
Habitat heterogeneity and intraguild interactions modify distribution and injury rates in two coexisting genera of damselflies
Summary
- Sublethal effects of predation can affect both population and community structure. Despite this, little is known about how the frequency of injury varies in relation to habitat, aquatic community characteristics or between trophically similar, coexisting taxa.
- In a tidal freshwater ecosystem, we first examined injuries (lamellar autotomy) of Enallagma and Ischnura damselfly larvae, which have unique behaviours and susceptibilities to predation, as a function of habitat type, body size and overall odonate density. We also examined relative abundance of these genera and potential anisopteran predators as a function of habitat type.
- The frequency of injury to Enallagma was high when larvae were small and overall odonate density was high. For Ischnura, however, the frequency of injury depended on habitat and was high for small larvae in less disturbed habitats low on the shore. Ischnura were most frequently found in more disturbed habitats high on the shore, whereas Enallagma were more frequently found in less disturbed habitats low on the shore.
- The relative importance of factors hypothesised to structure odonate communities varied between coexisting Enallagma and Ischnura. Distinctive distributions and patterns of injury for each genus provided new insights on the potential for intraguild interactions to modify habitat associations in tidal freshwater ecosystems.
Introduction
Predation is a well-studied biotic interaction that affects population and community structure and whose intensity varies according to environmental context (Power, 1992; Langellotto & Denno, 2006; Kovalenko, Thomaz & Warfe, 2012). Sublethal effects of predation, such as injury or altered behaviour, have received relatively little attention but can have broad effects on populations and communities (Schmitz, Krivan & Ovadia, 2004; Schmitz, 2008). Injuries are likely to increase subsequent mortality, alter antipredator behaviour and reduce fitness (Bowerman, Johnson & Bowerman, 2010; Bateman & Fleming, 2011; McCauley, Rowe & Fortin, 2011). Behaviourally mediated effects induced by the presence of predators can reduce prey foraging time (Lima & Dill, 1990; Rudolf, 2006; Ferrari, Wisenden & Chivers, 2010) and even initiate trophic cascades (Schmitz, Beckerman & O'Brien, 1997; Steffan & Synder, 2010).
Sublethal interactions can also affect intraguild predation and cannibalism in aquatic ecosystems (Crumrine & Crowley, 2003; Rudolf, 2006; Rudolf & Armstrong, 2008). The relative strength of these effects across species and communities, however, is not well known (Miller & Rudolf, 2011). In particular, little is known about the context in which sublethal injuries occur and how injury rate relates to the distribution and abundance of predators. For example, among larval odonates, factors that alter the intensity of intraguild predation and cannibalism may also alter the frequency and severity of injury. Smaller odonate larvae are more vulnerable to intraguild predation, cannibalism and injury (Baker & Dixon, 1986; Claus-Walker, Crowley & Johansson, 1997), and these risks increase with odonate density (Robinson et al., 1991; Johnson et al., 1995; Claus-Walker et al., 1997). In addition, habitat choice is thought to be crucial in mitigating interactions between odonates and fish, as physically complex habitats can reduce the intensity of intraguild and fish predation (Crowder & Cooper, 1982; Finke & Denno, 2006; Janssen et al., 2007; Flynn & Moon, 2011).
Here, we tested the influence of habitat type, body size and odonate density on the relative abundance and sublethal injury rates of Enallagma and Ischnura larvae in a tidal freshwater stream. These two damselfly genera have been well studied in the context of intraguild predation, because they have distinctive life histories (McPeek, 1996, 1998, 2004). Ischnura is a mobile habitat generalist that grows quickly, but is vulnerable to both invertebrate and fish predation, whereas Enallagma grows slowly and has behaviour that reduces its vulnerability to fish. Since members of each genus are frequently found together, McPeek (1996, 1998, 2004) hypothesised that these differences facilitate their coexistence.
We quantified lamellar loss, a common damselfly injury, in larval Ischnura and Enallagma as an index of sublethal interaction intensity between damselflies and other odonates (Baker & Dixon, 1986; McPeek, 1990b; Stoks, 1998) and modelled these as a function of habitat type, size structure and odonate density. Analogous to tail autotomy in lizards, autotomy of lamellae in damselflies is an effective form of predator avoidance, but also reduces fitness (Robinson et al., 1991; Althoff & Thompson, 1994; Stoks, 1999; Stoks & De Block, 2000; Bateman & Fleming, 2009). To complement this analysis, we examined the relative abundance of Enallagma, Ischnura and other Anisoptera in different habitat types. High densities of vegetation, invertebrates and fish (Thorp, Jones & Kelso, 1997; Kraus & Jones, 2012), combined with tidally fluctuating water levels, generate distinct habitat types, to which the two genera of damselflies may respond differently. We predicted that tidally disturbed, upper intertidal areas should disproportionately benefit Ischnura, because it is a habitat generalist and would benefit from situations where predation risk from fish and other invertebrates is reduced. We expected that Enallagma, which is relatively invulnerable to fish, would predominate in less disturbed, lower intertidal habitats.
Methods
Study site and survey methods
This study was conducted in Thompson Creek (38.654°N, 77.194°W), a slow moving, small, tidal freshwater tributary of the Potomac River, Fairfax County, Virginia, U.S.A. We focussed on a 300-m stretch near the mouth of the creek that could be navigated by canoe. Emergent marsh vegetation in Thompson Creek was dominated by Pontederia cordata, while submersed vegetation was dominated by Hydrilla verticillata (Rybicki & Landwehr, 2007). Water level changes by approximately 1 m between mean low tide and mean high tide, twice per day (NOAA, http://tidesandcurrents.noaa.gov). We selected three habitat types to sample based on vegetation patterns in relation to water level at low tide. Upper intertidal habitats were defined as areas with emergent vegetation and contained monocultures of P. cordata with small patches of submersed vegetation. These habitats were partially or completely drained at low tide. Lower intertidal habitats were defined as areas without emergent vegetation and with submersed vegetation. Lower intertidal habitats comprised of a band between 1 and 5 m wide, surrounded by emergent vegetation and deeper subtidal areas. These habitats were partially drained and formed mats of submersed vegetation at low tide, and were visually judged to have the highest structural complexity (i.e. vegetation surface area). Subtidal habitats were defined as offshore areas with submersed vegetation that bordered the creek channel. These habitats were not drained and did not form mats of submersed vegetation at low tide. Fish had access to subtidal habitats at all tide stages but were excluded from upper and lower intertidal habitats at progressively lower tidal stages.
To quantify the distribution and abundance of odonates, we employed a stratified random sampling design defined by our three habitat types. Sample sites were chosen without replacement from a random selection of 300 locations that were divided equally among the three habitats. All sites had submersed vegetation and some standing water at low tide. One sample from each habitat type was collected weekly from 26 June 2009 to 30 August 2009, except for the week of 7 August, for a total of 27 samples and a total of nine samples per habitat type (i.e. three samples per week for 9 weeks). To avoid sampling at different tidal heights in upper and lower intertidal habitats, we constrained sampling to a 3-hour window around high tide and randomised the habitat order on each round. Sampling around high tide in those habitats minimised the matting of submersed vegetation, which potentially would increase lamellar injuries when sampling. These mats were not a concern in subtidal habitats, which were sampled around mid-tide (flood or ebb) due to the need to sample the other two habitats at high tide. Invertebrates were collected by sweeping a dip net (0.5 mm mesh size) through submersed vegetation, and sampling continued in a habitat until 50 odonates were found or until 3 h had passed. These sampling procedures allowed us to estimate relative odonate abundances in subtidal habitats, where densities were typically 25–35% of other habitats, and ensured that upper and lower intertidal habitats could be sampled within one tidal stage. All invertebrates were preserved in 70% ethanol in the field and returned to the laboratory for analysis. Odonates were counted and identified using Westfall & May (1996) and Needham, Westfall & May (2000). The head capsule widths and missing lamellae of Ischnura and Enallagma were measured and counted under a Leica MZ12.5 dissection microscope (Leica Microsystems, Buffalo Grove, IL, U.S.A.) with the aid of ImageJ software (National Institutes of Health, Bethesda, MD, U.S.A.).
We used the number of missing lamellae of Enallagma and Ischnura as a measure of sublethal injury. Larval damselflies have three caudal lamellae that are used for respiration, swimming, predator evasion and communication between conspecifics (Erkisen, 1986; Robinson et al., 1991; Corbet, 1999). These lamellae are frequently lost in antagonistic encounters with aquatic invertebrates, especially other odonates (McPeek, 1990b; Robinson et al., 1991; Corbet, 1999). Because lamellae regenerate after several moults (Corbet, 1999), missing lamellae indicate that an individual had been attacked recently. We did not include leg injuries in our analysis because they were less common than lamellar injuries, cover different functions and have weaker associations with swimming and predator evasion (Robinson et al., 1991).
Analysis
To examine the relative importance of habitat type, body size and odonate density for the rate of lamellar injury, we compared a series of proportional odds multinomial logistic regression models with a logit link (Agresti, 2002). The number of missing lamellae on each damselfly was treated as the response variable in the regression models. The categorical nature of this variable (zero, one, two or three missing lamellae) allowed us to model the frequency of injury (present or absent) and injury severity (number missing).
The total number of odonates collected in each sample was standardised by sampling duration to produce relative densities (no. h−1). Density, head capsule width (mm, an index of body size) and habitat type (three categories: upper intertidal, lower intertidal and subtidal) were used as explanatory variables in the injury model. Habitat type was treated as a categorical variable, which allowed for contrasts between habitats. Head capsule width, rather than odonate length, was chosen because it is a better predictor of trophic position (Wissinger, 1992) and does not become distorted when specimens are preserved.
For both Ischnura and Enallagma, we compared 18 models to determine the relative importance of the explanatory variables and their interactions for the frequency and severity of lamellar injury, using sas (PROC LOGISTIC, SAS Institute, 2009). The 18 models comprised all possible combinations of primary effects and interaction terms. To find the model with the fewest parameters that described the most variation, we compared differences in log likelihood (Δ −2logeL) among models significantly better than the null model (P < 0.05). The chosen model was also tested against the full model as a measure of lack of fit.
To determine whether the relative abundances of Ischnura, Enallagma and Anisoptera depended on habitat, the total number of each taxa collected in each habitat was compared using a goodness-of-fit G-test (P < 0.05). The G-statistic from the overall goodness-of-fit test was then partitioned using four single degree of freedom G-tests to provide additional information about the strength and nature of the association (Agresti, 2002).
Results
A total of 1357 odonates were collected in the 27 samples obtained in the 10-week survey period. All zygopterans captured were in the genera Enallagma or Ischnura. Of those, I. verticalis and E. signatum were dominant, comprising more than 80% of each genus, although I. posita, E. durum and E. civile were also found. Epitheca princeps was the dominant anisopteran, comprising more than 80% of all dragonflies found. Mean (±SD) odonate density (no. h−1), a predictor in our injury models, was 46.82 ± 10.38 in the upper intertidal, 61.13 ± 15.06 in the lower intertidal and 14.59 ± 11.87 in subtidal habitats. Mean (±SD) head width, a second predictor in our injury models, was 2.14 ± 0.62 mm in the upper intertidal, 1.93 ± 0.62 mm in the lower intertidal and 1.78 ± 0.76 mm in subtidal habitats for Ischnura, and 2.23 ± 0.58 mm in the upper intertidal, 2.28 ± 0.62 mm in the lower intertidal and 2.19 ± 0.76 mm in subtidal habitats for Enallagma. Temporal effects due to seasonal emergence patterns (not presented here) did not alter model selection or overall distribution results.
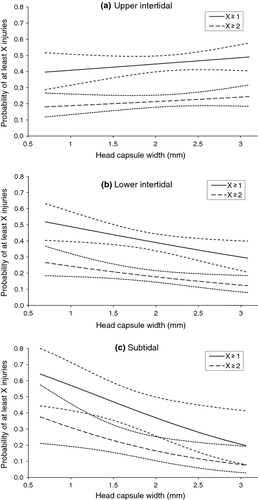
A total of 792 Ischnura were collected, and 338 of these had at least one lamellar injury. The model that contained habitat, head width and the habitat–head width interaction was chosen as having the best fit. In this model, there was a progressively higher frequency of injury for small individuals in the lower intertidal and subtidal habitats, but frequency of injury was unrelated to odonate density (Table 1; Fig. 1). Of the original 18 models, the six models that contained the habitat, head width and habitat–head width interaction performed significantly better than the null model (P < 0.05; Table 2). Of those six models, none performed significantly better than the model with those three coefficients alone, so no additional coefficients were added (Table 2). The full model did not perform better than the chosen model, which indicated adequate model fit (Table 2). In the lower intertidal and subtidal habitat, the odds of injury decreased by 0.676 and 0.449 times per 1-mm increase in head width, respectively (Table 1). In the upper intertidal, the relationship between injury and size was the weakest and insignificant (Table 1).
| Parameter | b i | SE | P | OR (+1 mm) |
|---|---|---|---|---|
| Size (upper intertidal) | 0.163 | 0.156 | 0.296 | 1.177 |
| Intercept (injury ≥ 1) | −0.522 | |||
| Intercept (injury ≥ 2) | −1.665 | |||
| Intercept (injury = 3) | −2.556 | |||
| Size (lower intertidal) | −0.392 | 0.178 | 0.028 | 0.676 |
| Intercept (injury ≥ 1) | 0.317 | |||
| Intercept (injury ≥ 2) | −0.710 | |||
| Intercept (injury = 3) | −1.468 | |||
| Size (subtidal) | −0.802 | 0.340 | 0.018 | 0.449 |
| Intercept (injury ≥ 1) | 1.056 | |||
| Intercept (injury ≥ 2) | 0.023 | |||
| Intercept (injury = 3) | −0.772 |
- Odds ratios (OR) are given are for a + 1-mm increase in head width.
| Ischnura | Verses null model (intercept only) | Verses chosen model (H, S, H × S) | ||||
|---|---|---|---|---|---|---|
| Injury candidate models | Δ −2LogeL | d.f. | P | Δ −2LogeL | d.f. | P |
| H, S, H × S * | 14.572 | 5 | 0.012 | – | – | – |
| H, S, D, H × S | 16.112 | 6 | 0.013 | 1.540 | 1 | 0.215 |
| H, S, D, H × S, S × D | 16.171 | 7 | 0.024 | 1.599 | 2 | 0.450 |
| H, S, D, H × S, H × D | 17.033 | 8 | 0.030 | 2.461 | 3 | 0.482 |
| H, S, D, H × S, H × D, S × D | 17.068 | 9 | 0.048 | 2.496 | 4 | 0.645 |
| Full model | 21.594 | 11 | 0.028 | 7.022 | 6 | 0.319 |
- Variables: H, habitat; S, size (head width, mm); D, density (no. captured h−1).
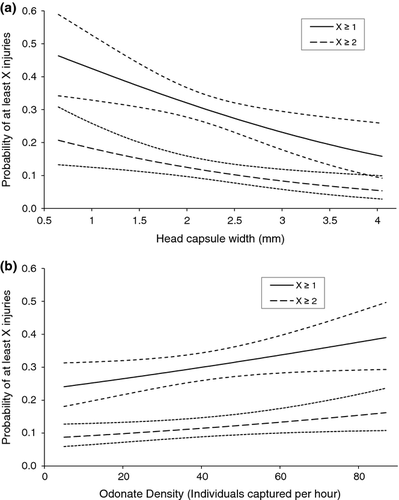
A total of 467 Enallagma were captured, and 141 of these had at least one lamellar injury. The model that contained head width and density was chosen as having the best fit. In this model, injury frequency increased with overall odonate density, but declined as head width increased (Table 3; Fig. 2). Injury frequency was unrelated to habitat (Table 3; Fig. 2). Of the original 18 models, the 14 models that contained the head width coefficient performed significantly better than the null model (not shown). Of these 14 models, four performed significantly better than the size-only model (P < 0.05; Table 4). Each of these models contained the head width and density coefficients, but none performed better than the model with those two coefficients alone, so no additional coefficients were added (Table 4). The full model did not perform better than the chosen model, which indicated adequate model fit (Table 4). In the model of best fit, the odds of injury increased by 1.187 times for each increase of 20 individuals per hour and decreased by 0.639 times per 1-mm increase in head width (Table 3).
| Parameter | b i | SE | P | OR (+1 mm/+20 ind) |
|---|---|---|---|---|
| Size | −0.449 | 0.158 | 0.005 | 0.639 |
| Density | 0.009 | 0.004 | 0.045 | 1.187 |
| Intercept (injury ≥ 1) | −0.191 | |||
| Intercept (injury ≥ 2) | −1.387 | |||
| Intercept (injury = 3) | −2.391 |
- Odds ratios (OR) are given for a + 1-mm increase in head width and +20 individual increase in the no. of odonates captured h−1.
| Enallagma | Verses size-only model (S) | Verses chosen model (S, D) | ||||
|---|---|---|---|---|---|---|
| Injury candidate models | Δ −2LogLe | d.f. | P | Δ −2LogLe | d.f. | P |
| S, D * | 3.982 | 1 | 0.0460 | – | – | – |
| S, H, D | 8.225 | 3 | 0.0416 | 4.243 | 2 | 0.120 |
| S, D, H, S × H | 12.168 | 5 | 0.0326 | 8.186 | 4 | 0.085 |
| S, D, H, S × D, S × H | 12.833 | 6 | 0.0458 | 8.851 | 5 | 0.115 |
| Full model | 14.462 | 10 | 0.1529 | 10.480 | 9 | 0.313 |
- Variables: H, habitat; S, size (head width, mm); D, density (no. captured h−1).
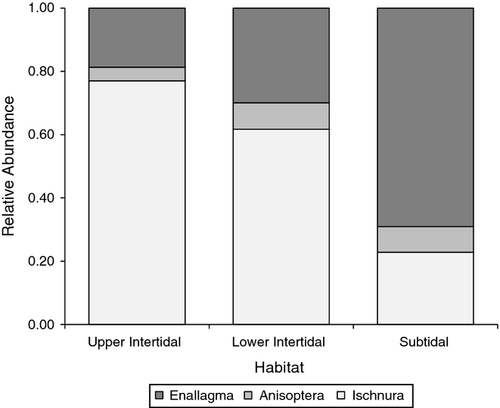
The overall goodness-of-fit G-test indicated that the relative abundance of Ischnura, Enallagma and Anisoptera depended on habitat (G = 253.20, d.f. = 4, P < 0.0001; Fig. 3). We began partitioning by comparing Enallagma and Anisoptera, because the relative abundance of both groups increased in lower intertidal and subtidal habitats and then finished by comparing those two groups combined with Ischnura (Fig. 3). The relative abundances of Enallagma and Anisoptera did not differ significantly in upper and lower intertidal habitats (G = 0.45, d.f. = 1, P = 0.5010; Fig. 3), but the relative abundance of Enallagma was significantly higher than Anisoptera in subtidal habitat compared with upper and lower intertidal habitats combined (G = 10.89, d.f. = 1, P = 0.0010; Fig. 3). The relative abundance of Ischnura was higher than that of both Enallagma and Anisoptera combined in lower intertidal habitat compared with subtidal habitat (G = 123.34, d.f. = 1, P < 0.0001; Fig. 3) and in upper intertidal habitat compared with lower intertidal and subtidal habitats combined (G = 118.52, d.f. = 1, P < 0.0001; Fig. 3).
Discussion
Both Ischnura and Enallagma are similarly sized, generalist predators that prefer complex, vegetated habitats (Corbet, 1999; Zimmer, Hanson & Butler, 2000; Tolonen et al., 2003). While differences in behaviour, growth and susceptibility to predation have been suggested to explain their coexistence (McPeek, 1998), our results show that the distributions of these two genera vary at small scales and suggest that the risk of injury in part explains differential responses to habitat and other ecological factors for each genus.
Lamellar loss reduces damselfly foraging and growth rates, inhibits swimming and escape behaviour and increases cannibalism and intraguild predation risk (Robinson et al., 1991; Stoks, 1999; Stoks et al., 1999; Stoks & De Block, 2000). The frequency of injuries and potential fitness losses, however, differ between the two genera in different habitats and circumstances. Previous experiments have found that interodonate predation, cannibalism and injury rate increase with higher odonate density (Anholt, 1990; Robinson et al., 1991; Claus-Walker et al., 1997). In our field study, however, increased odonate density was a strong predictor of injury frequency for Enallagma only (Table 3; Fig. 2), which suggests that the relative importance of odonate density differs between the two genera. The absence of a specific habitat effect in the injury model (Table 3) indicated that habitat characteristics, such as vegetative complexity, were relatively weak predictors of injury for Enallagma. This result fits the particular behavioural response of Enallagma to remain motionless in habitats where fish are present (McPeek, 1998). Remaining motionless can aid in avoiding detection by fish, but limits escape opportunities from other odonates that crawl on vegetation (Pierce, Crowley & Johnson, 1985; McPeek, 1990a). Whereas fish are presumed to consume damselflies whole and not to leave injuries, other odonates are expected to account for a higher frequency of sublethal encounters with damselflies (Stoks & De Block, 2000).
The relationship between habitat and size-structured interactions among predators has received considerable attention. For instance, habitat complexity has been shown to lower predation pressure on intraguild prey and reduce cannibalism (Finke & Denno, 2006; Langellotto & Denno, 2006; Janssen et al., 2007), while disturbance intensity in ponds alters odonate assemblages by creating opportunities for habitat generalists (McCauley, 2007). Our study extends body size and habitat associations with injury and highlights potential impacts of tidal disturbance and height on odonate assemblages. The correlation between size and injury was similar across habitats for Enallagma (Table 3; Fig. 2), but smaller Ischnura were less vulnerable to injury higher on the shore (Table 1; Fig. 1). Similar relative abundance and injury patterns suggest that Ischnura could be responding to increased injury or predation risk in the less disturbed, deep water habitats or that they are more effective competitors in tidally impacted, shallow habitats that pose fewer injury risks. The probability of encountering not only Enallagma and Anisoptera, which may injure or consume Ischnura, but also fish predators increases further down the shore (Fig. 3). Robles & Desharnais (2002) similarly predicted that predation risk from Piaster sea stars on predation-susceptible Mytilus bivalves would create a gradient where smaller individuals experience greater predation risk lower down the shore.
Because lamellae are removed in both aggressive encounters and failed in predation attempts, the injuries observed are probably attributable to encounters between damselflies and conspecifics or predatory invertebrates (McPeek, 1990b; Robinson et al., 1991; Corbet, 1999). Tidal freshwater streams, however, have low invertebrate diversity (Odum, 1988). Odonates were extremely common in Thompson Creek, while non-odonate insect predators, such as coleopterans or notonectids, were rare. This suggests that interodonate interactions are an important source of injury, which is consistent with previous studies measuring the frequency of lamellar injuries in field populations and enclosures (McPeek, 1990b; Stoks, 1998; Stoks et al., 1999). Although we cannot disregard the possibility that crayfish, or even small fish, caused the injuries observed, we did not focus on these because crayfish were relatively uncommon in samples, and it is uncertain whether small fish cause lamellar injuries (Stoks & De Block, 2000).
We found that the relative abundance of Ischnura and Enallagma could be predicted by height and distance from the shore (Fig. 3), which corresponds to two contrasting gradients – the potential for fish predation offshore and disturbance and desiccation risk near shore. Both of these pressures can structure odonate communities in unique ways. Large fish would not leave injuries and are expected to have a disproportionate effect on odonate abundances through predation, which creates communities dominated by fish-adapted taxa (Morin, 1984a,b; Stoks & McPeek, 2003). Fish, however, have limited foraging opportunities in the intertidal and thus have more potential to structure odonate communities in deeper habitats (Kneib & Wagner, 1994; Halpin, 2000; Ellis & Bell, 2004, 2008). Increased disturbance frequency and desiccation risk can cause intolerant species to migrate or become displaced, which benefits tolerant species that have settled or can persist in disturbed areas (McCauley, 2007). This is especially true in Thompson Creek, as individuals can move between habitats or closer to the bottom of macrophytes in response to the changing tide. We did not find any evidence, however, that odonates were moving frequently between habitats, as fish might. Once settled, larvae are not likely to move long distances, and disturbance-intolerant species are even less likely to disperse (Corbet, 1999; McCauley, 2007). Our results are consistent with these expectations: Ischnura had higher relative abundance in low-predation, more disturbed habitats, whereas Enallagma had higher abundances in less disturbed habitats with more fish access.
A growth and mortality trade-off has been proposed as an underlying mechanism that facilitates coexistence between Enallagma and Ischnura damselflies (McPeek, 1998); our results support this hypothesis and provide taxon-specific mechanisms that can predict both injury and distribution. Given the importance of intraguild interactions in structuring ecological communities (Holt & Polis, 1997; Arim & Marquet, 2004; Miller & Rudolf, 2011; Ohlberger et al., 2012) and the impact that injury can have on odonate fitness, our examination of lamellar injury rates in this system helps define the relative importance of mechanisms postulated to structure odonate communities. As we observed for two odonate genera, height on the shore and tidal disturbance have the potential to modify the within-stream distributions of coexisting predators. The mechanisms entailed characteristic responses to predation and intraguild interactions, which manifested in sublethal injuries that varied by genus. Our results highlight the potential for intraguild interactions and disturbance to modify habitat associations among coexisting predators in tidal freshwater systems.
Acknowledgments
We would like to thank Drs. Larry Rockwood, Scott Sillett and Marlene Cole, as well as three Freshwater Biology reviewers for their comments on earlier versions of the manuscript. We would also like to thank Anna Braum and Bradley Pratt for their assistance in collecting and sorting field data. This study was completed in partial fulfilment of M.S. degree requirements for J.W.W. and was funded in part by the Washington Biologists Field Club and Virginia Academy of Science. The views expressed in this article are those of the author and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. This article is Contribution 1767 of the U.S. Geological Survey Great Lakes Science Center.




