Temperature tracking by North Sea benthic invertebrates in response to climate change
Abstract
Climate change is a major threat to biodiversity and distributions shifts are one of the most significant threats to global warming, but the extent to which these shifts keep pace with a changing climate is yet uncertain. Understanding the factors governing range shifts is crucial for conservation management to anticipate patterns of biodiversity distribution under future anthropogenic climate change. Soft-sediment invertebrates are a key faunal group because of their role in marine biogeochemistry and as a food source for commercial fish species. However, little information exists on their response to climate change. Here, we evaluate changes in the distribution of 65 North Sea benthic invertebrate species between 1986 and 2000 by examining their geographic, bathymetric and thermal niche shifts and test whether species are tracking their thermal niche as defined by minimum, mean or maximum sea bottom (SBT) and surface (SST) temperatures. Temperatures increased in the whole North Sea with many benthic invertebrates showing north-westerly range shifts (leading/trailing edges as well as distribution centroids) and deepening. Nevertheless, distribution shifts for most species (3.8–7.3 km yr−1 interquantile range) lagged behind shifts in both SBT and SST (mean 8.1 km yr−1), resulting in many species experiencing increasing temperatures. The velocity of climate change (VoCC) of mean SST accurately predicted both the direction and magnitude of distribution centroid shifts, while maximum SST did the same for contraction of the trailing edge. The VoCC of SBT was not a good predictor of range shifts. No good predictor of expansions of the leading edge was found. Our results show that invertebrates need to shift at different rates and directions to track the climate velocities of different temperature measures, and are therefore lagging behind most temperature measures. If these species cannot withstand a change in thermal habitat, this could ultimately lead to a drop in benthic biodiversity.
Introduction
The long-term persistence of species in the face of climate change depends on the ability of populations to keep pace with moving climates or adapt to changes in situ (Burrows et al., 2011). In particular, shifts in the distributional ranges of populations and communities have been frequently observed in response to these changes (e.g. Parmesan & Yohe, 2003; Helmuth et al., 2006; Dulvy et al., 2008), but only a few studies have examined whether these shifts allow species to keep pace with climate change (e.g. Hiddink et al., 2012; La Sorte & Jetz, 2012; Pinsky et al., 2013). Furthermore, as most marine ectotherms are thermal range conformers, they tend to occupy fully their thermal niche and are therefore more responsive to warming than their terrestrial counterparts (Sunday et al., 2012). For marine species responding to climate change, rates of distribution shifts are, on average, consistent with those required to track mean ocean surface temperature changes (Poloczanska et al., 2013). Nevertheless, similar analyses on the tracking of minimum and maximum temperature by species responding to climate change are lacking (Warren & Chick, 2013), yet extremes rather than mean temperatures may be the primary drivers of distribution shifts (Sunday et al., 2012). Improving our current understanding of the factors governing range shifts is crucial for climate change conservation because, by changing the identity of biological communities, they are expected to alter importantly ecosystem function and structure under future anthropogenic climate change (Dawson et al., 2011).
The climate variability hypothesis proposes that species' latitudinal ranges reflect their thermal tolerance as defined by the highest summer and coldest winter temperatures within their ranges, whereby species would retreat from areas that become too warm and expand into areas that were previously too cold under climate change (Sunday et al., 2012). Therefore, assuming that species ranges are limited by maximum and minimum temperatures at their equatorial trailing edge and pole-ward leading edge, respectively, predictions from changes in temperature extremes might reflect more accurately observed shifts at range edges whether mean temperatures might be a better predictor of overall range centroid shifts.
Under the expectation of pole-ward shift responses to a warming climate, shifts in temperatures and distributions are often expressed simplistically along the latitudinal axis (e.g. Chen et al., 2011). While this procedure might be acceptable for regions with a predominantly north–south temperature gradient, it can otherwise lead to severe underestimations of the fingerprint of climate change (VanDerWal et al., 2013). Local differences in climate velocities, defining the rate and direction of isotherm shift through space (Loarie et al., 2009), are emerging as a consistent predictor of the directions and rates of shift in marine species (Pinsky et al., 2013). Taking into account both the rate and directionality of change in thermal conditions is necessary to fully characterize the shift of species tracking their thermal niche (Burrows et al., 2014). Such is the situation in the North Sea where mean annual and maximum temperatures increase along a northwest to southeast gradient, while minimum temperature decreases (Otto et al., 1990). This results in the south-east of the North Sea having the largest annual temperature range of over 10 °C while parts of the northern North Sea experience an annual range of less than 2 °C. The complex oceanography of the North Sea, generating effectively opposite directions of movement for those species tracking maximum and minimum temperatures, is particularly suitable for studying and disentangling the effect of different temperature variables on the distribution of species.
If species are not shifting at the trailing edge of their distribution (warm boundary) at the rate dictated by climate change, they will build-up an ‘extinction’ or ‘climatic’ debt (Jackson & Sax, 2010; Devictor et al., 2012; Hylander & Ehrlen, 2013). This will be the case, for example, where adults are long lived or reproduction continues at a rate slightly below that required for persistence. It can be assumed that shifts at the trailing edge are conditioned by its sensitivity to increasing temperatures (as no shift is expected if conditions are still within the suitable range of the organism), while failure to shift results in the build-up of an extinction debt. Alternatively, species could adapt physiologically or through micro-evolution. Few marine studies have recorded range contractions at the trailing edge of species distribution ranges defining their warm, equatorial boundary (Lima et al., 2007; Wethey & Woodin, 2008), suggesting that many marine species may be building up an extinction debt. On the other hand, shifts at the leading edge (the cold, polar boundary) are related to the ability of a species to disperse into areas that were previously too cold, and are likely to relate to the dispersal and settling capability of the species. A failure to colonize newly available habitat at the leading edge will result in a ‘colonization lag’ (Jackson & Sax, 2010). By evaluating the temperatures experienced by a species in a period of climatic warming, we can obtain an insight into its ability to track their thermal niche and the build-up of extinction debts and immigration lags.
The North Sea is one of the fastest warming continental shelf seas (Burrows et al., 2011) and has been experiencing changes in the distribution and composition of fish species. For example, fish species richness has increased, with small southerly species increasing and large northerly species decreasing in abundance and range (Hiddink & ter Hofstede, 2008; Simpson et al., 2011). The ranges of many fish species have also moved northwards and towards deeper water (Perry et al., 2005; Dulvy et al., 2008). Because most species of fish are mobile and migratory, they are likely to adapt quickly to changing temperature patterns. However, most species of benthic invertebrates are less likely to be able to keep pace with rapid climate shifts as they cannot move large distances as adults despite having pelagic eggs and larvae (but see Hiddink et al., 2012). Existing studies on the effect of climate change on range shifts on marine benthic invertebrates have largely focused on intertidal and rocky shore species (e.g. Helmuth et al., 2006; Keith et al., 2011), but no evidence of range shifts for off-shore soft-sediment invertebrates yet exists. Soft-sediment invertebrates are important because many are foundation species or ecosystem engineers with an important role in marine biogeochemistry, and constitute an important food source for commercial fish species, mobilizing organic carbon back to the pelagic realm (Snelgrove, 1999).
Here, we (1) analyse distribution shifts in the geographic (the centroid of the distribution as well as the leading and trailing edges) and depth ranges of 65 benthic invertebrates occurring in the North Sea between 1986 and 2000, (2) evaluate if observed distribution shifts match those corresponding to their thermal niches, using the velocity of climate change (VoCC) as a predictor, and (3) estimate whether these species are effectively maintaining their thermal niches over time or accumulating extinction debts or immigration lags. To do so, we examine thermal niches of the species in terms of minimum, mean and maximum sea bottom temperature (SBT) and sea surface temperature (SST). SBT is the temperature experienced by the adults of these organisms, while SST is the temperature at which most primary production will occur, and to which the larvae of the species may be exposed. Specifically, we test the hypotheses that (H1) benthic invertebrates in the North Sea are shifting their distribution in response to ocean warming induced by climate change, (H2) the direction and magnitude of observed range shifts can be predicted from that of temperature change (i.e. the VoCC), (H3) shifts in the distribution of species occurring at leading/trailing edges are better determined by changes in minimum and maximum temperatures, while those corresponding to the overall distribution are better described by changes in mean temperature, and (H4) species are tracking their thermal niche without extinction debts or immigration lags.
The outcome of this study has important implications for conservation. Because changes in different temperature parameters induced by climate change have different directions and magnitudes, it is important to understand which parameters are best tracked by organisms if we are to predict the effect of climate change on patterns of marine biodiversity. If species fail to keep pace with climate change, or shift in the wrong direction, and cannot withstand the resulting change in thermal conditions, this could ultimately lead to a drop in benthic biodiversity.
Materials and methods
Outline
Benthic invertebrate distribution data were obtained from the North Sea Benthos Project for all stations across the North Sea sampled in 1986 and 2000 (Rees et al., 2007). Geographic and depth range shifts were quantified for the common species, and compared to the expected shifts based on the SBT and SST observed temperature changes in the North Sea. We then examined if the observed shifts match those of their thermal niches predicted using the VoCC over the same period, and if species were eventually trailing, tracking or overcompensating their thermal niches, with regard to minimum, mean and/or maximum temperature.
Sea bottom and surface temperature
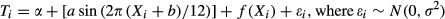 (1)
(1)Velocity and direction of climate change
The estimated 0.2° resolution maps for the minimum, mean and maximum sea bottom and surface temperatures in 1986 and 2000 were used to calculate corresponding maps of the VoCC giving the speed and direction with which organisms need to move to stay at the same temperature. Loarie et al. (2009) developed a method to calculate the velocity of temperature change (km yr−1), derived locally as the ratio between the rate of temperature increase (°C yr−1) and the spatial temperature gradient (°C km−1), with the direction of shifts given by the direction of spatial gradient (Burrows et al., 2011). This index represents the instantaneous local velocity needed by a species to maintain constant temperatures. As the spatial gradients and temporal changes in temperature are different for minimum, mean and maximum bottom and surface temperature, the direction and magnitude of the VoCC will also be different for each temperature parameter.
North Sea benthos distribution
The effect of temperature change on North Sea benthic macroinvertebrates (benthos) was analysed using distribution records from the North Sea Benthos project (http://www.vliz.be/vmdcdata/nsbp). Benthic invertebrates were sampled at several hundreds of stations in the North Sea in 1986 and 2000. Most of the 1986 and 2000 surveys were carried out from spring to early summer of the corresponding year, but some stations were sampled in adjacent years during the same period. At each station the macrofauna was sampled either by 0.1 m2 van Veen grab, box corers (0.068 and 0.25 m2), or 0.1 m2 Day and Hamon grabs, and some stations were sampled with more than one gear. Generally, three replicates were taken per station (Rees et al., 2007; Kroencke et al., 2011). Because grabs and corers of different sizes were used within and between sampling years, and the samples sieved using different mesh sizes, abundances were not compared and the analysis was performed using presence–absence data only. The databases do not give data for individual grabs samples and do not specify which gear was used at each station in each year, or what number of grabs was taken. This means that it was not possible to test for significant differences in the spatial distribution of gear types across stations between the 2 years to examine if the possible use of different sampling gears between years may be confounded with the effect of climate change. Despite the use of the different gears, imposed by the characteristics of the benthic habitat to be sampled (e.g., a box corer does not work on coarse sediments where a Hamon grab does) and differences in their depth profiles (Riddle, 1989), all of these gears do effectively sample the top layer of the sediment (>5 cm) providing comparable results. For example, Beukema (1974) found a good correlation between the number of invertebrates between grabs and corers. About 86% of all benthic invertebrate individuals are found in the top 5 cm of the sediment in the North Sea (Dauwe et al., 1998), and the species that were included in our analysis (Table S1) are all species that occur in this surficial sediment, with the exception of the mud shrimp Callianassa subterannea. Rees et al. (2007) compared the community composition in the two surveys at colocated stations, and found that the distributions of ranked densities were nearly identical but that the species richness was slightly but consistently lower in 2000. They concluded these broad comparisons of data structure provide reassuring evidence of the integrity of the dataset, and that it provides a sound basis for an evaluation of the status of North Sea benthic assemblages in 2000, and of any changes since 1986, while recognizing that sampling and analytical influences must also be accounted for in ecological interpretations.
We standardized both datasets (1986 and 2000) to balance the spatial distribution of the sampling stations by only selecting stations that were sampled in both years for further analysis. Two sampled locations were defined as the ‘same’ station in 1986 and 2000 when they were within 60 km of each other and when no other stations were closer to either of the pair. In total, 143 stations were selected for analysis with 90% of stations being within 16 km of each other; the density of stations in the southern North Sea being higher than in the northern North Sea (see Fig. S2 for a map of the sampling stations). On average, stations in 2000 were positioned 3.4 km south and 1.5 km east of the stations in 1986. This bias is comparatively smaller and in the opposite direction to most of the reported range shifts (see 3), and therefore cannot have caused the observed changes.
Sixty five benthos species that were found on at least 10 stations in both years were selected for analyses (42 ± 22 occupied stations; mean ± 1SD). This approach limited the analysis to common and widespread species where stochastic effects (i.e. false absences) are likely to be smaller. We expect stochasticity to increase variation, but it should not introduce biases. Data with a higher temporal resolution are not available to explore the relative importance of stochasticity. Nevertheless, it is likely that some species were not recorded at stations where they are present because of the relatively small size of the sampling gears (<1 m2). About 1/3 of examined species did not have their northern or southern latitudinal limits within the North Sea (11 species northern boundary, 31 species southern boundary, see Table S1). However, species having their regional distribution limits beyond the study area will be present at the most northerly and southerly stations sampled and therefore our reported distribution shifts are likely to be conservative. Our methodology to detect range shifts (see below) does, however, not rely on the presence of a simple latitudinal boundary as it has the ability to detect regional changes in distribution of species within a specific geographical setting. Nevertheless, reported range shifts are based on data from two single years. This approach assumes implicitly that these 2 years are representative of a long-term trend (Fig. S1). A regime shift across several trophic levels was observed in the North Sea at the end of the 1980s as a result of climate change (Beaugrand, 2004) and the change in distribution is therefore likely to represent a long-term change (deYoung et al., 2008). In any case, abnormal years causing short-term variations in the spatial distribution of benthos would decrease the predictive power of VoCC for predicting range shifts. Reported results are therefore likely to underestimate the predictor capacity of VoCC. Because of the limitations of the dataset our results should be interpreted with care. However, since no other surveys of benthos at a similar spatial scale are available, these are therefore limitations that must be acknowledged but accepted if we want to study range shifts of this important group of organisms.
Depth niche shifts
The minimum, mean and maximum depth occupied by each species in 1986 and 2000 were estimated using station depths extracted from the 30 s resolution bathymetry (Becker et al., 2009). To avoid stochasticity in the presence of species at stations having a large effect on the estimated depth boundaries, the minimum and maximum depth limit of the distribution were estimated as the 10% and 90% quantiles of the depths of stations at which a species was present (rather than the lowest and highest depth recorded).
Geographic range shifts
Geographic range shifts were quantified using three different measures: the centres of gravity of (1) the distribution (COGD), (2) the leading edge expansion (COGE) and (3) the trailing edge contraction (COGC). Although the COGD corresponds to the overall mean latitude and longitude of all the stations with records of a species, the COGC and COGE are defined by the mean latitude and longitude of those stations at which the species was respectively lost and gained between 1986 and 2000. Leading edge expansions and trailing edge contractions of the distribution were studied, because they were assumed to relate to the minimum and maximum temperatures that species can tolerate, whereas shifts in the COGD were expected to be more related to changes in mean temperatures. This approach was taken because it is important to examine shifts in the boundaries of the distribution as well as the centre of the range. Because boundary shifts are unlikely to be simple north–south shifts but have also an east–west component, we developed a method that can capture the direction of the shift of the boundaries. The stations into which a species expanded (i.e. present in 2000 but absent in 1986) and from which it contracted (i.e. present in 1986 but absent in 2000) were identified by subtracting the 1986 range from the 2000 range. Distance and direction of shifts from the original distribution (the COG of all stations at which the species was present in 1986) was then calculated for the centres of gravity of the leading edge expansion and the trailing edge contraction. This direction was inverted for the contraction to indicate the actual direction of movement of the range. Contrary to the COGD, the distance of the COGE and COGC cannot be interpreted as the rate of movement of the leading and trailing edges, because they are defined relative to an arbitrary origin and therefore related to the extent of the distribution of a species. However, they allowed us to test for differences between observed and VoCC-predicted expansion and contraction shifts (see below).
Comparison between observed and predicted geographic range shifts
 (2)
(2)This method of comparison resulted in the predicted shift always being positive. Comparison between the magnitudes of predicted shifts and observed shifts in the predicted direction were done using linear regression.
Thermal niche tracking
We defined the thermal niche of a species between 1986 and 2000 as the mean, minimum (10% quantile) and maximum (90% quantile) of the mean temperatures recorded at the stations where a species was present in a year. These temperatures were extracted from the interpolated temperature maps calculated for 1986 and 2000. To see whether a species managed to track its temperature niche between 1986 and 2000, we defined a temperature threshold beyond which it was assumed that a species was not tracking its temperature niche (because small variations in the experienced temperature are likely regardless of climate change). A threshold of 0.25 °C was selected because, for North Sea 0.2° grid cells, over 80% of SBTs and 100% of SSTs showed an increase of more than 0.25 °C. A change of 0.25 °C over 14 years corresponds with a VoCC of 0.5–7.7 km yr−1 using the observed temperature gradients for SST and SBT.
Results
Patterns of temperature and VoCC in the North Sea
Both sea surface and bottom temperatures registered the highest minimum values in the northern region of the North Sea, while the lowest values were registered in the south–east (Fig. S3). In contrast, highest mean and maximum temperatures were found in the south-eastern North Sea. The lowest mean and maximum values concentrated in the central North Sea for SBTs and in the northern North Sea for SSTs. Mean temperature increased by 0.31 °C for SBT and 0.42 °C for SST between 1986 and 2000 with all three temperature parameters significantly increasing in the entire North Sea (paired t-test, t32 = 12.3 and t32 = 27.4, P < 0.001 for respectively the three SBT and SST comparisons, Fig. S3), but the velocity and direction of climate change differed among them (Fig. S4; Table S2). Generally, velocities were higher for minimum SBT with a dominant south-easterly direction, while mean and maximum velocities were lower and mainly directed either north-west or east (Figs S4A, B and S5). In contrast, SST velocities were higher for mean temperature (Fig. S4E) and lower for maximum temperature (Fig. S4F), both generally directed either north-west or north-east (Fig. S6). Minimum SST velocities registered a predominantly south-easterly direction (Figs S4D and S6). Overall, mean surface velocities were considerably higher than bottom velocities (Table S2). To check the consistency of our VoCC estimates with those used in Burrows et al. (2011), we compared our CTD-based VoCC with that calculated using the HadISST 1.1 global sea-ice and SST dataset produced by the UK Met Office Hadley Centre (Rayner et al., 2003). Resulting VoCC patterns were in general agreement, both in magnitude and direction (Fig. S7), with the main deviations in the patterns concentrated around Shetland.
Geographic range and depth shifts
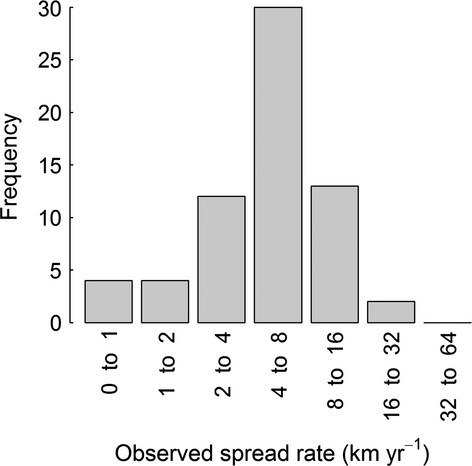
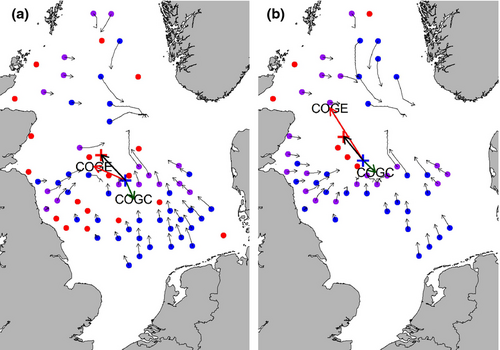
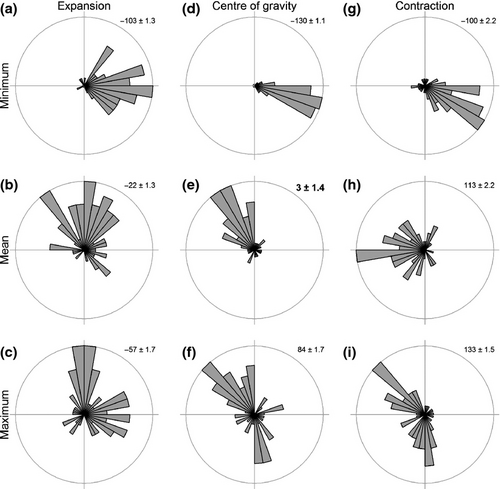
The centre of the distribution, the contraction of the trailing edge, and expansion of the leading edge of most species moved towards the north-west (Fig. 1). The direction of these shifts deviated significantly from random (COGE: W = 0.4, P < 0.01, COGD: W = 0.5, P <0.001, COGC: W = 0.24, P < 0.05). Most species shifted their centre of the distribution at a rate of 3.8–7.3 km yr−1 (interquantile range, Fig. 2), which is slower than most of the VoCC measures (Table S2). Figure 3 illustrates the range shift for two of the examined species, the polychaetes Amphictene auricoma and Levinsenia gracilis. The centre of the distribution of A. auricoma moved north-west (316°) at a rate of 7.8 km yr−1, while the centre of the distribution of L. gracilis moved north-west (321°) at a rate of 6.8 km yr−1.
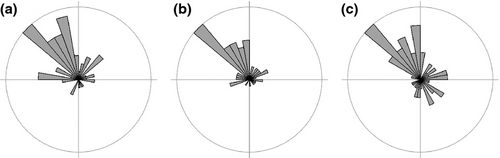
On average, species significantly deepened in their distribution, particularly at the deeper end of their range (0.99 ± 1.37 m yr−1 compared with 0.16 ± 0.44 m yr−1 at the shallow end; Fig. 4, t-test, minimum depth: t64 = −3.0, P = 0.003, mean depth: t64 = −7.2, P < 0.0001, maximum depth: t64 = −5.3, P < 0.0001).
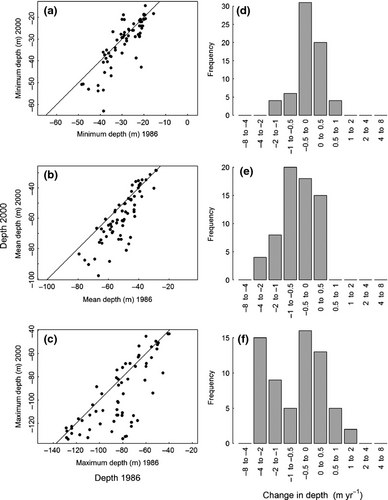
Comparison of observed with predicted range shifts
Sea bottom temperature (Figs 5 and S8) was a worse predictor of observed distribution shifts than surface temperature (Figs 6 and S9). Of the three parameters tested, the velocities for the mean (COGD) and maximum (COGC) SST were the only ones accurately predicting the direction (Fig. 6; Table S3, mean W = 0.1, P > 0.10 and maximum W = 0.2, P > 0.05) and correlating significantly to the magnitude of shift (Fig. S9; Table S3, mean R2 = 0.08, F1,63= 5.72, P = 0.020 and maximum R2 = 0.09, F1,63= 6.06, P = 0.017). No significant differences in shift direction were found between observed and predicted COGE shifts using the VoCC of mean and maximum SST (Fig. 6), but the relationship with the magnitude of the shift for the leading edge expansion was not significant (Fig. S9). Velocities based on SBT parameters failed to predict accurately distribution shifts with the sole exception of the mean SBT for the COGD shifts (Fig. S8; Table S4, W = 0.1, P > 0.10), though the relationship between observed and predicted magnitudes of shift was nonsignificant R2 = 0.04, (F1,63 = 2.91, P = 0.093).
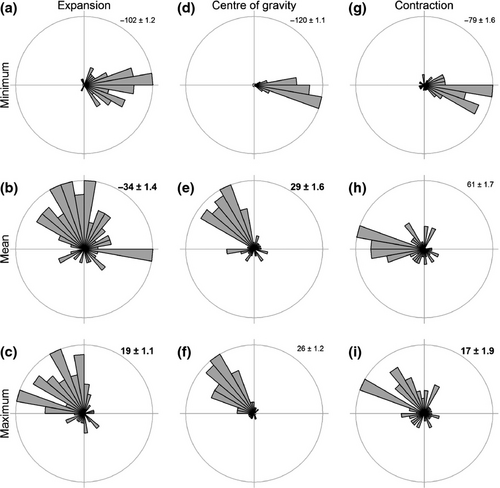
Overall, the best predictor of the overall distribution shift (COGD) was mean SST, while maximum SST best predicted the shift of the trailing edge (COGC). Even so, the magnitude of these shifts predicted by the VoCC seems to have underestimated the magnitude of the observed COG shifts (Fig. S9C, D). None of the three temperature parameters for neither SST nor SBT predicted accurately both the direction and the magnitude of the COGE shifts at the leading edge of the distribution.
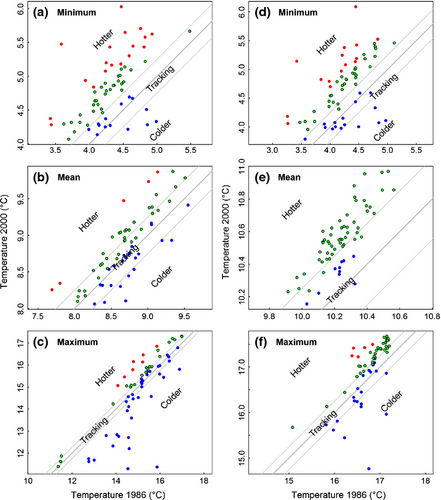
Thermal niche tracking
A large percentage of species (>32%) effectively did not shift their geographical distribution (i.e. the temperature at the stations occupied in 2000 was within 0.25 °C of the temperature that the species would have experienced if they had not moved since 1986; green symbols in Fig. 7). Many of these species were therefore experiencing a SBT (33–77% of species; depending on the temperature parameter) and SST (63–78% of species) in 2000 that was higher than in 1986 (Table S5, categories ‘static and lagging’ and ‘shifting against temperature’). However, not all species (SBT: 11.0 ± 11.4, SST: 9.3 ± 3.8) needed to move to track their temperature niche (i.e. green points within the grey lines in Fig. 7). Among the three temperature parameters, a higher percentage of species tracked mean SBT (57%, ‘static and tracking’ and ‘shifting and tracking’) than either minimum or maximum temperatures. In contrast, more species (24%) tracked maximum SST than the minimum or mean (Table S5). Some species overcompensated (SBT: 24.7 ± 7.8, SST: 15.3 ± 4.7), and ended up at lower temperatures in 2000 than in 1986 (Fig. 7, blue symbols below the grey lines), while other species (SBT: 11.3 ± 8.5, SST: 7.0 ± 8.2) moved in the opposite direction from that required to track temperature shifts and ended up in warmer waters than those they would have experienced if they had remained stationary, in particular for minimum temperature (Fig. 7, red symbols).
Discussion
Our results give strong evidence in support of our first hypothesis, that species of benthic invertebrates have shifted their distribution in response to the increasing trend in minimum, mean and maximum SST and SBT across the North Sea between 1986 and 2000. North-westerly range shifts and deepening were recorded for many species over this time period. Similar depth and distribution shifts have been observed for North Sea fish (Perry et al., 2005; Dulvy et al., 2008). While depth shifts were more pronounced at the deep than the shallow end of the depth range, distribution shifts were greater at the leading edge (cold boundary) of the distribution of species, implying that the extinction debts are likely to be larger than immigration lags (Jackson & Sax, 2010). Species could not physically track all temperature parameters simultaneously because the shifts in parameters had divergent directions due to the oceanography of the North Sea. Previous studies have generally only examined tracking of a single temperature parameter, e.g. minimum winter temperature (La Sorte & Jetz, 2012) or mean annual temperature (Bertrand et al., 2011). Our results show that the choice of temperature parameter is likely to affect the perceived extent to which species can track their temperature niche. Without prior information on what climate parameters are limiting the distribution of a species under climate change, it is hard to justify choosing one parameter above the others.
Our second (H2) and third (H3) hypotheses were partly supported: that the direction and magnitude of range shifts can be predicted from the velocity and direction of isotherm movements; and that mean temperatures are better predictors of the overall distribution shift, with minimum and maximum temperatures better predicting leading and trailing edge shifts. Contrary to expectations, the expansion of the leading edge was better predicted by the velocity of mean and maximum SST rather than minimum temperatures. The north-west expansion of the leading edge experienced by many species cannot be explained by an increase in minimum temperatures to above the lower thermal tolerance limit, because the minimum temperature increases with latitude in the North Sea. As species are not expanding into areas that were previously too cold, the climate variability hypothesis that proposes that thermal tolerance at a global scale is related to latitudinal range (Sunday et al., 2012), seems not to apply at finer spatial scales where the relationship among temperature parameters is more complex. This observation does not disprove the climate variability hypothesis for minimum temperatures, but instead indicates that other factors than increases in minimum temperature can explain the north-westerly expansion of the distribution of benthic invertebrates. It is possible that species range shifts respond to shifting temperatures in idiosyncratic ways, and that the shift in mean temperatures will best fit the average of the species shifts (Pinsky et al., 2013). This does not imply that the mean temperature is the most important determinant of distributions but just indicates that the average temperature is most likely to correlate with most measures of temperature. It is also likely that different thermal windows are important for each species. For example, northern barnacles are particularly sensitive to spring temperatures at the southern end of their range (Poloczanska et al., 2008) and this is neither the minimum nor the maximum temperature. As such, it may be worth repeating the analysis by examining VoCC for all 12 months individually in future studies. These results add to the mounting evidence on the ability of the VoCC to predict the magnitude and direction of shifts in marine species (Pinsky et al., 2013; Poloczanska et al., 2013), and introduces the potential usefulness of considering extremes as well as the mean for improving the prediction of distribution shifts.
Strong evidence in support for our last hypothesis (H4), that species will track their original thermal niche with no extinction debts or immigration lags, was only found for mean SBT. Even for this temperature parameter, we found that many species did not shift their ranges fast enough to keep pace with climate change. Depending on the temperature parameter examined, 25–78% of species were static and lagging behind temperature changes between 1986 and 2000, even though all three temperature parameters changed substantially (mean increase of 0.31 and 0.41 °C for mean SBT and SST respectively) in almost the whole North Sea over this period. It is therefore likely that many species built up extinction debts because of a failure to track temperatures, i.e. a large fraction of species are not tracking and end up warmer than before (Jackson & Sax, 2010). These results seem to contradict those of the VoCC analysis, where mean SBT only succeeded in predicting the direction but not the magnitude of the geographic shifts, yet mean and maximum SST predicted well both the direction and magnitude of geographic shifts. This may be related to the underestimation of the magnitude of the observed shifts of species by about 2 km yr−1 using the SST VoCC (Fig. S9). Immigration lags related to failure to track minimum temperature are also likely to develop as hardly any species are shifting in the right direction to track this parameter. Basic physiological principles dictate that environmental temperature affects the scope for activity, energy use, growth and other physiological processes for ectothermic organisms. Ongoing temperature changes may yet be within the thermal tolerance of these species, and their distribution therefore limited by other factors. Several studies have also shown that species can adapt quickly to the locally changing conditions through phenotypic plasticity and adaptive microevolution (Parmesan, 2006; Bellard et al., 2012), which would make them less sensitive to temperature changes. However, species with higher thermal tolerance are often more susceptible to climate change because of a trade-off between thermal tolerance and acclamatory capacity (Stillman, 2003; Krenek et al., 2013). Species that may adapt well to short-term temperature increases may therefore not cope well with ongoing climate change. As 14 years between 1986 and 2000 is longer than the life span of most species studied here, the only obvious exception being the ocean quahog Arctica islandica (Witbaard & Bergman, 2003), adaptive microevolution may also have played a role. A final explanation could be that these species are building up an extinction debt where populations are thriving, either because adults are long lived or because population mortality is only slightly higher than new recruitment, but will not be viable in the long term (Jackson & Sax, 2010; Sax et al., 2013).
Apart from climate change, the most important factor influencing the abundance, biomass and species richness of benthic invertebrates in the North Sea is bottom trawling. Trawling could result in changes in the distribution of benthos if the distribution of the trawling effort changes, and could explain our results if the relative trawling effort increased in shallow and south-easterly areas of the North Sea between 1986 and 2000. Nevertheless, there is no evidence that such a divergence in the spatial allocation of fishing effort occurred in this period (Jennings et al., 1999). On the other hand, primary production is generally assumed to be limiting the carrying capacity of the ecosystem for benthic invertebrates, and changes in primary production can therefore also result in changes in the distribution of the benthos (Hiddink et al., 2006). The two main drivers for changes in primary production are likely to be climate change and eutrophication. The effects of changes in primary production induced by climate change cannot be separated from those of temperature changes in this study, but no clear trends in primary production with climate change have been recorded for the North Sea (Beaugrand & Reid, 2003). Reductions in eutrophication could led to reduced primary production in coastal areas, and subsequently to the observed relative decrease in benthos in the southern North Sea, but cannot explain the observed expansions of species ranges into the northern North Sea. Finally, the abundance of major benthic predators, commercial fish species, decreased over the study period across the North Sea, releasing the benthos from predation pressure (Heath, 2005). There is however no reason to assume that this would have led to a change in the spatial distribution of benthic species.
In conclusion, although geographical and depth shifts were observed for many benthic invertebrates in the North Sea at the same time as ongoing changes in sea surface and bottom temperatures, only a minority of species did actually track effectively their thermal niches over time for most temperature measures. The exception was mean bottom temperature which was tracked without extinction debts or immigration lags by 57% of species. The distribution of most benthic species was lagging behind both bottom and surface temperature and many species were therefore experiencing higher temperatures in 2000 than in 1986. In contrast, the only predictors of the direction and magnitude of shifts of the distribution of benthos was the VoCC of mean surface temperature, while maximum surface temperature predicted accurately the expansion of the leading edge. Benthic invertebrates that are lagging behind climate change will run out of suitable thermal habitat in the long run, and part of the population is likely to be already occupying unsuitable thermal habitat at the moment. It is hard to envisage conservation strategies that could negate such effects beyond minimizing the effect of other disturbances and therefore ensure maximum reproductive potential, which would result in enhanced production and therefore dispersal of eggs and larvae.
Acknowledgements
We thank all involved in NSBS 1986 and NSBP 2000 for carrying out the surveys and making the data available. M.T.B. and J.G.M. were supported by the UK Natural Environment Research Council grant NE/J024082/1.




