
Evidence That Wild Salmonids Seek Cool Water Refuges to Reduce Parasite Virulence: The Proliferative Kidney Disease Case
Funding: This study was supported by Fischereiabgabe Baden-Württemberg.
Sarah Oexle and Albert Ros contributed equally to this study.
ABSTRACT
- Conservation of cold-stenothermic freshwater fish populations relies on local management to mitigate effects of climate change. With limited opportunity to migrate, freshwater fish are increasingly exposed to excessive warmth during summer heat waves and are susceptible to temperature-associated diseases. As a case example, a significant proportion of salmonids are infected with a salmonid parasite that causes proliferative kidney disease (PKD), which is associated with high mortality when water temperatures remain above 15°C for several weeks. We hypothesised that wild trout would actively migrate to cooler water when temperatures exceeded such temperature levels. Such ‘chill behaviour’ would allow infected fish to survive periods of intense heat by providing a means of controlling the virulence of PKD in diseased fish.
- Three locations were selected at the confluence of a stream with summer water temperatures of 15°C–24°C and a 1°C–7°C cooler tributary. The selected streams are known to harbour PKD. In these streams, wild brown trout were individually tagged with passive integrated transponders (PIT). Fish movements at the confluence were monitored from summer to autumn. Fish were recaptured in late autumn and checked for PKD and its causative parasite using inspection of the kidney and qPCR.
- Movement tracking revealed that the greater the temperature difference between the main stream and the cooler tributary, the more likely fish were to migrate to the cooler water downstream of the tributary and/or into the cooler tributary. Independent of this temperature effect, the level of PKD infection was found to be positively associated with trout migration from warm into cooler water.
- The study represents the first report of a ‘behavioural chill’ response in diseased wild fish. Such a response is in contrast and opposite to the more widely known ‘behavioural fever’ response. We suggest that this chill behavioural response is important for surviving PKD during periods of warm weather.
- Our results strongly underscore the importance of protecting accessible cool-water refuges in river systems for the conservation of healthy cold-stenothermic fish populations in the face of progressing climate change.
1 Introduction
Headwater streams in the temperate climate zone play an important ecological role in providing spawning grounds and habitat for keystone cold-stenothermic fish species such as salmonids (Haidvogl et al. 2015). This ecosystem is threatened by anthropogenic stressors such as water extraction for human use, alterations to flow regime, habitat fragmentation by damming (Dudgeon et al. 2006; Brinker et al. 2018), and, increasingly, by the effects of global warming (Daufresne and Boët 2007; Borgwardt et al. 2020). Consequently, many populations of cold-stenothermic fish species, salmonids in particular, are in severe decline (Zimmerli et al. 2007; Borgwardt et al. 2020). Salmonids not only perform key ecosystem functions (Vanni et al. 2013) but also provide significant social and economic value (Winfield 2016; Arlinghaus et al. 2020). Thus, substantial conservation efforts are directed towards restoring their populations (Dauwalter et al. 2020). These restoration actions can be categorised as short- and long-term conservation measures and management strategies that may be carried out simultaneously. For example, stocking is often used as a measure to restore a fish population, but without addressing habitat deficiencies, the effects are often temporary (Cowx 1998; Näslund 1998; Baer et al. 2007; Cowx et al. 2010). Approaches to address the root causes of population decline aim for long-term improvement of the fish stock (Burkhardt-Holm et al. 2002; Ros et al. 2022; Mejia et al. 2023) by promoting naturally reproducing fish populations. Such approaches might include restoration of river connectivity, reducing riverbed colmation, and ensuring adequate flow regimes (Foote et al. 2020; Bartholomew et al. 2023). The current study investigates the importance of heterogeneous stream microclimates with cool-water refuges for promoting the health and resilience of resident salmonid populations during warm summer periods.
Global warming has become a major concern in river ecosystem conservation (Johnson et al. 2024). In particular, periods of extreme heat and drought during summer months have far-reaching impacts, causing severe thermal stress in sensitive freshwater fish species (Dauwalter et al. 2020) and intensifying the spread and virulence of diseases (Marcogliese 2008). This phenomenon has already induced shifts in species distribution ranges, with specialist cold-water stenothermic fish being displaced by more tolerant generalist species (Basen et al. 2022). Even the unlikely best-case climate scenarios predict local riverine climates to undergo substantial further warming (Hari et al. 2006; Kędra 2020; Borgwardt et al. 2020). Without adaptive management, this will inevitably lead to significant damage to endemic biota, even when the climate attains a new stable state (Liu et al. 2015). Therefore, local microclimate mitigation management is becoming increasingly crucial for the conservation of cold-water stenothermic fish species communities, including economically important fish such as brown trout, which rely on cold headwaters throughout much of their life cycle.
European brown trout populations have been in decline since the end of the 20th century (Burkhardt-Holm et al. 2002; Zimmerli et al. 2007; Ros et al. 2021) despite intensive stocking and concerted efforts across Europe to restore river habitat (Zingraff-Hamed et al. 2020; Cucherousset et al. 2021). The temperature-associated salmonid proliferative kidney disease (PKD) that is caused by the myxozoan parasite Tetracapsuloides bryosalmonae (Hedrick et al. 1993; Canning et al. 2000; Ghittino et al. 2003; Okamura et al. 2011; Waldner et al. 2021) has been identified as one of the chief causative factors in the decline of salmonid populations (Sterud et al. 2007; Wahli et al. 2008; Arndt et al. 2019; Waldner et al. 2020; Ros et al. 2021). The incidence of this disease is likely to intensify with the expected future rise in water temperature (Okamura et al. 2011; Lauringson et al. 2021; Ros et al. 2021) because higher temperatures promote both growth of bryozoan hosts and proliferation of T. bryosalmonae in bryozoans (Tops et al. 2006, 2009) that release spores infective to salmonids (Grabner and El-Matbouli 2008; Strepparava et al. 2020). As it has been demonstrated that diseased fish can regenerate the kidney and fully recover in water cooler than 15°C (Schmidt-Posthaus et al. 2012; Strepparava et al. 2018), and that such parasite exposed and recovered fish show some resistance to development of PKD in the following summer (Okamura et al. 2011; Ros et al. 2023), climate mitigation measures are currently the only known defence against the spread of PKD in wild populations.
During periods of high-water temperature, salmonids have been found to actively seek out refuges with cooler water. For instance, brown trout will congregate in cool-water refuges when water temperature rises (Elliott 2000; Mejia et al. 2023), and Atlantic salmon Salmo salar are able to maintain a body temperature below mean river temperature by exploiting the river's microclimate (Moore et al. 2012). These local observations raise the question of whether brown trout will actively migrate to cooler water during heat waves, including between rivers, and whether this behaviour might be triggered by temperature-driven diseases, such as PKD. In a controlled laboratory study with brown trout infected with PKD, evidence was found for such an active thermotactic ‘chill behaviour’ that might enhance health (Ros and Brinker 2024). Studying the occurrence and function of such disease-induced thermotactic behaviour in a natural setting is vital for identifying priorities in effective adaptive management strategies to enhance the resilience of river ecosystems to global warming.
The current field study investigated the active migration of the brown trout Salmo trutta, a keystone species in salmonid streams, into cool-water refuges. In the study area, a significant proportion of salmonids are infected with the PKD parasite, and brown trout especially are subject to stock losses from PKD mortality (Ros et al. 2021). We hypothesised that wild trout would actively migrate to available cooler water when temperatures in the main stream exceed 15°C, the threshold for clinical PKD. Such ‘chill behaviour’ would allow healthy fish to survive periods of intense heat as well as provide control of the virulence of PKD in diseased fish. This was tested by tagging wild brown trout with passive integrated transponders (PIT) and observing fish movements at the confluence of a warm main stream with a cooler tributary in three different stream locations.
2 Methods
2.1 Study Area and Design
The study was conducted in three locations in headwater stream systems of the catchment of the Rivers Wutach, Argen and Neckar. These streams currently provide suitable salmonid habitat. However, at higher summer temperatures these local brown trout populations are expected to become increasingly affected by PKD caused by the presence of the myxozoan parasite Tetracapsuloides bryosalmonae in these rivers (Ros et al. 2021). Higher temperatures result in a double edged sword, increasing both the release of infective spores from infected bryozoan populations (Tops et al. 2006; Strepparava et al. 2020) and the severity of PKD (Okamura et al. 2011). Within each stream system, a confluence with a cooler tributary (ΔT > 1°C) was identified in a state wide survey the previous summer and these locations were selected based on the feasibility of installing fixed antenna stations to monitor fish movements. The locations were Wutach, at the confluence of the River Gutach with the tributary Langenordnach (N 47°55′15.6576, E 8°11′39.786); Argen, at the confluence of the River Haslach with the tributary Rhone (N 47°41′52.2528, E 9°43′56.352); and Neckar, at the confluence of a millrace with a tributary spring channel (N 48°23′25.6308, E 8°40′30.72).
At each confluence, three stationary passive integrated transponder (PIT) monitoring antennas were installed in June 2022 (Figure 1) and removed at the end of the experiment in September 2023, resulting in two sampling periods spanning most of July through September in both years. As brown trout migrate primarily near the stream bottom (Heggenes and Saltveit 1990; Armstrong et al. 1996), variations in water depth above the antennas due to rainfall and discharge levels were not expected to significantly influence detection rates (Ritter et al. 2020). Each antenna loop was mounted to the stream bottom as a swim-over detector covering the entire stream width (Armstrong et al. 1996; Ritter et al. 2020). In the main stream, one antenna was installed directly upstream of the confluence (warm site) and one downstream of the confluence (mixed site). The third antenna was installed at the entry of the tributary (cool site) (Figure 1). Each antenna station consisted of a PIT tag reader (Single Antenna HDX Reader; Oregon RFID, Portland OR, USA) with a tuner (Manual Tuner; Oregon RFID, Portland OR, USA). Antennas were powered from nearby household electricity networks using a DC converter (230 V AC to ~17 V DC, FEAS GmbH, Ahrensberg, Germany) with a passive line noise filter (Oregon RFID, Portland OR, USA). Antenna stations were run with a 50 ms charge and 50 ms listen pulse for nearby PIT tags at 10 scans per second. Although antennas were placed in areas of rapid current without cover to prevent fish from staying in the detection area for a prolonged period of time, some individual fish remained there for hours or even days.
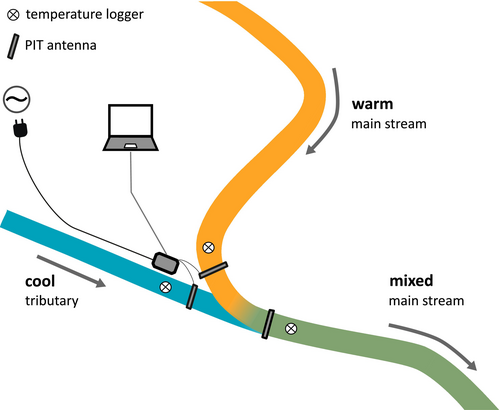
A temperature logger (HOBO Pendant; Onset Computer Corporation, Bourne, MA) was placed in the proximity of each antenna station to continuously monitor water temperature (Figure 1). Oxygen levels in the turbulent and fast-flowing streams (monitored intermittently) were near saturation in a range of 7.5–10.8 mg O2 L−1 and above critical levels for salmonids (6 mg O2 L−1, Ellis et al. 2002). Thus, differences in oxygen between the main stream and cool tributary, which may potentially affect fish aggregations (Elliott 2000), were not a factor. At each site and location, a 1-h search for the presence of the primary bryozoan hosts of the parasite T. bryosalmonae was conducted (methods: Ros et al. 2021). 10°C was used as the threshold for freshwater bryozoan development. European freshwater bryozoans can survive lower temperatures (Tops et al. 2009); however, 10°C was reported to be approximately the temperature above which bryozoans hatch in spring and below which most bryozoan colonies collapse in autumn (Vohmann et al. 2009; Fontes et al. 2017).
2.2 Fish Capture and PIT Tagging
Within a radius of ~200 m of the confluence, brown trout were captured in each stream site using single anode electrofishing gear (EFGI 650 Bretschneider GmbH; Germany). At each location, the mixed site of the main stream was sampled first, followed by the upstream warm site and the cool tributary. Fishing was conducted depending on local weather changes in spring of each sample year (June 2022, May 2023) for tagging the fish and in autumn of each sample year (October 2022, September 2023) for PKD assessment and assessing site fidelity of tagged fish. Trout were haphazardly captured from each stream and anaesthetised in a clove oil Caryophylli aetheroleum bath at 0.1 mL L−1 for about 1 min (Anderson et al. 1997; Pirhonen and Schreck 2003; Wagner et al. 2003). A small incision was made in the abdominal cavity through which a PIT tag with an individual identification code was injected (Vatland and Caudron 2015). The length of the tag (HDX+ PIT Tags, Oregon RFID, Portland OR, USA) was ~10% fish total length, as this dimension is well tolerated by trout (Ombredane et al. 1998; Acolas et al. 2007; Vatland and Caudron 2015). Total length of the fish ranged from 7 to 50 cm: 128 fish were < 15 cm; 209 were 15–30 cm, and 63 were 30–50 cm. Handling time was 10–15 s after which the fish were placed in an oxygen-aerated tank and recovery was monitored. When the effects of the anaesthetic were no longer apparent, fish were released into the site from which they were captured. The N-value for tagging was based on an ad-hoc prediction that 5% of the marked fish would establish in or around the detection antennae over the observation period and that a regression analysis with PKD and temperature as factors would require at least 20 individuals. Therefore, in total, 400 fish were tagged: Gutach, 53 in 2022 and 60 in 2023; Neckar, 61 in 2022 and 43 in 2023; Haslach, 93 in 2022 and 90 in 2023.
2.3 Kidney Sampling and Analysis of PKD
A total of 187 fish (Table 2) were anaesthetized with clove oil at 0.5 mL L−1 and killed by gill incision. Weight and total length were measured and kidney hyperplasia, scored on an ordinal index of severity from 0 to 5, was documented (Clifton-Hadley et al. 1986; Bruneaux et al. 2017). A portion of the kidney was removed and tested for T. bryosalmonae in the laboratory using molecular techniques (Bettge et al. 2009; Strepparava et al. 2018): 25–55 μg kidney tissue was homogenised (Bead Ruptor 4; OMNI International) and digested for 3 h at 55°C (Mixer HC; Starlab). DNA was extracted using a standard tissue kit following the manufacturer's instructions (PureLink Genomic DNA Mini Kit; Invitrogen, Carlsbad, CA, USA). DNA concentration was measured using a spectrophotometer (Nanodrop 2000c; ThermoFisher Scientific, USA) and eluted to get a final concentration in the PCR reaction mix of 30 ng μL−1 (recommended range: 10–100 ng). Quantitative real-time polymerase chain reaction (see Bustin et al. 2009) was employed for the detection of parasite DNA, using the primers PKDtaqf1 and PKDtaqr1 according to Bettge et al. (2009). The method applies a hydrolysis probe with carboxyfluorescein as a fluorescent reporter on the 5′ side and tetramethylrhodamine as a quencher at the 3′ side. Primers and hydrolysis probe were synthesised by Eurofins Genomics (Ebersberg, Germany). All analyses were carried out on a QuantStudio 3 (Applied Biosystems, USA) using TaqMan master mix (TaqMan Universal Master Mix II, no UNG; Applied Biosystems, USA). A maximum of 32 qPCR cycles was used as a threshold for analytical specificity, that is, an estimation of the limit of detection (LOD), as quantifications (Cq) at higher cycles may result from nonspecific amplification (Bettge et al. 2009; Strepparava et al. 2020). Repeatability of Cq values was 0.09 (SD). Reproducibility of Cq values was 0.21 (SD).
2.4 Data Analysis
All statistical results are summarised in Data S1. The nominal logistic fit and the contingency platforms of JMP Pro v.17.0.0 (JMP Statistical Discovery LLC, USA) were used to assess recapture rates. Other statistical analyses were carried out in R. All tests were two-sided with the threshold of significance set at alpha = 0.05. Families for GLM were selected based on visual inspection of qq plots of residual values of the models. Degrees of freedom are reported in square brackets after the test statistic. Differences in water temperature among locations and between years were assessed using nonlinear generalised additive models (package mgcv: Wood 2001). GLMM (package lme4: Bates et al. 2015) with Gaussian distribution was used for piecewise regression to test the significance of differences in the relationship between temperature or degree days (cDD) and movements of tagged fish. The breakpoint was found by iteration of a breakpoint variable in the model, and the best model was identified using the corrected Akaike information criterion. Site, location, and year were considered random effects in this analysis. GLMM with Bayesian correction for singularity (package blme: Chung et al. 2013) was used to calculate effects of temperature and parasite load on daily movements. A haphazard selection criterion was used to select trout with more than 1000 detections recorded in at least two different months. This resulted in 73 tracked fish. Individual (tag) was used as a random effect. Parasite prevalence was analysed with GLM (package basic, no random effect). Parasite intensity and kidney hyperplasia were quantified in fish that tested positive for T. bryosalmonae and analysed with GLM (package basic, no random effect). Marginal means and Cohen's D effect sizes were calculated using the package emmeans (Lenth 2023) and marginal R2 values were calculated using the package performance (Lüdecke et al. 2021). In multifactor comparisons, the maximum D is reported. Figures were drawn using marginal means and 95% confidence intervals (CI) with ggplot2 (Wickham 2016) and formatted in Inkscape (v. 1.2.2, 2022 Inkscape Developers). Results of the statistical models are given in Data S1.
3 Results
3.1 Abiotic Conditions and Risk Factors for PKD
Water temperature profiles of the warm site in the main stream differed significantly among locations (gam: location F[2.0,2158.9] = 65.5, p < 0.001; location * annual pattern, F[18.12158.9] = 16.0, p < 0.001; D = 0.90, R2 = 0.92), with the Gutach being the warmest and the Haslach being the coolest stream during summer months (Figure 2A). Annual patterns differed significantly between years (gam: F[1.0,2158.9] = 9.35, p = 0.0022), although variation was low (D = 0.086) due to a decrease in temperature in the warm site of the Neckar (degree days Figure S1), likely the result of lower water discharge in 2023 than in 2022. The weather during the study period was on average 3.1°C warmer and 3.7% drier than the climate equilibrium in the study area (BadenWürttemberg, Germany, reference period 1961–1990; DWD). Water temperature profiles at the cool site differed significantly among locations (gam; location: F[2.32163.2] = 361.5, p < 0.001; location * annual pattern: F[13.82163.2] = 622.2, p < 0.001, D = 5.72, R2 = 0.93) with no significant year effect (gam: F[1.0,2163.2] = 0.21, p = 0.65; D = 0.019). The temperature profile of the cool site of the Neckar in particular differed from the other sites, as it consisted entirely of groundwater with little annual temperature variation (Figure 2C). Summer temperatures ranged from 10.7°C ± 0.2°C in the cool site of the Neckar to 16.7°C ± 0.4°C in the Haslach and Gutach (Figure 2C). During the observation period, temperatures in the cool sites were 0.45°C, 3.03°C, and 6.27°C lower than upstream of the confluence in the Haslach, Gutach, and Neckar, respectively (Tukey test, all comparisons p < 0.001) (Figure 2D). Downstream of the confluence, mixing of the streams resulted in 2.4°C cooling in the Gutach (−12.8%), 0.51°C (−2.4%) in the Neckar (Tukey test Gutach vs. Neckar: p < 0.001), and 0.32°C (−2.0%) in the Haslach (Tukey test Gutach vs. Haslach: p < 0.001, Neckar vs. Haslach: p = 0.17) (Figure 2E).
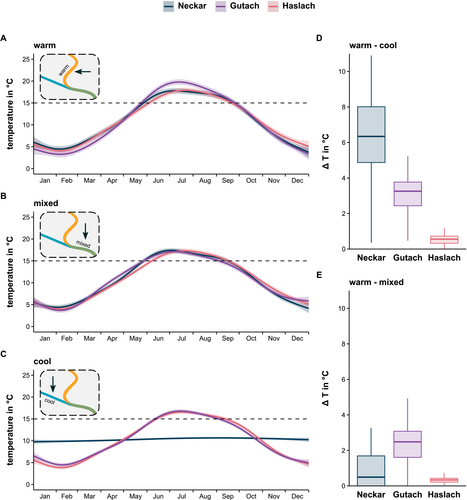
Colonies of bryozoans were found in all main streams at or near the detection sites. The 10°C threshold for bryozoan development was reached in the final week of April in all streams (Haslach and Neckar, April 22; Gutach, April 24) (Figure 2A). No colonies of bryozoans were found in the cool tributaries to the Neckar and the Gutach, but several colonies were found in the tributary to the Haslach. In all three main streams (warm and mixed sites), the 15°C threshold indicating the onset of risk of developing clinical disease from T bryosalmonae infection (PKD) was reached mid-May to mid-June (Gutach, May 25; Neckar, May 29; Haslach, June 5) with return to sub-15°C levels in the last week of September (Gutach, September 23; Neckar, September 23; Haslach, September 20). The PKD risk period was longest in the Gutach at 17.3 weeks, followed by the Neckar at 16.8 weeks and the Haslach, 15.2 weeks. Mean summer water temperatures reached highest values in the Gutach (mean ± CI = 19.78°C ± 0.61°C), with summer water temperatures similar in the Haslach; (17.90°C ± 0.62°C) and Neckar (17.79°C ± 0.60°C) (Figure 2A).
The period of PKD risk was 4 to 5 weeks shorter in the cool sites compared to the main stream sites of the Haslach and Gutach (11.5 and 12.1 weeks), and 15°C was never reached in the cool site of the Neckar. During the observation periods in summer, the average difference in water temperature between the warm and cool sites was highest in the Neckar (2022: 5.94°C; 2023: 5.11°C), intermediate in the Gutach (2022: 2.36°C; 2023: 3.28°C) and lowest in the Haslach (2022: 0.48°C; 2023: 0.34°C).
3.2 Recapture and Site Fidelity
The percentage of tagged fish recaptured in autumn was 46% (183 of 400). This percentage was independent of fish size at capture (logistic regression: χ2[1] = 0.92, p = 0.34, D = 0.28). There were significant differences with respect to location logistic GLM: χ2[2] = 6.60, p = 0.037, D = 0.30 (36% at Haslach vs. 52% in Neckar and 55% in Gutach), and temperature site (logistic regression: χ2[2] = 18.66, p < 0.001, D = 0.52). Recapture was highest in fish tagged in the cool tributary (61% vs. 34% in mixed and 36% in warm site). Most trout were recaptured in autumn from the site in which they were tagged in spring (Table 1, χ2[4] = 227.7, p < 0.001; sum diagonal: 90.1% in same site).
| Tagging site in spring | Recapture per site in autumn: n (%) | |||
|---|---|---|---|---|
| Cool | Warm | Mixed | Total | |
| Cool | 94 (93) | 3 (3) | 4 (4) | 101 (100) |
| Warm | 0 (0) | 25 (89) | 3 (11) | 28 (100) |
| Mixed | 5 (9) | 3 (6) | 46 (85) | 54 (100) |
- Significance: Values in bold are number and percentage of trout recaptured in the same site as they were tagged.
3.3 Temperature Effect on Movements of Tagged Individuals
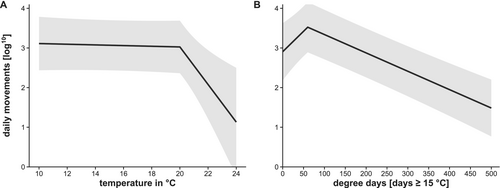
In the range of 10°C to 20°C, the mean daily frequency of fish detection within the main stream was relatively stable (piecewise GLMM, t[126] = −0.83, p = 0.41) (Figure 3A). At 20°C (breakpoint: χ2[1] = 7.6, p = 0.0058) and above, a steep drop in detection was observed in the main stream (piecewise GLMM, t[125] = −2.77, p = 0.0064, R2 = 0.026). Degree days (≥ 15°C) in the main stream increased linearly from mid-May to mid-September (Figure S1). The highest number of degree days in the 2 years of observation was reached in the Gutach and the lowest in the Haslach (mean of 2022 and 2023: Gutach, 438; Neckar, 382; and Haslach, 258). The frequency of fish detection at warm and mixed sites showed a significant breakpoint at 60° days (breakpoint: χ2[1] = 14.4, p < 0.001, Figure 3B) after which detection was significantly negatively related to degree days (piecewise GLMM, t[199] = −3.85, p < 0.001, R2 = 0.117, Figure 3B).
3.4 Temperature-Influenced Habitat Choice
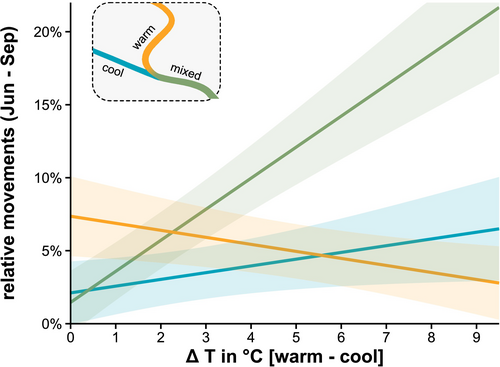
Normalised data per individual revealed significant differences in fish detection among sites relative to water temperature (GLMM: site * ΔT: χ2[2] = 119.5, p < 0.001; Full model R2 = 0.47; Figure 4). Individual fish detection decreased in the warm site and increased in the cool site proportional to site temperature difference (Figure 4). This increase with temperature was predominantly shown in the mixed site downstream of the cool tributary.
3.5 Parasite Detection, Quantification, and Kidney Pathology
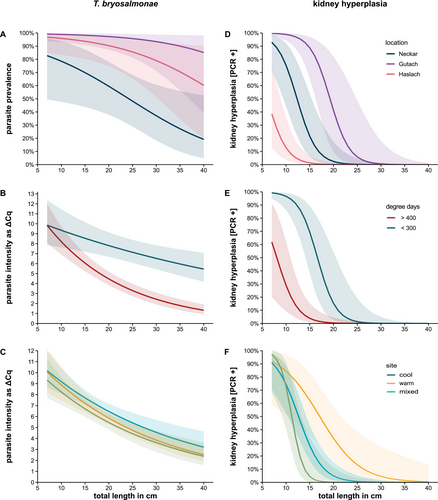
Parasite prevalence differed significantly among locations (GLM, χ2[2] = 29.1, p < 0.001, post hoc Tukey test Neckar vs. Gutach and Haslach, p < 0.05) (Figure 5A). More than 90% of sampled fish (Table 2) tested positive for T. bryosalmonae in the Gutach (92.2%) and Haslach (96.2%), whereas 45.6% tested positive in the Neckar. Parasite prevalence was negatively related to total length (GLM, length: χ2[1] = 7.1, p = 0.0075; length * location: χ2[2] = 0.25, p = 0.88) (Figure 5A). Parasite intensity (kidney qPCR threshold LOD (32) minus Cq value) was significantly negatively related to total length (GLM, length, χ2[1] = 48.7, p < 0.001) (Figure 5B,C). The relationship was steeper in the three cases in which summer temperatures in the warm site of the main stream were highest (GLM, degree days: χ2[1] = 11.9, p < 0.001; degree days * length: χ2[1] = 20.1, p < 0.001) (Figure 5B and Figure S1). Parasite intensity did not differ between warm and cool sites (GLM, site: χ2[2] = 5.0, p = 0.082; site * length: χ2[2] = 0.50, p = 0.78).
| Location | Site | Bryozoans present | Total (n) sampled | n with % parasites detected | qPCR delta Cq | n (%) with kidney hyperplasia |
|---|---|---|---|---|---|---|
| Neckar | Cool | No | 32 | 10 (31) | 7.52 ± 2.70 | 3 (30) |
| Mainb | Yes | 25 | 16 (64) | 5.45 ± 2.97 | 4 (25) | |
| Gutach | Cool | Noa | 23 | 23 (100) | 8.06 ± 2.62 | 12 (52) |
| Mainb | Yes | 30 | 28 (93) | 7.40 ± 2.98 | 19 (68) | |
| Haslach | Cool | Yes | 32 | 31 (97) | 7.37 ± 1.98 | 6 (19) |
| Mainb | Yes | 45 | 40 (89) | 7.01 ± 2.20 | 7 (17) | |
| Total | 187 | 148 (79) | 7.19 ± 2.56 | 51 (34) |
- Note: Delta Cq was calculated as the threshold for detection (i.e., 32) minus measured CTq value. Expressed as mean ± SD.
- a Upstream no suitable habitat for bryozoans existed and sampling did not show fish infected with T. bryosalmonae (Ros et al. 2021).
- b Warm and mixed sites.
Kidney hyperplasia (PKD) prevalence differed significantly among locations (GLM, χ2[2] = 35.2, p < 0.001) (Figure 5D) with the highest prevalence in the Gutach (post hoc Tukey test) vs. Haslach and Neckar (p < 0.01). As found for parasite intensity, PKD decreased significantly with total length (GLM, χ2[1] = 59.6, p < 0.001) (Figure 5D–F). PKD prevalence was significantly lower in locations with higher number of degree days (GLM, degree days: χ2[1] = 18.4, p < 0.001; length * degree days: χ2[1] = 0.14, p = 0.70) (Figure 5E). The highest percentages of fish with total length > 13 cm (Figure 5F) exhibiting PKD were found in the cool and mixed sites (GLM, site: χ2[2] = 13.9, p < 0.001, site * length, χ2[2] = 6.6, p = 0.037) (Figure 5F). The total GLM models explained 39%, 33% and 53% of variation in parasite prevalence, intensity and hyperplasia, respectively (R2).
3.6 Parasite-Influenced Habitat Choice
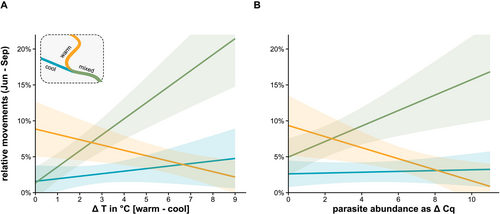
The analysis of fish detection showed a significant interaction of site with T. bryosalmonae infection (GLMM: site * parasite abundance: χ2[2] = 7.54, p = 0.023) (Figure 6B). Highly infected fish were less frequently detected in the warm site and more often detected in the mixed site downstream of the cool tributary than were uninfected fish (Figure 6B). The PKD impact on fish location was independent of the temperature influence: at larger temperature differences between warm and cool sites, individual fish detection decreased in the warm and increased in the mixed site downstream of the cool tributary (GLMM: site * ΔT: χ2[2] = 8.74, p = 0.013; Figure 6A). In total, the GLM model explained 64% of variation in fish detections (R2). In the cool tributaries of the Neckar and Gutach (no bryozoans), 42 of 55 sampled trout were found to be infected with the parasite (Neckar: 10 of 32; Gutach: 23 of 23).
4 Discussion
The increasingly warming climate poses a major challenge for the conservation of cold-water stenothermic fish species via direct factors such as thermal stress and oxygen depletion. Additionally, less documented effects of indirect factors such as increased pathogen virulence may further decrease the climate resilience of salmonid and other cold-stenothermic fish. In this context, the current study followed individually tagged brown trout, a keystone salmonid species, during the summer period at three locations involving confluences of a main stream with a cooler tributary. The objective of this study was to establish whether a preference for cooler refuges exhibited by brown trout during warm periods would be exacerbated by a common and temperature-related salmonid disease, PKD. Our results provide novel empirical evidence of salmonids actively seeking health-promoting conditions to reduce the impact of temperature-driven diseases and highlight the importance of accessible cool-water refuges such as cooler tributaries or groundwater inlets during the warm summer period.
4.1 Effects of Water Temperature on Habitat Choice
Water temperature in the studied stream sites ranged from 10°C to 24°C in summer. Below 20°C, the optimal temperature range both for juvenile and adult brown trout in summer (Elliott 1981), no variation over time was found in the amount of movement of the fish. However, an abrupt and strong decrease in movement was observed when temperatures were above 20°C. This decrease in fish presence was likely not due to migration away from the study area, as 85%–93% were recaptured in cool conditions in autumn from the site in which they were tagged. Fish in the study area showed the expected degree of fidelity to the stream, which is consistent with studies of territorial behaviour (Deverill et al. 1999; Johnsson et al. 1999; Ayllón et al. 2010) finding high residency and site fidelity in brown trout (Bridcut and Giller 1993; Galinat et al. 2020; Ros and Brinker 2024). During the study period, maximum mean daily temperature reached 24°C (22°C–25°C), beyond which brown trout cannot survive for long periods (Elliott 1981; Elliott and Elliott 2010). Temperatures of 19°C–23°C can be tolerated by acclimatised brown trout (Elliott 1981), although it results in reduced swimming activity (Elliott 1976) and cessation of feeding and growth (Elliott 1975).
Temperature difference between the main stream and cool tributary was significantly positively associated with migration from the warm upstream site into the cool tributary and the mixed site downstream of the cool tributary. This constituted an active thermotactic response. The migration occurred during summer at temperatures > 15°C, at which laboratory experiments have shown that resident brown trout show negative thermotaxis (Cherry et al. 1977; Ros and Brinker 2024). All trout in this study were initially captured within 200 m of the confluences, and the observed migration distances lay well within in the described home range for trout of several hundred metres to several kilometres (Burrell et al. 2000; Zimmer et al. 2010). As salmonids (Braithwaite et al. 1996; White et al. 2017) and fish in general have excellent spatial memory (Broglio et al. 2003; Kolm et al. 2009), locating the confluence should be easy for the tagged fish. We thus propose that cool refuges should be available at short ranges as a vital habitat component to allow local trout populations to locate them while staying within their home range during critical heat periods.
4.2 PKD in the Study Area and Behavioural Chill
Infections with the PKD-causing myxozoan T. bryosalmonae and clinical PKD were detected at all three studied locations, as was expected from a prior census (Ros et al. 2021). Parasite prevalence, intensity and kidney hyperplasia strongly declined with total length of the sampled fish. This concurs with other central European studies showing that young of the year are most affected by PKD (Schubiger et al. 2003; Schager et al. 2007; Waldner et al. 2021) with adults being more resilient to PKD as a consequence of resistance through prior exposure to the parasite (Foott and Hedrick 1987; Strepparava et al. 2018; Bailey et al. 2019; Ros et al. 2023). Interestingly, we found a large percentage of large brown trout to be infected with the parasite with parasite prevalence and kidney hyperplasia to be lower in the warmer locations. This is contrary to the intuitive expectation, as bryozoan growth, and T. bryosalmonae development and production of spore-filled sacs is enhanced by higher temperatures (Tops et al. 2006, 2009), which would increase malacospore release and thus (re-)infection risk leading to increased parasite load in the kidney (Bailey et al. 2017; Strepparava et al. 2020; Lauringson et al. 2023). However, such a result is consistent with the prediction (Ros and Brinker 2024) and demonstration (this study) that infected fish move actively away from warmer locations.
After 60 days exposure to ≥ 15°C degree days, trout movements showed a steep decrease. This time frame is approximately the time in which infected trout develop clinical PKD (Strepparava et al. 2020), which might result in direct or indirect (e.g., predation) mortality (Borsuk et al. 2006). Considering that only PKD-resistant or parasite-free brown trout survive well in waters over 15°C (Rubin et al. 2019), the decrease in trout movements may suggest the disappearance of fish with PKD from the warmer parts in the stream (Carraro et al. 2017). Detailed analysis of individual movements proved that trout infected with T. bryosalmonae actively migrated from the warm site to the mixed site downstream of the cool tributary. This effect was in addition to the association of migration with temperature differences between the confluences. Moreover, we captured 33 brown trout with T. bryosalmonae infection in the cool tributaries of the Gutach and Neckar where no bryozoans were found during a visual inspection. Although this absence of bryozoans could be further confirmed by eDNA sampling (Duval et al. 2024), it strongly suggests that these infected fish must have been exposed to T. bryosalmonae spores prior to entering the cool tributary. Both observations provide evidence for the behavioural chill hypothesis (Ros and Brinker 2024): Parasite-infected trout actively seek cooler refuges when water temperatures exceed 15°C, the threshold for producing clinical PKD. This is the first direct evidence in wild fish of a behavioural chill response to parasites. It was postulated that actively seeking out cool water is beneficial for trout in periods of > 18°C water temperature, given that mortality due to PKD increases with water temperature (Waldner et al. 2021). Cool water suppresses the inflammatory response in the kidney, decreasing damage to haematopoietic tissue (Hedrick et al. 1993; Schmidt-Posthaus et al. 2015; Bailey et al. 2020). The behavioural chill is therefore an adaptive response to reduce the virulence of pathogens in poikilotherms (Macnab and Barber 2012; Landis et al. 2012; Ros and Brinker 2024) that functions opposite to a phenomenon called behavioural fever responses (Kluger 1979). In the latter, poikilotherms search for a warmer environment during sickness, and this has been widely demonstrated across vertebrate taxa (Cabanac and Laberge 1998; Boltana et al. 2018; Lopes et al. 2021; Sanhueza et al. 2021).
The importance of these results on chill behaviour in response to rising temperature and disease can be generalised to other salmonid species as most salmonids are susceptible to PKD (Ros et al. 2022), and the investigated salmonid species so far show indications of negative thermotactic responses (Salvelinus fontinalis: Biro 1998; O. mykiss & S. fontinalis: Baird and Krueger 2003; S. salar: Moore et al. 2012; S. fontinalis: Hitt et al. 2017; O. mykiss: Wang et al. 2020). Further research comparing the occurrence of salmonid fish and their T. bryosalmonae parasite intensity and severity of PKD (hyperplasia) between comparable river stretches with or without access to cold-water refuges should give valuable information about how these refuges may increase resilience to PKD. In such a project, the possibility of detection of T. bryosalmonae spores in nonlethal repetitive sampling of urine (Duval et al. 2021) from marked individuals offers a promising novel method to follow individual changes in health status. Finally, behavioural chill responses might be a mechanism for fish to cope with a wider range of sublethal pathogenic infections (Macnab and Barber 2012; Landis et al. 2012), further increasing the importance of cold-water refuges for the conservation of aquatic ecosystems.
4.3 Recommendations for Salmonid Conservation
Our study underscores the urgency of preserving heterogeneous stream microclimates to increase the resilience of trout populations in the face of the adverse effects of climate change (Ros et al. 2022) As heat waves and droughts will increase in frequency (Piccolroaz et al. 2018; Parasiewicz et al. 2019; IPCC 2023), groundwater reserves that provide cool-water influx into streams will be increasingly depleted through evaporation and human water use (Wu et al. 2020). Particularly in fragmented river landscapes, reduced cool-water influx impairs the ability of brown trout to freely migrate to cooler refuges during critical heat waves. The current data predict that this will significantly reduce the health of trout populations. River restoration efforts should aim to restore habitat connectivity both within a river network and on a smaller scale among habitat patches with crucial cool-water refuges (Comte et al. 2014; Isaak et al. 2015; Ros and Brinker 2024; this study). Thermal refuges such as groundwater-fed pools or cool plumes from colder tributaries should be identified and protected, as they allow fish to follow their preferred thermal niche and find temporary shelter from heat (Frechette et al. 2018; Wang et al. 2020) as well as allowing fish to exhibit chill behaviour to counteract temperature-influenced disease. To further buffer peak water temperatures and provide cooling effects, native riparian vegetation should be fostered and restored, as it provides natural shading from solar radiation (Bond et al. 2015; Kalny et al. 2017; Dugdale et al. 2018). Joint solutions should be researched to safeguard fresh water availability during heat waves and/or droughts, for example, by creating reservoirs for irrigation that might ease groundwater depletion and extraction (Staub et al. 2012). Restoring small scale heterogeneous stream microclimates together with increased hydrologic connectivity allowing fish access to cold-water refuges (O'Briain et al. 2020; Mejia et al. 2023; Kelly and Kelly 2024) could mitigate climate change effects to safeguard natural resilience of wild brown trout and of other salmonid species.
4.4 Conclusions
This is the first study to provide evidence for a behavioural chill response to disease in a wild fish. Cold-water stenothermic salmonids were found to actively make use of cool-water refuges to escape excessive heat and as an antidote to temperature-influenced PKD.
The observed large effect size suggests both behaviours to be crucial to maintain healthy wild salmonid populations in the face of ongoing warming. Therefore, restoring and protecting accessible cool-water refuges where fish may seek shelter from heat and parasitic infections is a promising measure to prevent population declines in cold-water stenothermic fishes such as salmonids in warming river systems.
Author Contributions
Sarah Oexle, Albert Ros, Alexander Brinker: conceptualisation, developing methods, data interpretation, writing. Sarah Oexle, Albert Ros: conducting the research, data analysis, preparation of figures and tables.
Acknowledgements
The authors thank Herr Schon, Ganter, and Niggl, Frau Feyrer, and the Fischereiverein Fischingen, Angelkameradschaft Neustadt, and Fischereiverein Wangen im Allgäu e.V. for their support of, and assistance during, the project; Sägewerk Ketterer, Oehler Wasserkraftwerke, Fam. Hölz, Fam. Birk and Fam. Goretzky for providing power access; Hans-Peter Billmann and Andreas Revermann for logistical assistance and assistance with fieldwork; Helga Bentele for carrying out qPCR assays; and Lucidus Consulting for language correction and improvement of the manuscript. Measuring, weighing, tagging and killing of the fish was carried out under anaesthesia. Permission for animal experimentation was granted by the Regierungspräsidium Tübingen, Referat Tierschutz (application LAZ 03/21 G) according to the German Animal Welfare Act (TierSchG). All fish were caught under the permission of the local fisheries administration (Fisheries administration RP Freiburg, Tübingen, Karlsruhe and Stuttgart) according to current fisheries law [LFischVO section 6 (4)]. Fish were anaesthetised with clove oil (Caryophylli aetheroleum, 0.5 mL L−1) and killed by gill incision according to the German Animal Protection Law (section 4) and the ordinance of slaughter and killing of animals (Tierschutzschlachtverordnung section 13).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data are available on Figshare (https://figshare.com/s/e651599438b19428dd86).




