
The neonate nutrition hypothesis: early feeding affects the body stoichiometry of Daphnia offspring
Summary
- Aquatic herbivores consume variable quantities and qualities of food. In freshwater systems, where phosphorus (P) is often a primary limiting element, inadequate dietary P can slow maternal growth and reduce body P content. There remains uncertainty about whether and how dietary effects on mothers are transferred to offspring by way of egg provisioning.
- Using the keystone herbivore Daphnia, we tested a novel explanation (the ‘neonate nutrition hypothesis’) to determine whether the early nutrition of newborns affects their elemental composition and whether the indications of differences in maternal P nutrition found previously might be overestimated.
- We thus examined the P content of mothers and their eggs from deposition through development to the birth of neonates. We examined further whether very short periods of ingestion (3 h) by the offspring alter the overall P content of juvenile Daphnia.
- We showed that strong dietary P effects on mothers were not directly transferred to their eggs. Irrespective of the supply of P in the maternal diet, the P content of eggs in different developmental stages and in (unfed) neonates did not differ. This indicates that Daphnia mothers do not reduce the quality (in terms of P) of newly produced offspring after intermittent periods (i.e. several days) of poor nutrition. In contrast, the P content of neonates reflected that of their food after brief periods of feeding, indicating that even temporary exposure to nutrient poor food immediately after birth may strongly affect the elemental composition of neonates.
- Our results thus support the neonate nutrition hypothesis, which, like differential maternal provisioning, is a possible explanation for the variable elemental quality of young Daphnia.
Introduction
Animals have the ability to alter their life history in response to changing environmental conditions by varying the allocation of resources (Stearns, 1992, 2000). For example, some Daphnia species produce larger eggs when food quantity is low, probably because neonates derived from larger eggs survive longer without food (Gliwicz & Guisande, 1992; Guisande & Gliwicz, 1992). Studies of life history traits like somatic growth, development time, age and size at maturation and reproductive effort bridge the gap between physiology at the individual level and demography at the population level (Urabe & Sterner, 2001) and are at the heart of theoretical predictions of population dynamics of consumers and their prey (Gurney et al., 1990; McCauley et al., 1990). Despite this importance, there remains much to be learned about how resource allocation from mother to offspring changes in response to poor food quality.
Uncertainty about resource allocation remains, even for the relatively well-studied aquatic herbivore, Daphnia. Past investigations of Daphnia have largely focussed on understanding how resources are allocated between somatic tissues and reproduction. While it is clear that the number or mass of eggs produced decreases with declining quality of the diet (e.g. Sterner & Hessen, 1994; DeMott, 1998), how phosphorus (P) flows into reproductive tissue and the production of eggs is less certain. In contrast, changes in embryo carbon (C) content during the ontogenesis of eggs are well described (Urabe & Watanabe, 1990; Glazier, 1991; Boersma, 1995). Data on the P content of Daphnia eggs are largely absent, particularly for mothers consuming diets varying in P content (see Faerøvig & Hessen, 2003). The few studies of the response of eggs and neonates to differences in P content of the maternal diet have reported quite variable results. While some have found a constant P content in eggs (although usually only a single egg development stage has been studied) with decreasing P supply (e.g. Faerøvig & Hessen, 2003; Becker & Boersma, 2005), others have observed a reduced P content in the recently released offspring of P-stressed Daphnia (DeMott, Gulati & Siewertsen, 1998; Boersma & Kreutzer, 2002; Frost et al., 2010). Here, we examine an additional explanation (the ‘neonate nutrition hypothesis’) for the variable P content of neonate Daphnia. Our objective was to determine the effects of early nutrition on the body P content of newborns before and just after release.
Because offspring receive nutrients directly from their mother during egg production (Rossiter, 1996; Mitchell & Read, 2005), differences in the P content of neonates have been assumed to result from maternal nutrition (e.g. DeMott et al., 1998; Frost et al., 2010). However, the strength of those effects (i.e. the P content of neonates being determined by maternal nutrition) could also reflect the nutritional environment of neonates immediately after birth. If so, the effect of maternal nutrition might be less than previously reported (e.g. Frost et al., 2010) or not always manifested by different P allocation in deposited eggs. Therefore, we tested three components of our ‘neonate nutrition hypothesis’ (NNH) : (i) over short time periods (3 days), the P supply in the maternal diet would affect the mother's own somatic tissue but not that of her eggs, (ii) egg P content would not change during egg development, but egg C content would. This would lead to recently described differences between the P : C ratio of eggs and neonates [compare e.g. Faerøvig & Hessen (2003) and Becker & Boersma (2005) with DeMott et al. (1998) and Boersma & Kreutzer (2002)], and (iii) fresh neonates exposed to different dietary P supply would have different body P content. This last component would not apply to neonates hatched under starved conditions because they would not ingest any food that would alter their body chemistry. We suggest our hypothesis as a means for resolving, at least partially, the differences between direct measurements on eggs and these on newborns. Therefore, we examined whether Daphnia magna alters P provisioning to the different tissues (somatic or egg tissue) when the dietary P supply decreases over a 3-day period. More specifically, we analysed the body P content and P : C ratio of eggs (in the first egg stage) and somatic tissues of Daphnia fed different amounts of dietary P for 3 days. Further, we investigated the carbon and phosphorus content of eggs from development until birth and, finally, examined whether the very short periods of feeding (3 h) can influence the overall body P content of the newly released animals.
Methods
Organisms
The stock culture of Daphnia magna was raised in filtered lake water (0.2-μm pore-sized membrane filter) with saturating amounts of the P-replete cultured green alga Scenedesmus obliquus (SAG 276-3a, culture collection Goettingen, Germany) provided for food. For the growth experiment, the highly ingestible and non-toxic cyanobacterium, Synechococcus elongatus (SAG 89.79) (von Elert, Martin-Creuzburg & Le Coz, 2003), was used as food for D. magna. Synechococcus elongatus (SYN) was cultured in aerated 2-L flasks containing WC medium with vitamins (Guillard, 1975) and diluted daily (dilution rate 0.2 day−1) to ensure nutrient and phosphorus (P) repletion. SYN was maintained at an illumination of 40 μmol m−2 s−1 using a 16-h/8-h light/dark cycle. For P-limited S. elongatus (SYN P-), an aliquot of previously P-sufficient cultured SYN was transferred to P-free WC medium and cultivated until molar P : C ratios reached at least 1 mmol mol−1. KCl (100 μmol L−1) was added to P-free WC medium in order to prevent a limitation by potassium (K) due to the omission of the K and P containing stock solution (50 μmol L−1 K2HPO4). Only SYN P- was used in the experiments and pulsed with P, according to Rothhaupt (1995) and Boersma (2000), shortly before preparation of the different food P levels to prevent potential indirect P effects. Such could be changes in fatty acid composition or different digestibility, caused by different culture conditions (Boersma, 2000; Ravet & Brett, 2006) which occur especially in green algae. The effect of direct P limitation could then be much lower than that of indirect P limitation (Ravet & Brett, 2006). All organisms were raised at 20 °C.
To classify eggs at different development stages, we used a system developed by Threlkeld (1979). This system classifies five different stages of Daphnia egg development in which the embryos develop from a spherical stage I, without any morphological differentiation, to stage II when they lose their egg membrane and build up their antennae. After the embryos develop two red eyes in stage III, these eyes change colour to brown in stage IV and merge into a single eye when they are completely developed neonates in stage V.
Experimental procedure
We used third-clutch neonates for our experiments, fed on cyanobacteria with different P content. The cyanobacteria lacked sterols and polyunsaturated fatty acids (PUFA) and, to ensure growth and reproduction of Daphnia (Sperfeld & Wacker, 2011), were enriched with liposomes containing cholesterol (final concentration 14.2 μg cholesterol per mg C) and eicosapentaenoic acid (EPA, final concentration 6.4 μg EPA per mg C). The liposomes were prepared according to Wacker & Martin-Creuzburg (2012). Throughout the experiment, Daphnia were transferred daily into jars with renewed food suspensions.
To analyse the P content of maternal somatic tissue and eggs separately, we dissected eggs from the brood pouch using a gentle stream of water from an extended Pasteur pipette that was carefully inserted into the brood pouch of the animals (Wacker & Martin-Creuzburg, 2007), which were not injured by the procedure. The eggs of all mothers of each replicate (a replicate consisted of a 300-mL jar with three mothers each) and the mothers themselves were transferred separately into pre-weighed aluminium boats. After drying for 48 h at 50 °C, Daphnia and eggs were weighed on an electronic balance (±1 μg; CP2P, Sartorius, Goettingen, Germany) and the dry mass determined. After weighing, the aluminium boats of mothers and eggs of each replicate were used for the analysis of P content.
Preparation of food
For the experiments on tissue P content and the effects of short-term ingestion by adults, we used filtered lake water containing little dissolved P, which was further depleted by adding (and later removing) a P-limited culture of green algae to the water (Lukas, Sperfeld & Wacker, 2011). For the experiment on egg provision and neonate nutrition, we used P-free artificial Daphnia medium (ADaM, Kluettgen et al., 1994). Before the daily preparation of food suspensions, SYN P- (P : C < 1 mmol mol−1) was incubated for 40 min with P (75 μmol L−1 K2HPO4). An incubation time of 40 min was sufficient for SYN P- to obtain constant high P : C ratios (Lukas, Sperfeld & Wacker, 2011) resulting in a change from P-deficient into P-sufficient SYN (hereafter designated as SYN P*). After P incubation, P concentrations of SYN P- and SYN P* were subsequently determined daily on duplicate saved samples. Target P : C ratios were achieved by mixing SYN P- and SYN P* (P : C ratios in Table 1) in calculated proportions using the previously determined P concentrations. Thereby, the daily mixed P : C ratios varied by no more than 5% among daily preparations and did not differ from the target P : C ratios, which we tested twice during the experiment. To integrate the dietary P concentration over the whole experiment, we calculated the mean of the daily mixed P : C ratios.
| P : C SYN P- (mmol mol−1) | P : C SYN P* (mmol mol−1) | |
|---|---|---|
| Tissue P contents (duration n = 9 days) | 0.71 ± 0.05 | 7.46 ± 0.58 |
| Egg provision and neonate nutrition (duration n = 6 days) | 0.91 ± 0.06 | 6.40 ± 0.29 |
| Short-term (3-h) ingestion (duration n = 3 days) | 0.70 ± 0.03 | 7.16 ± 0.37 |
The daily prepared food suspensions started with an equal food concentration (2 mg cyanobacterial C L−1), which was estimated from photometric light extinction (800 nm) using previously determined carbon-extinction equations. Before preparation of food suspensions, samples of SYN P- and SYN P* were filtered onto pre-combusted glass fibre filters (25 mm, GF/F, Whatman) and dried for later analysis of particulate organic carbon using a carbon analyser (HighTOC+N, Elementar, Hanau, Germany) to calculate actual P : C ratios.
Tissue P content
Daphnia used for the experiment were raised in lake water with S. obliquus for 6 days from birth. When they released eggs of their first clutch into the brood pouch, we transferred these animals into six dietary P levels of SYN for 3 days. Because their resources had recently been allocated to reproduction alone when animals had very recently released their new eggs (Tessier & Goulden, 1982), and the almost empty ovaries could be investigated visually under a stereomicroscope, we assumed that the second clutch (used for our analysis) had largely been based on our experimental diets. The three lower P treatments were replicated four times, the higher P treatments three times. Each replicate started with three animals per separate jar containing 250 mL of food suspension. The experiment was terminated after 3 days, when the neonates of the first clutch were released and mothers released the second clutch into the brood pouch. These eggs of the second clutch, which were all in the spherical egg stage I (Threlkeld, 1979), and their mothers were then analysed for P content as described below.
Egg provision and neonate nutrition
Daphnia used for this experiment were raised in lake water with S. obliquus from birth for 6 days until they released the eggs of the first clutch into the brood pouch. The animals released their first clutch between zero and 12 h; therefore, we checked Daphnia twice a day, initially, when the first animals approached first clutch release and again when all animals had released their eggs. Subsequently, we transferred the mothers to three different P levels of the diet (P- c. P : C 1.5 mmol mol−1, P med c. P : C 2.8 mmol mol−1, P+ c. P : C 6.4 mmol mol−1) for 3 days. Each P treatment was replicated 19 times, starting with three animals in each jar containing 250 mL of food suspension. We used four replicates each for every egg stage tested (I, III/IV, V and neonates). Three further replicates of each P treatment were used to test the effect of the short-term ingestion by neonates. When mothers released the second clutch into the brood pouch (the neonates of the first clutch were released previously), we sampled the first four replicates of each P treatment and collected the eggs in egg stage I and the respective mothers (see 2.2 for further information). The mothers in the remaining jars were kept at their respective P supplies until we collected eggs in egg stage III/IV and egg stage V over the following days. To determine the effect of the short-term feeding by neonates on their P content, we starved mothers carrying the last egg stage (three replicates of each P treatment). Consequently, neonates that hatched between time zero and 6 h could not have ingested any food for the previous 3 h, on average. At the same time, mothers in four replicates of each P treatment were allowed to release their neonates with (maternal) food present; hence, these neonates were allowed to consume food for an average of 3 h each. We then analysed the P and C contents of the eggs and the neonates as described below.
Short-term (3-h) ingestion
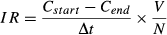 (1)
(1)To estimate the amount of carbon ingested, we used the difference in Daphnia P : C ratios between the two subsamples (animals with treatment food vs. animals with P-deficient food for 3 h) at each P level in the food. Using this difference, and the associated C content of the animals, we calculated the amount of P ingested. This amount of P and the respective food P : C ratio were used to calculate the amount of C ingested for each dietary P treatment (see Appendix S1 in Supporting Information for further information).
Analysis
We analysed the P concentration of aliquots of SYN P- and SYN P* filtered onto membrane filters (Tuffryn, 25 mm, 0.45 μm, Pall Corporation, NY, U.S.A.) and samples of dried Daphnia. Phosphorus concentration was determined using the molybdate blue reduction method (Murphy & Riley, 1962) after dissolving tissues with sulphuric acid and an oxidative hydrolysation using K2S2O8 at 120 °C and 120 kPA by autoclaving.
The carbon content of animals and eggs was calculated using carbon dry mass ratios determined prior to the experiment (0.41 and 0.52 μg C μg−1 dry mass, respectively) or was directly measured using a carbon analyser (Elemental Analyser Euro EA, HEKAtech, Wegberg, Germany).
All statistical analyses including one-way and two-way anovas, two-way ancovas, Tukey's HSD post hoc tests and two-tailed t-tests were performed using the statistical software package R v.2.5.1., which is under general public licence (R Development Core Team, 2007). We log-transformed the data of the dietary P supply for the two-way ancova for the experiment on effects of short-term ingestion, where we tested whether there was a difference between the P : C ratios of animals that ingested P-deficient food for 3 h and those that were collected and measured directly from their treatments.
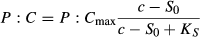 (2)
(2)Results
Tissue P content
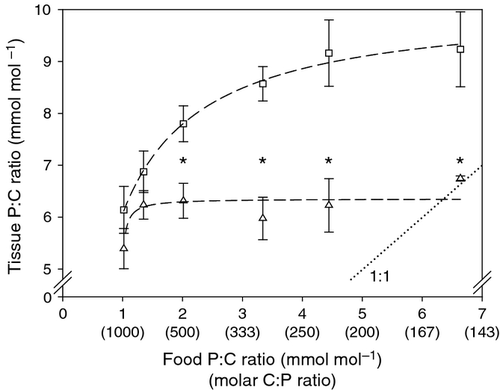
P : C ratios of eggs and soma from Daphnia consuming different dietary P content differed strongly. Both tissue types generally lay above the 1 : 1 line between tissue and food P content (Fig. 1). While the P content of Daphnia mothers (soma without eggs) decreased with decreasing dietary P, egg P content showed almost no response to different P diets and remained at 6.3 ± 0.4 mmol mol−1 (mean ± SD, n = 15) across nearly all the P : C ratios of the diets provided. We found that the P content of the eggs was reduced only at the very lowest dietary P availability (two-sample t-test: t16 = 3.6, P = 0.002). In contrast, the P : C ratio of the somatic tissue of the animals was substantially higher than those of eggs when food P : C ratio was 2.0 mmol mol−1 or higher.
Egg provision at different egg stages
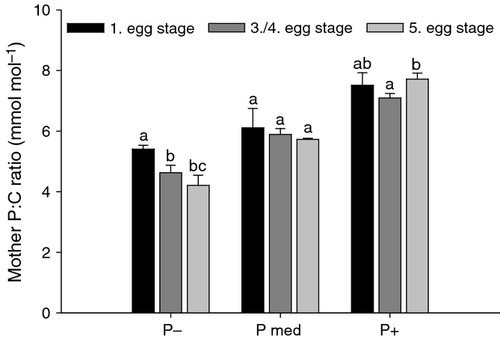
The P : C ratio of mothers was strongly influenced by the P content of the food (Fig. 2; two-way anova: P supply: F2,26 = 219.0, P < 0.001) but this was variable among the times that we collected the different egg stages (egg development stage: F2,26 = 8.5, P = 0.001). We analysed maternal tissues only until the eggs reached the fifth egg stage to avoid fluctuations associated with subsequent egg production. In addition to the decrease in maternal P content when the P content in their food decreased, body P content decreased further longer they were fed on P-deficient food. In contrast, the P content of mothers fed ‘P med’ and P-sufficient food remained more or less stable (see post hoc tests in Fig. 2). These differences in body P content of mothers with contrasting diets were evident from the significant interaction in the two-way anova (P supply × egg development stage: F4,26 = 5.8, P = 0.002).
Contrary to the mothers, the P : C ratio of eggs did not respond to P availability in the food, but increased during egg development (Fig. 3a; two-way anova, P supply: F2,32 = 0.7, P = 0.50; egg development stage: F3,32 = 65.2, P < 0.001; egg development stage × P supply: F6,32 = 3.2, P = 0.012). The significant interaction in this anova indicates that the increase in the P : C ratio of the eggs varied with P content of the diet. In the following, we used the ‘C or P per egg’ unit, which allowed us to distinguish the separate effects on P and C contents. In other words, we were interested in the decline in carbon due to respiration or other carbon consumptive processes. The P content per egg did not vary throughout egg development or among different dietary P treatments (Fig. 3b; two-way anova, egg development stage: F3,32 = 1.5, P = 0.23; P supply: F2,32 = 0.1, P = 0.87; egg development stage × P supply: F6,32 = 1.8, P = 0.13). Therefore, the reason for the changes in the P : C ratio of eggs was based on changes in C content. This decreased from 3.9 (±0.33) to 2.6 (±0.40) μg C per egg (mean ± SD, N = 12, respectively N = 9) during development from egg stage one to neonate (two-way anova, egg development stage: F3,32 = 46.8, P < 0.001; P supply: F2,32 = 0.1, P = 0.94; egg development stage × P supply: F6,32 = 1.1, P = 0.40). Since in both two-way anovas (for P and C contents of the eggs), the effect of P supply as well as the interaction between egg development stage and P supply was not significant, we conclude that there was no influence of dietary P supply on the P : C ratio of eggs in any of the different egg stages.
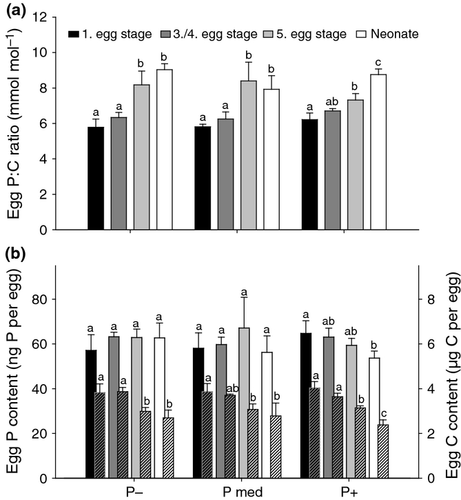
Neonate nutrition
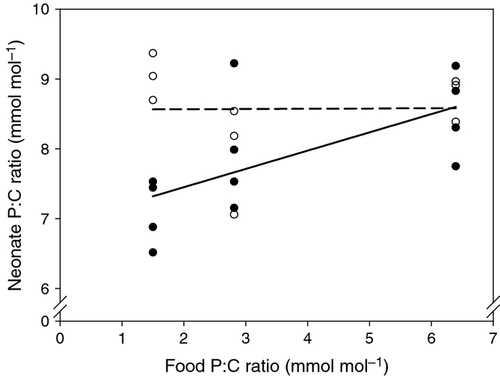
To determine the potential effect of ingested food on neonate body P content, we starved a first subsample of mothers carrying the last egg stage, while a second subsample of mothers released their neonates while provided with food. Consistent with the results from previous egg measurements, the P : C ratio of starved neonates did not vary under changing P supply (Fig. 4). However, the P : C ratio of neonates hatching during an exposure time of 3 h (on average) to the food of their mothers increased with greater P availability in the food (Fig. 4; Table 2). This indicates that relatively short periods (3 h on average) of feeding are sufficient to alter the body P content of newborn Daphnia. This effect was strongest under P-limited conditions, because food P : C ratio differed most strongly from the P : C ratio of the neonates (compared with almost equal P content of neonates and food under P-saturated conditions).
| Starved neonates | Neonates in food | |
|---|---|---|
| r² | <0.01 | 0.42 |
| n | 9 | 12 |
| Slope ± SE | 0.004 ± 0.115 | 0.261 ± 0.098 |
| 95% CI | (−0.269–0.277) | (0.043–0.478) |
| t-value | 0.1 | 2.7 |
| P-value | 0.98 | 0.024 |
| Intercept ± SE | 8.56 ± 0.48 | 6.93 ± 0.40 |
| 95% CI | (7.43–9.69) | (6.03–7.83) |
| t-value | 18.0 | 17.2 |
| P-value | <0.001 | <0.001 |
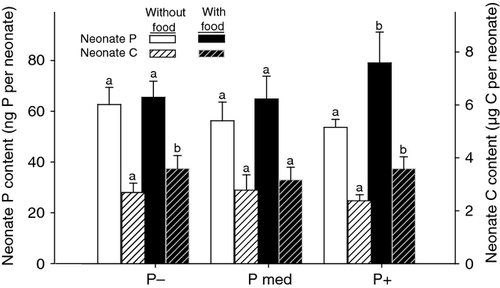
Changes in neonate P : C ratio after feeding across the dietary P gradient were derived from changes in both the P and C contents per neonate (Fig. 5). First, providing food affected neonate P content, but only when food was P-rich (increase by 31–42% compared with starved animals, one extreme of 80%). Second, supplying food increased the C content per neonate, both in animals fed P-rich (increase between 29 and 60% compared with starved neonates) and P-deficient food (increase between 8 and 49% compared with starved neonates), but not in animals grown under medium P conditions.
Short-term (3-h) ingestion
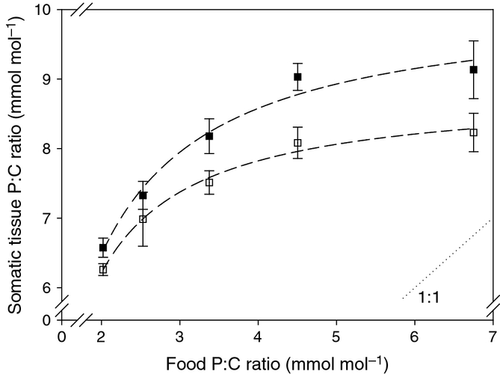
We measured lower P : C ratios in adult animals which had been fed P-deficient food for 3 h prior to collection and compared these to animals that had been collected directly from the different P treatments of 9 days pre-conditioning (Fig. 6). The positive relationship between body P content of D. magna with increasing dietary P supply was still obvious but had diminished, indicating that a 3-h period of ingestion could influence the P content of the body. We found further that when more dietary P was available, there was a greater difference between the P : C ratios of animals which had ingested P-deficient food for 3 h and those collected directly from the treatment food they had been pre-conditioned on (i.e. without further opportunity to ingest food) (two-way ancova with log-transformed food P : C ratios, pre-conditioning P supply: F1,36 = 210.8, P < 0.001; animals directly collected vs. animals collected after additionally feeding on P-deficient food: F1,36 = 31.8, P < 0.001; interaction: F1,36 = 4.8, P = 0.035).
For each P treatment, we used the difference in P : C ratios between animals collected from the treatment food they had been pre-conditioned on and those which had ingested P-deficient food for 3 h prior to collection to calculate the amount of carbon ingested within 3 h. The calculated amount of C ingested was not different from the measured amount ingested within 3 h (see Table S3, two-way ancova, P supply: F1,35 = 0.6, P = 0.45; calculated vs. measured C: F1,35 = 1.7, P = 0.20; P supply × calculated vs. measured C: F1,35 = 2.6, P = 0.11). Furthermore, we found that the proportion of the amount of C ingested to the overall carbon content of the whole animal was 15.4 ± 3.1% and did not vary with changing P supply (see Fig. S1).
Discussion
We found that the effects of phosphorus (P) deficient food on mothers were not transferred to their offspring by way of reduced P allocation to eggs. Eggs and fresh neonates, for the most part, did not vary in P content in relation to the supply of P in the maternal diet. On the other hand, we found that the P : C ratio of Daphnia eggs increased during embryonic development because the carbon content decreased within this time. Interestingly, P : C ratio of the newborn offspring varied strongly with varying P supply of their own food, indicating that ingestion, and probably also assimilation, by the neonates is probably the primary cause of the variable neonate body P content observed here.
There was evidence supporting the first component of our hypothesis that maternal feeding for 3 days on a P-poor diet changed mothers' own somatic tissue, but not the P content of their eggs. In our experiments examining the tissue P content of mothers, P content of the diet affected maternal P : C ratio strongly, which is consistent with other studies on cladocerans (DeMott et al., 1998; Acharya, Kyle & Elser, 2004; DeMott & Pape, 2005). We suggest that below a certain dietary P : C threshold, close to that previously found (P : C ratio of 3.7 mmol mol−1, Lukas et al., 2011), maternal tissue P : C ratio decreases with a declining supply of P in the diet. Moreover, this threshold P : C ratio is in the range of threshold elemental ratios for growth reported previously (Sterner & Hessen, 1994; Anderson & Hessen, 2005).
In contrast to the variable maternal P : C ratio, the P : C ratio of eggs in the first stage showed almost no response to dietary P and thus is consistent with reports of a constant P content of eggs (in stage one) with decreasing P supply (Faerøvig & Hessen, 2003; Becker & Boersma, 2005). This largely corroborates the first part of our hypothesis that there is no effect of different P supply in the maternal diet on egg P content. These results are for mothers experiencing acute P starvation (i.e. with a P-deficient diet in the 3 d before egg deposition), which differs from studies of mothers grown under chronic P starvation (e.g. DeMott et al., 1998; Frost et al., 2010), where dietary P was limited from birth. Hence, whether the P content of deposited eggs varies with maternal diet may depend on the duration of P-stress experienced by the mother. Moreover, rapid changes in P depletion in dynamically mixing water columns (e.g. within 48 h in Einarsson et al., 2004) may determine the duration of nutritional stress on zooplankton across short timescales. Hence, speed of appearance, duration and speed of disappearance of P limitation appear to interact with its severity and should be studied further.
We found the P content of embryos to remain stable during development through the five egg stages. This corroborates the second component of our hypothesis that there would be no differences in egg P content during egg maturation. However, the P : C ratio of newly deposited eggs (5.9 ± 0.4 mmol mol−1, mean ± SD, n = 12) was quite low compared with neonates (8.6 ± 0.7 mmol mol−1 mean ± SD, n = 9) (present study, see also Faerøvig & Hessen, 2003). This pattern of an increasing P : C ratio during egg development is likely to have resulted from the respiratory loss of carbon within the embryo (Urabe & Watanabe, 1990; Glazier, 1991; Boersma, 1995). Assuming a respiratory quotient of 1.16 (Lampert & Bohrer, 1984; Bohrer & Lampert, 1988), we converted our measured carbon loss during egg development until birth into a respiration rate, following Urabe & Watanabe (1990). This estimate of respiration was 6.4 μL O2 mg C−1 h−1 during this time period. This is slightly less than the respiration rate of D. galeata embryos found by Urabe & Watanabe (1990) and a little above that found for D. magna embryos by Glazier (1991) and for D. galeata by Boersma (1995). A higher estimate of respiration may be due to the inclusion of non-respiratory losses, such as the shedding of egg membranes (Glazier, 1991), and possibly an increased carbon loss during starvation of neonates. As eggs receive no further resources from the mother after deposition, our results indicate that P, but not C, is retained efficiently in the developing embryo.
Once neonates start to ingest food, ambient food quality should reassert itself as a determinant of neonate body stoichiometry. If so, the longer the newly hatched individuals are exposed to the environmental food source, the more the body P content of the neonates at the time of sampling should diverge from their original body P content. This difference would originate from the uptake of food of contrasting P content into the small-sized body of a Daphnia neonate. Neonates released when food is high in P might increase their P : C ratio rapidly, whereas the P : C ratio of neonates taking P-deficient food might decrease. We assume that young Daphnia can quickly assimilate P from the food ingested because: (i) Daphnia has a high P assimilation efficiency under P limitation (e.g. DeMott et al., 1998) and (ii) the amount of unassimilated food in the gut is a negligible fraction of that ingested over 3 h (Lampert, 1977a,b). Our study showed that as long as neonates do not experience the maternal food with a different P content, eggs and neonates of D. magna have the same P content per capita, independently of their egg development stage and the maternal dietary P supply. In contrast, the P : C ratio of neonates exposed to the maternal food supply declined with reduced P availability in that food. This resulted because of an increased body C content but no change in body P content. However, since both P and C increased in equal proportion when neonates were fed P-rich food, we found no difference between the P : C ratio of starved neonates and those which ingested food. Consequently, our results indicate that differentially nourished mothers can provide eggs with the same amount of P, and the differences observed recently in the P content of newborn offspring of Daphnia (DeMott et al., 1998; Boersma & Kreutzer, 2002; Frost et al., 2010) may partly be caused by the ingestion of the maternal food differing in P content rather than solely by maternal (provisioning) effects. In these latter studies, neonates were allowed to ingest the maternal food for a period of between 3 and 24 h.
Both the neonate nutrition experiment (Figs 4 & 5) and the short-term (3 h) ingestion experiment with adults (Fig. 6) suggest that food ingested within a short period after birth contributes to the total P and C contents of neonates. While Daphnia P : C ratio was strongly affected by P-limited food in the neonate nutrition experiment, we found the strongest effect on Daphnia P : C for P-saturated food in the short-term ingestion experiment. This result is likely to reflect differences in methods between the two experiments. In the neonate nutrition experiment, neonates ingested food (with different P supply) for the first time in their lives. Because neonates actually were P saturated (see P content of eggs), their P content was affected more strongly by ingesting P-deficient food rather than P-rich food (which was similar to their own body P content). In contrast, in our short-term ingestion experiment, adult Daphnia were pre-conditioned on different dietary P availabilities and then were transferred to P-deficient food for 3 h. Here, the P content of new food matched the P content of Daphnia fed previously on P-limited food. Hence, we found no effect for the P-limited cultured animals. Rather, the effect was high when animals had been fed on P-saturated food during pre-conditioning (which was largely different to the new P-limited food). Consequently, both experiments clearly show the strong effect of short periods of ingestion on the elemental composition of small animals such as Daphnia.
The differences between the P content of adult animals that had fed on P-deficient food for 3 h and those which were collected directly from their treatment food (see short-term ingestion experiment) were used to estimate the carbon ingested as a proportion of the overall carbon body content. This yielded an estimate of about 15% of total C of an adult Daphnia that was ingested within 3 h. We cannot state whether all of the carbon ingested was also assimilated, but the assimilation efficiency of S. elongatus is quite high (Arnold, 1971; Lampert, 1977a,b). Assuming this high assimilation efficiency, and abundant food, a Daphnia would typically ingest about 100% of its body mass per day (e.g. DeMott et al., 1998; Darchambeau, Faerøvig & Hessen, 2003). Hence, 3 h of feeding should equal ingestion of about 15% of body mass of food, which agrees with our results.
We used a two-member mixing model, following Frost, Hillebrand & Kahlert (2005), to see whether we can transfer our result derived from adult D. magna to neonates (see Appendix S2 in Supporting Information). We conclude that at least 20%, and up to 30%, of the mass of a neonate could be derived from ingestion during the first 3 h. This was corroborated by the results of our neonate nutrition experiment in which the carbon content of a neonate increased by a minimum of 8% to a maximum of 60% (32 ± 19%, mean ± SD, N = 12), when the neonates fed for a mean time of 3 h. From our estimation of ingestion rates (30% and 15% of body mass within 3 h for neonates and adults, respectively), we expect that the early nutrition of neonates might have an even greater influence on body P content.
Our study provides good evidence that Daphnia does not change P allocation to individual eggs, even as a maternal P content is depleted within a 3-d period. This time period is shorter than used in other studies, for example, in DeMott et al. (1998) and Frost et al. (2010), which makes direct comparison difficult. Nevertheless, our results reveal that very short periods of ingestion (3 h after birth) affect the offspring P content, which was non-variable during development before birth. While DeMott et al. (1998) concluded that a female diet strongly influenced the specific P content of the offspring and Frost et al. (2010) assumed maternal effects to be the primary reason for lower P content in the offspring of P-stressed Daphnia, we suggest that early neonate nutrition could contribute substantially to the overall effect of maternal food P content on neonates. However, the extent to which feeding after birth or direct maternal effects themselves lead to the subsequent effects on growth and reproduction shown by Frost et al. (2010) remains unclear and should be examined by future work.
In conclusion, our results show the importance of two issues. First, any measurements of substances in the body (here P, but also possibly other components of the food, such as other minerals, fatty acids and sterols) might be altered by the ingestion of food which differs from the experimental diet. This effect could be especially significant for relatively small animals and hence should need to be accounted for in future studies. However, the ratio of carbon to the compound measured would determine the strength of overestimation (i.e. a low dietary P : C ratio would lead to lower biased measurements compared with food with higher P : C ratios). Second, acute periods of P limitation are usually not sufficient to reduce the P content of deposited eggs of Daphnia and may not produce the maternal effects previously reported.
Thus, it appears that the early nutritional environment (immediately after birth) of neonates can strongly influence their elemental composition (and perhaps their growth rate) and we conclude that apparent differences in P content of Daphnia offspring may have resulted, at least to some extent, from variable neonate nutrition. This result indicates that the location of neonate release by mothers within the water column could have very strong effects on neonate performance (i.e. if food P : C ratios vary spatially). Such spatial heterogeneity of phytoplankton in lakes occurs within short periods of time, forced by internal and environmental driving factors (Serra et al., 2007; Caron et al., 2008; Alexander & Imberger, 2013). Assuming that spatial heterogeneity in phytoplankton abundance and species composition could be correlated with the spatial distribution of nutrients (Dickman, Stewart & Servantvildary, 1993), different P : C ratios in Daphnia food are very likely. Future work should examine fine-scale patterns in food P : C ratios in lakes and whether Daphnia mothers can vary the location of neonate release. Moreover, our results indicate that Daphnia mothers do not reduce quality of the eggs after a 3-d period of poor nutrition. Acute P starvation can thus be mediated by female Daphnia by: (i) reducing the number of offspring in response to nutritional constraints and/or (ii) depleting their own body P to the benefit of their offspring. These strategies may be ecologically worthwhile by postponing negative effects of low P supply onto the next generation when P supply remains poor. Instead, when P supply recovers quickly, the strategies are even more promising to sufficiently compensate the gap in P supply.
Acknowledgments
We thank S. Heim and E. Sperfeld for technical assistance and advice. We also thank A. Hildrew and two anonymous reviewers for valuable comments on this manuscript. This study was supported by the German Research Foundation (DFG, WA 2445/4-1).




