Genetic diversity is related to climatic variation and vulnerability in threatened bull trout
Abstract
Understanding how climatic variation influences ecological and evolutionary processes is crucial for informed conservation decision-making. Nevertheless, few studies have measured how climatic variation influences genetic diversity within populations or how genetic diversity is distributed across space relative to future climatic stress. Here, we tested whether patterns of genetic diversity (allelic richness) were related to climatic variation and habitat features in 130 bull trout (Salvelinus confluentus) populations from 24 watersheds (i.e., ~4–7th order river subbasins) across the Columbia River Basin, USA. We then determined whether bull trout genetic diversity was related to climate vulnerability at the watershed scale, which we quantified on the basis of exposure to future climatic conditions (projected scenarios for the 2040s) and existing habitat complexity. We found a strong gradient in genetic diversity in bull trout populations across the Columbia River Basin, where populations located in the most upstream headwater areas had the greatest genetic diversity. After accounting for spatial patterns with linear mixed models, allelic richness in bull trout populations was positively related to habitat patch size and complexity, and negatively related to maximum summer temperature and the frequency of winter flooding. These relationships strongly suggest that climatic variation influences evolutionary processes in this threatened species and that genetic diversity will likely decrease due to future climate change. Vulnerability at a watershed scale was negatively correlated with average genetic diversity (r = −0.77; P < 0.001); watersheds containing populations with lower average genetic diversity generally had the lowest habitat complexity, warmest stream temperatures, and greatest frequency of winter flooding. Together, these findings have important conservation implications for bull trout and other imperiled species. Genetic diversity is already depressed where climatic vulnerability is highest; it will likely erode further in the very places where diversity may be most needed for future persistence.
Introduction
Climate change is an escalating conservation problem that threatens many populations with extirpation and species with extinction (Walther et al., 2002; Thomas et al., 2004). In response, managers are urgently assessing the vulnerability of native species to climatic changes to effectively prioritize where, when, and how conservation actions are implemented (Williams et al., 2008; Isaak et al., 2012). Most studies forecasting the potential impacts of climate change have focused on patterns of species occupancy (Pearson & Dawson, 2003; Huntley et al., 2010; Araújo & Peterson, 2012) or measures of habitat quality (e.g., Halpern et al., 2007; Wade et al., 2013) to identify populations or regions that may be more susceptible to climatic warming. Unfortunately, few studies have measured how climatic variation influences genetic diversity within populations or how genetic diversity is distributed across space relative to future climatic vulnerability.
Genetic diversity describes a population's evolutionary potential and therefore resiliency to environmental change (Sgrò et al., 2011). Genetic diversity also integrates multiple components of population demography that influence population growth rate and viability, namely stochastic processes due to population size (i.e., drift) and dispersal among populations (i.e., gene flow) (Allendorf et al., 2013). Populations suffering from low abundance or reduced connectivity often have low genetic diversity and therefore reduced capacity for responding to future environmental change via natural selection and increased probability of negative inbreeding effects (Willi et al., 2006; Hoffman et al., 2014).
Vulnerability, defined as the degree to which a system is susceptible to adverse effects of climate change, is a function of (i) exposure (magnitude of climate change), (ii) sensitivity (likelihood of adverse response to change), and (iii) adaptive capacity (ability to cope with change) (IPCC, 2007). Genetic diversity, integrating both demographic and evolutionary factors, directly relates to adaptive capacity in the vulnerability framework. Despite the critical importance of considering a species’ adaptive capacity in response to climatic changes (Engle, 2011) and the potential value of using genetic data in this role, there are very few instances where it has been used in such contexts (but see Landguth et al., 2014).
In the United States, freshwater temperatures are increasing (e.g., Kaushal et al., 2010; Isaak et al., 2011), river flow regimes are shifting in timing and magnitude (Stewart et al., 2005; Barnett et al., 2008; Luce & Holden, 2009), and stream habitats are impacted by multiple human actions (e.g., dams, timber harvest, and water withdrawal). These environmental changes have significant impacts on aquatic organisms and the structure and function of freshwater ecosystems (Ficke et al., 2007; Heino et al., 2009; Woodward et al., 2010). Salmonid fishes may be at particular risk to climate warming because they are ectothermic, have relatively narrow thermal ranges, their life history events are adapted to the timing and magnitude of stream flow events, and their dispersal patterns are restricted to stream networks (Crozier et al., 2008; Williams et al., 2009). Moreover, understanding how climate change may impact salmonid fishes is a major priority for conservation management because of their economic, ecological, and cultural value (Willson & Halupka, 1995; Gende et al., 2002; Hilborn et al., 2003; Middleton et al., 2013).
Bull trout (Salvelinus confluentus) – a federally listed species under the United States Endangered Species Act – are habitat specialists that require cold water (Selong et al., 2001; Dunham et al., 2003; McMahon et al., 2007) and large, connected, high-quality, and complex habitats for growth, survival and reproduction (Rieman & McIntyre, 1995; Baxter et al., 1999; Dunham & Rieman, 1999; Baxter & Hauer, 2000). In addition to being limited by warm temperatures, bull trout are also negatively impacted by high winter flow events that can dislodge and destroy their incubating embryos (i.e., redd scouring) (Shellburg et al., 2010). Consequently, climate warming poses considerable risk for these fish (Rieman et al., 2007; Wenger et al., 2011; Jones et al., 2014; Wenger et al., 2013) as water temperatures and the frequency and magnitude of winter flooding increase throughout the next century (Stewart et al., 2005; Goode et al., 2013).
Natal homing during spawning drives precise site-fidelity in bull trout, resulting in strong genetic differentiation among spawning populations (Spruell et al., 1999, 2003; Whiteley et al., 2004; DeHaan et al., 2011). Whereas demographic rescue can occur from nearby populations, population dynamics appear to be more strongly regulated by habitat and climatic features operating at local spawning sites (Dunham & Rieman, 1999). Furthermore, anthropogenic habitat fragmentation likely limits the degree of genetic exchange among many populations (Rieman & McIntyre, 1995). As a result, within-population genetic diversity is likely to be strongly influenced by interactions between local habitat features and sources of climatic variation that influence long-term demography and persistence (Neville et al., 2006; Ozerov et al., 2012).
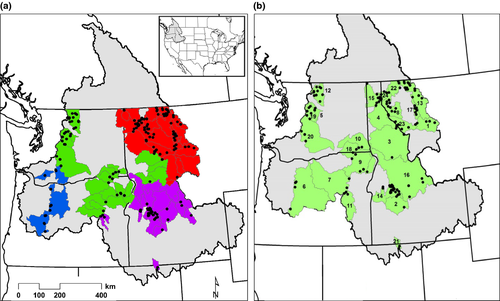
In this study, we address two major objectives: (i) quantify how climatic and physical habitat variations are related to bull trout genetic diversity within local populations; and (ii) measure the degree to which genetic diversity among watersheds covaries with watershed-scale climatic vulnerability. To address the first objective, we used genetic data from 130 bull trout populations across the Columbia River Basin, USA, including 24 watersheds (i.e., ~4–7th order river subbasins) nested within four conservation recovery units (United States Fish & Wildlife Service, 2010) (Fig. 1), to quantify relationships between population genetic diversity and habitat and climatic factors. This information provides insight into long-term (average effective population size [drift] and connectivity [gene flow] over time) and recent processes (population bottlenecks) influencing demography (Luikart et al., 1998; Allendorf et al., 2014). Based on bull trout ecology, we hypothesized that genetic variation would be negatively associated with climatic stressors (e.g., high summer temperatures and winter flooding) and positively associated with habitat patch size and complexity.
To address the second objective, we evaluated bull trout vulnerability at a watershed level based on exposure to forecasted climatic conditions, and sensitivity to those forecasts on the basis of current habitat complexity, but excluded adaptive capacity. We then compared vulnerability estimates to average genetic diversity among populations within a watershed. If vulnerable populations or groups of populations (metapopulations within a watershed) have relatively high genetic diversity, there may be adaptive potential in the face of rapid environmental change and low contemporary risk of negative inbreeding effects. Conversely, the opposite relationship would indicate that populations or metapopulations most susceptible to climate change are at additional risk from low genetic diversity, both in terms of evolutionary resiliency and reduced fitness owing to reductions in genomic variation. Overall, the results from this study provide a needed example of how genetic diversity can be used to describe the impacts of climatic variation on evolutionary processes and inform inferences about species’ resilience to climatic and habitat changes at large spatial scales.
Materials and methods
Bull trout ecology
The bull trout is an iteroparous salmonid that exhibits resident and migratory life histories (i.e., fish either grow to maturity in their natal stream or migrate to rivers or lakes to grow before returning to natal streams to reproduce) (Dunham et al., 2008). Bull trout typically spawn in August–October in low-gradient, unconfined alluvial valley stream segments that exhibit complex patterns of hyporheic exchange and groundwater upwelling (Baxter & Hauer, 2000). Embryos incubate in stream gravels until the following spring when they emerge. Juvenile bull trout typically spend 1–4 years in their natal stream before migrating to larger rivers or lakes where they grow to maturity, or remain residents until maturity (usually 5–7 years old) (Al-Chokhachy & Budy, 2008; Johnston & Post, 2009).
Bull trout samples and genetic data
Bull trout were sampled in 139 locations throughout the Columbia River Basin, USA. All genetic samples were collected from fish in their spawning habitats, and therefore, these samples represent local spawning populations (here forward we use population and location synonymously) (Ardren et al., 2011). Each sample was comprised of multiple age classes and, in some cases, multiple years of collections. All individuals were genotyped at 14 microsatellite loci (loci are described in Ardren et al., 2011). There is no evidence that these microsatellite loci suffer from null alleles (Patrick DeHaan USFWS, unpublished data).
Individuals with missing genotypes at four or more loci, as well as genetically identified bull trout x brook trout (S. fontinalis) hybrids, were removed from the dataset. Hybrids were identified with species diagnostic microsatellite loci (DeHaan et al., 2010). To avoid issues of sampling error and confounding factors, we removed two populations that had 15 or fewer sampled individuals, and seven populations that were completely isolated from other bull trout populations. The remaining database included 6086 individuals from 130 populations. The number of individuals per population ranged from 17 to 195 (median = 41). Population samples were generally in Hardy–Weinberg proportions and deviations, when they occurred, appeared to be due small effective population sizes as opposed to genotyping error or cohort effects (described in Ardren et al., 2011).
We used a sample size corrected estimate of allelic richness (Kalinowski, 2005) (here forward allelic richness = AR) as our measure of genetic diversity as it may better predict long-term adaptive potential and is more sensitive to demographic variation than other measures of genetic variation (e.g., heterozygosity) (Luikart et al., 1998; Caballero & García-Dorado, 2013; Allendorf et al., 2014). Additionally, AR values for the bull trout populations used in this study are highly correlated with other measures of genetic diversity such as expected heterozygosity (r = 0.92; P < 0.00001).
The 130 sampled bull trout populations encompass four major conservation recovery units: Coastal; mid-Columbia; upper-Snake; and upper-Columbia (Fig. 1a, Table S1) (United States Fish & Wildlife Service, 2010; Ardren et al., 2011). Nested within the four units, there were 24 watersheds (hydrological subbasins) where at least two populations were sampled (Fig. 1b). Watersheds followed current management boundaries for bull trout core areas – areas that contain interconnected habitat with one or more independent bull trout spawning populations (United States Fish & Wildlife Service, 2010). There were, however, some instances where we deviated from exact core area boundaries given the spatial distribution of the population samples (Table S1). The watershed delineations likely approximate the scale at which bull trout function, at least to some degree, as metapopulations (Dunham & Rieman, 1999; Spruell et al., 2003).
Covariates
We hypothesized that genetic diversity in bull trout is positively associated with habitat patch size, habitat complexity, and summer base flow, and negatively associated with summer temperature and winter flooding occurrence. Several variables were used to represent these habitat and climatic features (see Table S2 for details regarding methods and data used to generate the predictor variables). We calculated the following variables to represent habitat patch size at each location: the total length of stream designated as critical spawning and rearing habitat (based on empirical observation; United States Fish & Wildlife Service, 2010) and total stream length within each spawning drainage (e.g., Ozerov et al., 2012; D'Angelo & Muhlfeld, 2013). We used two variables to represent habitat complexity: drainage density and the proportion of unconfined valley bottom habitat (Wenger et al., 2011; Nagel et al., 2014) relative to the total spawning and rearing habitat. The latter variables act as index of the relative amount of alluvial stream habitat in spawning and rearing areas; alluvial areas are often composed of greater stream sinuosity (i.e., higher habitat to drainage area proportions) and hyporheic exchange, landscape features that correspond well with the reproductive distribution of bull trout (Baxter & Hauer, 2000).
Observed or predicted stream temperatures at local spawning sites were unavailable for the entire Columbia River Basin. Therefore, mean and maximum summer (July 15–September 15) air temperature was used to represent the thermal regimes experienced by bull trout populations during the summer (e.g., Rieman et al., 2007; Wenger et al., 2011). In general, air temperatures are strongly correlated with water temperatures during summer months (Isaak et al., 2010; Jones et al., 2014), but the degree of correlation can vary across space (Luce et al., 2014) or due to groundwater inputs (O'Driscoll & DeWalle, 2006). The latter can strongly buffer stream temperatures from ambient air temperatures. Temperatures were averaged from 1980 to 1999 to avoid inference from climatic anomalies. We used the predicted (Wenger et al., 2010) average summer base flow and average frequency of high winter flow events (the number of days that exceeded the 95th percentile of annual flows) to represent stream flow conditions.
To normalize covariates with count values (e.g., critical habitat patch size and total drainage area), we added 1.0 to each value and took the natural logarithm. All predictor variables were standardized prior to analyses by subtracting the mean value and dividing by the standard deviation.
Data analysis
Climatic and landscape features associated with genetic diversity
We used standard linear and linear mixed effects modeling (Zuur et al., 2009) to identify variables related to bull trout genetic diversity and quantify relationships. The first step of our analysis focused on removing poorly supported and redundant variables. For each major hypothesis (i.e., patch size, habitat complexity, flow, and temperature), we used standard linear models to separately test for relationships between AR and each covariate, including its quadratic form (variable + variable2). Quadratic effects were included to allow for potential ‘peaked’ relationships or threshold changes. To remove correlated predictor variables (Table S3) from further model selection and parameter estimation (i.e., avoid multicollinearity; Dormann et al., 2012), we then selected the variable (or quadratic effect) with the lowest AIC value and removed the other less supported variables representing each hypothesis.
Bull trout in the Columbia River Basin exhibit strong genetic differentiation at the conservation recovery unit – assemblages of core areas that retain ecological and genetic integrity – and subbasin scale (Spruell et al., 2003; Ardren et al., 2011), and both levels of structuring are used in management and recovery for this species (United States Fish & Wildlife Service, 2010). These groups (conservation recovery units and watersheds) were used as random intercepts in linear mixed models to account for correlations in genetic diversity due to missing covariates (e.g., presence or absence of invasive species, habitat degradation, or fragmentation due to dams; Link & Barker, 2009), and evolutionary or demographic history (Ardren et al., 2011).
Under each grouping (i.e., random effect) strategy, we fit a global model including all covariates that were retained after filtering in step 1 and interactive effects of a priori interest. We hypothesized that there could be interactions between habitat measures and climate such that large, complex habitats may have increased buffering capacity, whereas small, low complexity habitat patches may be particularly susceptible to climatic variation. All predictor variables and interactive terms were treated as fixed effects. We then considered all possible subsets of the global model (only one random effect was used at a time) and used AIC to compare model support (Burnham & Anderson, 2002).
For AIC model comparisons, we used Program R (The R Core Development Team, 2012) and the package nlme (Pinheiro et al., 2014) to fit linear mixed models using maximum likelihood estimation. The package MuMIn (Barton et al., 2014) was used to run each possible configuration of the global model and retain models with AIC values within 10.0 of the best supported model. We then eliminated models that did not reduce AIC by at least 2.0 relative to a less parameterized model (Arnold, 2010). Restricted maximum likelihood was used to obtain parameter estimates from the best supported models (Zuur et al., 2009). Marginal and conditional R2 values (Nakagawa & Schielzeth, 2012) were obtained from MuMIn. Finally, anova models were used to test whether there were differences in average allelic richness between the conservation recovery units and watersheds.
Relationship between genetic diversity and climatic vulnerability
Climatic variation and habitat complexity have well defined roles in influencing bull trout demography and occupancy (e.g., Rieman & McIntyre, 1995; Baxter et al., 1999; Baxter & Hauer, 2000; Dunham & Rieman, 1999; Dunham et al., 2003; Rieman et al., 2007; Wenger et al., 2011; Jones et al., 2014; Wenger et al., 2013), and both have been used to project bull trout range dynamics under future climate change (Wenger et al., 2013; Jones et al., 2014). Thus, we assessed bull trout vulnerability to climate change at the watershed scale using future stream temperature and flow projections as metrics of exposure, and existing proportion of valley bottom habitat (habitat complexity) as a proxy metric for sensitivity to climate exposure. We compared vulnerability (exclusive of adaptive capacity) to observed patterns of genetic diversity to assess the degree to which they are correlated across space. If genetic diversity is negatively related to exposure and sensitivity, bull trout may have reduced adaptive capacity and increased risk of inbreeding depression in the face of environmental change.
We focused our efforts at the watershed scale (as opposed to local populations) because it is congruous with current conservation designations for bull trout core areas (United States Fish & Wildlife Service, 2010), and climatic projections were available at this scale for the entire Columbia River Basin (Wu et al., 2012a). To quantify exposure metrics, we used future projections of maximum summer stream temperature and frequency of high winter flows events (number of days exceeding the 95th percentile of annual flows) at the mouth of each watershed for which we had genetic data (genetic data described above and shown in Fig. 1b). For this partial vulnerability assessment, we used simulated stream temperature and flow values for a future climate scenario (30-year average 2030–2059; i.e., ‘2040s’) obtained from a coupled stream flow and water temperature model (see Wu et al., 2012b for details) developed within the Riverscape Analysis Project (RAP) (Whited et al., 2012). Future flow and temperature projections were based on the middle-of-the-road A1B IPCC AR4 scenario (IPCC (International Panel on Climate Change), 2007). To compare future projections with contemporary conditions, we also obtained flow and temperature data from 1980 to 1999 (corresponding with the genetic data, see above). The RAP dataset was available across the entire Columbia River Basin, but at a coarse resolution inappropriate for developing models of AR as a function of climatic and habitat covariates, described above. Nevertheless, the RAP values appear to adequately represent climatic patterns occurring at local scales. The average values of maximum summer temperature, winter flood frequency, and proportion valley bottom habitat across local spawning populations within each watershed were correlated with watershed-scale climatic and habitat values obtained from RAP (r = 0.42–0.86; P < 0.04).
Bull trout sensitivity to climate conditions was approximated on the basis of habitat complexity, estimated as the proportion of unconfined valley bottom (Nagel et al., 2014) relative to the total spawning and rearing habitat (United States Fish & Wildlife Service, 2010) in each watershed. Sensitivity can be considered a ‘dose–response’ relationship between climate exposure and a species’ reaction, but it is difficult to measure empirically and is often represented through habitat proxy metrics (sensu Halpern et al., 2007; Wade et al., 2013; Falke et al., 2014). Habitat complexity is clearly related to bull trout ecology and resilience specifically (Baxter et al., 1999; Baxter & McPhail, 1999; Baxter & Hauer, 2000; Bowerman et al., 2014) and is an important buffer for salmonid fishes more generally (e.g., Torgersen et al., 1999). Fish in locations with higher proportions of valley bottom habitat were assumed to have lower sensitivity to changing climatic conditions.
To summarize vulnerability, the values for each metric of exposure and sensitivity were linearly rescaled from 1 to 100, with 100 always representing the ‘worst’ value (i.e., highest temperature, highest winter flood frequency, and lowest habitat complexity). We added the rescaled exposure and sensitivity metrics (maximum stream temperature + winter flood risk + habitat complexity) for each watershed to obtain an overall vulnerability value. The vulnerability index was also rescaled from 1 to 100, with 100 representing the watershed with the highest vulnerability (excluding adaptive capacity). To quantify how watershed-scale climatic vulnerability covaries with bull trout genetic diversity, we calculated Pearson correlations between vulnerability values and observed genetic diversity across watersheds (see Fig. S1 for a schematic of this process). Average AR value across spawning populations within a watershed was used as our genetic diversity metric at the watershed scale.
Importantly, other variables associated with exposure, sensitivity, or adaptive capacity (e.g., population abundance, invasive species, habitat connectivity, and other human impacts) were not included in these analyses. As such, we acknowledge that the vulnerability projection is oversimplified. However, our intent was not to conduct a precise vulnerability assessment, but rather to estimate which bull trout watersheds may be most susceptible to projected climate conditions across the Columbia River Basin and to assess whether bull trout vulnerability to climatic change may be buffered or compounded by genetic diversity given its current distribution across the landscape.
Results
Climatic and landscape features associated with genetic diversity
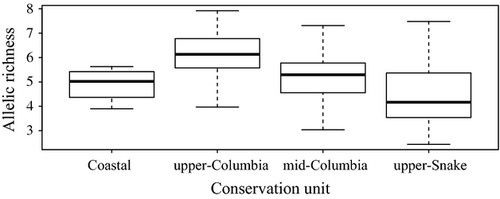
Allelic richness (AR) within bull trout populations across the Columbia River Basin ranged from 2.44 to 7.92 (mean = 5.43, SD = 1.20). AR varied significantly among conservation recovery units (F(3, 126) = 21.50, P < 0.001; Fig. 2) and generally increased from west to east. Specifically, AR was lowest in the upper-Snake and Coastal, moderate in the mid-Columbia, and highest in the upper-Columbia Recovery unit. (Fig. 2). Similarly, there was a significant variation (F(23, 102) = 6.86, P < 0.001) in AR across watersheds, where those watersheds located most northerly and to the east generally had the highest genetic diversity (Fig. 3d). Populations of bull trout in the Jarbidge River had the lowest average AR (3.55, SE = 0.75), whereas the highest average AR was found in the Swan River (6.88, SE = 0.55).
Preliminary tests (Table S4) were used to select the following variables for predicting bull trout AR using linear mixed models: total stream length (SL; representing patch size); proportion valley bottom habitat (VB; habitat complexity); frequency of winter flooding (WF); summer base flow (SF); and maximum summer air temperature (Tmax). We retained variables for both summer and winter flow because they represent different environmental stressors to bull trout, were not substantially correlated (r = −0.22), and have been used to predict the presence of bull trout across the same region (Wenger et al., 2011). There was evidence for quadratic effects of SF and Tmax on AR using linear models, and those terms were retained for subsequent modeling.
Thus, the global mixed model included a random intercept for spatial grouping (conservation recovery unit or watershed), five main effects (SL, VB, WF, SF, and Tmax), quadratic effects for SF and Tmax, and interactions between habitat complexity (VB) and climatic variation (WF, SF, and Tmax). When using conservation recovery unit as a random intercept, we found evidence that VB, SL, WF, and Tmax were related to bull trout AR within local populations across the Columbia River Basin (Table 1). There was no evidence for SF, interactive, or quadratic effects in any of the best supported models. The parameter estimates from leading models (Table 2) supported our predictions; bull trout genetic diversity was positively related to SL and VB, and negatively related to WF and Tmax (Fig. 4a–d).
| Random effect | Model | AIC | ΔAIC | Weight | R2 marginal | R2 conditional |
|---|---|---|---|---|---|---|
| Recovery unit | SL + Tmax | 365.5 | 0.0 | 0.41 | 0.10 | 0.41 |
| VB + Tmax | 365.5 | 0.0 | 0.41 | 0.10 | 0.37 | |
| SL + WF | 368.8 | 3.3 | 0.08 | 0.07 | 0.38 | |
| VB | 369.5 | 4.0 | 0.06 | 0.07 | 0.35 | |
| T max | 370.6 | 5.1 | 0.03 | 0.05 | 0.39 | |
| SL | 371.9 | 6.4 | 0.02 | 0.05 | 0.37 | |
| WF | 375.1 | 9.6 | 0.00 | 0.03 | 0.37 | |
| Full | 368.7 | 3.23 | 0.16 | 0.41 | ||
| Int | 377.9 | 12.4 | 0.36 | |||
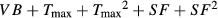 *
*
|
377.8 | 12.3 | 0.28 | |||
| Watershed | VB | 347.5 | 0.0 | 0.61 | 0.04 | 0.44 |
| SL | 348.7 | 1.2 | 0.25 | 0.03 | 0.49 | |
| Int | 350.3 | 2.9 | 0.14 | 0.48 | ||
| Full | 351.9 | 4.4 | 0.15 | 0.47 | ||
| VB + SF + SF 2 * | 366.9 | 19.4 | 0.19 |
- SL, total stream length; VB, proportion valley bottom habitat; WF, winter flood frequency; SF, mean summer flow; Tmax, maximum summer air temperature; and Int = model with random intercept only and Full = global model. Models with an * are the best supported models when the random effect for population grouping is not included in the model. R2 marginal is the variance explained by the fixed portion of the model, while R2 conditional is the total variance explained by the model.
| Random effect | Model | Parameter | ||||
|---|---|---|---|---|---|---|
| Int | SL | VB | WF | T max | ||
| Recovery unit | SL + TM | 5.22 ± 0.36 | 0.24 ± 0.09 | −0.25 ± 0.09 | ||
| VB + TM | 5.21 ± 0.33 | 0.25 ± 0.09 | −0.22 ± 0.98 | |||
| SL + WF | 5.26 ± 0.35 | 0.26 ± 0.09 | −0.21 ± 0.10 | |||
| VB | 5.18 ± 0.34 | 0.29 ± 0.09 | ||||
| TM | 5.22 ± 0.37 | −0.27 ± 0.09 | ||||
| Watershed | VB | 5.39 ± 0.17 | 0.22 ± 0.10 | |||
| Int | 5.39 ± 0.19 | |||||
- Int, intercept; SL, total stream length; VB, proportion valley bottom habitat; WF, winter flood frequency; and Tmax, maximum summer air temperature.
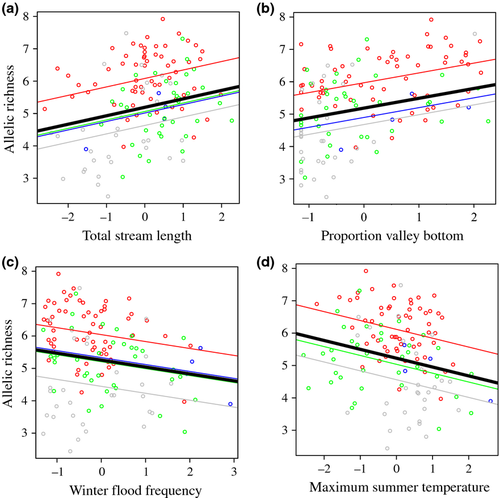
There was strong evidence that habitat and climatic variables were related to genetic diversity, as the best supported models decreased AIC by 12.4 relative to a model that only included the random intercept. However, there was considerable uncertainty surrounding the best supported model, as the top four models, which accounted for 96% of the Akaike weight, were all within 4.0 AIC units of one another. This was largely due to the fact VB and SL, and WF and Tmax appear to explain the same variation in AR. Indeed, their relationships with AR were quite similar (Table 2), and the variables were moderately correlated with one another (r = 0.49 for VB and SL; r = 0.57 for WF and TM). Also, combining VB and SL or WF and Tmax in a model never led to a substantive reduction in AIC (>2.0) relative to models with each variable in isolation. There was some evidence that TM better predicted AR than WF, as models with TM had more slightly support than models with WF. A random intercept structure (i.e., spatial heterogeneity in AR) based on conservation recovery units was well supported by the data. The ΔAIC between the best supported model with and without the random effect was 12.3, and the variation explained by the random portion of the model exceeded the variation explained by the fixed portion of the model (Table 2).
Bull trout recovery units with the lowest AR tended to have smaller habitat patch sizes, lower habitat complexity, higher summer air temperatures, and increased risk of winter flooding. While clearly supported by the data, the random intercept accounted for some of the habitat and climatic variation found across the Columbia River Basin from the fixed effects portion of the model. The effect sizes for SL, VB, and WF were greater, especially for VB, when the random effect was removed (Table S6). The random effect term accounts for this spatial variation and other historical effects, and in so doing reduces the estimated effect sizes for the fixed predictor variables.
When watershed was used as the random grouping factor, there was less support for relationships between AR and the habitat and climatic variables. Under this random effect structure, the only variable with any support was VB (Table 1). Similar to the results obtained when using conservation recovery unit as a random effect, VB was positively related to AR (Table 2). Climatic variables were less supported when using watershed as a random effect because maximum summer temperature and winter flood frequency across watersheds (from RAP metrics) negatively varied with average allelic richness (r = −0.49 and −0.48, respectively; see also vulnerability section below). The random effect structuring, which accounts for this spatial variation, removed this variation from the fixed portion of the model. Diagnostic tests for models using the recovery unit or watershed grouping in mixed effects models did not appear to violate major model assumptions (e.g., heteroscedasticity and normality; Figs S2–S6).
Implications for vulnerability to climate change
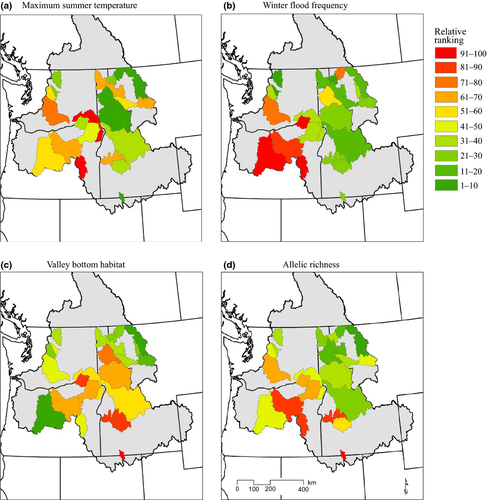
Projected maximum summer stream temperatures and winter flood risk (i.e., climatic exposure) generally increased from east to west and north to south for watersheds where multiple spawning populations of bull trout were sampled (Fig. 3a,b). Proportion of valley bottom habitat (i.e., inversely related to sensitivity) and average AR generally had the opposite pattern (Fig. 3c,d). Maximum summer temperature and winter flood frequency were predicted to increase in nearly every watershed (23 of 24 for temperature and 24 of 24 for flooding) from present to the 2040s (Fig. S6). On average, watersheds were predicted to be 1.15°C (SD = 0.57) warmer and have 2.80 (SD = 2.01) more winter flood days per year compared to current conditions. Relative to current conditions, the predicted shift in flow is much greater and represents an increase of 82%, while the change in temperature is only a 5% increase. Expected trends in temperature and stream flow were generally consistent with projections using different methods (CIG (Climate Impacts Group), 2008; Mantua et al., 2010).
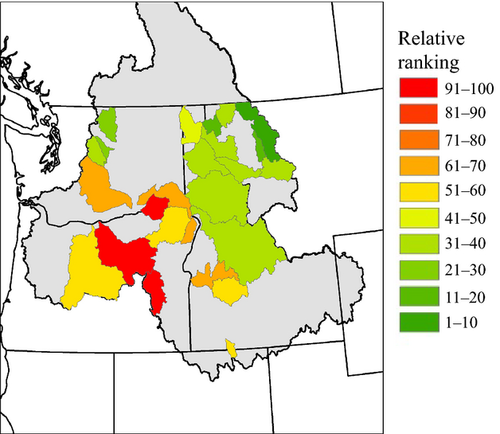
Climatic vulnerability (maximum summer stream temperature + winter flood risk + habitat complexity, but exclusive of adaptive capacity) at the watershed level was highest in the southwest portion (e.g., John Day and Malheur watersheds) and lowest in the headwaters (e.g., Flathead and Swan watersheds) of the Columbia River Basin (Fig. 5a). Given relationships between AR and climatic conditions and/or habitat complexity described above, it follows that there were negative relationships between average genetic diversity across populations within watersheds (mean AR) and projected vulnerability. The correlation (r) between average AR and vulnerability was −0.767 (P < 0.001). Thus, projected vulnerability was highest in those watersheds where genetic diversity was lowest (Figs 3d and 5).
Discussion
Although genetic diversity is critical for long-term viability, especially in rapidly changing environments (Hoffman & Sgro, 2011), our understanding of how climatic variation is related to population genetic diversity and the implications of such relationships for species’ susceptibility to climate change is limited. Here, we showed that genetic diversity at large spatial scales is associated with climatic drivers likely to shift substantially over the next century. Specifically, genetic diversity in bull trout populations throughout the Columbia River Basin was positively related to habitat patch size and complexity, and negatively related to maximum summer temperatures and frequency of winter flood events. These physical habitat and climatic variables are similarly related to bull trout occupancy, survival, and habitat selection (e.g., Rieman & McIntyre, 1995; Dunham & Rieman, 1999; Baxter & Hauer, 2000; Selong et al., 2001; Wenger et al., 2011), indicating that demographic risk for this species may be positively associated with evolutionary risk from climate change.
We also found that genetic diversity of bull trout populations was generally lowest in portions of the Columbia River Basin where we estimated future vulnerability (exposure + sensitivity) to climate change was highest. This pattern may be consistent for other vertebrate species threatened by climate warming (but see also Bálint et al., 2011 for a different pattern in European insects), especially ectothermic organisms whose population dynamics are strongly influenced by climatic conditions. Additional studies focused on identifying relationships between genetic diversity and climatic variability across large spatial scales could help determine the ubiquity of this pattern, and enhance conservation decision-making by identifying those areas of a species range where populations are threatened demographically and/or evolutionarily.
Climatic and habitat features associated with genetic diversity
After accounting for spatial heterogeneity in genetic diversity at multiple scales, the best supported predictor variable describing bull trout AR was the proportion of unconfined valley bottom stream length within critical spawning and rearing habitats. This variable acts as an index of habitat complexity, where stream reaches in unconfined valley areas typically have expansive channel networks (sinuosity) with extensive hyporheic exchange and groundwater upwelling (Stanford & Ward, 1993; Stanford et al., 2005). Such areas are preferentially used by bull trout for reproduction and juvenile rearing (Baxter & Hauer, 2000), as groundwater exchange appears to directly influence bull trout embryonic survival and recruitment (Baxter et al., 1999; Bowerman et al., 2014). Unconfined valleys also help buffer juvenile bull trout from flow and temperature variation (Shellberg et al., 2010; Goode et al., 2013). Thus, streams with greater valley bottom habitat likely maintain larger numbers of successfully breeding adults and therefore greater average genetic diversity. For bull trout conservation, this finding highlights the need to protect critical habitat areas, especially those in unconfined valley reaches, by avoiding land and water use practices that can adversely impact stream and riparian habitats (Baxter et al., 1999; Dunham & Rieman, 1999; Ripley et al., 2005). This is particularly true given our findings that genetic diversity is also related to climatic variables, such as stream flow and water temperature, which are likely to change substantially over the next century.
As predicted, genetic diversity within bull trout populations was negatively related to maximum summer air temperatures, a finding similar to previous distributional studies (Rieman & McIntyre, 1995; Wenger et al., 2011). Because bull trout are among the most temperature-sensitive salmonid species in North America (Selong et al., 2001), populations exposed to higher temperatures likely have lower, or more variable, adult abundances, both of which influence the genetic effective population size (Kalinowski & Waples, 2002; Waples, 2002) and therefore the rate at which genetic diversity is lost (Allendorf et al., 2013).
Similarly, genetic diversity was negatively related to the frequency of winter flooding, a pattern that also mirrors results for spatial occupancy (Wenger et al., 2011). However, effects of summer temperature and winter flow were apparently confounded; there was little support for models that contained the additive effects of both variables, but there was considerable support for models that included each variable separately. These results suggest that streams that are warm during the summer also have higher risk of winter flooding and vice versa, a fairly common pattern in stream environments (e.g., Mantua et al., 2010; Arismendi et al., 2013). There was little evidence that summer base flow influenced AR; while this may be an accurate representation of nature, it could also reflect the fact that hydrologic predictions of summer base flow are relatively poor compared to other seasons (Wenger et al., 2010).
Our results suggest that much of the climatic variation across the Columbia River Basin occurs at the watershed scale, likely driven by topographic and local controls (Chang & Psaris, 2013). This pattern is quite evident from the correlations between bull trout vulnerability and average AR within watersheds (described below) and the fact that climatic variables were poorly supported when watershed was used as a random effect. This finding may imply that our parameter estimates for the fixed climatic effects are conservative because the spatial random effect is removing systematic differences in climate and/or habitat across space. Alternatively, spatial patterns in AR across watersheds may be driven by other anthropogenic stressors such as human land use intensity (e.g., fragmentation) or evolutionary history that are correlated with differences in climate across the Columbia River Basin.
Natural and anthropogenic barriers to movement can have strong impacts on bull trout genetic diversity (Costello et al., 2003; Meeuwig et al., 2010). To avoid the confounding effects of fragmentation on genetic diversity, we did not include data from single isolated populations. Founder effects during postglacial colonization can also influence the distribution of standing genetic diversity across space, where areas of glacial refugia typically have higher genetic diversity (Bernatchez & Wilson, 1998; Costello et al., 2003). However, conservation recovery units that were putative glacial refugia (i.e., mid-Columbia, upper-Snake, and Coastal; Fig. 1a) (Haas & McPhail, 2001) had substantially lower genetic diversity than the upper-Columbia recovery unit, an area that was colonized during the retreat of the Wisconsinan glacial period (Ardren et al., 2011). Moreover, 8 of the 11 watersheds with above average allelic richness were partially or completely covered during the maximum extent of the Cordilleran ice sheet (Ardren et al., 2011). Thus, it seems unlikely that evolutionary history alone can explain the observed patterns. Instead, the fact that climate and habitat are implicated in patterns of bull trout occupancy throughout this region supports the notion that these variables influence genetic drift within populations and thus the distribution of genetic diversity across the landscape.
Habitat and landscape factors influencing the numbers of effective breeders (and effective population size, Ne) are likely responsible for the patterns in genetic diversity, as local Ne in bull trout is often quite low (Ardren et al., 2011). These low effective sizes translate to high rates of genetic drift and thus considerable genetic stochasticity; this may explain why our models explained less than 50% of the variation in genetic diversity across populations. It is likely, however, that some combination of factors, including habitat fragmentation, habitat degradation, evolutionary history, low genetic effective sizes, and other important variables (e.g., invasive species) (Fredenberg, 2002; Rieman et al., 2006), are responsible for the variation not explained by habitat complexity or climatic effects. Despite these complications, we still observed climatic and habitat effects on genetic diversity, indicating that these results, which are supported by other ecological data, are real (e.g., Rieman & McIntyre, 1995; Dunham & Rieman, 1999; Baxter & Hauer, 2000; Selong et al., 2001; Wenger et al., 2011). Regardless of underlying source, there is a clear gradient in genetic diversity for bull trout across the Columbia River Basin with diversity increasing from west to east and south to north. This spatial pattern in contemporary patterns of genetic diversity has important implications for current and future susceptibility of bull trout, and likely many other species, to climatic and other anthropogenic stressors.
Implications for bull trout vulnerability to climate change
Given relationships described above, the strong gradients in both climate and habitat complexity across watersheds in the Columbia River Basin, and how we defined vulnerability, it is not surprising that the values for climatic vulnerability (exclusive of adaptive capacity) were highly correlated with genetic diversity. However, the finding is salient because it strongly suggests that bull trout populations are potentially limited in their capacity to adapt to climate change where they are most at risk of extirpation due to projected future climatic patterns. Additionally, the negative demographic effects of reduced genomic diversity (Willi et al., 2006; Hoffman et al., 2014) are likely to occur in areas where climatic vulnerability is greatest.
The fact that genetic diversity at a watershed scale is related to climatic and habitat variation such that its distribution across space may provide little, or at least less, buffering is disconcerting for bull trout conservation. Moreover, given the relationships between genetic diversity and climate at a population scale, it seems quite likely that genetic diversity will decrease in the future, further reducing evolutionary potential in the face of rapid environmental change, while simultaneously increasing the probability of negative inbreeding effects (Willi et al., 2006). Although our vulnerability assessment was simple and coarse scale, the overall patterns of vulnerability relative to genetic diversity are of considerable interest for conservation and management of this threatened species. At a minimum, it is clearly necessary that managers consider the concept of adaptive capacity in addition to measures of exposure and sensitivity when conducting species’ vulnerability assessments.
Watersheds with the highest potential vulnerability and lowest genetic diversity were located in the southern range of their current distribution in North America. These peripheral populations may have evolutionary value (Lesica & Allendorf, 1995; Haak et al., 2010) with unique adaptations to conditions such as warm temperatures or winter flood frequency (e.g., Narum et al., 2010, 2013). However, the ability to conserve such populations will become increasingly difficult as climate warming continues and bull trout retreat to higher elevation thermal refugia (Isaak & Rieman, 2013), creating challenging conservation decisions with respect to resource allocation (i.e., fringe populations vs. interior populations). Additionally, shifts in suitable thermal habitats will result in increased thermal isolation (Jones et al., 2014), which will further exacerbate losses of genetic variation for bull trout populations (Landguth et al., 2014). Indeed, the spatial patterns in AR observed across the Columbia River Basin may reflect some degree of thermal isolation already occurring in warmer watersheds lower in the basin.
Notably, several metapopulations with the most suitable environmental conditions and highest average genetic diversity located in the upper-Columbia are currently threatened by extirpation from nonnative species, especially lake trout (Fredenberg, 2002; D'Angelo & Muhlfeld, 2013). Given that these areas have the lowest vulnerability and likely contain populations with the greatest adaptive potential, conservation efforts should focus on eliminating threats of invasive species and habitat loss, and conserving ecological and evolutionary diversity of bull trout across this region.
Study limitations
Given that climate projections are inherently variable, we acknowledge uncertainty in our assessment of bull trout vulnerability. Future projections of precipitation, including volume or type (i.e., rain vs. snow), are particularly uncertain (IPCC (International Panel on Climate Change), 2007), yet precipitation is the predominant driver in stream temperature, flow, and groundwater-surface water exchange (Scibek & Allen, 2006). Coupled with the spatial and temporal complexities of groundwater processes themselves, this uncertainty prohibits our ability to accurately predict changes in future hyporheic exchange and groundwater at the scale of our analyses (Scibek & Allen, 2006; Jyrkama & Sykes, 2007). As such, habitat complexity in our vulnerability assessment is temporally static. Although unconfined valley stream reaches are unlikely to change substantially (Goode et al., 2013), it is unclear how groundwater and habitat characteristics that are important to bull trout populations may be affected by climatic change. It is clear, however, that the hyporheic zone and groundwater specifically is critical for spawning, rearing, and as thermal refugia for salmonid fishes (e.g., Torgersen et al., 1999; Baxter & Hauer, 2000); thus, our vulnerability assessment may be conservative, particularly in areas where future climate change may limit buffering provided by habitat complexity. Future landscape genetic and vulnerability work at finer spatial scales that explicitly consider within-basin and within-stream thermal heterogeneity would substantially improve our understanding of how climatic change may influence bull trout and other riverine organisms.
Conclusions
Genetic diversity can vary strongly along physical and climatic gradients, with important implications for how climate change will impact biodiversity and for how populations might respond to rapidly changing environmental conditions (Pauls et al., 2013). For conservation and management, relationships between genetic diversity and climatic variation can be used to augment other sources of information (e.g., bioclimatic envelope models and physiological tolerances) to better understand current threats to population persistence and potential vulnerability to climatic change. At present, a major source of uncertainty is how neutral or adaptive genetic diversity influences adaptive potential on ecological time scales (Sgrò et al., 2011; Harrison et al., 2014). However, microevolution in response to climate change can occur rapidly in salmonid fishes (Crozier et al., 2011; Kovach et al., 2012), highlighting that genetic diversity can offer resiliency in the face of climatic change. Nevertheless, genetic diversity remains neglected in conservation and management decision-making worldwide (Laikre, 2010), especially in the context of climate change. To address this deficiency, additional efforts are needed to describe how genetic diversity is distributed across the landscape relative to climatic and habitat variation so that we can ultimately understand and predict the ecological and evolutionary consequences of climatic change for global biodiversity.
Acknowledgements
This work was funded by the Department of the Interior Northwest Climate Science Center, the Great Northern Landscape Conservation Cooperative, and NASA. A USGS Mendenhall Fellowship partially supported RPK. GL and RPK were also partially supported by NSF-DEB 1258203. We would like to thank the numerous biologists and technicians from various natural resource agencies who provided genetic samples used in this study as well as the USFWS and Montana Fish, Wildlife and Parks laboratory technicians who provided genotyping assistance. This manuscript benefitted greatly from helpful comments provided by Jason Dunham and three anonymous reviewers. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.




