, 2009). This is showed by the higher NH4-N concentrations in RF at the beech than at the Scots pine stand at the HN, while no difference was found for NO3-N concentrations (Fig. 1). Fluxes relative to the 2011 growing season indicated that at the beech stand, NH4-N is the dominant component of wet Ndep, while NH4-N and NO3-N contributed almost similarly to wet deposition at the Scots pine (Table 2). These results are in line with the data from long-term monitoring within the ICP forest network (Table 1), which showed that Thetford is among the sites receiving the highest Ndep in the United Kingdom (Vanguelova et al., 2010; RoTAP, 2012), mostly in the reduced form, coming mainly from the intensive livestock farms (in particular pigs and chickens). Records over more than 10 years also suggest that the overall total Ndep at the Thetford pine site has decreased over time, because of reductions in wet (in both forms NH4-N and NO3-N) rather than dry deposition (Vanguelova et al., 2010), confirming the national trend (RoTAP, 2012).
The relative contributions of dry vs. wet Ndep at the site-level were broadly in agreement with modelled deposition rates obtained at the 5 × 5 km scale (RoTAP, 2012, cf., Fig. S1). For example, the modelled data suggested similar values for the total (wet plus dry) oxidized N forms (NO3-N, NO2-N and HNO3-N) at the HN vs. LN sites, which is consistent with a similar impart of traffic-derived emissions at these sites. The large difference in dry NHx deposition between LN and HN is also consistent with the effects of the numerous pig and chicken farms at HN. In addition, the rates of modelled NOx and NHx wet deposition were similar to the long-term measurements (Table 1) in RF at both the HN and LN sites. However, the modelled values of dry NOx and NHx deposition at both the HN and LN sites were substantially higher compared to the estimated dry deposition as difference between RF and TF. While this suggests significant canopy uptake at the HN sites, it must be remembered that the model estimates also include NH3-N together with NH4-N deposition, and NO2-N-HNO3-N together with NO3-N, which were not directly account for in either the data previously published and reported in Table 1 or the current study. In addition, it is possible that the 5 × 5 km model of RoTAP (2012) fails to capture the small-scale variability in Ndep, especially in dry deposited NH3.
Isotopic signatures measured in NO3− and NH4+ in RF (Figs 2 and 3a, b) at our sites were in the same range found in previous analyses of monthly rainfall samples from several sites in the United Kingdom (Heaton et al., 1997; Curtis et al., 2012; T.H.E. Heaton, unpublished data; Table 3). Overall, δ15N values in NH4+ measured across the United Kingdom ranged from negative to slightly positive values (−12.6‰ to +2.8‰), with a mean of −4.3‰. The positive values observed at the Thetford sites are likely reflecting the contribution of NH4/NH3 emissions coming from the intensive chicken farms. Indeed, Heaton et al. (1997) reported that the δ15N value of TF ammonium in part of a Scots pine plantation artificially fumigated with ammonia gas was 17‰ higher than the value for TF in the nonfumigated part of the plantation. Moreover, in a recent study, Yeatman et al. (2001) measured δ15N values of +13.5‰ in aerosol NH4+ sampled near chicken, cow and pig livestock enterprises, and positive δ15N values in bulk precipitation were also reported by Emmett et al. (1998) for two conifer stands near livestock feed lots in the Netherland.
| Isotope | Total range | Mean | Interquartile range | N |
|---|---|---|---|---|
| δ15N-NO3 | −8.2 to +4.3‰ | −2.0‰ | −3.8 to −0.5‰ | 117 |
| δ18O-NO3 | +50 to +82‰ | +69‰ | +65 to +73‰ | 117 |
| δ15N-NH4 | −12.6 to +2.8‰ | −4.3‰ | −6.2 to −2.8‰ | 86 |
The δ15N values of NO3− were similar to those reported in the study by Heaton et al. (1997). However, a high range of values was measured across the United Kingdom (−8.2‰ to +4.3‰) (Table 3), with a mean δ15N-NO3 values of −2‰. A similar range of δ15N values in NO3− from −11 ‰ to +3.5 ‰ was reported in studies across the USA (Kendall, 1998; Elliott et al., 2007; Kendall et al., 2007), while Tobari et al. (2010) measured δ15N values in bulk precipitation ranging from −7 to +15.4‰ across different watersheds in Japan. Moreover, a number of studies in the literature used δ15N to assess the anthropogenic NOx source. For instance, very negative (−13‰ to −2‰) δ15N-NOx values were reported in the case of emissions coming from traffic, while positive values (between 4 and 16‰) were measured for emissions from coal-fired power plants (Heaton, 1990). Similar values of δ15N-NO3 in RF at HN and LN sites in our study suggest a similar anthropogenic NOx source, most likely emissions coming from local road traffic, consistent also with the absolute concentrations measured in RF at both HN and LN. This is confirmed also by the similar values we measured for δ18O-NO3 in RF, irrespective of site. Moreover, Δ17O in RF at the HN was 2‰ higher than at the LN sites, possibly suggesting that NOx went through slightly different oxidation processes (Michalski et al., 2003). Δ17O values measured at our sites (ranging from 22 to 26‰) were similar to those reported by Costa et al. (2011) for NO3− in rain samples (23.1 ± 1.8‰) collected in Michigan and by Michalski et al. (2004) in aerosol (26 ± 3‰) sampled in Southern California.
Processes affecting throughfall N at contrasting Ndep levels: canopy retention, dry Ndep and biological transformation
Our data showed that at the LN TF-N fluxes were lower than RF N fluxes (Table 2), suggesting that most of the atmospheric N was retained by tree canopies, as observed also in other studies (Lindberg et al., 1986; De Schrijver et al., 2004; Staelens et al., 2007; Fenn et al., 2013; Ferretti et al., 2014; Houle et al., 2015). Epiphytic lichens, fungi and micro-organisms on the canopy may contribute to the higher N retention and subsequent processing at the LN sites, a possibility supported by the significant increase in DON concentrations in TF (Fig. 1c; Table 2), as also reported in other studies (Woods et al., 2012).
By contrast, at the HN sites, NH4-N and NO3-N concentrations and fluxes were higher in TF than in RF, irrespective of tree species (Fig. 1 and Table 2). We also found higher NH4-N in TF underneath beech than Scots pine, with the former receiving higher NHx-N atmospheric inputs than the latter, while both NO3-N and NH4-N TF fluxes increased underneath the Scots pine. These last results are in line with previous studies in the literature (Lovett & Lindberg, 1993; Fenn et al., 2000; Vanguelova et al., 2010; De Vries et al., 2014), and they suggest that in areas with high dry Ndep, canopy filtering and rain washing will contribute to increasing the N inputs to TF and hence to the soils, compared to areas subjected to low atmospheric N loads, in particular dry Ndep. Nevertheless, the different proportion of the N compounds in TF underneath the two forests could also be related to species-specific canopy N retention, which, however, is difficult to quantify by looking only at the difference between TF and RF.
The more positive values for δ15N in NH4+ collected in TF are consistent with the dry NHdep washed-off the canopies and contributing to increasing NH4-N in TF at the Thetford site (Figs 1 and 2). Indeed, the δ15N values of NH4+ in dry deposition tend to be higher than those measured in bulk precipitation (Heaton et al., 1997), suggesting that a fraction of the measured TF originated from dry Ndep. Interestingly, while the NH4-N concentration did not vary significantly from RF to TF and the N fluxes were lower in TF vs. RF at the LN forests, a fingerprint of dry Ndep was still detected by the 15N enrichment in NH4+ underneath the canopies.
The higher NO3-N in TF at the HN sites for both Scots pine and beech could in principle result from a combination of dry deposition and canopy nitrification processes. As in the case of NH4+, higher values of δ15N of NO3− in TF compared to RF could be expected (Heaton et al., 1997), but were not found at these sites (Fig. 3a). Nitrification of NH4+ leads to the production of 15N-depleted NO3− leaving behind more 15N-enriched NH4+ (Högberg, 1997). Indeed, we measured more negative (but not significantly so) δ15N-NO3− in the TF–RF differentials at the HN compared to the LN site. The 15N depletion of NO3− in TF was particularly detected for Scots pine, but not for beech, at the HN site (Figs 3a and 4a). A decrease in δ15N in NO3− from RF to TF was reported in studies in a spruce forest in Germany by Sah & Brumme (2003) and in a montane rainforest in Ecuador by Schwarz et al. (2011), explained in both cases by isotope fractionation during nitrification of NH4+ to NO3− in the canopy leaves. However, none of these previous studies could unequivocally attribute the shifts in 15N-NO3 to biological NH4+ nitrification. In this study, evidence of nitrification occurring within the canopy was clearly provided using two independent methods, based on Δ17O and δ18O. Our results showed that although atmospheric NO3− was the dominant source of NO3− in TF at the Scots pine stand, a considerable proportion (varying between 17 and 38%, depending on which isotope was employed for the mass balance) derived from biological nitrification. The two approaches broadly agreed, but Δ17O lead to higher fAtm estimates than those obtained by δ18O (Fig. 5). Significantly, both methods detected the contribution of biologically derived NO3−.
Interestingly, similar values of δ15N-NO3 in TF and RF did not provide a clear signal of canopy transformation for the Beech at the HN (Fig. 4c). In contrast, the mass balance approach using Δ17O and δ18O proved that biological activity contributed to higher NO3− underneath beech canopies, with quite different estimate of fBio although. Indeed, based on Δ17O, biologically derived NO3 was as much as from atmospherically derived NO3 (Fig. 5a), whereas the mixing model based on δ18O estimated that 90% of the NO3− in TF derived from the atmosphere and only a small fraction from nitrification (Fig. 5b).
Moreover, the significant increase in DON concentrations in TF at both Scots pine and beech sites provide evidence of transformation of dissolved inorganic N to DON within tree canopies (Gaige et al., 2007). Higher DON concentrations in TF can be related to leaching from leaves and needles and/or release by bacterial epiphytes in the phyllosphere (Müller et al., 2004).
The fact that only in the case of the Scots pine, we found consistency between δ15N-NO3− and the mixing model approaches based on δ18O and Δ17O could be partially related to differences between species in the canopy structure and phenology. Conifers are more efficient in scavenging aerosol and atmospheric deposition than broadleaf species (Augusto et al., 2002; De Schrijver et al., 2007) due to the greater canopy surface area and roughness. Furthermore, conifer evergreen phenology implies a higher canopy retention capacity than in deciduous species (De Schrijver et al., 2000), as in the case of Scots pine, whose needles can remain in the canopy for 2–3 years. This means that atmospheric N deposited onto tree canopies and cumulated over multiple growing years and not taken up by needles could undergo several biological transformations, which imply isotope fractionations leading to a distinct isotopic signature between the atmospheric N source and the final produced N specimen. However, assessing the differences between two forests at HN for canopy N transformation goes beyond the aim of this study, due to low replicates per species. Nevertheless, our results certainly shade light on species-specific dynamic of biological activity in tree canopies, which deserves further investigation.
Effectiveness of the two mass balance approaches based on δ18O and Δ17O
The use of Δ17O in nitrate was successfully applied to assess the contribution of atmospheric vs. microbiologically derived NO3− in a forest catchment (Costa et al., 2011) and lately, in combination with δ18O, in an urban environment (Riha et al., 2014). Both studies looked at the isotopic composition in N specimens in the run-off water vs. RF. Processes occurring in the soil, with particular reference to nitrification, and isotope fractionations associated with them, are very well described (see among the others, Högberg, 1997). While canopy N retention and transformation are widely acknowledged as important pathways for trees to acquire N (Sparks, 2009; Pennisi, 2015), the underlying mechanisms are still not understood. This is particularly true for the isotope fractionations, which may occur during nitrification in the canopies or N uptake. One potential limitation of the mixing model based on δ18O is in the estimation of the δ18ONitr (see 2). First, precipitation intercepted by tree canopies might be subjected to evaporation, which, in turn affects the δ18O of the precipitation-derived water available for nitrification (e.g. water remaining on the canopy might be more 18O-enriched than precipitation itself). Second, the assumptions underlying the use of Eqn 5 may not be always valid. In some environments, oxygen isotope exchange between a nitrification intermediary, nitrite and water may invalidate the two-third and one-third proportions of Eqn 5. In addition, the possible influence of an equilibrium isotope fractionation during NO2− and H2O exchange at the enzyme (+14‰) and an inverse kinetic isotope effect during NO2−oxidation into NO3− have also been proposed and would lead to higher δ18O values than those predicted by the simple isotope mass balance model (Casciotti et al., 2010, 2011; Snider et al., 2010; Buchwald et al., 2012). Third, N deposited onto canopies could be more reactive and subject to further transformations before being processed within the canopies or washed-off (e.g. NH3 volatilization, NO reaction with ozone or denitrification). Thus, improper calculation of δ18ONitr might affect the estimation of fAtm and fBio. While mass-dependent isotope fractionations related to NO3− transformation can significantly affect the δ18O, they have no effect on Δ17O. For this reason, using Δ17O seems a more robust approach, leading to a better estimate of fAtm vs. fBio (Michalski et al., 2003). However, more studies are needed to assess the Δ17O of wet vs. dry Ndep and how they change over time.
Synthesis
Our results partially confirmed the initial hypotheses (1) that at the LN sites, ion concentrations in TF and their respective isotopic signatures reflected the input of atmospheric N as derived from RF. However, isotope data revealed that even with a low atmospheric N load, canopies played an important role in intercepting and retaining dry Ndep (with particular reference to the reduced N form), which represents an additional (but often overlooked) N source relative to wet Ndep as assessed through RF. Differences in the RF and TF fluxes together with an increase in TF DON concentrations provided evidence of canopy N retention and possible uptake. At the HN sites, the passing of atmospheric N through canopies affected both ion concentrations and their isotopic signature (which confirmed our hypothesis 2). The occurrence of dry deposition explained the higher NH4-N concentrations and 15N enrichment in NH4+ measured below the canopy in TF water vs. RF. As for the higher NO3-N in TF vs. RF, the isotopes δ15N and δ18O could not provide clear indications of its origin, even though for Scots pine δ15N-NO3− provided some indications of biologically derived NO3−. The unambiguous response came, however, from Δ17O, which allowed to detect that a consistent fraction of the NO3− recovered underneath the canopies derived from biological nitrification, with an especially large magnitude at the beech stand (where the other isotopes, particularly δ18O, failed to provide conclusive evidence).
We acknowledge that the conclusions of this study rely on a limited number of isotope measurements at each site and a limited selection of forest stands, which did not allow detailed investigations of the tree species-specific pattern of canopy N transformations. However, by combining multiple isotopes, the study identified canopy processing of atmospheric deposition (and especially canopy biological nitrification) as a major process that should not be neglected and needs further exploration. This has important implications for policy-related emission abatement strategies, which aim to manage forests and landscape not only for enhancing C-sequestration, but also for atmospheric N capture.
Acknowledgements
RG acknowledges Newton International Fellowship Alumni follow-on travel funding (2013-2015) from the Royal Society, following the NIF Grant No. NF082365 (2009-2011) funded by the Royal Society, the British Academy and the Royal Academy of Engineering. MM acknowledges funding from NERC project grant NE/G00725X/1 and NERC project grant IP-1205-1110. GM acknowledges funding from the National Science Foundation DEB 0918708. Funding for this work was also provided by Forest Research and acknowledgments go to Forest Research technical team for sample collection and the research laboratory for carrying out all chemical analysis. Rona Pitman and Sue Benham provided help with the research and the final edits of this manuscript. We acknowledge the anonymous reviewer for valuable suggestions and comments on the manuscript.




