Long-term dynamics of mycorrhizal root tips in a loblolly pine forest grown with free-air CO2 enrichment and soil N fertilization for 6 years
Abstract
Large-scale, long-term FACE (Free-Air CO2 enrichment) experiments indicate that increases in atmospheric CO2 concentrations will influence forest C cycling in unpredictable ways. It has been recently suggested that responses of mycorrhizal fungi could determine whether forest net primary productivity (NPP) is increased by elevated CO2 over long time periods and if forests soils will function as sources or sinks of C in the future. We studied the dynamic responses of ectomycorrhizae to N fertilization and atmospheric CO2 enrichment at the Duke FACE experiment using minirhizotrons over a 6 year period (2005–2010). Stimulation of mycorrhizal production by elevated CO2 was observed during only 1 (2007) of 6 years. This increased the standing crop of mycorrhizal tips during 2007 and 2008; during 2008, significantly higher mortality returned standing crop to ambient levels for the remainder of the experiment. It is therefore unlikely that increased production of mycorrhizal tips can explain the lack of progressive nitrogen limitations and associated increases in N uptake observed in CO2-enriched plots at this site. Fertilization generally decreased tree reliance on mycorrhizae as tip production declined with the addition of nitrogen as has been shown in many other studies. Annual NPP of mycorrhizal tips was greatest during years with warm January temperatures and during years with cool spring temperatures. A 2 °C increase in average late spring temperatures (May and June) decreased annual production of mycorrhizal root tip length by 50%. This has important implications for ecosystem function in a warmer world in addition to potential for forest soils to sequester atmospheric C.
Introduction
Rising atmospheric CO2 could alter ecosystem function in ways that significantly affect C exchange between the land and atmosphere. This has important implications for projecting future climates because the balance of C influx and efflux from ecosystems will determine whether terrestrial biomes will ameliorate or exacerbate ongoing atmospheric CO2 enrichment. Unfortunately, large-scale experiments documenting ecosystem responses to long-term exposure to elevated atmospheric CO2 have proven highly variable. In some cases, the commonly observed CO2 fertilization effect on photosynthesis and net primary productivity is reduced or eliminated over time because the supply of fixed C eventually exceeds the availability of mineral nutrients (Luo et al., 2004; Johnson, 2006; Körner, 2006; Menge & Field, 2007; Norby et al., 2010). The most often discussed nutrient that becomes progressively limiting in CO2-enriched atmospheres is soil N [PNL, (progressive nitrogen limitation); Luo et al., 2004; Johnson, 2006]. In other cases, higher net primary productivity (NPP) in CO2-enriched environments is sustained in spite of soil nutrient limitations (Langley et al., 2009; McCarthy et al., 2010; Drake et al., 2011). The reasons for these variable ecosystem responses to elevated CO2 remain unknown. The elucidation of a mechanistic explanation of long-term ecosystem responses would be useful for predicting the fate of unstudied, and often disparate, ecosystem types in a CO2-enriched and warmer world and for accurately modeling future climates (Norby & Zak, 2011).
It has been suggested that responses of ectomycorrhizal fungi (ECM) will be particularly important for controlling ecosystem responses to long-term exposure to elevated CO2 because they link canopy carbon relations with soil nutrient cycles (Drake et al., 2011; Cairney, 2012). Mycorrhizal root tips, often referred to as short roots, are particularly important for tree nutrition because they absorb the majority of mineral nutrients taken up by trees (Pregitzer et al., 2002; Guo et al., 2004, 2008). In some plants, as much as 80% of tree N originates from ECM symbionts (Van der Heijden et al., 2008). Globally, it is estimated that 1/7 of all the N contained in terrestrial vegetation can be found in fine-root systems (Yuan et al., 2011), and ectomycorrhizal root tips account for as much as 40–50% of total fine-root biomass in some trees (Ostonen et al., 2005; Helmisaari et al., 2007, 2009). Understanding how ECM respond to atmospheric CO2 enrichment, therefore, might prove to be the key for predicting long-term ecosystem response to environmental changes.
The types of mycorrhizal relationships that dominate a given plant system might explain variable ecosystem responses to CO2 enrichment (Phillips et al., 2013). Systems dominated by ECM may be less prone to progressive nutrient limitations to sustained higher NPP in CO2-enriched atmospheres compared to systems dominated by plants colonized by vesicular arbuscular mycorrhizae (Drake et al., 2011). Talbot et al. (2008) suggested that mycorrhizal fungi, particularly ECM which have been shown to function as partial saprotrophs, may be effective in decomposing organic matter when allocation of carbohydrates to mycorrhizal roots is high, as is typically the case when forest canopies are fumigated with extra CO2. Meta-analyses have suggested that exposure of plants to 2x ambient CO2 normally elicits a 35–50% increase in mycorrhizal abundance (Treseder, 2004; Alberton et al., 2005). Increased activity of enzymes important for decomposition of soil organic matter, such as chitinase, may also accompany atmospheric CO2 enrichment (Dorodnikov et al., 2009). Increased allocation of resources to mycorrhizal fungi under CO2-enriched conditions could accelerate the rate of SOM decomposition thereby enhancing nutrient availability, which, coupled with more extensive colonization of fine roots by ECM fungi, could improve nutrient uptake into plants and ameliorate PNL (Phillips et al., 2011, 2013).
In addition to their importance for forest nutrient relations, responses of ECM fungi to elevated CO2 are also an important component of the soil C pool. For instance, it was estimated that 10–20% of NPP may be allocated to support maintenance and growth of ECM in pine forests (Vogt, 1982; Johnson & Gehring, 2007; Cairney, 2012), and in some cases production of extramatrical mycelia (EMM) of ECM fungi may account for more than half of the C that is added to soil (Godbold et al., 2006; Cairney, 2012; Clemmensen et al., 2013). The net effect of C allocation to mycorrhizal tips and EMM on soil C sequestration, however, will depend largely on the rate of turnover of these structures, which has not yet been adequately characterized. For example, estimates of mycorrhizal root tip life span range from as short as a few months to 6 years (Cairney, 2012). For coniferous trees, median longevity of 70–210 days has been reported in several studies (Majdi & Nylund, 1996; Rygiewicz et al., 1997; Pritchard et al., 2008a; McCormack et al., 2010). It is widely recognized that our understanding of belowground C cycling is the weakest link in understanding C and nutrient flow through the terrestrial biosphere, and this is largely attributable to our lack of understanding of mycorrhizal dynamics (Cairney, 2012; Fransson, 2012; Pickles et al., 2012).
We quantified mycorrhizal root tip production, mortality, standing crop, and root tip dimensions with minirhizotrons at the Duke FACE (Free-air CO2 enrichment) experiment approximately monthly over the final six growing seasons (2005–2010) corresponding to years 10–15 of the experiment. This study was established in 1996 with the primary goal of quantifying the long-term effects of atmospheric CO2 enrichment on ecosystem C transfer and storage processes. Published data from this experiment have indicated a persistent increase in NPP by CO2 enrichment for at least a decade with no indications of PNL (McCarthy et al., 2010; Drake et al., 2011). The beginning of this study corresponded to the date that the plots were split and a N fertilization treatment was initiated (spring, 2005). We previously published data on mycorrhizal production from minirhizotrons for the period prior to the N fertilization treatment (1998–2004; Pritchard et al., 2008a). We hypothesized a negative relationship between soil N availability and mycorrhizal production and biomass. Furthermore, we hypothesized that CO2 enrichment would mitigate these effects on mycorrhizal production due to increased carbohydrate availability, and that these enrichment effects will increase during the course of the study as soil N pools are transferred into living biomass.
Materials and methods
Study site description
In 1996, the FACTS-1 FACE experiment was established in a 15-year-old loblolly pine plantation in the Duke Forest in Orange County, North Carolina, USA. The forest was established from 3-year-old loblolly pine (Pinus taeda, L.) seedlings planted in 1983 at a 2.4 × 2.4 m spacing. Since then, several species of deciduous trees have become established including red maple (Acer rubrum), winged elm (Ulmus alata), sweetgum (Liquidambar styraciflua), tulip poplar (Liriodendron tulipifera), and redbud (Cercis canadensis). Density of loblolly pine trees, however, represents more than 98% of the total basal area in this forest. The soil series is Enon loam (fine, mixed, thermic Ultic Hapludalfs).
Briefly, the FACTS-1 experiment is a randomized block design with three replications of each of two CO2 concentrations maintained with FACE technology (Hendrey et al., 1999). CO2-enriched plots are maintained at approximately 200 ppm above ambient atmospheric CO2 levels (Taneva et al., 2006). Each of the six experimental plots is 30 m in diameter. Beginning August, 1996, fumigation was constant except when temperature dropped below 5 °C or the wind speed was higher than 5 m s−1 for more than 5 min. Fumigation was limited to daylight hours only beginning in 2003.
During late fall and winter of 2004 (after 9 years of CO2 fumigation), each FACE plot was split and a N fertilization treatment was implemented on half of each ring. The plots were split so that the litter production rates and pine biomass increment of each half were most similar. A 70-cm-deep trench, which is deeper than the zone in which roots are typically found in this forest (Matamala & Schlesinger, 2000), was dug to physically separate the two halves. Impermeable plastic sheeting was then inserted into the trenches to block passage of roots, nutrients, and water. A total of 112 kg ha−1 yr−1 of NH4NO3 was applied in two applications of 56 kg ha−1, which occurred in March and April of 2005 and in one yearly application (March) for the remainder of the experiment. This fertilization rate is typical for the forest industry in the region and exceeds the local rate of atmospheric deposition by an order of magnitude.
Minirhizotron analysis of root dynamics
The methods of mycorrhizal root analyses for this experiment have been previously described (Pritchard et al., 2001, 2008b). Briefly, a total of 72 minirhizotrons (12 per plot) were installed into each of the six FACE rings from June 23 to 26, 1998. Minirhizotrons are clear plastic tubes (OD = 56 mm) that allow repeated, noninvasive measurement of root growth. These clear tubes were installed at an angle of 45° from vertical to a vertical depth of ca. 32 cm. FACE rings were divided equally into four sectors; three tubes were installed at random with respect to vegetation into each of the four sectors. The portion of the minirhizotron tube extending above the ground was covered with a closed cell polyethylene sleeve, and the end was sealed with a rubber cap to exclude light and minimize heat exchange between the air and the tube. A PVC cap was then installed over the end to protect the rubber cap from UV damage, and to further protect and insulate the tube. To prevent minirhizotron tubes from moving, aluminum brackets were clamped to tubes and anchored into the ground with 40 cm stainless steel rods. Data collection for this study began 7 years after tube installation allowing ample time for roots to colonize the tube surface, and to allow soil adjacent to minirhizotrons to equilibrate with bulk soil (Strand et al., 2008).
From spring 2005 through November 2010, a BTC-2 ICAP system (Bartz Technology Corp., Carpinteria, CA, USA) was used to record images of ectomycorrhizal root tips growing along the soil/minirhizotron tube interface. Tubes were imaged approximately monthly (52 sessions). The camera was equipped with an indexing handle that allowed precise and consistent camera placement (Johnson & Meyer, 1998) enabling us to track the fate of individual fungal structures through time. We collected and analyzed all images collected from each minirhizotron (an average of 34 locations/frames for each of the 67 access tubes that remained functional for the duration of the study resulting in a total of ca. 119 065 analyzed images). Images were 13 × 17 mm in dimensions. Animations were played for each of the 34 locations for each minirhizotron tube to ensure that files were named correctly and that excessive image shift did not occur.
Data were extracted from digital images using Rootfly software (Wells and Birchfield, Clemson University). At each date, tip diameters, total length of live ECM tips, new tip production, and tip length and diameter mortality were recorded. ECM tips were considered dead when they either disappeared from view, or upon their structural disintegration. In some cases, nonfunctional structures may have been classified as alive as long as they remained present and intact, and thus errors would be overestimations of ECM tip longevity, standing crop, and mortality rate.
We calculated mycorrhizal tip NPP by calculating the biomass for each mycorrhizal tip using specific root length (SRL) values derived from intact soil monoliths harvested in November, 2010 and then converting from two-dimensional minirhizotron images to three dimensions using an empirically derived 0.78 mm depth of field (Taylor et al., 2013). To determine SRL, between 10 and 14 categories of roots of diameters ranging from 0.3 mm and 39 mm were established for three replicates for each CO2 × N treatment combination. We did this in order to create a continuous relationship between SRL and root diameter as in Iversen et al. (2008) to determine if changes in root density for a root of a given diameter were present. The smallest category of fine roots was composed of the most distal, first-order mycorrhizal root tips. Length and average diameter of roots in each group were recorded using WinRhizo (Regent Instruments, Quebec, Canada). Each category was then dried to a constant mass at 65 °C and dry weights were recorded. SRL was calculated as the total length of all roots in a size category divided by the total mass of the roots in that size category. The values from each diameter category were then used to establish a continuous relationship between average diameter and SRL. A yearly turnover index was calculated as annual ECM tip production divided by average annual mycorrhizal tip standing crop after Nadelhoffer (2000). This turnover index was then used to estimate average tip longevity to validate estimates of longevity determined from Kaplan–Meier survivorship curves.
Statistical analyses
The experimental design was a split plot design with three replications (three elevated plots and three ambient plots). Three statistical blocks were created by pairing one ambient and one elevated CO2 treatment plot with similar N mineralization rates. Each ring was further split into fertilized and unfertilized plots. Each combination of CO2 and fertility contained six subsample minirhizotron access tubes. Minirhizotron frames were grouped into two depth classes, 0–16 and 17–32 cm. Statistical analyses were done using repeated measures anova to test for effects of CO2 (two levels), depth (two levels), and N, and their interactions on yearly production, mortality, standing crop, and diameter (Proc mixed, SAS version 9.1; SAS Institute Inc., Cary, NC, USA). In all cases, plot means were used in statistical analyses. Data were checked for normality by visual inspection. Because of lack of statistical power characteristic of large-scale, minimally replicated, FACE experiments, statistical significance was designated when P < 0.10 (Filion et al., 2000).
We used Cox's proportional hazards model to test for main effects of treatment, depth, N and CO2, as well as interactions of these factors, on survivorship of mycorrhizal tips over the 6 year experiment. The Kaplan–Meier method along with Log-rank and Wilcoxon's tests were then used to compare homogeneity of survivorship curves between treatment combinations of interest determined from results of the Cox's proportional hazards test as we have done previously (Pritchard et al., 2008a, 2010). All root tips that were present at the beginning of the experiment (at the time of initiation of the N treatment) were omitted from analysis of standing crop and survival analyses to facilitate a better understanding of the N treatment. All mycorrhizal tips still alive at the termination of the experiment were treated as right censored for all survivorship analyses.
Results
Mycorrhizal tip length and diameter
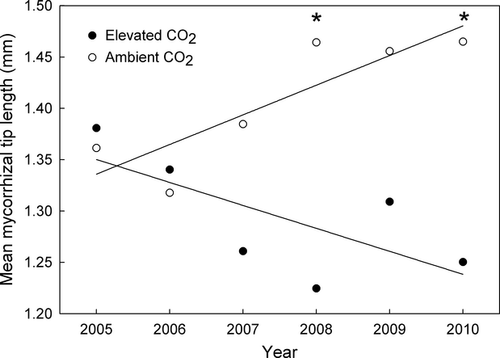
Although no main effect of CO2 was observed, CO2-enriched plots generally had shorter (median and mean) mycorrhizal tips than control plots under N-fertilized conditions (1.22 vs. 1.54 mm; CO2 × N, P = 0.03) (Fig 1). This decrease in tip length was greatest in deeper soil where tips in the CO2-enriched fertilized plots were 30% shorter than tips in ambient fertilized plots. A main effect of N fertilization was also noted (P = 0.04); tips in fertilized plots were 13% longer than tips in unfertilized plots. Similarly, the two statistical blocks with the highest N mineralization rates had 18% longer tips than the block with the lowest N mineralization rates. Interestingly, mycorrhizal tips that were produced in ambient plots tended to increase in length with increasing duration of the experiment, whereas tips produced in CO2-enriched plots were progressively shorter (Fig. 2).
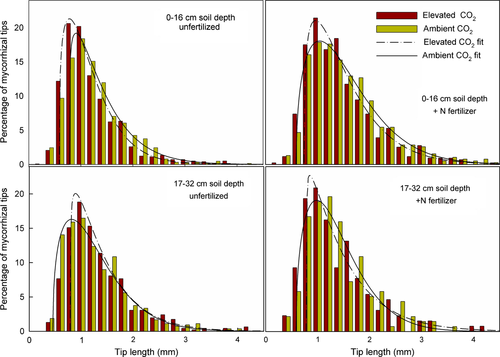
No significant main effects of depth, CO2, or N on diameter of mycorrhizal tips were observed. A CO2 × depth interaction was noted (P = 0.02), however; tips produced in shallow soil were 13% thicker than tips in deeper soil in CO2-enriched plots (0.36 vs. 0.32 mm), while tip diameter did not vary with depth in ambient plots (data not shown).
Mycorrhizal tip survivorship
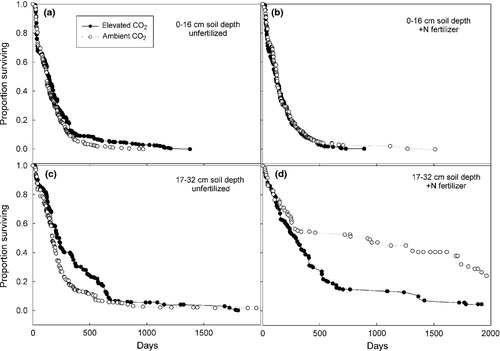
Mean and median survivorship of mycorrhizal root tips were 255 and 151 days, respectively. Tip longevity calculated from the turnover index (derived from tip diameter annual production and mean annual diameter standing crop) was 235 days. Tips produced in shallow soil exhibited a mean longevity of 181 days compared to 396 days in deeper soil (P < 0.0001). Similarly, tip longevity derived from the turnover index indicated that tips in shallow soil survived 167 days compared to 388 days in deep soil (P < 0.0001).
The Cox proportional hazards test indicated a significant CO2 × N × depth interaction for survivorship (P < 0.0001) (Fig. 3). In unfertilized plots, CO2 enrichment increased mean longevity of mycorrhizal tips by 108 days in deep soil (335 vs. 247 days) and 45 days in shallow soil (204 vs. 159 days). Under fertilized conditions, elevated atmospheric CO2 had no significant effect on mycorrhizal tip survivorship in shallow soil (mean longevity of 186 days in ambient vs. 159 days in elevated plots) whereas survivorship was decreased greatly by CO2-enrichment in deep soil of fertilized plots (451 vs. 978 days). Fertilization increased survivorship of mycorrhizal root tips in deep soil (705 days vs. 298 days), but had no impact on survivorship in shallow soil (N × depth; P < 0.0001). Although not significant, longevity calculated from the turnover index suggested that survivorship in deep soil of fertilized plots was 21% higher than in deep soil of unfertilized plots (data not shown).
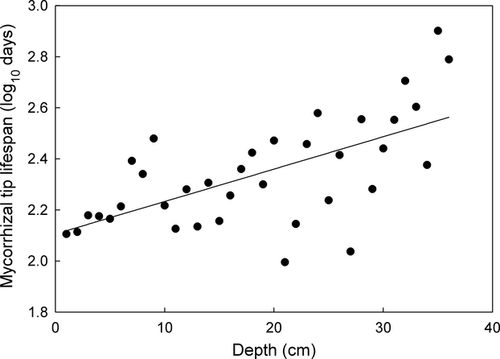
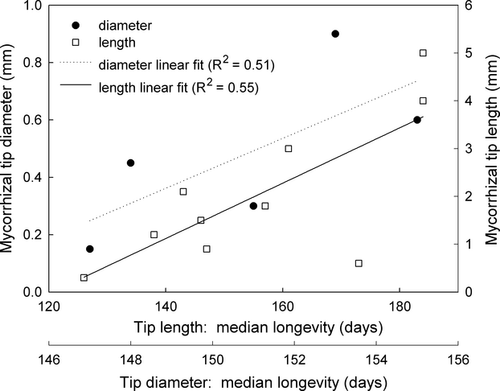
Tip longevity increased significantly with increasing soil depth in a log-normal fashion (R2 = 0.40) (Fig. 4). Longevity of tips also increased with increasing tip diameter (R2 = 0.57) as well as increasing tip length (R2 = 0.51) (Fig. 5).
Mycorrhizal tip production and mortality
We observed significant main effects of N fertilization and depth on mycorrhizal tip diameter and length production (Figs 6 and 7). Averaged over the entire 6 years of the experiment, annual production of root tips diameters and lengths was 54% greater in unfertilized plots than in fertilized plots (P = 0.02). Averaged across all treatments, production was 177% greater in shallow compared to deeper soil (P = 0.005 for the main effect of depth; data not shown). Production also varied significantly from year to year as described in more detail below (P = 0.004).
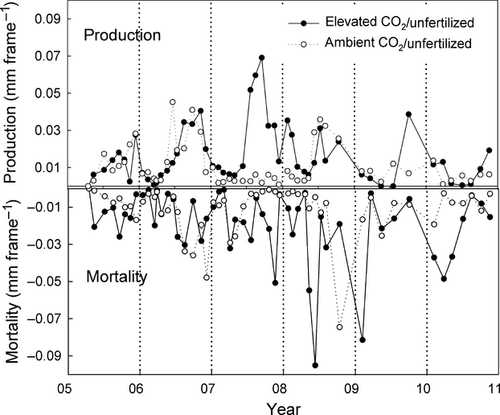
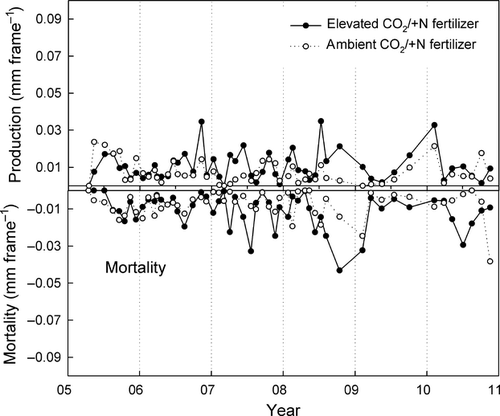
A significant CO2 × nitrogen × year interaction for annual production was also noted (P < 0.05; Figs 6 and 7); significantly more production was observed in CO2-enriched compared to ambient plots under unfertilized conditions, but only in 2007 (P = 0.002). Effects of N and CO2 were more variable during the other years.
Ectomycorrhizal tip production converted into annual NPP values based on SRL, and our empirically derived depth of field for the minirhizotron camera, mirrored production of tip length and diameter production values presented in Figs 6 and 7. Based on average forest NPP values reported previously for this site (McCarthy et al., 2010), NPP of mycorrhizal tips accounted for only ca. 1% of total forest NPP; average NPP for all 6 years for ambient and CO2-enriched plots was 17 and 27 g m−2 yr−1 in unfertilized plots and 15 and 20 g m−2 yr−1 in N-fertilized plots (Table 1). Production of mycorrhizal tips accounted for ca. 10% of the total fine-root NPP as determined from a parallel minirhizotron study focused on root production (data not shown). The proportion of total fine root NPP attributed to ECM tips was unaffected by atmospheric CO2 enrichment (data not shown). Although not statistically significant, the percent of total fine-root NPP accounted for by ECM tip NPP was 22% greater in unfertilized plots (11.2%) compared to those receiving supplemental N (8.7%; P = 0.18).
| Ambient | Elevated | Fertilized | Elevated × fertilized | |
|---|---|---|---|---|
| 2005 | 21.8 (5.9) | 19.0 (4.2) | 22.0 (8.1) | 14.7 (8.4) |
| 2006 | 34.6 (16.4) | 40.4 (13.7) | 20.1 (7.2) | 28.4 (10.1) |
| 2007 | 9.3 (0.9) | 64.9 (32.2) | 18.9 (5.1) | 27.1 (11.4)) |
| 2008 | 23.3 (6.0) | 21.9 (2.2) | 11.2 (4.1) | 26.5 (9.5) |
| 2009 | 6.6 (2.4) | 5.7 (4.2) | 3.4 (1.5) | 7.8 (4.0) |
| 2010 | 7.6 (2.4) | 8.7 (3.0) | 16.1 (5.9) | 18.4 (14.7) |
| Mean | 17.2 | 26.8 | 15.3 | 20.5 |
- Units are g biomass m−2 (±SE) of ground area to a depth of 32 cm.
Patterns of mortality were similar to production. Annual mortality was 66% higher in unfertilized compared to fertilized plots (P = 0.02; Figs 6 vs. 7) and 130% greater in shallow compared to deep soil (P = 0.003). There was generally more mortality in CO2-enriched plots compared to controls through much of the study (+61%), but this effect was not significant because of high variation and low replicate number. We did observe a CO2 × year interaction; CO2-enriched plots had higher mortality than ambient plots in 2008, the year following the increase in production in 2007 as noted above.
Mycorrhizal tip standing crop
No main effects of CO2 or N were observed for standing crop established subsequent to the initiation of the N fertilization treatment in spring, 2005, until the end of the 6-year study.
Standing crop averaged over the 6-year study was 91% greater in unfertilized CO2-enriched compared to unfertilized ambient plots. But the increase in standing crop in unfertilized subplots was not maintained during the entire study duration. This is reflected by a significant CO2 × N × year interaction (P = 0.02). The increase in standing crop with elevated CO2 under unfertilized conditions was only significant in 2007 and 2008 (Fig. 8). Standing crop decreased in CO2-enriched unfertilized plots during 2008 and 2009 as mortality rates exceeded production until the middle of 2010 when no further treatment effects were observed.

Environmental controls of mycorrhizal tip production and mortality
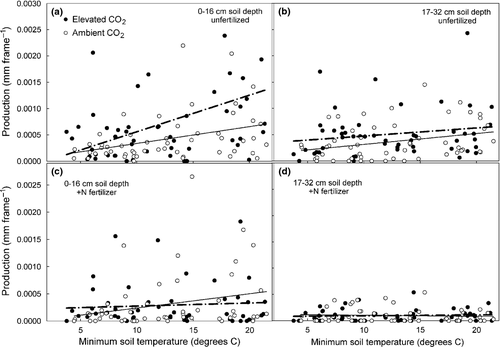
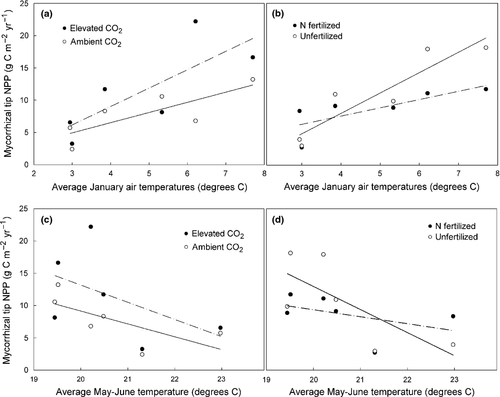
The best predictor of mycorrhizal tip production during a given month (sampling interval) was minimum soil temperature (Fig. 9); soil moisture and air temperatures were of less predictive value (data not shown). Soil temperatures, however, were significantly and linearly correlated with monthly production only in shallow soil of unfertilized plots. Production in fertilized subplots was largely uncoupled from temperature. The best predictor of annual NPP of ECM tips was average of January and May/June air temperatures (Fig. 10). Net primary productivity exhibited a linear increase with increases in mean January air temperatures while increases in average air temperatures during May and June significantly decreased NPP. As was the case with minimum monthly soil temperatures, the relationship between NPP and winter and late spring air temperatures was largely uncoupled with the addition of N fertilization.
Discussion
At the Duke FACE experiment, where a loblolly pine forest was grown with elevated CO2 for 14 years, CO2 enrichment stimulated ecosystem NPP for over a decade with little evidence of progressive nutrient limitations (Finzi et al., 2007; Jackson et al., 2009; McCarthy et al., 2010; Drake et al., 2011). In this demonstrably N-limited forest, the sustained increase in NPP was accompanied by significantly higher uptake rates of soil N by trees. Additional N was presumably made available through a combination of faster rates of soil organic matter turnover in CO2 enriched plots coupled with higher rates of uptake by roots or mycorrhizae (Matamala & Schlesinger, 2000; Garcia et al., 2007; Pritchard et al., 2008a,b; Jackson et al., 2009). In fact, in spite of the input of an additional 1000 g C m−2 yr−1 in CO2-enriched plots over this period (an addition equal to 33% of the total soil C pool), no net accumulation of C in the mineral soil pool was observed (Drake et al., 2011). The lack of C accrual in soil, coupled with consistent and widening effects of CO2-enrichment on soil respiration (Jackson et al., 2009), along with greater rates of exudation and activity of enzymes involved in decomposition (Phillips et al., 2009, 2011, 2013), suggest acceleration of SOM decomposition.
Our data indicate a small direct contribution of mycorrhizal tips NPP to total ecosystem NPP. For example, average annual ECM root tip NPP was only ca. 10 g C m−2 yr−1 (7.8 and 11.4 g in ambient and CO2-enriched plots, respectively). This represents only ca. 10% of annual NPP of the entire fine-root pool (<2.0 mm; data not shown) and only ca. 1% of total forest NPP at this site (McCarthy et al., 2010). Assuming mycorrhizal tips are 30% C (Harley, 1971), the contribution of fungal mantle tissue to total ecosystem productivity is small and, in this plant system, is unlikely to directly influence soil C sequestration potential especially when taking into account the relatively rapid turnover of these structures. The flow of plant-derived C through mycorrhizal root tips, and subsequent incorporation of that C into extraradical soil mycelia (not reported here), however, is likely to contribute to greater soil C inputs to some unknown extent (Wallander et al., 2001, 2003; Fransson, 2012; Clemmensen et al., 2013).
In a recent belowground synthesis, Drake et al. (2011) argued that the increased N uptake by trees in CO2-enriched plots was likely facilitated by a stimulation of ECM fungi associated with dominant P. taeda trees. It was further suggested that forests dominated by ECM fungi are less likely to experience PNL than systems dominated by AM fungi based on the saprotrophic nature of ECM. The assertion that a stimulation of ectomycorrhizal relationships in FACE plots in the Duke forest have facilitated faster decomposition, higher rates of soil N uptake and therefore long-term enhancement of NPP with CO2 enrichment (Drake et al., 2011) is contingent upon the demonstration of long-term stimulation of ECM fungi. For the first half of the experiment (1998–2004), data did indeed suggest a substantial stimulation of ECM at Duke FACE, at least in deep soil (no effect was noted in shallow soil). This previous report, however, also suggested a decrease in the observed CO2 effect by the end of 2004. (Pritchard et al.,2008a). Other data from Duke collected during 2005 and 2006 showed only a modest 14% increase in mycorrhizal colonization in CO2-enriched compared to ambient plots (Garcia et al., 2007). Parrent & Vilgalys (2007) found no evidence for an effect of CO2-enrichment on soil fungal mycelial production using ingrowth bag techniques during 2004 and 2005. As 83.5% of soil fungi at Duke are ECM, the lack of a CO2 effect likely reflects responses of extraradical mycelia of ECM in this forest (Parrent & Vilgalys, 2007). Detailed analyses of mycorrhizal tip numbers and percent colonization of fine roots by mycorrhizae from destructive harvests of 8000 cm3 soil monoliths at the termination of the experiment in Fall, 2010, also indicated small, if any effects of CO2 enrichment on mycorrhizae (K.V. Beidler, A.E. Strand, B.N. Taylor, E.R. Cooper, M. Schonholz & S.G. Pritchard, unpublished data). Finally, differences in natural abundance of 15N in plant tissues in CO2-enriched plots also do not indicate a significant role of ECM in uptake of additional N at Duke FACE (Hofmockel et al., 2011). Because N transferred from mycorrhizae to plants is generally depleted in 15N (Emmerton et al., 2001), one would expect this depleted 15N signature to be reflected in CO2-enriched tissue. On the contrary, Hofmockel et al. (2011) instead detected an increase in 15N in litter of CO2 enriched plots. Collectively, data at Duke FACE suggest a smaller than expected role of mycorrhizal tips and mycelium in obtaining additional N in CO2-enriched conditions and a greater than expected contribution of faster turnover of SOM pools (Phillips et al., 2011, 2013).
It is difficult to predict how important transient stimulations in mycorrhizal populations, as were observed in this experiment, will be for long-term forest function. We only noted a positive effect of elevated CO2 on production of mycorrhizal root tips during a single year (2007), and then only in unfertilized subplots. That increase in production resulted in a higher standing crop through much of 2007 and 2008 until high rates of root tip mortality returned standing crop to levels observed in control plots by 2009. Interestingly, Phillips et al. (2011) reported a large surge in exudation during 2007 in unfertilized CO2-enriched compared to unfertilized ambient subplots (but no effect of CO2-enrichment in fertilized plots). In the desert FACE experiment, a similar transient increase in standing crop of fine roots was noted during a particularly wet year (Ferguson & Nowak, 2011). We were unable to attribute the transient CO2 fertilization effect observed during 2007 in our study to abiotic environmental conditions. The biological importance of temporary periods of mycorrhizal stimulation by CO2 enrichment and the potential for such episodic stimulation to influence long-term patterns of NPP, remain unknown and in need of further study.
It appears that transient periods of enhanced root tip production and standing crop are unlikely to completely explain the increase in ecosystem N uptake in CO2 enriched plots at Duke. It is likely that additional C allocation belowground with CO2-enrichment (Drake et al., 2011) is stimulating nutrient uptake by one or more alternative mechanisms. Phillips et al. (2011) reported a 55% increase in exudation rates by terminal fine roots <1.0 mm in diameter over three growing seasons at Duke FACE (2007–2009). Furthermore, this increase in dissolved C inputs to soil under CO2-enriched conditions was significantly linked to the release of enzymes involved in breakdown of organic N by soil microbes. These data suggest that increased nutrient uptake was likely driven by priming of the soil food web causing faster SOM decomposition and an increase in rate of release of mineralized N, often in the absence of large increases in density of mycorrhizal structures in soil. Such a mechanism would explain the increase in tree N uptake per unit of fine-root biomass reported by Drake et al. (2011). It is clear that resolving the controls of C and N exchange in the rhizosphere will require a more detailed analysis of molecular events within both roots and soil microbes that accompany the shifts in stoichiometry of available fixed C to organic and inorganic N quantity and quality in soil. Functional genomic approaches hold considerable promise for informing these processes.
Increased N uptake in the absence of equivalent increases in root tip density in soil can also be explained by changes in short root tip morphology. Thinner fine roots and mycorrhizal tips (with high SRA and SRL) generally exhibit higher rates of uptake per unit of tissue constructed compared to thicker fine roots (Ostonen et al., 2007a, b). Although the magnitude of the effect of CO2 enrichment on mycorrhizal root tip density was smaller than that reported in other ecosystems (Lukac et al., 2003), it is interesting to note the effect that CO2-enrichment had on the dimensions of mycorrhizal tips. Tips were generally shorter in CO2-enriched plots than in controls and this effect increased in magnitude with increasing study duration. This may suggest that species composition of mycorrhizae was changed by long-term CO2 enrichment. Just such an effect was noted previously; Tylospora species were less abundant and Russula species were significantly more abundant in CO2-enriched compared to ambient conditions (Parrent et al., 2006). A change in the relative importance of colonizing ECM fungi might explain the progressively larger decrease in mycorrhizal tip length in CO2-enriched plots observed here.
The significant decrease in mycorrhizal root tip production in N-fertilized plots confirms many previous studies (Wallander & Nylund, 1991). When supplied with nonlimiting quantities of N, total and proportional C allocation belowground generally decreases, which, in turn, slows carbohydrate transfer to mycorrhizal fungi (Litton et al., 2007). Hasselquist et al. (2012) found, for example, that respiration by ectomycorrhizal hyphae was greatly reduced and sporocarp production was nearly eliminated by N fertilization in a boreal forest.
Few data exist on changes in production, morphology, and survivorship of mycorrhizal roots caused by a combination of environmental conditions including atmospheric CO2 enrichment, N fertilization, and temperature variation over long time periods (Ostonen et al., 1999, 2007a,b; Ostonen et al., 2011). We observed a negative relationship between average May and June temperatures and annual mycorrhizal NPP; a 2 °C increase in average spring temperatures resulted in a 50% decrease in mycorrhizal root tip production in unfertilized plots (P = 0.02). This result is consistent with previous work by others showing a significant decline in mycorrhizal root tip abundance along North to South latitudinal gradients (Ostonen et al., 2011). For example, Norway spruce growing at higher latitudes (cooler climate) produce more mycorrhizal root tips and greater biomass and root tips tend to be thinner and longer (Ostonen et al., 2007a, b). Root tip density of Scotts pine is also greater in northern compared to southern latitudes (Helmisaari et al., 2009). Decreased production during warm years is difficult to reconcile, however, with the positive relationship between mean annual temperature and total belowground C flux that seems to hold true for most plant systems (reviewed in Litton & Giardina, 2008). It is likely that during warmer years, rates of N mineralization in soil are stimulated and nutrients are more readily available (Melillo et al., 2002; Allison & Treseder, 2008). A meta-analysis showed that warming increased soil respiration by 20% and net N mineralization by 46%, for example (Rustad et al., 2001). Improvements in plant nutrition could then lead to a smaller tree dependence upon mycorrhizae for uptake.
Although warm temperatures during May and June tended to decrease tip production, tip NPP exhibited a positive relationship with average January temperatures. This could be linked to additional C fixed by loblolly pine trees during years with warm winter months, increased activity by soil microorganisms involved in mineralizing nutrients during warmer winters, alterations in temperature-dependent whole-tree C allocation patterns, or a combination of these mechanisms. Interestingly, we noted that interannual variation in January and late spring temperatures had a more dramatic influence on mycorrhizal production in unfertilized relative to N-fertilized plots; when environmental conditions were optimal for mycorrhizal tip growth (warm winters and cool springs), mycorrhizal production tended to be higher in unfertilized compared to fertilized plots. When conditions were suboptimal (cold winters and warm springs), tip NPP was either unaffected by the N treatment or was higher in fertilized plots. This response likely reflects a greater dependence of trees on temperature-dependent mineralization processes in unfertilized conditions. When N fertilizer is added, the dependence of trees on soil organisms becomes weakened as plant-available N is abundant and C allocation to symbionts is simultaneously decreased (Wallander & Nylund, 1991). Regardless of the physiological phenomena that induced the change, the temperature dependence of mycorrhizal production has significant implications for forest function in a world that is generally warmer and that also experiences greater variation in temperatures as has been projected (IPCC, 2007).
Although previous studies have speculated about the potential for atmospheric CO2 enrichment, soil N fertility, and interannual variation in temperature and precipitation to influence the life spans of fine roots and ECM tips, few data actually address this issue and those data remain equivocal (Pritchard, 2011). Past research has shown that elevated atmospheric CO2 increases or decreases survivorship of fine roots, including mycorrhizal tips, with about equal frequency (Fitter et al., 1997; Thomas et al., 1999; Arnone et al., 2000; Johnson et al., 2000; Milchunas et al., 2005; Pritchard et al., 2008b; McCormack et al., 2010; Pritchard, 2011). For example, McCormack et al. (2010) reported that elevated atmospheric CO2 decreased median survivorship of mycorrhizal tips from 185 to 139 days in longleaf pine. Previously, we reported that CO2 enrichment reduced survivorship of mycorrhizal tips from 15 to 30 cm soil depth but increased survival in shallower soil (Pritchard et al., 2008a). In this study, we observed a complex three-way interaction between fertilization, CO2, and soil depth. In unfertilized plots, CO2 enrichment increased longevity of mycorrhizal tips. Such an increase in longevity might be linked to greater carbohydrate availability to support maintenance respiration as has been suggested previously (Eissenstat et al., 2000). The increase in longevity of tips under unfertilized conditions might contribute to the increase in N uptake per unit of fine-root production noted previously (Drake et al., 2011). In our previous work conducted over the period from 1998 to 2004, we noted that mycorrhizal tips in this forest turned over more quickly (median longevity was 104 d) than they did in our current study (median longevity was 151 d). We attribute this to a severe drought experienced in 2002 which induced dieback of both fine roots and soil fungi during 2003 and 2004 (Pritchard et al., 2008a).
In conclusion, we found increased production of mycorrhizal tips in CO2-enriched plots at Duke FACE during only one (2007) of the final 6 years of the experiment, resulting in a higher standing crop during two of those years (2007 and 2008). This effect was only found in unfertilized conditions, however, with no CO2 effects found in N-fertilized subplots. Increased soil nutrient uptake in CO2-enriched plots is, therefore, more likely attributable to a combination of more exudation leading to priming of the soil food web and faster SOM decomposition (Hofmockel et al., 2011; Phillips et al., 2011) coupled with architectural shifts in fine-root branch systems (K.V. Beidler, A.E. Strand, B.N. Taylor, E.R. Cooper, M. Schonholz & S.G. Pritchard, unpublished data), although the potential for short-term (seasonal to annual) changes in root and mycorrhizal activity to impact longer term nutrient balances and productivity should also be further explored. More work will be required to determine if changes at the cellular level, within both roots and their fungal partners, also contributed to higher rates of N uptake in CO2-enriched plots within this forest. A shift in community composition favoring more efficient species of ectomycorrhizal fungi, however, cannot be ruled out (Parrent et al., 2006; Parrent & Vilgalys, 2007). As expected, soil N fertilization decreased tree dependence on mycorrhizal fungi, a result consistent with a substantial body of theoretical and empirical literature. Mycorrhizal production was greatest when average spring temperatures were cool and average January temperatures were warm. We observed an approximately 50% reduction in annual mycorrhizal root tip production for a 2 degree increase in average late-spring temperatures. This has important implications for ecosystem function in a warmer world in addition to potential for forest soils to sequester atmospheric C.
Acknowledgements
The authors would like to thank R. Oren, and R. Nettles for maintenance of the FACE experiment and Will Cook for statistical advice. This research was supported by the Office of Science (BER), U.S. Department of Energy, Grant No. DE-FG02-95ER62083, and the National Science Foundation, award number DE-FC02-06ER64156.




