
Resource Quality and Quantity Exert Distinct Influences on Trophic Interactions and Food Web Pyramids Under Different Nutrient Conditions
Funding: This work is supported by the National Key Research and Development Program of China (2021YFC3201004), the National Natural Science Foundation of China (52471276, 52239005 and 52388101) and the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2019ZT08L213) and the Nansha Key Scientific and Technological Project, Guangdong Province (2023ZD012).
Zhenmin Yang and Fen Guo contributed equally to the article.
ABSTRACT
- Resource quality and quantity are critical drivers in shaping trophic interactions and food web structures in aquatic ecosystems. However, the lack of clarity on how these drivers distinctly influence energy flow and trophic pyramids represents a significant gap in understanding the mechanisms governing ecosystem stability and productivity.
- This study explored how resource quality and quantity influenced trophic interactions and food web pyramids across a spatial gradient of aqueous nutrient levels. Resource quality was assessed by omega-3 (ω3) long-chain polyunsaturated fatty acids (LC-PUFA), while resource quantity was evaluated based on biomass. Food web components were collected, including algae (phytoplankton and periphyton) and their consumers (zooplankton, macroinvertebrates and fish).
- Our results showed that higher ambient nutrient concentrations significantly boosted phytoplankton biomass, leading to a bottom-heavy biomass pyramid in the low-quality food group. However, this increase in quantity was accompanied by a notable reduction in ω3 LC-PUFA in primary producers, resulting in a distinct FA stock pyramid with a narrowed base and middle. This pattern suggests that despite high phytoplankton biomass, poor food quality created a resource quality bottleneck that constrained the transfer of essential fatty acids.
- This reduction in resource quality simplified the transfer pathways of ω3 LC-PUFA to piscivorous fish, limiting their dietary options and weakening trophic connections. Notably, the unusual role of planktivorous fish in accumulating FA at the second trophic level in the study area, in contrast to typical trends observed in other regions, highlights how variations in species composition and resource quality can reshape trophic structure and influence energy flow.
- Our findings emphasise that declines in resource quality exerted a greater influence on food web dynamics than declines in resource quantity. Our study underscores the importance of considering resource quality, alongside quantity, to maintain ecosystem stability and resilience in nutrient-enriched aquatic systems.
1 Introduction
Both the quantity and quality of basal resources are crucial in determining trophic interactions and the structure of trophic pyramids (Shipley et al. 2024). While extensive research has highlighted the importance of resource quantity (e.g., primary productivity), increasing evidence points to resource quality (i.e., biochemical composition) being a key regulator of consumer communities and carbon transfer across trophic levels (Huang et al. 2024; Yan et al. 2024). However, it remains unclear how changes in the quantity and quality of basal resources differentially impact the flow of dietary energy through food webs and shape the structure of trophic pyramids.
Long-chain polyunsaturated fatty acid (LC-PUFA) analysis is an advanced method that enables in-depth exploration of food quality and aquatic trophic interactions. In aquatic ecosystems, diatoms, dinoflagellates and cryptophytes are recognised as high-quality food resources for consumers due to their greater contents of omega-3 (ω3) LC-PUFA, particularly eicosapentaenoic acid (EPA, 20:5ω3) and docosahexaenoic acid (DHA, 22:6ω3) (Guo, Kainz, Sheldon, and Bunn 2016; Guo, Kainz, Valdez, et al. 2016). These two fatty acids are physiologically indispensable, playing critical roles in maintaining membrane fluidity, neural development and reproductive performance in aquatic animals (Luo et al. 2025; Wu et al. 2025; Yan et al. 2023). Conversely, macrophytes, cyanobacteria and green algae lack ω3 LC-PUFA, rendering them lower-quality food resources for consumers. Most aquatic consumers cannot synthesise ω3 LC-PUFA de novo; thus, they depend on the dietary acquisition of these essential nutrients (Taipale et al. 2016). Furthermore, since dietary ω3 LC-PUFA are transferred to consumers with little or no modification in consumers' adipose tissues, they also serve as tracers for nutrient transfer in trophic dynamics (Guo et al. 2021; Mathieu et al. 2022; Wu et al. 2025).
However, algal ω3 LC-PUFA are highly sensitive to environmental change, particularly aqueous nutrients, which influence the ability of microalgae to synthesise ω3 LC-PUFA (Calderini et al. 2023; Taipale et al. 2019; Wu et al. 2025). In oligotrophic lakes, primary producers are usually characterised by high-quality algae, such as diatoms, cryptophytes, or chrysophytes (Pilecky et al. 2024; Yan et al. 2023). However, as aqueous nutrient concentrations increase, phytoplankton biomass tends to rise, but this is often accompanied by a compositional shift from diatoms to cyanobacteria, resulting in a significant reduction in ω3 LC-PUFA content of primary producers (Fujibayashi et al. 2018; Perhar and Arhonditsis 2009). Notably, some mesotrophic lakes may support phytoplankton communities with moderate biomass but relatively higher ω3 LC-PUFA content compared to either oligotrophic or eutrophic systems (Taipale et al. 2016).
Changes in phytoplankton FA consequently affect zooplankton FA and biomass (Keva et al. 2021; Calderini et al. 2023). Zooplankton consuming diatom-rich diets typically contain high levels of ω3 LC-PUFA, thereby effectively promoting the conversion of consumed phytoplankton carbon into their own biomass (Keva et al. 2021; Müller-Navarra et al. 2000). In contrast, the poor nutritional quality of cyanobacteria leads to inefficient carbon transfer to zooplankton, even when their biomass is high, resulting in low zooplankton biomass (Brett et al. 2009; Taipale et al. 2016). The addition of ω3 LC-PUFA emulsions to green or cyanobacteria-based diets can improve food quality, resulting in zooplankton FA compositions similar to those of zooplankton fed directly with ω3 LC-PUFA-rich cryptophytes (DeMott and Müller-Navarra 1997; Weers and Gulati 1997).
Changes in phytoplankton FA also affect the FA composition of higher trophic level fish (Keva et al. 2021; Calderini et al. 2023). Previous research has shown that high levels of phytoplankton ω3 LC-PUFA lead to higher ω3 LC-PUFA in perch muscle, with a strong correlation between DHA contents in perch and dinoflagellates and chrysophytes (Taipale et al. 2016). Furthermore, additional studies have demonstrated that forage fish, such as pelagic planktivores like alewife (Alosa pseudoharengus), typically contain higher DHA levels, and this elevated lipid content can provide essential ω3 LC-PUFA reserves for top predators (Happel et al. 2017). However, eutrophication can shift phytoplankton communities from high-quality to lower-quality taxa, reducing zooplankton survival and abundance, and subsequently decreasing ω3 LC-PUFA availability in juvenile fish (Taipale et al. 2018).
Changes in the FA and biomass of phytoplankton may impact trophic pyramids across various trophic levels (Brett and Müller-Navarra 1997; Keva et al. 2021). Current research has mainly focused on community biomass changes across trophic levels, often illustrated by biomass pyramids (De Ruiter et al. 2005; Nagelkerken et al. 2020; Thormar et al. 2016). These pyramids typically have a triangular shape, with a wide base representing the large biomass of primary producers and progressively narrower tiers for herbivores and predators. Furthermore, FA stock pyramids could be calculated by multiplying the average biomass of each trophic level by their corresponding average EPA and DHA content, allowing the potential to assess the distribution of high-quality food across the food webs (Keva et al. 2021). In waters with abundant low-quality food, FA stock pyramids may become top-heavy with a smaller base, while in waters with abundant high-quality food, they tend to be bottom-heavy, with a larger base of high-quality resources. However, it remains unresolved how these dynamics manifest, particularly under conditions of high nutrient levels in aquatic ecosystems.
To date, most current studies have been primarily conducted for pelagic food webs in temperate and arctic lakes with relatively low nutrient concentrations (Calderini et al. 2023; Chaguaceda et al. 2024). These studies often cover only limited food web components and fail to map complete energy transfer pathways in natural settings. This restricts the comparison of transfer pathways under different environmental pressures, hindering a comprehensive understanding of trophic energy dynamics. It is unclear whether similar changes occur in lakes with high nutrient levels, particularly those in warmer regions. While phytoplankton has received considerable attention in studies of lake food webs and FA transfer, other basal resources, such as periphyton, remain underexplored. Periphyton, which dominates benthic primary production in littoral zones, may also contribute significantly to consumer nutrition (Luo et al. 2024; Huang et al. 2025). Furthermore, few studies have explored how eutrophication affects the biomass and FA composition of periphyton in lake littoral zones, which are also an important food source for consumers.
Therefore, our study aimed to investigate how resource quality and quantity would affect trophic interactions and food web pyramids across contrasting aqueous water nutrient levels. We analysed ω3 LC-PUFA of food web components for food quality assessment, measured the biomass of algae (phytoplankton and periphyton) and their consumers (zooplankton, macroinvertebrates and fish) to quantify resource availability and calculated the average biomass and FA stock of each trophic level to assess changes in trophic pyramids. We hypothesised that:
H1.Phytoplankton and periphyton biomass increases with aqueous nutrient concentrations, while their respective EPA and DHA contents decline, resulting in corresponding changes in the biomass and FA composition of zooplankton and macroinvertebrates.
H2.The decrease in EPA and DHA levels of basal food sources simplifies the pathways of EPA and DHA transfer to piscivorous fish.
H3.High food quantity and low food quality lead to a bottom-heavy trophic-level biomass pyramid, but a bottom-narrow FA stock pyramid.
2 Methods
2.1 Study Area
This research was conducted in Lake Taihu, located at the Yangtze River Delta region of eastern China (30°55′40″ N–31°32′58″ N, 119°52′32″ E–120°36′10″ E) (Figure 1). Lake Taihu covers an area of approximately 2338 km2, with a catchment area of 36,500 km2 (Qin et al. 2010). The region has a subtropical monsoon climate, with an average annual temperature of 15°C–17°C and annual precipitation of 1010–1400 mm (Lin et al. 2021). The lake is fed by a dense network of rivers with an annual runoff of approximately 57 × 108 m3. This lake serves as a major drinking water source for 30 million people residing in the surrounding cities of Wuxi, Suzhou, Changzhou and Huzhou, and supports tourism, fisheries and shipping. Since the mid-1980s, accelerated urbanisation and continuous population growth have led to increased nutrient loadings, resulting in intensifying eutrophication in parts of Lake Taihu, particularly in the northern and northwestern regions (Xiao et al. 2019; Xu et al. 2010). In recent years, multiple restoration measures have been implemented to control eutrophication. As a result, water quality in the eastern part of the lake has improved significantly and is now used as a drinking water source (Zhang 2016). In contrast, the northern and northwestern regions still exhibit high nutrient concentrations, while the northeastern and southwestern regions show intermediate values (Kang et al. 2024; Xiao et al. 2019). These spatial differences in aqueous nutrient levels create a clear nutrient gradient across the lake, which may lead to variation in the nutritional quality of basal resources, particularly algal communities.
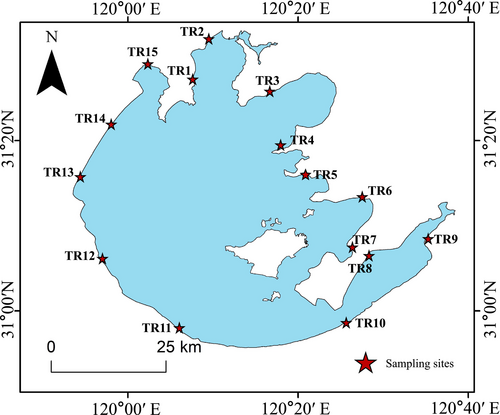
2.2 Field Sampling
In August 2022, we selected 15 sampling sites along the littoral zone of Lake Taihu, spanning a gradient of aqueous nutrient concentrations. At each site, we collected the main food-web components, including phytoplankton, periphyton, zooplankton, macroinvertebrates and fish. For each biological group, we collected two parallel sets of samples: one set for FA analysis and the other for assessing community composition and biomass.
Phytoplankton and zooplankton were collected using plankton nets (phytoplankton: mesh size 64 μm; zooplankton: mesh size 112 μm), with five replicates sampled at each site, primarily for FA analysis (Luo et al. 2025; Yan et al. 2024). These samples were immediately frozen in the field using a −20°C portable freezer. To assess phytoplankton biomass and community composition, 1 L of surface water (~0.5 m depth) was collected using a water sampler and preserved with 15 mL of Lugol's solution. Zooplankton biomass and community structure were assessed by collecting 1 L of surface water for protozoa and rotifers, and filtering 20 L of surface water through a 64 μm mesh net for cladocerans and copepods. All biomass samples were stored in light-shielded coolers with ice packs and transported to the laboratory for further analysis.
Periphyton samples were also collected at each site for FA analysis and biomass/community composition assessment. For FA analysis, periphyton samples were collected at each site with five replicates. In each replicate, five rocks with relatively even visible periphyton coverage were randomly selected from the littoral zone. The periphyton was gently brushed from the rock surfaces using a toothbrush and transferred into a clean container. These samples were immediately frozen in the field using a portable −20°C freezer. For biomass and community composition analysis, quantitative periphyton samples were also collected. At each site, five replicate samples were collected, each consisting of three rocks randomly selected from the littoral zone. A plastic ring (2.5 cm radius) was placed on each rock to define the sampling area. To avoid contamination, periphyton outside the ring was first removed, and the periphyton within the ring was then carefully brushed into sampling bottles. The five replicate samples (from a total of 15 rocks) were pooled into a single container, adjusted to a final volume of 500 mL, and preserved with Lugol's solution (1.5% of the total sample volume). Quantitative samples were stored in light-shielded ice boxes with ice packs and transported to the laboratory as soon as possible.
Macroinvertebrates were collected using both hand nets and D-shaped nets (mesh size 250 μm), depending on habitat conditions at each site. Individuals were identified and sorted in situ, then transferred into labelled plastic vials and immediately frozen at −20°C in the field for subsequent fatty acid analysis. To assess species composition and biomass, three replicate macroinvertebrate samples were collected at each site, covering a total area of 1 m2. These samples were sieved through a 60-mesh (0.25 mm) screen to remove sediment and debris. All specimens were then preserved in 75% ethanol for later identification, enumeration, and biomass determination in the laboratory.
Fish samples were collected using a multi-mesh gill net and a customised series of collapsible traps, which were deployed at each sampling site. The nets were retrieved after 12 h. All captured fish were identified to the species level, and the total length (mm), standard length (mm), and weight (g) of each fish were recorded. Dorsal muscle tissue was extracted from each fish, placed into pre-labelled plastic vials, and immediately flash-frozen in liquid nitrogen for subsequent FA analysis.
Water samples were collected from each site using two 1 L bottles and kept at 4°C prior to analysis. Before chemical determination, the samples were filtered through 0.45 μm membrane filters to remove particulate matter (State Environmental Protection Administration 2002). The filtered water samples were then used to measure total nitrogen (TN), total phosphorus (TP), ammonium nitrogen (NH4–N), nitrite nitrogen (NO2–N), nitrate nitrogen (NO3–N), and phosphate phosphorus (PO4–P).
Electrical conductivity (EC), dissolved oxygen (DO) and pH were measured in situ using an Aquaread AP5000 multi-parameter water quality analyser (UK, Aquaread Ltd). The latitude and longitude of the sampling sites were recorded using a handheld GPS navigator and, when necessary, verified with cartographic data.
2.3 Laboratory Analysis
2.3.1 Fatty Acid Analysis
For FA analysis, samples were stored in a −80°C freezer (DW-86L416G, Haier, Qingdao). They were subsequently freeze-dried (LGJ-18, Songyuan Huaxing, Beijing) and homogenised using a glass rod and/or a food processor. Thereafter, phytoplankton and periphyton (10 mg each), macroinvertebrates (5 mg), zooplankton (5 mg) and fish muscle tissues (5 mg) were transferred into glass tubes. These samples were then subjected to FA extraction, following the methods described in Guo et al. (2021). FA methyl esters (FAME) were analysed using a gas chromatograph (Thermo Scientific Trace 1310) equipped with a flame ionisation detector (FID), temperature-programmable injector and an autosampler. The chromatograph was set with the following parameters: FID at 250°C, carrier gases: He at 1 mL/min, H2 at 35 mL/min, N2 at 30 mL/min, air at 350 mL/min and a temperature programme of 100°C (3 min)-3°C/min-200°C (3 min)-3°C/min-240°C (6 min) = 58.67 min. FAME were separated using a Supelco SP-2560 column (100 m, 25 mm i.d., 0.2-μm film thickness) and identified by comparing their retention times with known standards. Quantification was done using 7-point calibration curves based on known standard concentrations, and FA compositions were expressed as percentage values relative to total FA (FA%) and FA mass fractions (μg FA/mg dw).
2.3.2 Quantitative Sample Processing
Quantitative samples of phytoplankton, zooplankton, periphyton and macroinvertebrates were identified to the species level whenever possible. In cases where morphological features were insufficient for species-level identification, such as in early life stages or damaged individuals, organisms were classified to the genus level.
Phytoplankton were identified into six phyla (Cyanophyta, Chlorophyta, Bacillariophyta, Cryptophyta, Dinophyta and Euglenophyta), comprising 96 species (Chen and Wei 2006). Cell counts for each phytoplankton species were calculated under an optical microscope and subsequently converted to cells per litre of water sample, representing the phytoplankton density (cells/L) (Chen and Wei 2006). The wet weight of each phytoplankton species (mg/L) was estimated by multiplying the cell density (cells/L) by the average cell mass of that species (Hillebrand et al. 1999; Menden-Deuer and Lessard 2000). The wet weights of different phytoplankton species within the same phylum were then summed to obtain the total wet weight. The measurement of cell volume was based on the body shape of the algae, including length, height and diameter, which were measured according to the most similar geometric form and calculated using the corresponding volume formula (Hillebrand et al. 2022).
The identification of periphyton began with non-diatoms, followed by total diatom counts under a 400× optical microscope, with non-diatoms generally identified at the genus level. A 1–2 mL subsample of the periphyton suspension was then acid-treated and mounted on permanent slides for diatom identification and enumeration under a 1000× oil immersion lens, typically to the species or variety level (Chen et al. 2020). The abundance and fresh weight biomass assessments were similar to those for phytoplankton. However, periphyton density was reported as cells/cm2.
Rotifers, cladocerans and copepods were counted (ind./L) under an optical microscope using a plankton counting frame. For each zooplankton species, the wet weight (mg/L) of cladocerans and copepods was converted based on length-weight regression equations, while the wet weight of rotifers was determined using volume conversion methods (Jiang and Du 1979; Wang 1961; Zhang and Huang 1991).
The identification of aquatic oligochaetes and chironomid larvae was performed using a stereomicroscope (Olympus SZX16) under magnifications ranging from 10× to 100×, depending on the organism's size and diagnostic structures. Leeches and gastropods were also identified under the same stereomicroscope. Macroinvertebrates were identified to the genus or species level, with some chironomids to the subfamily level and a few coleopteran species to the family level (Liu 1979; Wang 1995). After identification, the number of individuals of each taxon was recorded, and the moisture on the surface of the animals was absorbed with absorbent paper and weighed using an electronic balance (Sartorius BCE224L1CCN), with a precision of 0.0001 g. For data analysis, densities (ind./m2) and wet weight (g/m2) were calculated per square metre based on the sampling area.
A total of 86 fish specimens were collected across all sites, representing 4 orders, 4 families, 19 genera and 24 species. These data provided the basis for functional classification. Cyprinidae was the most represented family, comprising 20 species, including Hemiculter bleekeri, Hypophthalmichthys molitrix, Aristichthys nobilis, Toxabramis swinhonis, Culter mongolicus and Cultrichthys erythropterus. The functional feeding group (FFG) of each fish species was determined using FishBase (https://www.fishbase.se/search.php) and supporting literature (Ni and Zhu 2004). Based on feeding habits, fish were categorised into five functional groups: planktivores (mean body length: 322.47 ± 90.39 mm; mean body weight: 805.96 ± 739.57 g), piscivores (180.62 ± 73.75 mm; 74.89 ± 145.33 g), omnivores (78.53 ± 51.44 mm; 32.06 ± 130.53 g), benthivores (173.73 ± 71.41 mm; 185.43 ± 181.35 g) and zooplanktivores (125.22 ± 46.79 mm; 40.82 ± 81.66 g). Given the limited number of individuals per site, the total wet weight of fish at each sampling site was calculated as the sum of individual fish wet weights (g).
2.3.3 Aqueous Nutrient Analysis
Nutrient analysis of lake water was determined using an automated flow system (Skalar San Plus Analyser) (Table 1). The detection method for NO3–N and NO2–N followed the ISO standard from 1996, identified as ISO 13395. PO4–P and TP were analysed according to ISO 15681, published in 2018. TN was measured following the ISO 29441 standard from 2010, and NH4–N was determined based on the ISO 11732 standard from 2005 (Jin and Tu 1990).
| Sampling sites | Longitude | Latitude | TP | TN | NO3–N | NO2–N | NH4–N | PO4–P | DO | EC | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| °E | °N | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | μS/cm | ||
| TR1 | 120.13 | 31.45 | 0.111 | 2.181 | 1.415 | 0.069 | 0.116 | 0.034 | 6.81 | 0.190 | 8.37 |
| TR2 | 120.16 | 31.53 | 0.104 | 0.650 | 0.010 | 0.007 | 0.031 | 0.008 | 8.88 | 0.190 | 8.99 |
| TR3 | 120.29 | 31.43 | 0.098 | 0.731 | 0.118 | 0.012 | 0.043 | 0.005 | 8.51 | 0.200 | 8.52 |
| TR4 | 120.31 | 31.32 | 0.100 | 0.542 | 0.013 | 0.000 | 0.049 | 0.005 | 5.94 | 0.190 | 7.73 |
| TR5 | 120.34 | 31.27 | 0.070 | 0.611 | 0.140 | 0.004 | 0.036 | 0.004 | 13.34 | 0.210 | 9.07 |
| TR6 | 120.46 | 31.22 | 0.096 | 0.916 | 0.013 | 0.006 | 0.127 | 0.004 | 11.17 | 0.160 | 9.27 |
| TR7 | 120.43 | 31.13 | 0.062 | 0.627 | 0.001 | 0.006 | 0.062 | 0.008 | 4.35 | 0.180 | 7.82 |
| TR8 | 120.48 | 31.12 | 0.057 | 0.891 | 0.031 | 0.029 | 0.083 | 0.004 | 7.96 | 0.180 | 8.40 |
| TR9 | 120.59 | 31.15 | 0.027 | 0.622 | 0.140 | 0.014 | 0.072 | 0.007 | 12.97 | 0.180 | 8.87 |
| TR10 | 120.43 | 30.98 | 0.054 | 0.502 | 0.000 | 0.009 | 0.028 | 0.003 | 8.25 | 0.200 | 8.71 |
| TR11 | 120.14 | 30.94 | 0.068 | 0.998 | 0.364 | 0.036 | 0.035 | 0.004 | 7.23 | 0.190 | 8.22 |
| TR12 | 119.97 | 31.07 | 5.104 | 25.076 | 0.018 | 0.009 | 0.206 | 0.029 | 6.58 | 0.200 | 7.82 |
| TR13 | 119.92 | 31.28 | 0.057 | 1.274 | 0.442 | 0.070 | 0.087 | 0.012 | 6.06 | 0.190 | 8.81 |
| TR14 | 119.98 | 31.38 | 35.745 | 224.705 | 0.001 | 0.016 | 8.843 | 1.732 | 5.00 | 0.200 | 8.00 |
| TR15 | 120.05 | 31.48 | 0.136 | 2.446 | 1.567 | 0.079 | 0.054 | 0.027 | 4.31 | 0.230 | 7.75 |
- Abbreviations: DO, dissolved oxygen; EC, electrical conductivity; NH4–N, ammonia nitrogen; NO2–N, nitrite-nitrogen; NO3–N, nitrate nitrogen; PO4–P, phosphate phosphorus; TN, total nitrogen; TP, total phosphorus.
2.3.4 Community Biomass Assessment
To better reflect biomass changes across different nutrient gradients and trophic levels, we converted the wet weight of the samples into dry weight biomass. Following Evans et al. (1998), phytoplankton, which contains about 75% water by weight, had their dry weight estimated at 25% of the wet weight. The average dry weight biomass of phytoplankton in each food nutritional group was calculated by summing the wet weights of each phylum across all sampling sites in the group, multiplying each total by 0.25, and then dividing by the number of sampling sites to obtain the average dry weight biomass for each phylum. Since water transparency and depth were not measured at each site, we used a standardised depth of 1 m to convert phytoplankton biomass from mg dw/L to mg dw/m2. This conversion was done to ensure consistency in units across all biological groups, allowing integrated comparisons and summation of total biomass and FA stock per square metre. Since water transparency and average depth at each sampling site were not measured, we standardised the depth to 1 m, converting mg dw/L to mg dw/m2 for FA production calculations.
The dry weight of periphyton was estimated to be approximately 8% of its wet weight, using the same method as for phytoplankton (Sládeček and Sládečková 1964). The dry weight of zooplankton was estimated at 10% of the wet weight, following Gladyshev et al. (2011) and Zuev et al. (2012).
The average wet weight of macroinvertebrates in each food nutritional group was first calculated by summing the wet weights of each genus or species across all sampling sites in the group and then dividing by the number of sampling sites. The dry weight biomass was then calculated based on Le Cren and Lowe-McConnell (1980), with Dreissena at 5% and other macroinvertebrate species at 15% of the wet weight, to obtain the average dry weight biomass.
The wet weight of fish was converted to total dry weight (dw, g) by multiplying by 24%, based on the dry-to-wet weight ratio of fish reported by Harvey et al. (2003) and Li et al. (2020). Community composition was calculated as the percentage of total biomass represented by specific groups, normalised.
2.4 Data Analysis
Before data analysis, relative FA values (% FA) were arcsine-square-root transformed for normal distribution approximation (Kelly and Scheibling 2012).
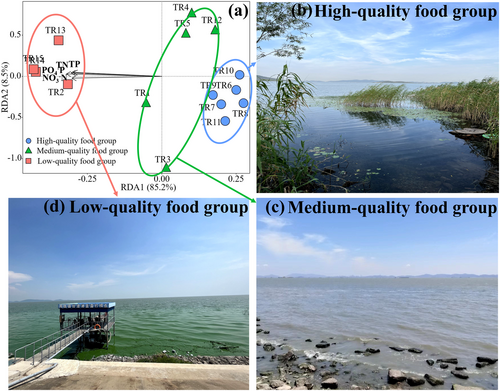
Redundancy Analysis (RDA) was employed to assess the influence of aqueous nutrient levels on the FA composition of phytoplankton. This analysis incorporated, for each sampling site, eight response variables of phytoplankton FA data (DHA, EPA, ALA, LIN, ARA, SAFA, MUFA, BAFA) and the explanatory variables comprising standardised environmental factors (TP, TN, NO3–N, PO4–P). We checked for multicollinearity among variables and conducted permutation tests to verify the robustness of the environmental impacts on FA compositions. Spatial variations in the FA composition of phytoplankton were visualised using the first two axes of the RDA. Based on the ordination patterns and clustering of sampling sites in the RDA space, which reflected underlying gradients in algal nutritional quality, we categorised all sampling sites into three food nutritional quality groups: high, medium and low. To validate this classification, we further examined the average EPA and DHA profiles of phytoplankton at each group, which corresponded well with the spatial FA gradients revealed by the RDA results (see Table 1 and Figure 2a) (Kang et al. 2024; Xiao et al. 2020).
To visualise the differences across the three food nutritional quality groups, the biomass, community composition and profiles of EPA and DHA of different aquatic organisms were plotted for phytoplankton, periphyton, zooplankton and macroinvertebrates. The mean FA values of samples at each site were used in calculating the community sum of EPA and DHA. One-way ANOVA was conducted to test the differences in biomass, community composition, and EPA and DHA profiles of these aquatic organisms among different food nutritional quality groups, followed by Tukey's HSD multiple comparisons.
Correlation analyses were used to assess the influence of basal resource quantity and quality on primary consumers. These analyses plotted the biomass and profiles of EPA, DHA and ω3 LC-PUFA (the sum of DHA and EPA) in phytoplankton and periphyton against the biomass and levels of EPA, DHA and ω3 LC-PUFA in associated consumers, including zooplankton and macroinvertebrates. The results included the Pearson correlation coefficient (r) and significance p-values.
Piecewise structural equation models (SEM) were used to estimate the transfer of ω3 LC-PUFA from basal food resources to predatory fish. Initially, a theoretical framework for energy flow from resources to higher trophic levels was developed, based on empirical knowledge and literature review. This framework defined causal relationships and path directions to guide the formulation of our model. The path framework was then translated into a series of linear models to analyse the relationships between multiple variables (Lefcheck 2016). Each linear model was fitted using restricted maximum likelihood estimation techniques (Shipley 2009). Model fit was assessed using Chi-Squared and Fisher's C statistics to determine if our model adequately captured all necessary relationships among variables (Grace 2006). A p > 0.05 in the chi-square test indicated support for the model's assumptions regarding conditional independence (Shipley 2009). Path coefficients were computed along with their significance levels (p-values), further enriching our analysis.
To visually display the changes in phytoplankton, zooplankton, periphyton, macroinvertebrates and fish ω3 LC-PUFA production and average community biomass across various trophic levels along the aqueous nutrient gradient, we created FA stock pyramids and biotic biomass pyramids. The biomass pyramid was generated by summing the average dry biomass of all species within each trophic level. Each level's total biomass was then used to visualise the relative distribution of biomass from primary producers to top consumers. For the FA stock pyramid, we used FA concentration data to calculate FA production per square metre at each trophic level. Specifically, we multiplied the mean dry mass biomass (mg dw/m2) of each species by its average FA concentration (μg FAME/mg dw) to obtain the species-specific FA stock. The total FA stock for each trophic level was then calculated by summing the FA stock of all species within that level. This approach enabled us to estimate ω3 LC-PUFA production at each trophic level under different nutrient conditions and visualise the trophic distribution of FA stock from base to top, forming the FA stock pyramid. The trophic levels were classified as follows: primary producers (phytoplankton, periphyton) at the first trophic level, primary consumers (zooplankton, macroinvertebrates, planktivorous fish) at the second, secondary consumers (omnivorous fish, zooplanktivorous fish, benthivorous fish) at the third, and tertiary consumers (piscivorous fish) at the fourth trophic level.
All statistical analyses were conducted using R version 4.2.1 (R Core Team 2022). The analysis involved the use of the “vegan” package for RDA (Luo et al. 2024), the “ggplot2” and “dplyr” for correlation analysis (Irawan and Putranto 2016) and the “piecewiseSEM” for SEM (Guo et al. 2018).
3 Results
3.1 Classification of Food Nutritional Quality Groups
The RDA results highlighted how phytoplankton FA composition varied with aqueous nutrients (TP, TN, NO3–N, PO4–P) across sampling sites. The analysis captured 85.2% of the total variance along the RDA1 axis (F-value = 12.831, p = 0.001); nutrient variables were strongly negatively correlated with site scores on RDA1, including TP (correlation coefficient: −0.560), TN (−0.616), NO3–N (−0.650) and PO4–P (−0.649). In contrast, the RDA2 axis explained only 8.5% of the variance (F-value = 1.209, p = 0.828). These findings suggest that RDA1 effectively captured a spatial gradient in algal nutritional quality. Based on the ordination patterns and clustering along RDA1, all 15 sampling sites were categorised into three food nutritional quality groups: high, medium and low (Figure 2a). Sites located on the far left of RDA1 (red), closely aligned with the nutrient vectors, were assigned to the low-quality food group, reflecting areas with high nutrient input. In contrast, sites on the far right (blue), situated opposite to the nutrient vectors, were classified as the high-quality food group, typically characterised by lower nutrient levels. Sites positioned between these extremes (green) were grouped as the medium-quality food group.
3.2 Changes in the Biomass and FA Profiles of Phytoplankton and Periphyton Across Food Nutritional Quality Groups
As expected, the highest phytoplankton biomass was observed in the low-quality food group (63.18 mg dw/L), followed by the medium- (1.72 mg dw/L) and high-quality food groups (1.65 mg dw/L), with significant differences among groups (F-value = 10.79, p = 0.002) (Figure 3a1). Additionally, the absolute biomass of cyanobacteria was significantly higher in the low-quality food group than in the high- and medium-quality food groups (F-value = 10.04, p = 0.003). Conversely, the highest periphyton biomass was observed in the high-quality food group (0.23 mg dw/cm2), with lower values recorded in the medium- (0.14 mg dw/cm2) and low-quality food groups (0.13 mg dw/cm2) (Figure 3b1); however, the differences among groups were not statistically significant (F-value = 3.69, p = 0.06).
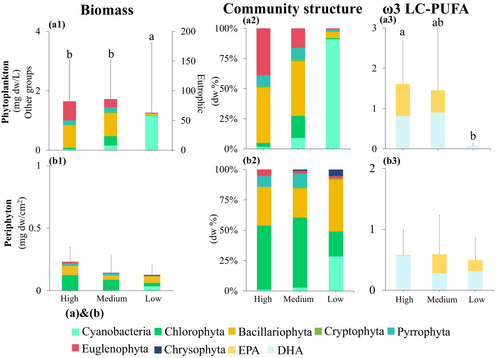
A significant difference in ω3 LC-PUFA content in phytoplankton was also observed across the food nutritional quality groups (Figure 3a3), with values near zero in the low-quality food group and substantially higher in the high- and medium-quality food groups (F-value = 4.08, p = 0.04). In contrast, periphyton ω3 LC-PUFA levels did not differ significantly across food nutritional quality groups (F-value = 0.41, p = 0.68) (Figure 3b3).
3.3 Changes in Biomass and FA of Zooplankton and Macroinvertebrates in Response to Changes in Resource Quantity and Quality
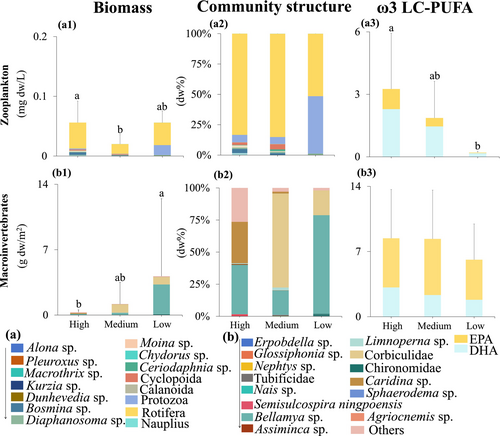
Our results revealed that zooplankton biomass remained consistently low across all food nutritional quality groups, with slightly higher values observed in the high-quality food group and the lowest in the medium-quality food group, showing significant differences among groups (F-value = 7.30, p = 0.008; Figure 4a1). Additionally, the ω3 LC-PUFA content of zooplankton declined significantly from the high- to low-quality food group (F-value = 8.59, p < 0.001; Figure 4a3). In contrast, macroinvertebrate biomass increased significantly across the food nutritional quality gradient (F-value = 5.10, p = 0.025; Figure 4b1), primarily driven by a higher abundance of gastropods such as Bellamya sp. (Figure 4b2). However, there were no significant differences in total FA content of macroinvertebrates across the three food nutritional quality groups (F-value = 0.696, p = 0.521; Figure 4b3).
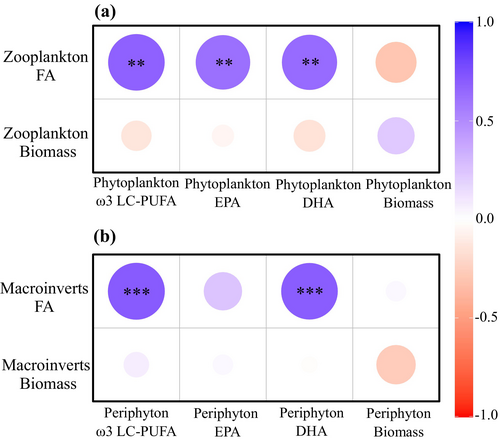
Correlation analysis indicated that neither phytoplankton nor periphyton biomass was significantly associated with the biomass or total FA content of zooplankton and macroinvertebrates (Figure 5). However, the EPA, DHA and ω3 LC-PUFA content in phytoplankton were significantly correlated with the corresponding FA content in zooplankton. In contrast, no significant correlations were found between periphyton FA profiles and macroinvertebrate biomass. Nevertheless, significant correlations were observed between DHA and ω3 LC-PUFA content in periphyton and those in macroinvertebrates (Figure 5).
3.4 Pathways of ω3 LC-PUFA From Basal Food Resources to Top-Level Consumers
Piecewise structural equation models (SEM) revealed that the number of ω3 LC-PUFA transfer pathways to top consumers declined from high- to low-quality food groups. (a) In the high-quality food group, three significant ω3 LC-PUFA pathways were identified: through planktivorous fish (r = 0.517, p < 0.001), benthivorous fish (r = 0.246, p = 0.007) and omnivorous fish (r = 0.408, p < 0.001); (b) In the medium-quality food group, two significant pathways involved omnivorous fish (r = 0.558, p = 0.01) and benthivorous fish (r = 0.467, p = 0.017); and (c) In the low-quality food group, only a single pathway remained, from omnivorous fish to piscivorous fish (r = 0.929, p < 0.001) (Figure 6).

3.5 Changes in the Biomass and FA Stock Pyramids in Response to Changes in Resource Quantity and Quality
The biomass pyramid exhibited a typical Eltonian structure in the high- and medium-quality food group, with biomass gradually decreasing from primary producers to top consumers, but shifted to a bottom-heavy shape with a reduced top in the low-quality food group (Figure 7a). In the low-quality food group, the base of the pyramid (phytoplankton) contributed 86% of the total biomass. The second trophic level accounted for only 12%, roughly one-third of that observed in the high- or medium-quality food group (34%). The third trophic level exhibited the highest biomass in the medium-quality food group (17%), followed by the high-quality food group (11%) and then nearly absent in the low-quality food group (< 1%). The fourth trophic level consistently represented a small proportion of total biomass across all groups.
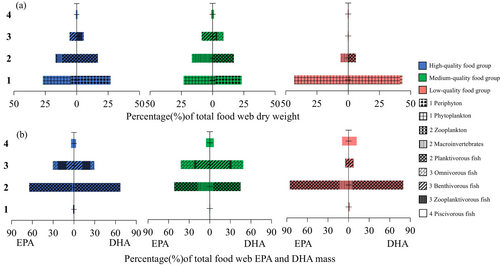
The FA stock pyramid revealed that the second trophic level (primary consumers) consistently contributed the largest share of total ω3 LC-PUFA stock across all food nutritional quality groups (Figure 7b). In contrast, both the first level (primary producers) and the fourth level (top predators) accounted for smaller proportions of the total FA stock. Notably, under the low-quality food group, FA stock at the fourth level increased compared to the high-quality food group, while the third level showed a marked decline. This redistribution led to a distinct hourglass-shaped FA stock pyramid under the low-quality food group.
4 Discussion
Our study comprehensively examined the effects of resource quality and quantity on trophic dynamics and food web pyramids across different food nutritional quality groups. We found that elevated aqueous nutrient concentrations reduced phytoplankton ω3 LC-PUFA, leading to a FA stock pyramid with a narrowed base and middle, indicating impaired transfer of essential fatty acids to higher trophic levels. As the nutritional quality of algae declined, the trophic pathways of ω3 LC-PUFA to top consumers were simplified, underscoring the importance of food quality in supporting higher trophic consumers. Our study provides evidence showing how nutrient increases in aquatic ecosystems affect food web dynamics through alterations in food quality. Our findings underscore that even under high aqueous nutrient conditions, ω3 LC-PUFA can still accumulate across trophic levels, indicating that consumers selectively retain these essential fatty acids despite the lower nutritional quality of basal food resources.
Higher aqueous nutrient concentrations were associated with elevated phytoplankton biomass but lower ω3 LC-PUFA, consistent with previous research (Calderini et al. 2023; Taipale et al. 2016). However, periphyton biomass and ω3 LC-PUFA showed limited variation across the high-, medium- and low-quality food groups, likely due to a combination of factors. Nutrient limitation and grazing pressure in the high-quality food group, along with light limitation from dense phytoplankton blooms in the low-quality food group, may have collectively limited periphyton growth (Cebrian et al. 2014). Additionally, habitat alterations around the study lake, such as artificial wetland creation, may have further reduced periphyton sensitivity to nutrient changes by submerging existing habitats and forcing regrowth in new zones (Que et al. 2021). Collectively, these findings underscore the complex interactive effects of resource quantity and quality, where reductions in ω3 LC-PUFA of basal food sources (phytoplankton and periphyton) significantly influenced ω3 LC-PUFA availability in herbivorous consumers (e.g., zooplankton and macroinvertebrates). However, no significant correlations were observed between the biomass of phytoplankton and zooplankton and between the biomass of periphyton and macroinvertebrates. These results illustrate that while resource quantity increased with aqueous nutrient levels, the concurrent decline in resource quality had more pronounced effects on trophic transfer.
Our findings also demonstrate that declining food quality simplified the transfer pathways of ω3 LC-PUFA to top consumers. Under high- and medium-quality food groups, high ω3 LC-PUFA levels in basal resources provided a variety of high-quality dietary options for piscivorous fish, thus supporting diverse trophic pathways (Gomes et al. 2016; Hayden et al. 2014). In contrast, the low-quality food group, characterised by reduced ω3 LC-PUFA in phytoplankton, limited the availability of high-quality food for consumers and weakened trophic connections. This simplification of pathways restricts dietary choices for top predators, potentially reducing their ω3 LC-PUFA levels (Taipale et al. 2016) and illustrating how diminished resource quality can disrupt traditional energy flow within food webs. The conflict between increased reliance on low-quality resources and highly selective acquisition of high-quality yet low-quantity resources under low food-quality conditions highlights the potential for degraded resource quality to drive fundamental shifts in food web dynamics. These findings underscore the importance of future studies in determining threshold food-quality levels beyond which reduced resource quality impairs energy transfer and simplifies food web structure.
The contrasting impacts of resource quantity and quality are further highlighted by the distinct pyramid structures we observed. While the low-quality food group produced a bottom-heavy biomass pyramid consistent with other studies on nutrient-rich systems (Heathcote et al. 2016), the FA stock pyramid displayed a unique pattern. In the high-quality food group, FA stock peaked at the second trophic level due to the presence of high-quality phytoplankton rich in ω3 LC-PUFA, facilitating efficient energy transfer to higher trophic levels (Feniova et al. 2021). At sites with lower food quality, however, the FA stock pyramid became constricted at both the basal and intermediate trophic levels, likely due to shifts toward lower-quality phytoplankton and the resulting decrease in ω3 LC-PUFA transfer. This constriction reflects a resource quality bottleneck, where the limited availability of high-quality food reduces the biomass and FA stock at intermediate levels, which in turn impacts piscivorous fish at the top of the pyramid. This pattern suggests that while increased resource quantity (biomass) can initially support a more extensive base, the loss of resource quality ultimately constrains energy flow through the ecosystem, ultimately disrupting trophic structure and function under low food-quality conditions.
Interestingly, while the FA stock pyramid in the medium-quality food group of sites showed similar proportions at the second and third trophic levels, the second level accounted for a relatively larger share of total FA stock in both high- and low-quality food groups—a pattern that contrasts with findings from subarctic lakes, where FA stock typically increases toward the top of the food web pyramid (Keva et al. 2021). This unique pattern in Lake Taihu can likely be attributed to several factors. Planktivorous fish, such as silver and bighead carp, dominate the second trophic level in Lake Taihu (Xie 2022) and contribute substantially to the FA stock due to their high biomass and ability to accumulate ω3 LC-PUFA efficiently. The 10-year fishing ban in the Yangtze River Basin has likely accelerated the recovery of these fish populations (Yin et al. 2022), reinforcing their dominance and increasing their impact on FA distribution. Additionally, these large-bodied species (Locher et al. 2022) are less susceptible to predation, which allows them to maintain higher biomass levels. Seasonal temperature increases may further promote their growth rates and metabolic activity (Cooke and Hill 2010), enhancing their contribution to the FA stock at this level. Together, these factors underscore the critical role of planktivorous fish in shaping FA distribution within low food-quality aquatic systems, demonstrating how shifts in both species composition and resource nutritional quality can profoundly influence trophic structure and energy flow.
5 Conclusions
Our findings demonstrate that resource quantity and quality differentially influence food web dynamics, with resource quality playing a particularly crucial role in trophic interactions and FA distribution. By elucidating how differences in aqueous nutrient levels alter both biomass and FA stock pyramids, this study provides a comprehensive view of the cascading impacts on aquatic food webs. Future research should focus on the resilience of these altered trophic structures, particularly in the context of changing nutrient regimes and other environmental pressures, to inform conservation strategies that prioritise the maintenance of resource quality alongside quantity in eutrophic ecosystems.
Author Contributions
Conceptualisation: F.G., S.E.B. Developing methods: Z.Y., F.G., M.J.K. Data analysis: Z.Y., F.G. Preparation of figures and tables: Z.Y. Conducting the research, data interpretation, writing: Z.Y., F.G., S.E.B., X.O., M.J.K., F.L., W.G., Y.Z.
Acknowledgements
This work is supported by the National Key Research and Development Program of China (2021YFC3201004), the National Natural Science Foundation of China (52471276, 52239005 and 52388101) and the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2019ZT08L213) and the Nansha Key Scientific and Technological Project, Guangdong Province (2023ZD012). We appreciate the field assistance from Han Zheng, Ziwei Xu, Qixiang Chen, Bangrun He and Yiduo Luo. We thank Ms. Fei Wang at the Analysis and Test Centre of Guangdong University of Technology for her assistance with FA analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon request.




