Interactive effects of elevated CO2 and nitrogen deposition on fatty acid molecular and isotope composition of above- and belowground tree biomass and forest soil fractions
Abstract
Atmospheric carbon dioxide (CO2) and reactive nitrogen (N) concentrations have been increasing due to human activities and impact the global carbon (C) cycle by affecting plant photosynthesis and decomposition processes in soil. Large amounts of C are stored in plants and soils, but the mechanisms behind the stabilization of plant- and microbial-derived organic matter (OM) in soils are still under debate and it is not clear how N deposition affects soil OM dynamics. Here, we studied the effects of 4 years of elevated (13C-depleted) CO2 and N deposition in forest ecosystems established in open-top chambers on composition and turnover of fatty acids (FAs) in plants and soils. FAs served as biomarkers for plant- and microbial-derived OM in soil density fractions. We analyzed above- and belowground plant biomass of beech and spruce trees as well as soil density fractions for the total organic C and FA molecular and isotope (δ13C) composition. FAs did not accumulate relative to total organic C in fine mineral fractions, showing that FAs are not effectively stabilized by association with soil minerals. The δ13C values of FAs in plant biomass increased under high N deposition. However, the N effect was only apparent under elevated CO2 suggesting a N limitation of the system. In soil fractions, only isotope compositions of short-chain FAs (C16+18) were affected. Fractions of ‘new’ (experimental-derived) FAs were calculated using isotope depletion in elevated CO2 plots and decreased from free light to fine mineral fractions. ‘New’ FAs were higher in short-chain compared to long-chain FAs (C20−30), indicating a faster turnover of short-chain compared to long-chain FAs. Increased N deposition did not significantly affect the quantity of ‘new’ FAs in soil fractions, but showed a tendency of increased amounts of ‘old’ (pre-experimental) C suggesting that decomposition of ‘old’ C is retarded by high N inputs.
Introduction
Humans are changing the global environment by increasing atmospheric carbon dioxide (CO2) concentrations and nitrogen (N) deposition, which ultimately leads to perturbations of global biogeochemical cycles and the climate system (Denman et al., 2007). In May 2013, atmospheric CO2 concentrations reached a landmark of 400 ppm and are still exponentially increasing (Bala, 2013), while atmospheric N deposition increased three- to fivefold within the last century and is expected to further rise in the future (Galloway et al., 2008). Combustion of fossil fuels and fertilizer application are the main sources of reactive N in the atmosphere (Davidson, 2009), which is deposited to terrestrial ecosystems and thereby fertilizes both plant and microbial communities. It is hypothesized that increased N deposition leads to allocation shifts during plant growth from below- to aboveground plant biomass and shifts in microbial community composition from fungal- to microbial-dominated communities (Janssens et al., 2010). The combined effects of elevated CO2 and N deposition have been shown to affect carbon (C) cycling in plants and soils (Ainsworth & Long, 2005; Reich et al., 2006; Hyvönen et al., 2007; Dieleman et al., 2010; Norby & Zak, 2011).
Soils play a key role in the storage of C in terrestrial ecosystems (Stockmann et al., 2013), but may also release large amounts of C into the atmosphere. It remains unclear how C cycling in the plant-soil system reacts to the environmental changes which are currently underway (Smith, 2012). This is largely due to the fact that the general mechanisms behind stabilization and destabilization of organic matter (OM) in soils are still under debate (Schmidt et al., 2011; Dungait et al., 2012; Cotrufo et al., 2013).
Soil OM is thought to be mainly stabilized by occlusion in soil aggregates and interaction with soil minerals (Sollins et al., 1996; Six et al., 2002; von Lützow et al., 2006). Physical soil fractionation has been frequently used to isolate distinct soil fractions to examine different stabilization mechanisms of soil OM (von Lützow et al., 2007). Density fractionation schemes separate bulk soil into ‘light’ fractions, in which OM is either physically unprotected or occluded in aggregates and into ‘heavy’ fractions, in which OM is associated with soil minerals (Golchin et al., 1994; Crow et al., 2007). Although soil density fractions have been frequently analyzed for their total organic C contents (e.g. John et al., 2005; Dorodnikov et al., 2011), the analysis of specific biomarkers in density fractions has only been undertaken in a limited number of studies (Glaser et al., 2000; Wiesenberg et al., 2010a; Griepentrog et al., 2014).
Biomarkers are organic compounds that can be attributed to a specific organism or groups of organisms (Brocks & Pearson, 2005). Ideally, they have the potential to be preserved in any kind of archive such as soils and sediments and can be used as markers for a specific vegetation type, plant part or environment. Therefore, biomarkers can be used as proxies for the source determination of soil OM, such as inputs from plant biomass and microorganisms (Harwood & Russell, 1984; Dinel et al., 1990; Kögel-Knabner, 2002; Amelung et al., 2008).
Fatty acids (FAs) can be used as biomarkers for plant and microbial-derived OM in soils (Dinel et al., 1990). Short-chain FAs (<C20) are ubiquitously produced by all living organisms, including plants and microorganisms (Harwood & Russell, 1984). Long-chain FAs (≥C20) are mainly synthesized by plants (Harwood & Russell, 1984) and are therefore frequently used as biomarkers for plant-derived OM in soils (e.g. Bull et al., 2000; Wiesenberg et al., 2004, 2008a, 2012; Otto & Simpson, 2005; Quénéa et al., 2006; Feng et al., 2010). Plant-derived fatty acids are an important energy source for other soil organisms and qualitative or quantitative changes in fatty acid composition can be hypothesized to influence the cycling of organic matter in soils by changing e.g. microbial community structure and decomposition processes.
Analyzing the composition and stable isotope signatures of specific biomarkers within distinct soil fractions should greatly enhance our understanding of the turnover and stabilization of OM in soils (Amelung et al., 2008; Feng & Simpson, 2011; Simpson & Simpson, 2012; Gleixner, 2013; Mendez-Millan et al., 2014). The stable isotope analysis of biomarkers in soil fractions is in particular helpful to improve our understanding with respect to different stabilization mechanisms and turnover of plant- and microbial-derived organic matter in soil aggregates and associated with soil minerals.
In this study, we used model forest ecosystems that were exposed to ambient and elevated (13C-depleted) CO2 concentrations in combination with two levels of N deposition. We investigated the molecular and isotope (δ13C) composition of FAs in above- and belowground plant biomass of beech and spruce, as well as in bulk soil and soil density fractions. We tested if elevated CO2 concentrations and increased N deposition affect the molecular and isotope composition of FAs in above- and belowground plant biomass and soil density fractions. Furthermore, we used the different isotopic signatures of ambient and 13C-depleted CO2 to isotopically trace the organic matter in different ecosystem compartments. By applying an isotope mixing model, we calculated the fraction of ‘new’ C in soil fractions that is derived from plant inputs throughout the 4 years of the experimental period. We hypothesized that mainly ‘new’ FAs (produced within the experimental period), are affected by N deposition, in contrast to ‘old’ FAs (that derived from before the experimental period).
Materials and methods
Experimental setup
We used archived plant and soil samples from a combined elevated CO2 and N deposition experiment that was conducted during four growing seasons between 1994 and 1998. A detailed description of the experimental setup can be found in Egli et al. (1998). In brief, model forest ecosystems were established in open-top chambers with lysimeters: an acidic soil with sandy loamy texture (Haplic Alisol) from a natural beech-spruce forest site was planted with beech (Fagus sylvatica) and spruce (Picea abies) trees as well as five typical understory plant species (Carex sylvatica, Geum urbanum, Hedera helix, Ranunculus ficaria, Viola sylvatica). At the start of the experiment, beech trees were 2–3 years old and spruce trees were 4 years old.
The model ecosystems were treated with ambient CO2 (370 μmol mol−1) and elevated CO2 (570 μmol mol−1) concentrations in combination with low (7 kg NH4NO3-N ha−1 yr−1) and high (70 kg NH4NO3-N ha−1 yr−1) levels of N deposition. The isotope value of the elevated CO2 treatment was depleted in 13C compared to the ambient CO2 treatment by Δδ13C = 16‰ (Hagedorn et al., 2003). The following treatment combinations were studied: ambient CO2 and low N; ambient CO2 and high N; elevated CO2 and low N; elevated CO2; and high N. Each of the four CO2 to N treatment combinations was replicated three times in the field.
After 4 years of treatment, soils were sampled from 0 to 10 cm depth prior to tree harvest. Tree biomass was sampled separately for above- and belowground plant compartments of each tree species including beech leaves, beech roots, spruce needles, and spruce roots. All plant and soil samples were dried at 60 °C for 48 h immediately after sampling. Samples were stored in sealed containers in a fully air-conditioned archive (17 °C) with low air humidity (<40%) until analysis.
Soil fractionation
Soils were physically fractionated following the principle concept of Golchin et al. (1994). The fractionation procedure aims at separating three organic matter fractions that conceptually relate to different stabilization mechanisms of organic matter in soil, which cover: (i) organic matter physically unprotected against degradation; (ii) organic matter protected against degradation by occlusion in soil aggregates; and (iii) by association with soil minerals.
Bulk soil was separated into free light fraction (fLF), occluded light fraction (oLF), and total heavy fraction (tHF) using density fractionation along with ultrasonic dispersion. The total heavy fraction was further separated by particle-size fractionation at 20 μm into a coarse heavy fraction (cHF) and a fine heavy fraction (fHF). The selection of density and ultrasonic dispersion energy was based on preceding experiments to maximize C content in light fractions (Cerli et al., 2012; Griepentrog & Schmidt, 2013). Results showed that a density of 1.6 g cm−3 and a dispersion energy of 250 J ml−1 were the most suitable parameters for our soil.
The fractionation procedure was previously described in detail (Griepentrog et al., 2014). In brief, sodium polytungstate (SPT 0, Tungsten Compounds, Grub am Forst, Germany) solution was used for density fractionation and SPT solutions were collected and recycled during the fractionation procedure (Six et al., 1999). Bulk soil samples (dried and sieved <2 mm) were suspended in SPT solution and centrifuged. The floating material (fLF) was collected, filtered, and rinsed with de-ionized water to remove residual SPT. The remaining soil material was resuspended in SPT solution and ultrasonically dispersed. The ultrasonic equipment was calorimetrically calibrated according to Schmidt et al. (1999). The floating material (oLF) was collected, filtered, and rinsed with de-ionized water to remove residual SPT. The remaining soil material (tHF) was rinsed three times with de-ionized water followed by subsequent centrifugation each time. Thereafter, the tHF fraction was particle-size fractionated at 20 μm into cHF and fHF fractions using wet sieving and subsequent sedimentation. All fractions were freeze-dried and milled with a ball mill prior to analysis. All soil samples were fractionated in triplicate and pooled for further analysis. On average, we recovered >99% of the initial sample masses (Griepentrog et al., 2014).
Bulk carbon and nitrogen analysis
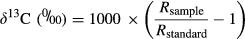 (1)
(1)where R = 13C/12C for both sample and standard. The Vienna Pee Dee Belemnite (VPDB) standard was used as a reference.
Fatty acid analysis
Total lipids were extracted from defined amounts of plant biomass, soil density fractions, and bulk soil with a mixture of dichloromethane/methanol (9/1; v/v) using microwave extraction for one hour at 1600 W (MARS Xpress™ Microwave Reaction System, CEM Corporation; cf. Ernst et al., 2013). After centrifugation, supernatant was collected and residue was washed again with solvent mixture. The procedure was repeated three times to maximize lipid recovery. Total lipid extracts were filtered through glass fiber filters, dried, and weighed.
Total lipid extracts were separated into neutral and FA fractions via solid phase extraction using silica gel with 5% potassium hydroxide (Silica 60 + 5% KOH, 63–200 μm, Margot Köhnen-Willsch, Jülich, Germany). Total lipid extracts were redissolved in dichloromethane and transferred to columns filled with the above-mentioned silica gel. Neutral lipids were eluted with dichloromethane and collected in round bottom flasks. Thereafter, the FA fraction was eluted with a mixture of dichloromethane/formic acid (99/1; v/v) and collected in round flasks. Individual lipid fractions were volume reduced by rotary evaporation, transferred to collection vials, and dried thereafter. The FA fractions were derivatized using boron trifluoride/methanol solution (BF3/MeOH). Prior to derivatization, deuteriated eicosanoic acid (C20D39) was added as an internal standard for quantification.
Compound identification and quantification was performed via an Agilent 6890 Series gas chromatograph (GC) coupled to a mass spectrometry detector (Agilent Technologies, Santa Clara, CA, USA). GC column was an Agilent DB-5MS with 50 m length, 200 μm inner diameter and 0.33 μm film thickness. GC oven temperature program was 50 °C for 2 min, ramp to 150 °C at 5 °C min−1, ramp to 310 °C at 3 °C min−1, hold at 310 °C for 20 min. Carrier gas was Helium flowing at 1 ml min−1.
Compound-specific stable isotope analysis (δ13C) of individual FAs was performed using a Thermo Trace GC Ultra connected to a Thermo Delta V Plus via a Thermo GC Isolink and a Thermo Conflo 4 (Thermo Fisher Scientific, Waltham, MA, USA). GC column was an Agilent VF-1MS with 60 m length, 250 μm inner diameter and 0.25 μm film thickness. GC oven temperature program was 45 °C for 1 min, ramp to 130 °C at 40 °C min−1, ramp to 320 °C at 6 °C min−1, hold at 320 °C for 20 min. Carrier gas was Helium flowing at 1 ml min−1. Reproducibility and stability of δ13C values were evaluated with pulses of CO2 reference gas and FA methyl ester standards of known isotope composition.
Isotope-based calculations
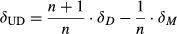 (2)
(2)where n is the number of C atoms in the underivatized FA and δUD and δD are the C isotope ratios of the underivatized and the derivatized FAs, respectively. δM is the C isotope ratios of the added methyl group (−43.7 ± 0.2 ‰). δM was determined by repeated measurement of both derivatized and underivatized C14 FA and rearrangement of Eqn 2.
 ):
):
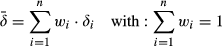 (3)
(3)where wi is the normalized mass proportion of the individual FAs and δi is the isotope ratio of the individual FAs (cf. Wiesenberg et al., 2004, 2008b). Examples of measured isotope ratios of individual FAs of plant biomass and soil fractions can be found in the supporting information (Figures S1 and S2).
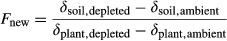 (4)
(4)where δsoil,depleted and δsoil,ambient are the δ13C values of total organic C, short-chain FAs or long-chain FAs in bulk soil or soil fractions for treatments with 13C-depleted CO2 and ambient CO2, respectively (cf. Wiesenberg et al., 2008b). Corresponding, δplant,depleted and δplant,ambient are the δ13C values of total organic C, short-chain FAs or long-chain FAs in plant biomass for treatments with 13C-depleted CO2 and ambient CO2, respectively. For δplant, mean isotope values of plant biomass (beech leaves, beech roots, spruce needles, spruce roots) were calculated using data from Rasse et al. (2005) to estimate the relative distribution of above- vs. belowground plant inputs. Based on Rasse et al. (2005), we assumed that after 4 years of experiment the plant input was divided into 55% aboveground plant litter and 45% roots in 0–10 cm soil.
Statistical analysis
The mean is given along with the standard error for replicate measurements (Webster, 2001). Means and standard errors are based on three field replicates. However, due to limited sample amounts, field replicates of plant biomass were pooled for lipid extractions. Here, the means and standard errors are derived from laboratory measurements of the available fractions. Two-way analysis of variance was used to test the significance of treatment effects (N deposition, elevated CO2) and their interactions (Webster, 2007).
Results
Total organic carbon and total nitrogen
Spruce needles had higher C/N ratios than beech leaves, but spruce roots showed lower C/N ratios than beech roots (Table 1). Only spruce needles were significantly affected by both elevated CO2 concentration and increased N deposition. Under elevated CO2, N concentrations decreased and C/N ratios increased. The opposite was true for high N deposition, which increased N concentrations and decreased C/N ratios. Four years continuous addition of 13C-depleted CO2 (Δδ13C = 16‰) led to a significant decrease of δ13C values of all plant tissues by circa 10‰ (P < 0.001, Table 1). The total organic C isotope values were similar for above- and belowground plant biomass and an identical incorporation of the isotope label was observed for different plant tissues (Fig. 2a).
| N deposition | CO2 concentration | Carbon (gCfr kgfr−1) | Nitrogen (gNfr kgfr−1) | C/N (–) | δ13C (‰) | |
|---|---|---|---|---|---|---|
| Beech leaves | Low | Ambient | 481 ± 4 | 18.7 ± 0.1 | 26 ± 0 | −30.2 ± 0.2 |
| Low | Elevated | 477 ± 3 | 18.4 ± 0.1 | 26 ± 0 | −41.8 ± 1.1 | |
| High | Ambient | 490 ± 3 | 19.8 ± 1.2 | 25 ± 2 | −30.7 ± 0.2 | |
| High | Elevated | 489 ± 2 | 19.4 ± 0.6 | 25 ± 1 | −39.5 ± 0.7 | |
| Significance | N | ** | ns | ns | ns | |
| CO2 | ns | ns | ns | *** | ||
| N × CO2 | ns | ns | ns | ns | ||
| Spruce needles | Low | Ambient | 497 ± 0 | 8.0 ± 0.1 | 62 ± 1 | −30.2 ± 0.1 |
| Low | Elevated | 496 ± 1 | 6.8 ± 0.1 | 74 ± 1 | −41.5 ± 0.9 | |
| High | Ambient | 503 ± 3 | 10.4 ± 0.6 | 49 ± 3 | −30.1 ± 0.0 | |
| High | Elevated | 494 ± 6 | 9.1 ± 0.0 | 55 ± 1 | −40.3 ± 0.6 | |
| Significance | N | ns | *** | *** | ns | |
| CO2 | ns | ** | ** | *** | ||
| N × CO2 | ns | ns | ns | ns | ||
| Beech roots | Low | Ambient | 491 ± 4 | 7.1 ± 1.0 | 73 ± 12 | −28.9 ± 0.2 |
| Low | Elevated | 461 ± 14 | 7.7 ± 1.1 | 64 ± 13 | −41.0 ± 1.2 | |
| High | Ambient | 468 ± 11 | 8.5 ± 1.9 | 62 ± 16 | −29.1 ± 0.1 | |
| High | Elevated | 474 ± 10 | 7.3 ± 0.5 | 66 ± 3 | −39.0 ± 0.6 | |
| Significance | N | ns | ns | ns | ns | |
| CO2 | ns | ns | ns | *** | ||
| N × CO2 | ns | ns | ns | ns | ||
| Spruce roots | Low | Ambient | 483 ± 7 | 9.8 ± 0.6 | 50 ± 3 | −29.1 ± 0.2 |
| Low | Elevated | 453 ± 7 | 9.2 ± 0.2 | 49 ± 1 | −39.1 ± 0.8 | |
| High | Ambient | 459 ± 13 | 11.2 ± 0.6 | 41 ± 3 | −28.7 ± 0.1 | |
| High | Elevated | 449 ± 12 | 9.3 ± 0.2 | 48 ± 1 | −40.2 ± 1.9 | |
| Significance | N | ns | ns | ns | ns | |
| CO2 | ns | * | ns | *** | ||
| N × CO2 | ns | ns | ns | ns |
Soil density fractions showed higher C/N ratios in light than in heavy fractions (Table 2). High N deposition significantly decreased C/N ratios in light fractions. Four years of continuous treatment with 13C-depleted CO2 significantly decreased δ13C values of total organic C in all soil fractions and bulk soil (P < 0.001, Table 2). Heavy fractions revealed higher δ13C values of total organic C compared to light fractions. Increased N deposition did not affect the δ13C values of total organic C in plant biomass, soil density fractions, and bulk soil (Table 4). More detailed information on the distribution of organic C and total N in soil density fractions and bulk soil of this experiment can be found in Griepentrog et al. (2014).
| N deposition | CO2 concentration | Carbon (gCfr kgfr−1) | Nitrogen (gNfr kgfr−1) | C/N (–) | δ13C (‰) | |
|---|---|---|---|---|---|---|
| Free light fraction (fLF) | Low | Ambient | 321 ± 7 | 9.9 ± 0.1 | 33 ± 0 | −29.1 ± 0.2 |
| Low | Elevated | 326 ± 7 | 9.7 ± 0.3 | 34 ± 2 | −35.4 ± 0.5 | |
| High | Ambient | 323 ± 14 | 11.8 ± 0.4 | 28 ± 1 | −29.1 ± 0.1 | |
| High | Elevated | 330 ± 5 | 11.4 ± 0.1 | 29 ± 1 | −35.4 ± 0.4 | |
| Significance | N | ns | *** | ** | ns | |
| CO2 | ns | ns | ns | *** | ||
| N × CO2 | ns | ns | ns | ns | ||
| Occluded light fraction (oLF) | Low | Ambient | 394 ± 16 | 12.5 ± 0.2 | 31 ± 1 | −28.7 ± 0.0 |
| Low | Elevated | 393 ± 16 | 11.8 ± 0.2 | 33 ± 1 | −30.9 ± 0.4 | |
| High | Ambient | 349 ± 17 | 12.1 ± 0.3 | 29 ± 1 | −28.5 ± 0.1 | |
| High | Elevated | 357 ± 6 | 12.7 ± 0.3 | 28 ± 0 | −30.7 ± 0.2 | |
| Significance | N | * | ns | *** | ns | |
| CO2 | ns | ns | ns | *** | ||
| N × CO2 | ns | * | ns | ns | ||
| Total heavy fraction (tHF) | Low | Ambient | 9.6 ± 0.1 | 0.7 ± 0.0 | 13 ± 0 | −26.0 ± 0.0 |
| Low | Elevated | 9.3 ± 0.1 | 0.7 ± 0.0 | 13 ± 0 | −27.1 ± 0.1 | |
| High | Ambient | 9.5 ± 0.2 | 0.8 ± 0.0 | 13 ± 0 | −26.0 ± 0.1 | |
| High | Elevated | 9.9 ± 0.3 | 0.8 ± 0.0 | 13 ± 0 | −27.4 ± 0.1 | |
| Significance | N | ns | ** | * | ns | |
| CO2 | ns | ns | ns | *** | ||
| N × CO2 | ns | ns | ns | ns | ||
| Fine heavy fraction (fHF) | Low | Ambient | 23.6 ± 0.3 | 2.2 ± 0.1 | 11 ± 0 | −26.3 ± 0.0 |
| Low | Elevated | 22.2 ± 0.7 | 1.9 ± 0.1 | 12 ± 0 | −27.2 ± 0.1 | |
| High | Ambient | 22.9 ± 0.3 | 2.0 ± 0.0 | 11 ± 0 | −26.2 ± 0.0 | |
| High | Elevated | 23.7 ± 0.4 | 2.1 ± 0.0 | 11 ± 0 | −27.3 ± 0.1 | |
| Significance | N | ns | ns | ns | ns | |
| CO2 | ns | ns | ns | *** | ||
| N × CO2 | * | ** | ns | ns | ||
| Bulk soil | Low | Ambient | 15.1 ± 1.0 | 1.0 ± 0.0 | 16 ± 0 | −26.9 ± 0.2 |
| Low | Elevated | 14.7 ± 0.6 | 1.0 ± 0.1 | 15 ± 0 | −29.1 ± 0.1 | |
| High | Ambient | 14.3 ± 0.8 | 1.0 ± 0.0 | 15 ± 0 | −27.0 ± 0.1 | |
| High | Elevated | 15.1 ± 0.2 | 1.0 ± 0.0 | 15 ± 0 | −29.2 ± 0.2 | |
| Significance | N | ns | ns | * | ns | |
| CO2 | ns | ns | ns | *** | ||
| N × CO2 | ns | ns | ns | ns |
- Mass distribution between soil fractions: fLF = 0.9 ± 0.02%, oLF = 0.2 ± 0.01%, tHF = 98.9 ± 0.1%, fHF = 31.1 ± 0.2%.
- Carbon distribution between soil fractions: fLF = 21.8 ± 0.6%, oLF = 6.9 ± 0.2%, tHF = 71.3 ± 1.0%, fHF = 62.6 ± 1.0%.
Total lipid and fatty acid composition
No significant effects of elevated atmospheric CO2 concentration and N deposition were observed for total lipid and FA composition and therefore the means of all four treatments are shown in Fig. 1 and Table 3. Total lipid extract yields were generally higher for plant biomass compared to soil fractions and bulk soil (Table 3). In plant biomass, higher total lipid extract yields were found in above compared to belowground plant biomass and in spruce compared to beech biomass. In soil density fractions, light fractions had higher total lipid extract yields compared to heavy fractions. The highest total lipid extract yields of all density fractions were found in occluded light fractions, which showed similar values as beech roots (Table 3).
| Lipid extract yield | Fatty acid molecular proxies | |||||
|---|---|---|---|---|---|---|
| (mg kg−1) | (g kgC−1) | C16:1/C16:0 | C18:1–2/C18:0 | LC/SC | ACL | |
| Plant materials | ||||||
| Beech leaves | 66 574 ± 3831 | 166 ± 10 | 0.03 ± 0.00 | 0.3 ± 0.0 | 1.0 ± 0.0 | 20.8 ± 0.1 |
| Spruce needles | 132 300 ± 5956 | 331 ± 15 | 0.14 ± 0.02 | 0.3 ± 0.1 | 1.5 ± 0.1 | 21.0 ± 0.1 |
| Beech roots | 28 011 ± 1808 | 70 ± 5 | 0.09 ± 0.01 | 2.8 ± 0.5 | 1.2 ± 0.1 | 19.6 ± 0.1 |
| Spruce roots | 41 878 ± 2660 | 105 ± 7 | 0.20 ± 0.04 | 3.9 ± 0.2 | 1.7 ± 0.1 | 19.9 ± 0.1 |
| Soil density fractions | ||||||
| Free light fraction (fLF) | 21 718 ± 671 | 67 ± 2 | 0.02 ± 0.00 | 0.5 ± 0.1 | 1.1 ± 0.1 | 20.4 ± 0.1 |
| Occluded light fraction (oLF) | 28 182 ± 932 | 75 ± 2 | 0.05 ± 0.01 | 0.3 ± 0.0 | 0.4 ± 0.0 | 18.7 ± 0.1 |
| Total heavy fraction (tHF) | 338 ± 9 | 35 ± 1 | 0.04 ± 0.00 | 0.3 ± 0.0 | 1.7 ± 0.1 | 21.4 ± 0.2 |
| Fine heavy fraction (fHF) | 770 ± 21 | 33 ± 1 | 0.12 ± 0.01 | 0.2 ± 0.0 | 1.5 ± 0.0 | 20.6 ± 0.1 |
| Bulk soil | 610 ± 16 | 41 ± 1 | 0.07 ± 0.01 | 0.7 ± 0.1 | 1.4 ± 0.1 | 21.1 ± 0.2 |
- LC/SC = ΣC20–32/ΣC16–19; ACL = Σ(zn × n)/Σ(zn).
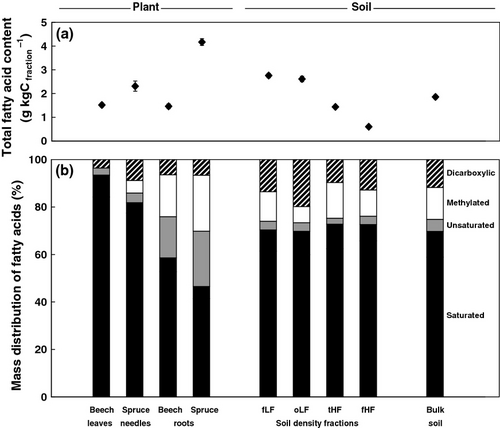
In plant biomass, higher contents of total FAs were found in spruce compared to beech biomass (Fig. 1a). Beech showed similar FA contents in roots and aboveground biomass, while spruce roots had higher FA contents compared to spruce needles. Distinct distribution patterns of FAs were found between roots and aboveground plant biomass. Roots of both tree species revealed higher contents of methylated and unsaturated FAs compared to aboveground biomass (Fig. 1b).
In soil density fractions, total FA contents decreased in the following order: free light fraction > occluded light fraction > total heavy fraction > fine heavy fraction (Fig. 1a). Distribution patterns of FAs were similar among soil fractions and bulk soil (Fig. 1b). However, occluded light fractions had slightly higher contents of dicarboxylic acids compared to the other fractions (Fig. 1b).
Fatty acid molecular proxies
Several molecular proxies have been proposed to assess the source of fatty acids in soils at the molecular level (Wiesenberg et al., 2010a). The ratio of unsaturated-to-saturated C16 FAs (C16:1/C16:0) is generally higher in microbial- compared to plant-derived OM, due to the common presence of C16:1 FAs in microbial tissue (Harwood & Russell, 1984). Furthermore, unsaturated C18 FAs mainly derive from plant biomass and only partially from microorganisms (Harwood & Russell, 1984). Consequently, high ratios of unsaturated-to-saturated C18 FAs (C18:1–2/C18:0) indicate fresh, mainly plant-derived OM and decreasing values reflect ongoing degradation from plant biomass toward soil OM (Wiesenberg et al., 2010a).
No significant effects of CO2 concentrations and N deposition on FA molecular proxies were observed and therefore the means of all four treatments are shown in Table 3. In plant biomass, higher ratios of unsaturated-to-saturated C16 FAs were determined in roots compared to aboveground plant tissues and in spruce compared to beech biomass (Table 3). The ratio of unsaturated-to-saturated C18 FAs was one on the order of magnitude higher in below- compared to aboveground plant biomass. In soil fractions, the ratio of unsaturated-to-saturated C16 FAs increased from free light toward fine heavy soil fractions, while the ratio of unsaturated-to-saturated C18 FAs decreased in the same order (Table 3).
Average chain lengths can be used as molecular proxy for the source of FAs (Wiesenberg et al., 2010a). Microbial-derived FAs are characterized by lower average chain lengths than plant-derived FAs, due to the absence of any long-chain fatty acids (>C19). In plant biomass, lower average chain lengths were found in roots compared to aboveground plant biomass (Table 3). In soil density fractions, occluded light fractions revealed extreme low values of long-chain vs. short-chain FAs (0.4 ± 0.0) as well as average chain lengths (18.7 ± 0.1) compared to other soil fractions and bulk soil.
Carbon isotope composition (δ13C) of fatty acids
Similar to what we found for total organic C, the treatment with 13C-depleted CO2 significantly decreased the δ13C values of FAs in plant biomass, soil density fractions, and bulk soil, compared to control samples (Table 4, P < 0.001).
| Number of replicates | Short-chain fatty acids (C16+18) | Long-chain fatty acids (C20−30) | ||||||
|---|---|---|---|---|---|---|---|---|
| Field | Analytical | N | CO2 | N × CO2 | N | CO2 | N × CO2 | |
| Plant materials | ||||||||
| Beech leaves | 1 | 4 | *** | *** | *** | *** | *** | *** |
| Spruce needles | 1 | 4 | * | *** | ** | *** | *** | *** |
| Beech roots | 1 | 4 | *** | *** | *** | *** | *** | *** |
| Spruce roots | 1 | 4 | ** | *** | ns | ns | *** | ns |
| Soil density fractions | ||||||||
| Free light fraction (fLF) | 3 | 4 | * | *** | *** | ns | *** | ns |
| Occluded light fraction (oLF) | 3 | 4 | *** | *** | *** | ns | *** | ns |
| Total heavy fraction (tHF) | 3 | 4 | ** | *** | ** | ns | *** | ns |
| Fine heavy fraction (fHF) | 3 | 4 | ns | *** | ns | ns | *** | ns |
| Bulk soil | 3 | 4 | * | *** | ** | ns | *** | ns |
Short-chain FAs (C16+18) in roots were characterized by higher δ13C values compared to aboveground plant biomass under both ambient and elevated CO2 concentrations (Fig. 2b). The δ13C values of short-chain FAs in light soil fractions revealed similar values to those of roots biomass for ambient and elevated CO2 concentrations. The δ13C values of short-chain FAs increased in the following order in soil fractions under both CO2 concentrations: free light fraction < occluded light fraction < total heavy fraction < fine heavy fraction. Increased N deposition affected the C isotope composition of short-chain FAs in plant biomass, soil density fractions, and bulk soil, except for the fine heavy soil fraction, which was not affected by increased N deposition (Table 4). However, there was a statistically significant interaction between increased N deposition and elevated CO2 concentrations, except for spruce roots and fine heavy fractions. Short-chain FAs were only affected by increased N deposition under elevated CO2 concentrations, leading to higher δ13C values. N deposition effects were larger in beech compared to spruce biomass.
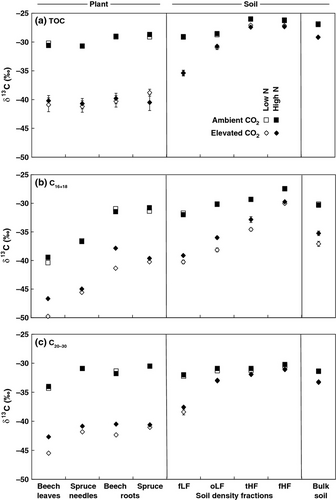
Long-chain FAs (C20−30) did not show distinct differences between the δ13C values of root and aboveground plant biomass for both ambient and elevated CO2 concentrations (Fig. 2c) in contrast to short-chain FAs (Fig. 2b). Similar to short-chain FAs, the δ13C values of long-chain FAs increased in the following order in soil fractions: free light fraction < occluded light fraction < total heavy fraction < fine heavy fraction (Fig. 2c). However, the increase was less pronounced under ambient compared to elevated CO2. Increased N deposition significantly affected the C isotope composition of long-chain FAs in plant biomass, except for spruce roots (Table 4). As observed for short-chain FAs, long-chain FAs also revealed a significant interaction between N deposition and atmospheric CO2 concentration. Increased N deposition only showed significant effects under elevated CO2 concentration, thereby leading to higher δ13C values of long-chain FAs (Fig. 2c). In contrast to short-chain FAs, long-chain FAs were not affected by increased N deposition in soil density fractions and bulk soils (Table 4).
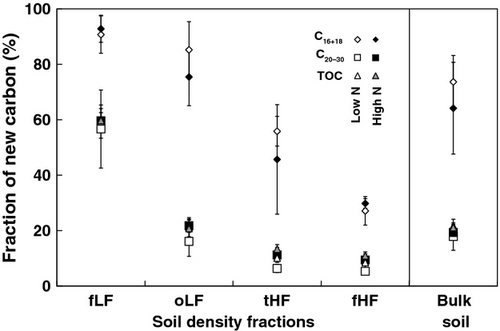
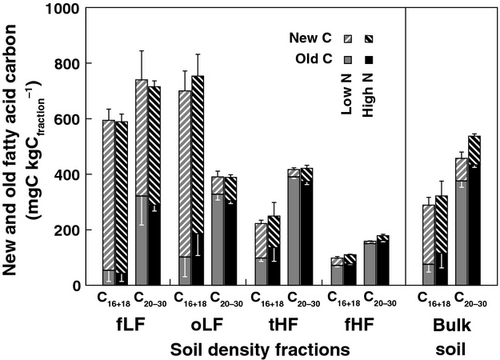
Estimation of new and old fatty acids in soil density fractions and bulk soils
For both short-chain (C16+18) and long-chain (C20−30) FAs in soils, fractions of new experimental-derived FA C decreased in the following order: free light fraction > occluded light fraction > total heavy fraction > fine heavy fraction (Fig. 3). Within all soil density fractions and bulk soil, long-chain FAs showed approximately equal fractions of new C compared with total organic C, while short-chain FAs generally showed significantly higher fractions of new C (Fig. 3). Although the fraction of new C is lower in long-chain compared to short-chain FAs, higher total amounts of new C were found in long-chain FAs (Fig. 4). However, in contrast to other soil fractions and bulk soil, occluded light fractions showed higher (double) amounts of short-chain compared to long-chain FAs (Fig. 4). Increased N deposition had no significant effects on the fractions of new C in short-chain and long-chain FAs in all soil fractions and bulk soil (Fig. 3). Also the total amounts of new short-chain and long-chain FAs were not affected by increased N deposition (Fig. 4). In contrast to new FA C, the amounts of old long-chain FA C were 7.8% higher in fine heavy fractions and 15.4% higher in bulk soil under increased N deposition compared to the control (Fig. 4). However, the effect only tended to be significant (P = 0.094 for fine heavy fractions; P = 0.075 for bulk soil).
Discussion
Bulk carbon and nitrogen
In plant biomass, C/N ratios varied between different plant tissues and species (Table 1), which has been commonly reported (e.g. Otto & Simpson, 2005). Elevated atmospheric CO2 concentration and increased N deposition influenced the N contents of spruce needles in opposite directions. While high N deposition increased N contents, elevated CO2 decreased N contents, with corresponding changes most evident in the C/N ratio of spruce needles (Table 1). Contrasting effects of elevated CO2 concentration and N deposition on the C and N composition of plant biomass have been observed previously (Saxe et al., 1998; Hyvönen et al., 2007) and are attributed to changes in photosynthesis (Ainsworth & Long, 2005) or OM allocation within the plant (Dieleman et al., 2010). The overall change in plant isotope composition (δ13C) by circa 10‰ due to 4 years of isotope labeling is similar to other free air CO2 enrichment experiments (e.g. Van Kessel et al., 2000).
In soil density fractions, high C/N ratios in light fractions reflect C/N ratios of plant biomass input, while lower C/N ratios in heavy fractions are attributed to an increased contribution of microbial-derived OM in mineral soil fractions (Golchin et al., 1994). N effects on C/N ratios in light fractions are consistent with effects observed for plant biomass, which is the main constituent of OM in light fractions (Crow et al., 2007; Wagai et al., 2009; Dorodnikov et al., 2011). An extended discussion on the distribution of C and N in soil fractions of this experiment can be found in Griepentrog et al. (2014).
Total lipid and fatty acid composition
Total lipid extract yields varied between different plant tissues and soil fractions (Table 3) and are in the range of values found in density fractions of a cropland soil (Wiesenberg et al., 2010a). Contents and distribution patterns of FA varied between the two tree species in our experiment (Fig. 1), which has also been observed for other plant species in forests (Otto & Simpson, 2005; Feng et al., 2010), in grassland (Wiesenberg et al., 2008a) as well as in cropland (Wiesenberg et al., 2004). Spruce roots showed significantly higher FA contents (Fig. 1) and lower average chain lengths than spruce needles (Table 3) and short-chain FAs of microbial origin might therefore be an additional source of root FAs (Harwood & Russell, 1984; Lichtfouse et al., 1995). In contrast to aboveground biomass, roots are generally associated with microbial communities, in particular mycorrhizal fungi (Jones et al., 2009) and might be therefore analyzed together. High amounts of methylated (Fig. 1) and mono-unsaturated (Table 3) FAs in root biomass furthermore point toward a considerable contribution of microbial-derived FAs in roots (Harwood & Russell, 1984).
Distribution patterns of FAs were similar among soil fractions and bulk soil (Fig. 1) and most likely reflect a mixed contribution of root, aboveground plant and rhizomicrobial FAs (Wiesenberg et al., 2012; Mueller et al., 2013). The decrease in FA contents from free light to fine heavy fractions (Fig. 1) might be due to high inputs of fresh plant-derived FAs into light fractions and a trend of increasing degradation toward minerals soil fractions. This is also consistent with the decrease in mainly plant-derived polyunsaturated C18 FAs from free light toward fine heavy fractions (Table 3). Low values of long-chain vs. short-chain FA ratios and average chain lengths in occluded light fractions (Table 3) point toward a high contribution of microbial-derived FAs, which is consistent with the prominent role of microorganisms in soil aggregate formation (Six & Paustian, 2014). Our results are in general agreement with those from a study of FAs in density fractions of a cropland soil (Wiesenberg et al., 2010a). However, the lack of FA accumulation relative to total organic C in fine mineral fractions (Fig. 1) contradicts observations made by Griepentrog et al. (2014) for microbial-derived amino sugars in this experiment. This suggests that FAs are not as effectively stabilized by association with soil minerals compared to microbial sugars or total organic C, which might be related to the fast incorporation of FAs in soils (Wiesenberg et al., 2010b).
Carbon isotope composition (δ13C) of fatty acids
Short-chain FAs of roots were characterized by higher δ13C values compared to aboveground biomass (Fig. 2b). Root C is derived from photosynthetically fixed C that was translocated within the plant (Hobbie & Werner, 2004). Metabolic intermediates (such as sugars) are fixed and released several times during transport to roots and then synthesized into root FAs (Hobbie & Werner, 2004), which causes isotope fractionation and mainly leads to the 13C enrichment of short-chain FAs in roots (Wiesenberg et al., 2004). In addition, microorganisms are generally associated with plant roots (Jones et al., 2009) and typically enriched in 13C compared to plant-derived OM, due to isotope fractionation during microbial uptake and biochemical transformation (Werth & Kuzyakov, 2010). Therefore, microbial-derived short-chain FAs could potentially also contribute to the observed 13C enrichment in roots. However, root samples were thoroughly washed before extraction and analysis and microbial remains should only be present in minor quantities, mainly as microorganisms living within the root tissues.
Short-chain FAs in light soil fractions revealed similar δ13C values to those in root biomass (Fig. 2b). Therefore, root-derived OM may contribute substantial quantities to plant-derived inputs of soil FAs (Wiesenberg et al., 2008b, 2012), as suggested for total soil organic C (Rasse et al., 2005). However, it is also possible that OM in light fractions might be derived from aboveground plant biomass that was altered during microbial degradation and had therefore undergone isotope fractionation (Nguyen Tu et al., 2004). The latter would also account for the progressive enrichment in 13C from free light toward fine heavy soil fractions.
In plant biomass, isotope compositions of both short-chain (C16+18) and long-chain (C20−30) FAs were significantly affected by increased N deposition (Table 4). However, N deposition effects only occurred under elevated CO2 concentrations, showing significant interactions between CO2 concentrations and N deposition (Table 4). The combined effects of increased N deposition and elevated CO2 concentrations may be related to changes in C isotope fractionation during photosynthesis (Huang et al., 1999). Under elevated atmospheric CO2 concentration, the ratio of partial pressures of CO2 concentrations within and outside the leaves changes, resulting in stomatal closure (Ainsworth & Rogers, 2007). However, N application stimulates plant growth and promotes photosynthesis, which increases the consumption of intercellular CO2 and reduces intercellular CO2 partial pressure (Huang et al., 1999). The combined effects of reduction in stomatal conductance and increase in intercellular CO2 consumption lead to a reduction in 13C discrimination during photosynthesis and therefore to higher δ13C values (Farquhar et al., 1989).
The observed isotope effects were larger for beech compared with spruce biomass (Fig 2b, c). Beech grows better on sites with high contents of available nutrients and high pH values, while spruce is more adapted to sites that are depleted in nutrients and characterized by low pH values (Spinnler et al., 2002). Also for our experiment, it could be shown that beech was more dependent on N availability than spruce (Hagedorn et al., 2002).
In soil fractions, only δ13C values of short-chain FAs were affected by increased N deposition. Here, N effects can be attributed to changes in δ13C values of plant biomass input, but probably also to N effects on microbial-derived FAs. The latter might be more important since no effects on plant-specific long-chain FAs could be observed in soil fractions (Fig 2c; Table 4). Furthermore, measurements of amino sugars as biomarkers for microbial residues support this assumption, since they indicated higher production of fungal residues under increased N deposition (Griepentrog et al., 2014). Therefore, our results suggest that N effects in soil fractions are mainly attributed to microorganisms and only in minor parts to changes in plant-derived OM composition, which was recently also suggested for soil OM genesis (Miltner et al., 2012).
New and old fatty acid carbon in soil fractions
Fractions of new FA C decreased from free light toward fine heavy soil fractions (Fig. 3) reflecting higher inputs of isotopically labeled plant biomass into light fractions. In heavy fractions, organic matter was altered by microorganisms, causing isotope fractionation (13C enrichment) as part of the microbial loop (Bonkowski, 2004; Hobbie & Werner, 2004), which in turn led to less incorporation of new C in mineral fractions (Fig. 2).
While long-chain FAs revealed equal fractions of new C compared with total organic C, short-chain FAs had significantly higher fractions of new C. Long-chain FAs are indicative for plant biomass, which substantially contributes to soil OM inputs and total organic C, and they are therefore expected to have similar fractions of new C. However, in previous studies it has also been reported that FAs have faster turnover times than total organic C (Wiesenberg et al., 2004, 2008b; Feng et al., 2010). This indicates that, in our experiment, also other compounds with similar turnover times to those of long-chain FAs have largely been incorporated into soil OM or that the comparatively short-term duration of the experiments of 4 years might have biased the results.
Short-chain FAs show higher contributions of new C compared to long-chain FAs throughout all soil fractions (Fig. 3), which confirms prior observations in grassland soils (Wiesenberg et al., 2008b). The main reason is the additional contribution of microorganisms to short-chain FAs and the faster incorporation of the isotope label into microbial-derived OM with a shorter turnover compared to plant biomass (Kramer & Gleixner, 2006). An additional process that could have partially contributed to the observed pattern is the rapid incorporation of plant-derived short-chain FAs incorporated via root exudates (Wiesenberg et al., 2010b).
Although increased N deposition altered the isotope composition of FAs (Fig. 2), N deposition did not significantly affect the fraction of new FA C in soil fractions and bulk soil (Fig. 3). The absence of significant effects might be attributed to large errors associated with the analysis, the calculation of new C fractions and the duration of the experiment of only 4 years. However, there is a (statistically insignificant) trend that high N deposition decreased the fraction of new C in short-chain FAs (by 10%) in occluded light and total heavy fractions as well as in bulk soil (Fig. 3). This could be attributed to N effects on microorganisms, leading to a decline in microbial biomass under increased N deposition (Treseder, 2008).
Amounts of old FA C were 7.8% (P = 0.094) and 15.4% (P = 0.075) higher in fine heavy fractions and bulk soil respectively, under high N deposition compared to control treatments. We attribute the weak significance level to the short duration of the experiment, the large stocks of C in soil and high uncertainty in calculating fractions of new and old C (Fig. 3 and 4). However, the higher amounts of old C in FAs are in agreement with other findings within the same experiment, showing that there is a retarded decomposition of old total organic C (Hagedorn et al., 2003) and old microbial residue C (Griepentrog et al., 2014) under high N deposition. The decrease in decomposition of old C under high N deposition could be attributed to reduced mining of native soil OM by microorganisms, if additional inorganic N is available (Fontaine et al., 2011). This process seems to be especially important in fine heavy fractions where OM is associated with soil minerals.
In summary, we were able to trace new vs. old above- and belowground plant-derived FAs into forest soil density fractions using compound-specific stable isotope analysis. Our results suggest a substantial contribution of root-derived FAs to the soil FA pool. In contrast to total organic C, FAs are apparently not preferentially stabilized by association with soil minerals. N deposition increased δ13C values of plant FAs under elevated CO2 concentrations, most likely due to lesser isotope discrimination during photosynthesis. However, in soil fractions only short-chain FAs were affected by increased N deposition, which could be related to a faster turnover throughout all soil fractions compared to plant-derived long-chain FAs. The absolute quantities of new C in FAs of soil density fractions and bulk soil were not significantly affected, at least partly due to the short experimental period of 4 years. However, we found a tendency of increasing amounts of old FA-C under high N inputs which is in agreement with previous findings that N deposition retards the decomposition of old soil C. Temperate forest ecosystems are the major ecosystems in Europe and the retarded decomposition of native soil OM under increased N deposition might affect the C balance of European soils, because it potentially increases soil C sequestration. However, at a global scale, the study of boreal and tropical forest ecosystems is a potential area of future research with high relevance to global soil C storage.
Acknowledgements
Daniel B. Montluçon (ETH) gave laboratory assistance during fatty acid extraction and compound-specific δ13C analyses. Ursula Graf (WSL) performed bulk elemental and δ13C analyses. Rolf T.W. Siegwolf (PSI) provided the plant samples. Kavita Srivastava (UZH) and Daniel B. Wiedemeier (UZH) gave constructive comments on outline and figures of the manuscript. The study was funded by the Swiss National Science Foundation (SNF).
Author contributions
F.H., M.W.I.S. and G.L.B.W. initially proposed the study. T.I.E. enabled the compound-specific δ13C analyses of fatty acids and gave conceptual and technical support. M.G. conducted the laboratory work, analyzed the data and wrote the manuscript. All authors contributed with constructive comments to the final version of the manuscript.




