
Importance of terrestrial subsidies for native brook trout in Appalachian intermittent streams
Summary
- Recent studies of perennial streams have shown that changes in riparian vegetation can reduce terrestrial invertebrate subsidies to streams and can cause trophic cascades through aquatic food webs. Intermittent stream food webs have received less attention but may be even more dependent on terrestrial invertebrate subsidies because of limited aquatic invertebrate resources.
- The objectives of this research were: (i) to quantify the abundance and biomass of aquatic, adult aquatic and terrestrial invertebrates in and entering two Appalachian intermittent streams to determine how these resources vary with environmental factors such as stream flow and (ii) to determine the effects of experimental reductions in terrestrial invertebrate subsidies on brook trout diet.
- Stream flow was the main factor driving total invertebrate abundance and biomass, and these resources decreased by 71% as the summer dry season progressed. Terrestrial invertebrates represented only 7% of total resource abundance, but made up 54% of brook trout diet by abundance.
- Experimental reductions in terrestrial invertebrate subsidies resulted in a 49% decrease in terrestrial invertebrate abundance, which resulted in a 55% decrease in terrestrial invertebrates in brook trout diet samples. In contrast to studies of other salmonids in perennial streams, brook trout in these intermittent streams did not switch to consuming more aquatic invertebrates when terrestrial invertebrates were experimentally reduced. Therefore, land use or vegetation changes that cause reductions in terrestrial invertebrate resources may adversely affect brook trout populations in intermittent streams by reducing caloric intake as fish prepare for autumn spawning.
Introduction
Intermittent headwater streams, which do not flow continuously along the length of their channel, are common in the Appalachian Mountains of the eastern United States due to the prevalence of infrequent, large debris flows that leave thick, highly porous deposits of coarse sediment (Eaton et al., 2003). This coarse sediment limits the persistence of surface flow (May & Lee, 2004) and exerts a primary control on channel morphology and fish habitat (May & Lisle, 2012). During summer low flow periods, fish habitat in these streams is limited to small isolated pools, which provide critical habitat for native brook trout (Salvelinus fontinalis) populations (Hudy et al., 2008). However, aquatic invertebrate food resources in isolated pools may be quickly depleted through the summer, and falling terrestrial invertebrates may therefore play a particularly important role in brook trout survival during low flow conditions in intermittent streams.
Terrestrial invertebrate subsidies are generally important components of headwater stream food webs (Baxter, Fausch & Saunders, 2005; Wipfli & Baxter, 2010). Studies in perennial Appalachian streams have shown that brook trout obtain 51–63% of their energy from terrestrial invertebrates (Webster & Hartman, 2005; Utz & Hartman, 2006, 2007; Utz et al., 2007; Sweka & Hartman, 2008). Land use or vegetation changes that reduce falling terrestrial invertebrate inputs into streams may have a negative impact on these systems. For example, streams with an intact forest and high canopy cover generally have greater inputs of terrestrial invertebrates with little to no flying ability (e.g. Lepidoptera larvae and some Coleoptera) than clear-cut forests or grassland streams, in part due to more overhanging vegetation (Kawaguchi & Nakano, 2001; reviewed by Baxter et al., 2005).
Experimental studies examining the impacts of reducing terrestrial invertebrate subsidies to streams have only been conducted recently and primarily in one small perennial stream in Japan (Baxter et al., 2005; Fausch, Baxter & Murakami, 2010). In that stream, experimental reductions in terrestrial invertebrate subsidies caused native Dolly Varden charr (Salvelinus malma) to switch their feeding from terrestrial to aquatic invertebrates when confined to experimental reaches, which resulted in trophic cascades within the stream community and reduced riparian insectivore populations (Nakano, Miyasaka & Kuhara, 1999; Baxter et al., 2004). When Dolly Varden were allowed to move, fish avoided reaches with experimental terrestrial invertebrate reductions and did not switch to consuming more aquatic invertebrates (Kawaguchi, Taniguchi & Nakano, 2003). Switching of fish foraging behaviour has been observed in other salmonids and may play an important role in determining whether stream and riparian communities are resistant or resilient to interruptions in terrestrial subsidies due to land use changes (Fausch et al., 2010); yet, consequences of this behaviour on food webs have rarely been investigated. Switching to consuming more aquatic invertebrates when terrestrial subsidies are reduced may not be possible for fish in intermittent headwater streams because of limited aquatic invertebrate resources; therefore, fish in intermittent streams may be even more dependent on terrestrial invertebrate subsidies than fish in perennial streams.
The objectives of this study were: (i) to quantify the abundance and biomass of aquatic and terrestrial invertebrates in and entering intermittent streams as flow decreases through the summer dry season to determine how food resources for brook trout vary with respect to time, discharge, pool size, spatial distribution of pools, canopy cover and fish density and (ii) to determine how experimental reductions in terrestrial invertebrate subsidies in intermittent streams affect brook trout diet. We hypothesised that: (i) low flow conditions associated with intermittent streams would reduce total invertebrate resources (aquatic, adult aquatic and terrestrial) potentially available for brook trout consumption; (ii) brook trout would be strongly dependent on terrestrial invertebrates subsidies and (iii) with experimental reduction in terrestrial invertebrate subsidies, brook trout would remain heavily dependent on this limited resource and not switch to consuming more benthic invertebrates because of limited benthic production.
Methods
Study area and pool selection
This study was conducted from mid-June to late August 2011 in the Dry River catchment in the George Washington National Forest, Virginia (Fig. 1). The headwaters of the catchment are heavily forested with secondary growth oak-hickory forest, and riparian areas are dominated by red-maple (Acer rubrum), hemlock (Tsuga canadensis) and black birch (Betula lenta). Soils are predominantly derived from sandstone. Many of the tributaries in the Dry River basin are dammed for flood control, and streams below the dams are stocked with non-native and native fish species. Streams above the dams provide critical habitat for small isolated native brook trout populations (Hudy et al., 2008). In these headwater streams, fish communities are limited to brook trout and mottled sculpin (Cottus bairdi).
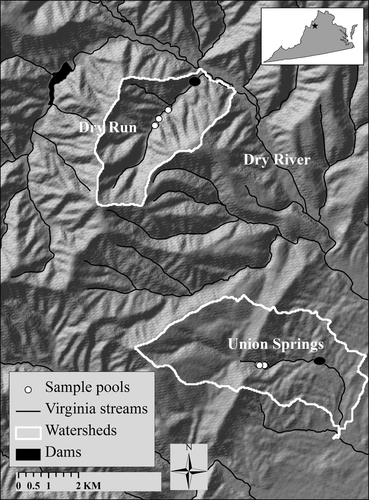
Two intermittent headwater tributaries of Dry River, Union Springs and Dry Run (Fig. 1), were selected for the study to complement a separate investigation in this catchment on the effective population size of brook trout (Whiteley et al., 2012). Both Union Springs and Dry Run have a step-pool morphology (Montgomery & Buffington, 1997) with a 4% channel gradient, mean bankfull width of 6 and 5 m and basin sizes of 18.3 and 12.2 km2, respectively. Six pools in each stream were chosen based on fish abundances, accessibility and size. Of seven persisting pools at Dry Run, one fishless pool was excluded from consideration. At Union Springs, there were many more persisting pools (n = 50), so a 0.3-km reach containing 16 pools was investigated. Within this reach, pools were selected based on size such that pool sizes were comparable between the two streams (Table 1).
| Stream | Treatment | Mean poolarea (m2) | Mean poolvolume (m3) | Mean density (fish per m3) | Mean length (mm) | Mean weight (g) | Mean sculpin density (fish per m3) | Total number of diets sampled | Total number of diets sampled with at least one prey item |
|---|---|---|---|---|---|---|---|---|---|
| DR | EN | 15 ± 4 | 5 ± 2 | 1.49 ± 0.46 | 165 ± 5 | 63 ± 3 | 5.07 ± 1.37 | 37 | 27 |
| N | 24 ± 12 | 7 ± 4 | 1.14 ± 0.40 | 160 ± 7 | 57 ± 2 | 6.22 ± 1.74 | 44 | 34 | |
| US | EN | 18 ± 4 | 4 ± 1 | 0.79 ± 0.21 | 156 ± 14 | 35 ± 2 | NA | 12 | 8 |
| N | 19 ± 5 | 7 ± 2 | 0.44 ± 0.09 | 151 ± 17 | 41 ± 3 | NA | 8 | 8 |
Study design
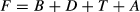
F = total invertebrates per pool; B = benthic invertebrates; D = drifting invertebrates (including aquatic, adult aquatic, and terrestrial); T = falling terrestrial invertebrates (including winged and crawling); A = returning adult aquatic invertebrates.
Total invertebrate resources (F) were considered the standing stock, and a statistical model was constructed to determine how the standing stock varied with respect to discharge, pool size, spatial distribution of pools, canopy cover and fish density.
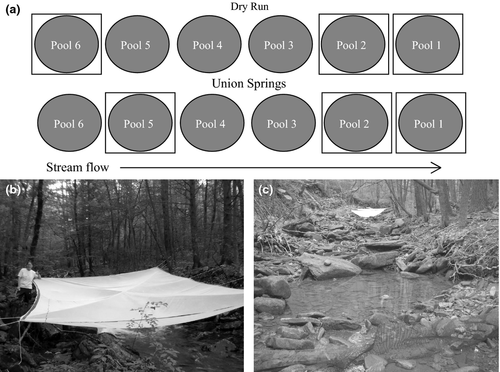
To address the second objective, experimental reductions in terrestrial subsidies were conducted from 18 July to 25 August when fish movement was restricted by low or no flow between isolated pools. During this 6-week period, terrestrial subsidies were experimentally reduced by placing mosquito netting (1.7 × 0.8 mm mesh) exclosures over three of the six pools at each stream (Fig. 2). Exclusion nets were supported with ropes to trees on the stream banks and were c. 1 m above the water surface. A 20 × 20 cm hole was cut in the centre of each exclosure to allow emerging aquatic invertebrates to escape. Exclusion nets covered the entire pool plus 3 m upstream of the pool. Due to logistical problems, randomisation of treatments for pools was not possible. Terrestrial exclusion nets could not be placed over the three pools with wood because the exclusion net could not cover the pool adequately with protruding in-stream wood. We further omitted the two largest pools because they were larger than the exclusion nets; however, there was no significant difference in mean pool area between treatments (t-test, t = 0.422, d.f. = 18, P = 0.678; Table 1).
Fish diet was assessed during this 6-week period to test the hypothesis that brook trout would be strongly dependent on terrestrial invertebrates and to test the hypothesis that brook trout would not switch to consuming more aquatic invertebrates to compensate for the reduction in terrestrial invertebrates. To enable brook trout diet composition to be compared with standing stock resources, and between pools with and without exclusions nets, approximately equal fish densities were necessary. Fish densities were equalised by redistributing fish in some pools on 21–25 July, so that a minimum of three adult brook trout were present in each pool. This was considered the lowest feasible number for statistical analysis of diets and the highest number that was practical for the available habitat given densities observed in other pools in these streams and other intermittent streams in the region. Mean densities of adult brook trout after manipulation were 0.62 ± 0.13 and 1.31 ± 0.28 fish per m3 at Union Springs and Dry Run, respectively (Table 1). There were only seven young-of-the-year brook trout present in the study pools at both streams combined, and their densities were not manipulated. Plastic mesh fencing (6.35 mm mesh) was placed at the downstream and upstream end of each pool on 18 and 19 July prior to fish density manipulation to prevent fish movement in the event of rainstorms and to compare standing stock food resources with actual brook trout diet. Fencing was cleaned as needed and still allowed passage of invertebrates.
Invertebrate sampling
Benthic invertebrates (B) were assessed using a Hess benthic sampler (36 cm diameter and 500-μm mesh). One sample per pool was taken on a representative area of stream bottom. An additional benthic sample was taken in the riffle upstream of each pool for the first sampling period because fish were still able to forage in these areas. After the first sampling period, reductions in flow made foraging in these areas impossible, and therefore, no subsequent benthic samples were taken in riffles.
To capture drifting invertebrates (D), one drift net was placed at the head of each pool for c. 24 h. Due to equipment constraints, two different sizes of drift nets were used (50 cm width × 31 cm height, 82 cm long, 500 μm mesh; or 45 cm width × 27 cm height 93 cm long, 500 μm mesh); however, net size was accounted for in all drift calculations. To determine the proportion of drifting invertebrates that were caught in the net compared with the total drifting invertebrates coming into a pool, current velocity was measured in the centre of the net and across the head of the pool at the time of net collection to calculate the volume of water flowing through the net relative to the total discharge. Once a pool became completely isolated, drift nets were no longer deployed. All pools became completely isolated after the third sample date with the exception of one pool at Dry Run and three at Union Springs that had flow for all five sampling dates.
To capture terrestrial invertebrates (T) and returning adult aquatic insects (A), one clear, tethered floating pan trap (53 × 35 cm area, 15 cm depth) was placed in each pool for c. 24 h. Pan traps were filled with c. 10 cm of water and a few drops of surfactant. A trace amount of generic tabasco sauce was also added to deter insectivores from feeding on the collection because our previous observations indicated that insectivore predation on the collections would be problematic. Due to pan trap design and placement, terrestrial invertebrates entering from stream banks may not have been captured. To capture emerging adult aquatic insects, one tethered floating pyramidal PVC trap (45 × 45 cm base, 38 cm height) covered on all sides except the base with mosquito netting (1.7 × 0.8 mm mesh) was placed in each pool for c. 24 h. Placement of pan traps and emergence traps was not randomised within a pool, but most pools were small with little leeway for trap placement.
Invertebrate sample analyses
To assess their importance for fish, the abundance and biomass of each taxon were determined. All invertebrates were preserved in 95% ethanol, and for each pool, aquatic invertebrates and adult aquatic insects were identified to family and terrestrial invertebrates were identified to order. Orders having both terrestrial and aquatic taxa were identified to the appropriate level to determine whether they were aquatic or terrestrial. All individuals were counted, and biomass of each individual in each taxon was measured as dry mass to the nearest 0.0001 mg after drying at 105 °C for 24 h. If a taxonomic group contained more than 20 individuals in total across all sampling methods, a random source (benthic, drift, pan trap or emergence), date, stream and pool were chosen, and all individuals in that sample were selected for measuring dry mass. Random samples were selected until a subsample of 20 individuals was obtained. Distributions of the subsamples were modelled using EasyFit (MathWave Technologies, www.mathwave.com) to determine the best-fit distribution for each taxon, and the theoretical mean of the best-fit distribution was used as the mass for all individuals in that taxon (see Supporting Information Table S1). Subsampling was used because weighing all invertebrates individually was impractical because many taxa had hundreds to thousands of individuals. This subsampling procedure was validated by comparing the mean Aranae weight obtained using 20 individuals to the mean Aranae weight using all individuals (n = 40). With fitting the same distribution, there was only a 7% change in the mean. This taxon was one of the most variable, and this result confirms that this was a mathematically robust approach for subsampling.
Invertebrate abundance and biomass per unit area per day were determined for each source (emergence, pan trap, benthic or drift). Emergence and pan trap results were easily converted to units of area per day. Benthic samples were area-constrained but had no time factor, so to convert to units of area per day, daily benthic abundance and biomass were assumed to be equal to the benthic abundance and biomass taken on a specific sampling date. Drift abundance and biomass were measured in units of volume (m−3 day−1), and drift abundance and biomass were estimated by dividing the total abundance or biomass of invertebrates retained in the net during a c. 24-h period by the discharge that flowed through the drift net during that time. A common unit of measure among sources was needed to determine the proportion that each source made up of the total standing stock. To enable this comparison, drift abundance and biomass by volume (m−3 day−1) were converted to area (m−2 day−1). First, the total daily input of drifting invertebrates per pool was determined by multiplying the abundance or biomass (m−3 day−1) by the total daily discharge at the head of each pool. Second, the total daily drifting invertebrate input per pool was divided by the pool area on each sampling date.
Factors determining standing stock invertebrate resources
Seven factors (discharge, pool volume, spatial distribution of pools, percentage canopy cover, relative sculpin density, adult brook trout density and stream) were assessed concurrent with invertebrate sampling to test the hypothesis that abundance and biomass of total invertebrate resources would decline with low flow conditions. Discharge was assessed for each pool during each invertebrate collection as described above. Pool volume was estimated by measuring the wetted width, length and depth of each pool on each sampling date. The spatial distribution of pools was assessed as the distance of each pool from the upstream most pool at each stream using ArcGIS (ESRI, Redlands, CA, U.S.A.). Percentage canopy cover of each pool was assessed with a convex spherical densiometer.
Fish densities in each pool were determined twice during the study on 21–25 July and 22–23 August after flows restricted movement, using three-pass depletion surveys with a backpack electrofishing unit. Brook trout and mottled sculpin inhabited Dry Run; however, pools in Union Springs only contained brook trout. Because bottom dwelling species, such as sculpin, are notoriously difficult to capture, our estimates should be considered relative values. A rough estimate of brook trout mortality was also obtained by comparing fish abundances during the first sample to the last sample. Because fish abundances were assessed only twice on 21–25 July and 22–23 August to minimise fish stress, but invertebrate availability was assessed five times during the study (13 June–25 August), assumptions were made about fish densities to determine how they influenced invertebrate resources. First, fish densities were not assessed prior to 21–25 July because spatially continuous stream flow allowed for fish movement among pools; therefore, fish abundances prior to 21–25 July were assumed to be equal to fish densities after pool isolation but before fish abundance was experimentally manipulated on this date for the diet component of this study. Because flow conditions prior to 21–25 July were already low, we think this is an appropriate assumption; however, this assumption may result in fish abundance being overestimated. Secondly, fish abundances between 21–25 July and 22–23 August were assumed to be equal to abundances on 21–25 July after experimental manipulation of fish abundances.
Brook trout diet sampling
To minimise shock, diet was assessed during two mid-day depletion surveys (21–25 July and 22–23 August) and once in the middle of the study on 8–9 August to test the hypothesis of brook trout dependence on terrestrial invertebrates and whether brook trout would switch to consuming more aquatic invertebrates to compensate for terrestrial invertebrate reductions. We acknowledge that single, mid-day diet samples only represent a snapshot in time of fish diet; however, multiple measurements of stomach contents over short time periods to assess total consumption were not feasible. A maximum of 10 adult brook trout caught from each pool was immobilised with tricaine methanesulfonate (MS-222), weighed to the nearest gram on a portable balance, and stomach pumped using gastric lavage (Light, Adler & Arnold, 1983). Stomach contents were preserved in 95% ethanol and identified to family or as aquatic or terrestrial if family could not be determined. While head capsules typically are counted in diet samples to determine prey abundance, in this study heads and wings were counted due to the low number of heads in the samples. Only the body part (either wings or heads) that was most abundant for each taxon in each sample was used to determine abundance. If wings were used to determine abundance, the total number was divided by the number of wings an individual in the taxon possesses. Individuals identified by wings made up 35% of the total number of individuals in diet samples. It should be noted that gut transit rate was assumed to be equivalent for wings and heads, although the true residence time for any of these structures is unknown. To determine the biomass of individuals in the diet samples, the abundance of each taxon was multiplied by the mean mass of that taxa calculated for invertebrates in the standing stock sampling (described above).
Statistical analysis
Factors determining standing stock invertebrate resources
A linear, mixed-effects model was developed to determine how total invertebrate abundance and biomass per square meter varied with respect to seven candidate explanatory variables: discharge, pool volume, spatial distribution of pools, percentage canopy cover, relative sculpin density, adult brook trout density and stream. Pool was specified as a random variable to factor out correlations of repeated observations for each pool, and all other variables were considered fixed. Stream was not considered a random variable because we were interested in examining differences between streams that may have resulted from differences in fish densities and the presence or absence of sculpin. No autocorrelation parameter was included in models to factor out possible correlations between observations closer together in time because no significant difference in model fit was identified when likelihood ratio tests were run with and without autocorrelations.
To select the most parsimonious, information-rich model that explained total invertebrate abundance and biomass, a best-fit model was selected using Akaike's information criterion corrected for small sample sizes (AICc). This information-theoretic method uses Akaike weights (wi) to assess the relative support for each model in a set of candidate models. Weights of all models sum to 1 such that the weight of each model is a proportion of the total weight of evidence (Burnham & Anderson, 2002). The support for each possible single variable model compared with the null (intercept only) was determined, and from these results, a candidate list of multiple variable models was constructed. This method was used instead of an a priori candidate model set because there was no combination of variables that was expected to have a better model fit than any other (Table 2).
| Candidate variables | Justification for inclusion |
|---|---|
| Discharge coming into pool | Discharge may directly affect drifting invertebrates and may indirectly affect benthic, falling terrestrial and returning adult aquatic invertebrates |
| Adult brook trout density | Brook trout consume invertebrates, and trout density may have an impact on population size of invertebrates |
| Relative sculpin densitya | Sculpin may compete with brook trout for invertebrates, and sculpin density may have an impact on the population size of invertebrates |
| Total fish density | Total fish density was the only way to incorporate the effect of sculpin in the analysis because sculpin density was strongly positively correlated with adult brook trout density (d.f. = 16, R2 = 0.605, P < 0.001) and confounded with stream (i.e. sculpin were present at Dry Run but not at Union Springs) |
| Pool volume | Pool volume may affect the amount of available habitat for benthic invertebrates and the surface area intercepting falling terrestrial and adult aquatic invertebrates |
| Percentage canopy cover | Percentage canopy cover may influence abundance of falling terrestrial invertebrates and aquatic invertebrate productivity |
| Spatial distribution of poolsb | Pools closer together may have similar characteristics that cause a correlation structure to invertebrate resources |
| Stream | Pools at one stream may be more similar to each other than pools between streams because of different environmental conditions present in each system |
- a Removed from final analysis due to strong correlation with adult brook trout density and stream.
- b Removed from final analysis due to extremely high ΔAIC for single variable model (i.e. low support).
Experimental reductions in terrestrial invertebrates
Terrestrial exclusion net efficiency was determined by placing two pan traps, described above, at each exclosure pool. One pan trap was placed immediately adjacent but outside of the exclosure and was used to quantify baseline flux of falling terrestrial invertebrates. A second pan trap was placed underneath the exclosure, and the abundance and biomass of falling terrestrial invertebrates were compared with the pan trap outside of the exclosure using paired t-tests. Abundance and biomass of terrestrial invertebrate fluxes were also compared between the pools with and without exclosures using t-tests, and Wilcoxon signed rank tests or Mann–Whitney U-tests were used if normality and heteroscedasticity assumptions could not be met after transformation. Additionally, these two pan traps allowed the observational and experimental components of this study to be conducted simultaneously, and the pan trap placed outside terrestrial exclusion nets was used to quantify baseline flux of falling terrestrial invertebrates for the model of factors determining standing stock invertebrate resources described above.
Brook trout diet
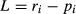
where ri is the relative abundance of prey type i in the diet (as a proportion of the total number of prey in the diet) and pi is the relative abundance of prey type i in the environment (Strauss, 1979). Possible values range from +1, which indicates perfect selection for a prey type, and −1, which indicates perfect selection against it. Because many benthic invertebrates in the study pools (e.g. burrowing taxa) may not have been accessible as prey, our results incorporate both availability and selectivity.
Logistic regression was used to determine whether terrestrial exclusion nets and sampling date had an effect on the probability of a fish having an empty stomach. Because results from this analysis indicated that terrestrial exclusion nets had no effect on the probability of a fish having an empty stomach, subsequent diet analyses were conducted only including fish with at least one prey item in their stomach at the time of sampling. To further determine the effects of terrestrial exclusion nets on brook trout diet, the mean number and mass of terrestrial, adult aquatic and aquatic invertebrates in diet samples were compared between treatments using mixed-effects ancovas with brook trout density as a covariate. One-tailed analyses were used for terrestrial analyses due to an a priori hypothesis of a reduction in terrestrial invertebrates in diet samples. Variance of fish diet within a pool did not differ across treatments, so to prevent pseudo-replication, the diet of fish averaged across all fish within a pool was used for analysis. To account for the non-independence of the three sample days at each pool, pool was considered a random effect. Fish weight was not included as a covariate because there was little variation in fish size and weight between treatments (Table 1), and there was no significant correlation between fish weight and diet after removing two large fish that were outliers (199.5 and 171.5 g, with most other fish weighing <100 g). With the exception of the mass of terrestrial invertebrates in diet samples, all response variables for all statistical tests in this study were non-normal and therefore were ln (x + 1)-transformed to meet normality and heteroscedasticity assumptions. All statistical analyses for the study were performed using R version 2.14.0 (R Development Core Team, 2011) with an alpha level of 0.05, and both mixed-effects analyses were conducted using nlme package (Pinheiro & Bates, 2000).
Results
During late July, flow was low enough at both streams to restrict fish movement to pools, but riffles still had some potential for providing drift (Fig. 3). Starting on 10–11 August, both streams became fully intermittent, and riffles between pools became completely dry with the exception of four pools, which maintained an average inflow of 0.005 m3 s−1 throughout the summer. Hurricane Irene ended the intermittent period on 27 August, resulting in 40 days of pool isolation and 17 days of full intermittency. Total summer rainfall during the summer was 295 mm, which is close to the 118-year average of 305 mm (Southeast Regional Climate Center, 2011). Mean pool area was 19 ± 3 m2, and on average, pool area shrank by 28 ± 9% and 55 ± 10%, during the late summer period (13 June–25 August) at Union Springs and Dry Run, respectively. Despite a high mortality rate (43% for both streams combined for 21 July–25 August), brook trout density increased because of the dramatic reduction in pool volume during the summer.
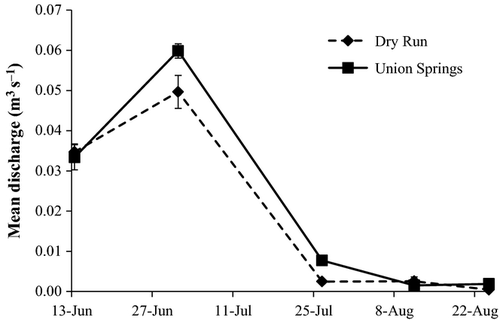
Invertebrate resources
Total standing stock resources declined considerably through the summer as flow declined (Fig. 4; Table S3). Trends in abundance and biomass of standing stock resources for brook trout (F) were similar; therefore, only biomass results are reported for all standing stock invertebrate analyses. When F = B + D + T + A was used to determine the relative contribution of each source of invertebrates across all sample dates, benthic (B), drifting (D), falling terrestrial (T) and returning adult aquatic (A) made up 82, 11, 4 and 2% of standing stock biomass, respectively, at Dry Run and 83, 5, 11 and 1%, respectively, at Union Springs (Fig. 4). Benthic invertebrates (B) were the most plentiful source of invertebrates at both Dry Run and Union Springs, but were quickly depleted once pools became isolated. Drifting invertebrates (D) were the second most important gravimetrically but also dramatically declined as flow diminished. For the first two sample dates, drift was an important source of terrestrials, making up 53 ± 21% and 85 ± 2% of the total terrestrial biomass at Dry Run and Union Springs, respectively; however, after the first two sampling dates, flows were so low that drift was almost negligible as a source of terrestrial invertebrates, making up 10 ± 5% and 4 ± 3% of the total terrestrial biomass at Dry Run and Union Springs, respectively. Falling terrestrial biomass (T) was low throughout the summer on a per square meter and per pool basis, with the exception of a pulse at Union Springs that was due to a few large Arachnids in the Phalangiidae family (Fig. 4). Returning adult aquatic (A) and emerging adult aquatic insect biomass also stayed low throughout the summer on a per square meter and per pool basis. The mean biomasses of returning and emerging adult aquatic insects were similar with a mean returning adult aquatic biomass of 2.1 and 1.5 mg m−2 day−1 and a mean emerging adult aquatic biomass of 2.5 and 2.0 mg m−2 day−1 for Dry Run and Union Springs, respectively.
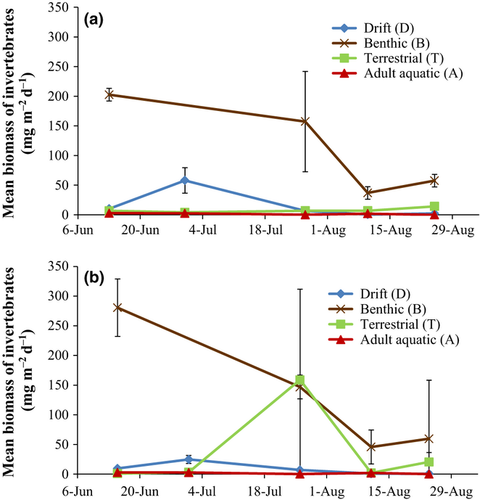
Over half of benthic invertebrates at both streams were from the family Chironomidae. This taxon was so abundant that it made up 43% of all standing stock invertebrates across all dates at both streams in terms of abundance and 17% in terms of biomass. Leptophlebiidae and Leuctridae were also important components of the benthos and the drift in terms of abundance and, respectively, made up 15% of total resources at Dry Run and 13% of total resources at Union Springs. These three taxa declined throughout the summer but made up the majority of resources even at the end of the study. Other important taxa in the drift in terms of abundance included Baetidae and Simuliidae, which, respectively, made up 61% of drift at Dry Run and 11% of drift at Union Springs. Common terrestrial taxa included Diptera, Coleoptera and Hymenoptera, which, respectively, made up 43, 12 and 12% of falling terrestrial abundance at both streams. Returning and emerging adult aquatic insects were mainly from the families Chironomidae and Leptophlebiidae, which, respectively, made up 50 and 21% of both resources in terms of abundance.
Factors determining standing stock invertebrate resources
The most parsimonious, information-rich model predicting total standing stock invertebrate biomass per square meter contained discharge, stream and adult brook trout density as explanatory variables. Other candidate models, including those that contained canopy cover (which ranged between 72 and 94%) or pool volume, had little support (Table 3). For every 0.01 m3 s−1 decrease in discharge, invertebrate biomass per square meter was 20% lower. Invertebrate biomass per square meter was 72% lower at Union Springs compared with Dry Run. For every 1 fish per m3 increase in adult brook trout density at Dry Run, total invertebrate biomass per square meter was reduced by 41%, but adult brook trout density at Union Springs had no effect on total invertebrate biomass.
| Model | Covariates included in each model | K | AICc | Δ AICc | w i |
|---|---|---|---|---|---|
| Model 1 | Discharge, streamabdensity | 6 | 226.1 | 0.0 | 0.93 |
| Model 2 | Discharge, streamabdensity, volume | 7 | 232.5 | 6.3 | 0.04 |
| Model 3 | Discharge, streamabdensity, canopy | 7 | 233.3 | 7.1 | 0.03 |
| Model 4 | Discharge, streamabdensity, volume, canopy | 8 | 239.7 | 13.6 | <0.01 |
| Global | Discharge, streamabdensity, streamacanopy, streamadistance, sdensity, volume | 12 | 276.0 | 49.9 | <0.01 |
- a Indicates that main and interaction effects were considered. bdensity and sdensity are adult brook trout and sculpin densities, respectively.
Experimental reduction in terrestrial invertebrates
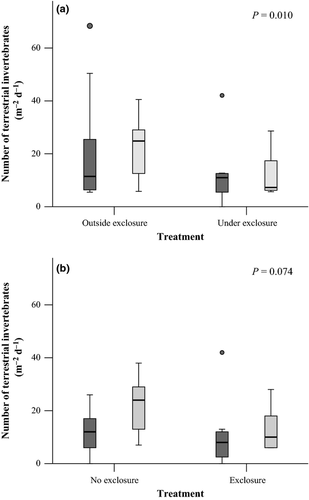
Terrestrial exclusion nets reduced terrestrial invertebrate abundances by 49% (11 invertebrates per square meter) when terrestrial invertebrate fluxes were compared between pan traps underneath terrestrial exclusion nets and traps immediately adjacent to but outside the nets (paired t-test, t = 2.9, d.f. = 17, P = 0.010; Fig. 5a). Terrestrial abundances between pools with and without nets were highly variable, and abundances were only marginally reduced (Mann–Whitney U-test, U = 105, Z = −1.795, d.f. = 34, P = 0.074; Fig. 5b). In contrast to abundance, falling terrestrial invertebrate biomass did not differ between pan traps underneath terrestrial exclusion nets and traps outside the nets (Wilcoxon Signed Rank test, Z = −0.806, d.f. = 17, P = 0.420). Falling terrestrial invertebrate biomass was greater in pools with terrestrial exclusion nets than in pools without nets (Mann–Whitney U-test, U = 254, Z = −2.5, d.f. = 34, P = 0.012). For both comparisons, biomass may not have followed similar trends as abundance, because structure provided by exclusion nets may have attracted and increased the abundance of a few large Arachnids in the Phalangiidae family; however, this taxon was not found in diet samples, so any artificially increased abundances of this taxon would not affect diet analysis.
Brook trout diet
Terrestrial exclusion nets did not affect the probability of a fish having an empty stomach (logistic regression, z = 1.108, d.f. = 100, P = 0.268); however, the percentage of trout with empty stomachs increased as summer progressed. The odds of an individual fish having an empty stomach increased by a factor of 14 when comparing the first (4% empty) and last (37% empty) diet samples, corresponding with a decrease in standing stock resources (logistic regression, z = 2.478, d.f. = 100, P = 0.013).
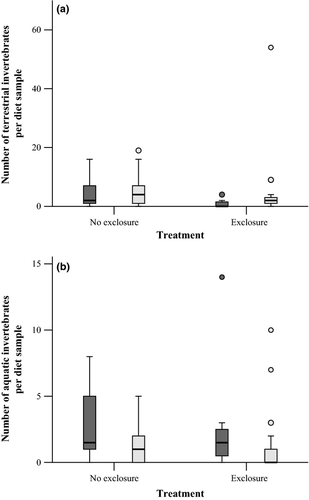
Terrestrial exclusion nets significantly reduced the mass of terrestrial invertebrates in brook trout diet samples and marginally reduced the number of terrestrial invertebrates in diet samples after controlling for differences in brook trout density between pools (Table 4 and Fig. 6a). Of the fish that had at least one prey item in their stomach, fish that were in pools with terrestrial exclusion nets had 55% less terrestrial invertebrate biomass in diet samples compared with fish in pools without terrestrial exclusion nets; however, diet samples indicated that fish did not switch to consuming more aquatic invertebrates or adult aquatic insects to make up for reductions in terrestrial invertebrates (Table 4 and Fig. 6b).
| Mean | F | P-value | ||
|---|---|---|---|---|
| EN | N | |||
| Terrestriala | ||||
| Abundance | 2.4 | 4.8 | 2.276 | 0.085 |
| Mass (mg) | 2.2 | 4.9 | 3.790 | 0.044 |
| Adult aquatic | ||||
| Abundance | 1.5 | 1.6 | 0.130 | 0.728 |
| Mass (mg) | 1.1 | 1.2 | 0.930 | 0.363 |
| Aquatic | ||||
| Abundance | 2.5 | 2.3 | 0.080 | 0.785 |
| Mass (mg) | 1.8 | 1.5 | 1.342 | 0.277 |
- a One-tailed analyses were used.
Despite experimental reduction in terrestrial invertebrate resources, brook trout preyed selectively on terrestrial invertebrates and selected against aquatic invertebrates across all three diet sampling dates regardless of experimental treatment (Table 5). Terrestrial invertebrates only made up 7% of the total invertebrate standing stock by abundance during this time period; however, they made up 54% of brook trout diet by abundance and 65% of brook trout diet by biomass. Important terrestrial taxa included Diptera, Hymenoptera, Homoptera and Coleoptera, and, with the exception of Diptera, reductions in terrestrial invertebrates in diet samples appeared to be due to reductions in consumption of these taxa (Tables S3 & S4).
| Type of invertebrate | Strauss selectivity index (L) | |||
|---|---|---|---|---|
| Dry Run | Union Springs | |||
| Exclosure | No exclosure | Exclosure | No exclosure | |
| Aquatic (B + D) | −0.82 | −0.68 | −0.27 | −0.57 |
| Adult aquatic (A + D) | 0.15 | 0.09 | 0.09 | 0.09 |
| Terrestrial (T + D) | 0.67 | 0.59 | 0.18 | 0.48 |
Discussion
Effects of diminishing flow
Stream flow intermittency was the over-arching factor affecting both the observational and experimental components of this study even though the period of intermittency was relatively short (17 days). As predicted, standing stock resources and effects of experimental reductions in terrestrial invertebrates on brook trout diet differed from previous studies in perennial streams (e.g. Baxter et al., 2004). Flow at Dry Run and Union Springs dramatically declined throughout the summer leaving most pools completely isolated from 10 to 27 August. During this study, total summer rainfall was approximately equal to the long-term average for the region; this suggests that these streams frequently become intermittent in the late summer, even during years of average rainfall conditions. If weather patterns become more erratic with climate change or as needs for water withdrawal increase, intermittent streams may become more common or the duration that streams are intermittent may increase (Milly, Dunne & Vecchia, 2005; Cowell & Urban, 2010).
Increased flow intermittency may negatively impact brook trout populations because, in this study, stream flow was the predominant factor influencing total abundance and biomass of invertebrate resources for brook trout and resources declined with declining flow. Reductions in total available resources with decreasing discharge were largely driven by declining benthic and drifting invertebrates. Benthic invertebrates were the dominant source of invertebrates; however, these resources were quickly depleted, presumably due to increased fish predation, adult aquatic insect emergence and shrinking habitat. Drift was a major source of terrestrial invertebrates before pools became intermittent, but after this, terrestrial invertebrate biomass declined by 62% leaving brook trout dependent on a low flux of falling terrestrial invertebrates.
Total falling terrestrial and returning adult aquatic invertebrates per pool did not decline through the summer despite an average reduction of 42% in pool surface area intercepting these fluxes. Temporal variability in fluxes of falling terrestrial invertebrates could explain this lack of decline, and correlations between discharge and terrestrial input could depend on whether terrestrial invertebrates are passively or actively entering the stream (Edwards & Huryn, 1995). Both returning and emerging adult aquatic insect fluxes stayed fairly constant throughout the summer. An average of 80% of emerging adult aquatic insects in this study returned to the stream, in contrast to studies in perennial streams that have shown that <1–60% of emerging insects return to the water (summarised by Jackson & Fisher, 1986). This may be due to isolated pools being hot spots for emergence or sites of adult aggregations (Iwata, 2006; Hagen & Sabo, 2012), but it is hard to quantify emergence owing to high temporal variability resulting from large, short-lived peaks (Nakano & Murakami, 2001). As flow declines, the proportion of this resource that is available for brook trout may increase, but may become largely unavailable for insectivorous riparian predators. Therefore, diminishing flows in intermittent streams may change the proportion of terrestrial and aquatic reciprocal subsidies for stream and riparian food webs (Hagen & Sabo, 2012).
Brook trout diet was affected by reductions in total standing stock resources as flow decreased and as the summer progressed empty stomachs were more common regardless of experimental treatment. This reduction in consumption may have had an effect on brook trout mortality, although mortality from predation may have been substantial. Mortality from 21 July to 25 August was 43% for both streams combined, indicating the potentially strong bottleneck caused by dry conditions during the summer. These findings are consistent with other decreases in brook trout consumption during summer low flows (Sotiropoulos, Nislow & Ross, 2006) and with other mortality estimates during drought periods and in intermittent streams in other regions (Hakala & Hartman, 2004; May & Lee, 2004).
Brook trout dependence on terrestrial invertebrates
While canopy cover was not an important factor influencing standing stock resources in this study, this may have been due to the low range of percentage canopy cover observed between pools (72–94%). Changes in riparian vegetation that reduce terrestrial invertebrates could still have a detrimental impact on stressed and isolated brook trout populations. Terrestrial exclusion nets did not exclude a large number or biomass of terrestrial invertebrates, probably because unmanipulated terrestrial abundance and biomass were already low. Reductions were particularly hard to detect at Union Springs due to the lower terrestrial resources at this stream; however, exclusion nets reduced falling terrestrial invertebrate abundance by 49% on average at both steams. This reduction was large enough to reduce the biomass of terrestrial invertebrates in brook trout diet samples by 55%. Similarly, when 50 and 90% of tree basal area was removed along 250-m reaches of perennial streams in West Virginia, Niles (2010) found that compared with reference reaches, there was 31 and 48% less terrestrial invertebrate biomass in brook trout diets in 50 and 90% removal reaches, respectively.
Reductions in diet sample invertebrate biomass of the magnitude reported in this study may result in negative growth during the summer months (Sweka & Hartman, 2008), and low fat stores may negatively affect overwinter survival and autumn spawning (Hutchings, 1994). Brook trout populations in intermittent streams in this study persisted despite low food resources associated with low flow conditions, and other studies have shown that these headwater populations are also impacted by low genetic variability [effective population size (Ne) <5 individuals at Dry Run in 2011; M. Hudy, unpubl. data], acid rain, climate change and habitat fragmentation (Hudy, Downey & Bowman, 2000; Hudy et al., 2008; Nislow et al., 2011). The total population size in our study streams was small (M. Hudy, unpubl. data), but brook trout densities were high compared with those in many perennial streams of the region (Utz & Hartman, 2009) because there were so few isolated pools available. The mechanisms behind survival in these unfavourable conditions are poorly understood, but terrestrial invertebrate resources appear to be a major energetic resource supporting this population, and reduction in this food resource through land use or vegetation changes can be expected to be detrimental.
Brook trout selectively preyed on terrestrial invertebrates regardless of experimental treatment. Even though terrestrial invertebrate resources only made up 7% of the total invertebrate standing stock by abundance, they made up 54% of brook trout diet. Similarly, terrestrial invertebrates have made up more than a third of fish diet in other systems where terrestrial invertebrates have only made up 10–15% of the drift (Hubert & Rhodes, 1989; Young, Rader & Belish, 1997). In our study, Diptera, Hymenoptera, Homoptera and Coleoptera were of particular importance in brook trout diet samples. Other studies in Appalachian Mountain streams have also found that terrestrial taxa are critical prey items for brook trout (e.g. Utz & Hartman, 2007); however, ours is the first study to compare total standing stock resources (benthic, drifting, falling terrestrial and returning adult aquatic) to brook trout diet samples.
Despite brook trout dependence on them, terrestrial invertebrate subsidies prior to experimental reduction were an order of magnitude lower than those in forested streams in other systems (reviewed by Baxter et al., 2005). Terrestrial invertebrate biomass at Dry Run was 8 mg m−2 day−1, which is more similar to streams with grassland vegetation than other forested streams (Baxter et al., 2005; Saunders & Fausch, 2012). Union Springs had higher biomass (37 mg m−2 day−1), but this was due to a few large Arachnids in the Phalangiidae family, which were possibly attracted to the exclusion nets, and without this taxon, mean falling terrestrial biomass was lower than Dry Run (7 mg m−2 day−1). Low terrestrial biomass may characterise streams in the Appalachian Mountains because other studies in this region have also found low terrestrial biomass (e.g. 17.3 mg m−2 day−1 see Romaniszyn, Hutchens & Wallace, 2007; Studinski, 2010); however, values from our study in the Ridge and Valley region of Virginia are a magnitude lower than similar-sized streams located in the Piedmont region of Virginia possibly due to the large differences in geology and vegetation between these two regions (Cloe & Garman, 1996). Additionally, intermittency can play a role in this regional variation because intermittent streams may have different vegetation or provide unique habitat or microclimate conditions that lead to low terrestrial biomass (Steward et al., 2011; Katz, Denslow & Stromberg, 2012).
No switching of fish foraging when terrestrial invertebrates were experimentally reduced
While other studies have demonstrated that salmonids, such as Dolly Varden charr, switch from feeding on terrestrial invertebrates to feeding on benthos when terrestrial invertebrate availability is reduced (Tippets & Moyle, 1978; Nakano et al., 1999; Kawaguchi et al., 2003; Baxter et al., 2004), brook trout in our study did not demonstrate this behaviour. This also contrasts with behaviour shown by brook trout in perennial streams, which have been shown to feed readily on the benthos (Benjamin, Fausch & Baxter, 2011; Lepori et al., 2012; Benjamin et al., 2013) and to increase aquatic invertebrate consumption when terrestrial invertebrate availability is reduced (Niles, 2010). Brook trout in the study by Niles (2010) may have increased aquatic invertebrate consumption because benthic resources increase when canopy cover is removed (Nislow & Lowe, 2006).
In our study, benthic resources were already the most abundant source of invertebrates, but despite this, aquatic invertebrates did not comprise an equally large part of brook trout diets. This is surprising because if aquatic invertebrates were accessible, brook trout in intermittent streams should be more likely to switch to this resource because fish were confined to isolated pools and could not migrate to areas with greater terrestrial inputs as seen with native charr in perennial streams (Kawaguchi et al., 2003). We suspect that the majority of benthic invertebrates in our study area were largely unavailable as prey because of their smaller size and hiding potential. For example, the majority of benthic resources in these streams were composed of Chironomidae, which may have been burrowers and unavailable for brook trout consumption, although some Chironomids were found in diet samples. Additionally, substratum characteristics were not quantified in this study, but intermittent stream substratum characteristics probably differ from perennial streams (e.g. high loads of course substratum contribute to making the streams in this study intermittent). Therefore, benthic taxa in these streams may be less susceptible to predation due to substratum that allows better concealment.
Brook trout also may have restricted foraging on aquatic invertebrates because of competition with sculpin, which feed solely from the benthos. Brook trout and sculpin may not compete for food resources in perennial streams (Cheever & Simon, 2009), but this could change when flows are reduced and brook trout can no longer feed on the drift. Competition with sculpin may have prevented brook trout from eating aquatic invertebrates when terrestrial invertebrates were reduced at Dry Run. Despite the absence of sculpin at Union Springs, brook trout diet samples did not indicate higher consumption of aquatic invertebrates in pools with exclusion nets either; however, when comparing both streams, diet samples at Union Springs had a higher percentage of aquatic invertebrates. The analysis of factors influencing standing stock provides additional evidence for competition with sculpin. Increasing brook trout density decreased total standing stock at Dry Run but not at Union Springs, possibly due to higher brook trout densities at Dry Run and/or sculpin acting as a driving factor reducing standing stock because sculpin densities were positively correlated with brook trout density at Dry Run.
Management implications
Increased flow intermittency that persists for even short durations may substantially affect brook trout food resources because for every 0.01 m3 s−1 decrease in discharge, total invertebrate biomass per square meter was 20% lower. In particular, terrestrial invertebrates made up a large proportion of brook trout diet even though terrestrial invertebrate biomass was low and declined with declining flow due to reductions in drifting invertebrates. This, combined with the inability of brook trout to switch from feeding on terrestrial invertebrates to the benthos, suggests that intermittent streams are heavily dependent upon terrestrial subsidies. Because of the close linkage between the terrestrial and aquatic ecosystem, land use or vegetation changes in the riparian zone could have a large impact on intermittent stream food webs. Despite this, intermittent streams currently receive less protection than perennial streams (Downing, Winer & Wood, 2003; ; Blinn & Kilgore, 2004; Wigington et al., 2006; Leibowitz et al., 2008). As basic knowledge of frequency and location of intermittent streams is lacking (Uys & O'Keefe, 1997; Hansen, 2001), more research is required so that best management practices such as water conservation and maintenance of riparian buffers can be determined and implemented to conserve these threatened ecosystems, which provide critical habitat for isolated native brook trout populations.
Acknowledgments
This research was conducted as the primary author's master's thesis, and we thank Mark Hudy, Andy Dolloff and Heather Griscom for serving as thesis committee members and for their advice on project design and methods. We thank Ben Stanley, Christina Anderson, Harrison Mohn and Zak Robinson for their assistance with field sampling. We thank Ricky Domangue and Patrice Ludwig for their statistical advice and Steven Hiner for his assistance with invertebrate identification. We would also like to thank the U.S. Forest Service for permission to conduct this research in the George Washington National Forest, and we thank two anonymous reviewers for their thorough evaluation of the submitted manuscript.




