
Effects of ice cover on the diel behaviour and ventilation rate of juvenile brown trout
Summary
- Winter ice conditions in boreal streams are highly variable, and behavioural responses by fish to river ice may affect overwinter survival rates. One type of ice, surface ice, stabilises water temperatures, reduces instream light levels and may provide overhead cover.
- Because surface ice is believed to afford protection against endothermic predators, we predicted that metabolic costs associated with vigilance would be lower under surface ice than in areas lacking surface ice. This potentially favourable effect of ice cover was tested by observing ventilation rates of juvenile brown trout (Salmo trutta) in a laboratory stream at dawn, during the day and at night in the presence and absence of real, light-permeable surface ice. Further, we offered trout drifting prey during daylight to test whether ice cover increased daytime foraging activity.
- Ice cover reduced ventilation rates during the day, but not at night or dawn. Moreover, fish made more daytime foraging attempts in the presence of ice cover than in its absence.
- We suggest that the most plausible explanation for these results is that fish experience a reduced perceived predation risk under surface ice.
Introduction
During winter, the ice regime of a stream may vary considerably, altering the availability of habitats and influencing fish behaviour (Prowse, 2001a,b; Huusko et al., 2007). Ice plays a dichotomous role, as different forms of river ice may have opposite effects (Brown, Hubert & Daly, 2011). Frazil and anchor ice form by primary or secondary nucleation in super-cooled water and may exclude fish from their winter refuges (Prowse, 2001b). Surface ice, on the other hand, prevents thermal heat loss to the air, which stabilises water temperature and hinders the development of frazil and anchor ice (Prowse, 2001a; Hicks, 2009). Therefore, stream fishes under surface ice have been suggested to have low metabolic costs associated with acclimatisation (Huusko et al., 2007; Linnansaari & Cunjak, 2010). Furthermore, surface ice reduces instream light intensities, in particular when the ice is covered with snow. Reduced light levels influence both activity patterns (Valdimarsson et al., 1997; Valdimarsson & Metcalfe, 2001) and metabolism (Huusko et al., 2007). For example, fish under simulated ice cover, with zero light penetration, decrease their metabolic rate, presumably because the cost of antipredator behaviour is reduced (Finstad et al., 2004; Helland et al., 2011). Moreover, surface ice may create new overhead cover (Meyers, Thuemler & Kornely, 1992; Young, 1995; Prowse, 2001b), which potentially increases the amount of suitable winter habitat available for lotic fish. The effects of overhead ice cover have been suggested to explain behavioural changes in juvenile stream salmonids, which have been shown to respond to stationary surface ice with increased site fidelity (Linnansaari et al., 2009) and daytime activity (Linnansaari, Cunjak & Newbury, 2008), a broad use of substratum sizes (Linnansaari et al., 2008, 2009) and low mortality (Linnansaari & Cunjak, 2010; Hedger et al., 2013).
In studies of the relationship between predator vigilance and metabolism in fish, the rate of opercular beating has often been recorded (Hawkins, Magurran & Armstrong, 2004b; Brown, Gardner & Braithwaite, 2005), as this measure is sensitive to predator cues (Hawkins, Armstrong & Magurran, 2004a; Hawkins, Magurran & Armstrong, 2007). It seems likely that fish view ice cover as a physical overhead barrier, which prevents aerial attacks from mammals and birds, even if the ice cover permits light penetration. If so, ice cover should reduce metabolic costs associated with vigilance and hence decrease opercular beating rates. Moreover, there is often a trade-off between foraging and avoiding predators (Lima & Dill, 1990). Several factors may influence this trade-off including perceived predation risk. A reduced perceived predation risk should increase feeding activity, and, for trout in cold water, this potential increase would be easiest to detect during daylight, the time of day during winter with the greatest predation risk in ice-free streams (Heggenes et al., 1993; Metcalfe, Fraser & Burns, 1999). It is not known whether ice cover affects opercular beating or foraging activity since direct observations are difficult to make, especially in the field. In this study, we address this difficulty using real ice in controlled laboratory stream experiments, where we observed the beating of the opercula, swimming activity, microhabitat selection, aggression and daytime foraging rates of juvenile brown trout (Salmo trutta) in ice-free and iced conditions. We predicted the following: (i) ice cover will decrease the rate of opercular beating and (ii) fish will make more daytime foraging attempts with ice cover than without ice cover.
Methods
Fish
Juvenile brown trout (n = 40; total length 74 ± 1.5 mm; mass 3.0 ± 0.16 g; means ± 1 SE) were collected in November 2011 by electrofishing in River Lillån, Västergötland, Sweden (57°50′23″N, 14°6′34″E), and the fish were transported to the aquarium facility at Karlstad University. Prior to the experiment, fish were held in an artificial indoor stream provided with instream cover and a natural photoperiod. Water in the holding stream tank was c. 4 °C, and trout were given maintenance rations of thawed drifting chironomid larvae during the day.
Stream tank
We created an experimental arena in a 1.2 × 0.54 m section of a 7-m-long stream channel. One side of the channel had a glass window for observing the fish. Fish were shielded from disturbance by plastic curtains outside the stream channel, and these curtains also prevented light from entering through the glass window. Surface ice blocks (70 × 60 × 7 cm) were created by freezing distilled water in plastic trays (Dilling, IKEA AB, Älmhult, Sweden). Two blocks of ice, placed adjacent to each other, were suspended with concrete blocks and reinforcing bar 10 mm above the water surface, thereby covering the surface of the entire experimental arena. The bottom of the stream channel was covered with 5- to 30-mm-diameter gravel. In the experimental arena, we created two microhabitats of approximately equal size: a shallow, fast-flowing upstream section (depth 0.12 m) and a deep, slow-flowing downstream section (depth 0.20 m). Water velocity at 0.6 of the depth was 0.14 and 0.08 m s−1 in the centres of the shallow and deep sections, respectively, and total discharge was 8 L s−1. In the deep section, we created instream overhead cover (16 × 10 cm) using a concrete block, which was supported by four iron legs. The legs were buried into the substratum, so that the concrete block was 5 cm over the bottom. The reason for having two different sections was to create one microhabitat where fish could feel relatively protected (deep with instream cover) and one where fish would be more exposed (shallow without instream cover). Fish were fed by pumping (Universal 1046, Eheim GmbH & Co KG, Deizisau, Germany) water to a funnel in which food was added. This funnel was connected to a hose, which released water and drifting prey at the upstream end of the experimental arena.
Infrared lamps enabled us to film fish in darkness and at low light intensities. Infrared light has been shown to be invisible to many vertebrate eyes, including those of salmonids (Ali, 1961; Rader et al., 2007). One infrared lamp (IR illuminator IP65 No 751649, Conrad Electronic GmbH & Co KG, Wels, Austria) was placed inside a submerged plastic box and two large infrared lamps (IR illuminator No 40748, Kjell & Co Elektronik AB, Malmö, Sweden) were placed outside the stream, pointing inwards through the glass window. Filming was performed using a digital video camera recorder (DCR-TRV16E, Sony Co, Tokyo, Japan) with the NightShot infrared mode. Over the stream channel, fluorescent lights (Master TL5HE 145 35W/830, Philips NV, Amsterdam, Netherlands) with adjustable light intensities made it possible to simulate dawn and day. The photoperiod was set to 12 : 12 L:D, with 90 min of low light intensity in the morning. Light regimes in the holding tank and the ice-free experimental arena were identical. Light intensities, measured with a lux meter (No 619999005, Windaus Labortechnik GmbH & Co KG, Clausthal-Zellerfeld, Germany) at the surface in the middle of the arena, were 17.7 ± 0.2 lx during 90 min of ‘dawn’ and 694 ± 22 lx at maximum light intensity (simulating day) (n = 20; means from each light level, pooled data from iced and ice-free conditions ± 1 SE). When the lights were not switched on (simulating night), light intensity was too low to be accurately measured (< 0.1 lx). To minimise disturbance to the fish, light intensities were not measured in the water during the experiment. Instead, measurements were made just outside the stream channel's glass window. After the experiment was finished, we created a conversion equation by simultaneously measuring instream light intensities at the bottom of the stream channel between the shallow and the deep water sections (depth 0.16 m) and just outside the glass window. Using this equation, calculated instream light intensities with and without surface ice, respectively, were 7.6 ± 0.4 lx and 14.2 ± 0.7 lx at dawn and 297 ± 14 lx and 581 ± 20 lx during the day (n = 10; means ± 1 SE). Thus, ice cover reduced light levels by about 50%. Water temperature was held between 3.8 and 3.9 °C throughout the experiment using a built-in chilling system and four external water chillers (two RA680 and two RA200, Teco Srl, Ravenna, Italy). Thawing surface ice did not affect temperature by more than 0.1 °C.
Experimental design and execution
The experiment was performed in March and April 2012. We used two treatments in the experiment: presence and absence of ice cover. Observations were made three times during the course of a day: (i) in darkness, before the light had been switched on, (ii) c. 45 min after the lights had been switched on with low light intensity and (iii) c. 45 min after the lights had been adjusted to maximum intensity. Hereafter, these three times of day are referred to as night, dawn and day. The day before a trial, two fish were carefully transferred from the holding stream to the experimental arena in which fish were not fed until the daytime foraging trials. Thus, the fish were starved for c. 24 hours before a trial. We used the same pair during the course of one day of observations (i.e. one night, one dawn and one day observation), and fish were only used once. In total, 20 fish pairs (length difference between fish in pairs 12 ± 1.9 mm; mean ± 1 SE) were used on 10 replicate days with and 10 days without surface ice, tested in randomised order.
For trials with ice cover, ice was placed over the stream more than 45 min before the first observation. On days without ice cover, we disturbed the fish in a similar way by putting a piece of polystyrene sheet over the stream and then removing it. A trial (i.e. observations at night, dawn or during the day) consisted of observing and filming (i) the beating of the opercula of the fish, (ii) whether the fish was swimming or resting on the substratum, (iii) the use of the two sections, including switches and (iv) agonistic behaviour. The beating rate of the opercula, henceforth referred to as ventilation rate, has been found to be a good proxy for oxygen consumption and therefore metabolic rate (Millidine, Metcalfe & Armstrong, 2008). Ventilation rates were recorded only when fish were resting, and we counted the number of beats per 30 s three times during each trial for each fish. Most often these three measures of ventilation rate were practically identical, but sometimes one value would be high in comparison with the other two. As behaviour of fish, prior to measuring ventilation rate, could have resulted in elevated ventilation rates, the median values of the three recordings of ventilation rate were used in the analysis. After having recorded ventilation rates, we observed the fish once every min during 10 min to quantify swimming activity (swimming or not swimming) and section use (deep or shallow), resulting in a combined score of 0 to 10 for swimming activity and section use, respectively. Additionally, the number of agonistic actions, such as nips, chases and aggressive displays, was counted during these 10 min.
After the last observation of the day (i.e. the observation at full light intensity) had been completed, we introduced three thawed chironomid larvae (c. 10 mm length) into the stream channel every min during 10 min. We never observed fish to catch more than one prey at a time. Therefore, the total number of foraging attempts made by each fish could range from 0 to 10. We defined a foraging attempt as an observed manoeuvre in which the fish left its station and chased after a prey. We did not consider whether the prey was in fact captured or not, as it was sometimes difficult to actually see whether an attacked prey was captured or missed.
Data analysis
We recorded measurements from each individual fish, but to avoid pseudoreplication, the mean of the ventilation rates and sums of the scores from each pair were used as response variables in the statistical analyses. We used repeated-measures anova to test for effects of ice cover and time of day (i.e. night, dawn or day) on ventilation rate. Ice cover was treated as a between-subject variable and time of day as a within-subject variable. Data from the observations of swimming activity, use of the deep section, the number of section switches, aggression and the number of daytime foraging attempts were not normally distributed. For the number of daytime foraging attempts, the effect of ice cover was analysed with a Mann–Whitney U-test. To test the effects of ice cover and time of day on swimming activity, use of the deep section, the number of section switches and aggression, we used generalised estimating equations (GEEs) with Poisson distributions applied with their respective log link functions. GEE extends generalised linear models to analyses of clustered or correlated observations, and GEE is therefore particularly suitable for longitudinal data analyses (Liang & Zeger, 1986). All statistical analyses were performed with PAWS Statistics 18 (IBM, Armonk, NY, U.S.A).
Results
Ventilation rate
The ventilation rates of individual fish ranged from 24 to 64 beats min−1 and were lower at night than at dawn and during the day (Bonferroni adjusted pairwise post hoc comparisons, P < 0.001) (Fig. 1). Ice cover did not affect ventilation rates equally over the course of a day, which was revealed by a significant ice cover × time of day interaction effect (Table 1). Ice cover reduced ventilation rates during the day, but not at night or at dawn (simple main effects of ice cover at night, dawn and during the day, P = 0.61, 0.17 and 0.018, respectively). Interestingly, mean ventilation rate under ice during the day, when instream light intensity averaged 297 lx, was 39 beats min−1, whereas that of fish under ice-free conditions at dawn, when light levels were considerably lower (14 lx), was 43 beats min−1.
| Variable | Source of variation | d.f. | F | P |
|---|---|---|---|---|
| Ventilation rate | Ice cover | 1, 17 | 4.52 | 0.076 |
| Time of day | 2, 34 | 8.69 | < 0.001 | |
| Ice cover × time of day interaction | 2, 34 | 3.99 | 0.025 |
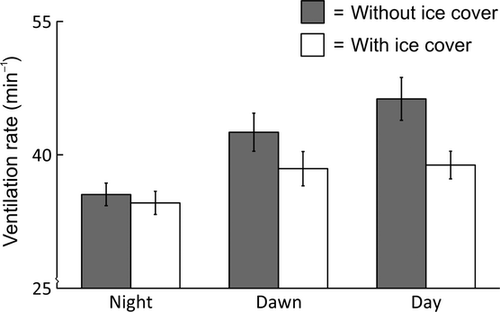
Foraging behaviour
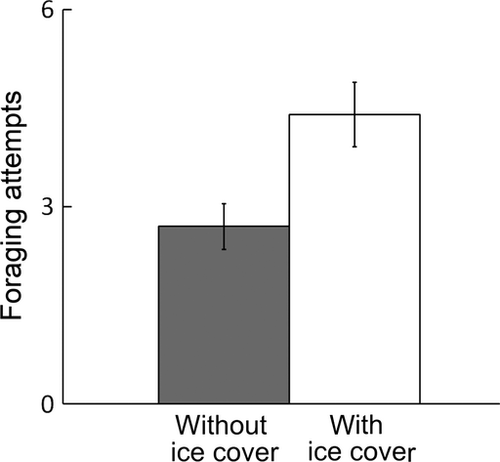
Fish typically used a sit-and-wait foraging tactic, where they mostly used a position in the deep downstream section as a focal point for their prey capture manoeuvres. In 17 of the 20 pairs, both fish foraged actively, and in the remaining three pairs, only one fish foraged. Of 10 encounters with prey, fish made from zero to eight foraging attempts (3.55 ± 0.34; mean ± 1 SE). There was an effect of ice cover on the number of foraging attempts (Mann–Whitney U-test, P = 0.009), so that fish made more foraging attempts with ice cover than without (Fig. 2).
Activity, section use and aggression
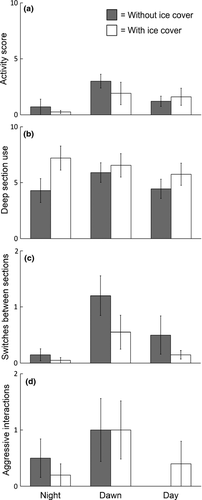
Fish tended to be more active at dawn than at night and during the day, but there was no effect of ice cover (Fig. 3a and Table 2). Section use did not vary with time of day. However, ice cover increased the use of the deep section (Fig. 3b and Table 2). Fish mostly switched sections at dawn, and the presence of ice cover reduced switching behaviour (Fig. 3c and Table 2). We observed very little direct aggression between fish, and only seven of the 20 pairs showed any agonistic behaviour at all. Most aggressive interactions were observed during dawn, but there was no influence of ice cover on aggression (Fig. 3d and Table 2).
| Variable | Source of variation | d.f. | χ2 | P |
|---|---|---|---|---|
| Swimming activity | Ice cover | 1 | 0.63 | 0.43 |
| Time of day | 2 | 20.46 | < 0.001 | |
| Ice cover × time of day interaction | 2 | 1.77 | 0.41 | |
| Deep section use | Ice cover | 1 | 7.94 | 0.005 |
| Time of day | 2 | 2.91 | 0.23 | |
| Ice cover × time of day interaction | 2 | 4.70 | 0.096 | |
| Section switches | Ice cover | 1 | 4.00 | 0.046 |
| Time of day | 2 | 23.22 | < 0.001 | |
| Ice cover × time of day interaction | 2 | 0.76 | 0.69 | |
| Aggressive interactions | Ice cover | 1 | 0.35 | 0.55 |
| Time of day | 2 | 7.71 | 0.021 | |
| Ice cover × time of day interaction | 2 | 0.66 | 0.42 |
Discussion
This study shows that the presence of surface ice reduces ventilation rates in juvenile brown trout, but this effect depends on the time of day, as the reduction in ventilation rates was only observed during the day. Ice cover also increases daytime foraging activity. Taken together, these findings suggest that ice cover may benefit fish by both reducing stress and by allowing increased energy intake during winter. The effects of winter processes on stream salmonid population ecology are not well documented (Jonsson & Jonsson, 2009) despite the fact that winter events may play an important role. For example, in their review of the winter ecology of stream salmonids, Huusko et al. (2007) reported overwinter survival rates of 2 to 91% for juvenile brown trout; this large variation in survival rates must have clear population-level implications. Our results provide one behavioural explanation for how the extent or duration of surface ice may affect stream fish populations. Below, we relate our results on ventilation rate and daytime foraging activity to previous research on winter behaviour, and we discuss how these results may help to explain some of the previously described patterns for salmonids during winter.
Ice cover may affect ventilation rates via effects on the light climate or by acting as overhead cover. When it concerns the light climate, it is known that stream salmonids avoid high light intensities in cold water (Valdimarsson et al., 1997; Valdimarsson & Metcalfe, 1998) and it is possible that low light levels reduce stress during winter. In our study, the lowest ventilation rates were indeed found at night, which probably reflects temporal differences in perceived predation risk. When light levels are low, ice cover should have little or no effect on ventilation rates because the fish's predators are mainly visual hunters, and darkness in itself may provide sufficient protection. Even if light may affect ventilation rates, our results suggest that decreased instream light levels under ice cover cannot alone explain the reduction in ventilation rates caused by ice. If this was the case, then one would have expected ventilation rates of fish under ice cover during the day, when instream light intensity was relatively high, to have been greater than the ventilation rates of fish in the absence of ice at dawn, when light intensity was low. Instead, we found that ventilation rates were slightly higher at the lower light level. Simulated ice cover has previously been shown to reduce resting metabolic rates for Atlantic salmon (Salmo salar) (Finstad et al., 2004), Arctic char (Salvelinus alpinus) and brown trout (Helland et al., 2011) in laboratory studies. These studies, however, simulated ice cover with total darkness, using an opaque cover, hence making it difficult to evaluate whether ice cover influences metabolic rates via effects on the light climate or as a form of overhead cover. Even in our study, we cannot differentiate between these two possibilities, although we found similar effects to those of Finstad et al. (2004) and Helland et al. (2011) when we used real ice that transmitted light. Furthermore, our study demonstrates that the effects of ice cover on ventilation rates are dependent on the time of day.
The results support our hypothesis that ice cover increases the foraging activity of the fish during the day. Several studies have shown that feeding motivation is largely state dependent (Orpwood, Griffiths & Armstrong, 2006; Finstad et al., 2010) and that daytime foraging is normally avoided if energy requirements are met through nocturnal foraging (Orpwood et al., 2006). However, the efficiency of nocturnal drift foraging during winter is generally low due to a reduced ability to detect prey in darkness (Fraser & Metcalfe, 1997) and difficulties associated with capturing prey in cold water (Watz & Piccolo, 2011). Thus, fish trade-off perceived predation risk and the use of storage lipids against foraging benefits during winter (Metcalfe & Thorpe, 1992; Bull, Metcalfe & Mangel, 1996). Our results support the hypothesis that perceived predation risk may be reduced under ice cover (Linnansaari et al., 2008), thereby allowing fish to forage actively during the day when prey are more easily detected. Thus, under ice cover, fish may meet their energetic needs with fewer foraging bouts and spend overall less time on foraging than would be the case if ice cover was lacking. Winter field studies, albeit difficult to perform, could shed light on this possible response to ice cover.
In our study, fish were more actively swimming, somewhat more aggressive, and switched sections more often at dawn than during the day, which is consistent with the crepuscular peaks in activity described in other studies (e.g. Greenberg & Giller, 2001; Ovidio et al., 2002). During the day, however, fish had the highest ventilation rates despite a relatively low overall activity. Therefore, it seems likely that high ventilation rates during the day in our study were not related to increased activity. Instead, the high ventilation rates may have been related to predator vigilance, as juvenile stream-dwelling fishes are vulnerable to attacks from birds and mammals, particularly in cold water (Huusko et al., 2007). Thus, the reduced metabolic rates of fish under ice cover indicate that the fish might view surface ice as overhead cover, even if the ice is light permeable, resulting in a reduced need for predator vigilance. Other studies have related metabolic costs to the presence or absence of cover, and, for example, Millidine, Armstrong & Metcalfe (2006) found that metabolic rates of juvenile Atlantic salmon in a respirometer chamber were 30% lower when the otherwise bare respirometer was provided with partially transparent ledge cover. In our study, using a semi-natural setting, ice cover reduced average ventilation rates by 15% during the day. Clearly, the need for overhead cover varies over the course of the day and the role of surface ice as overhead cover may be particularly important when suitable instream cover is rare (Linnansaari et al., 2009). The presence of ice cover increased the use of the deep section and reduced the number of switches between the shallow and deep section in our study. Water depth per se may be of minor importance for protection (as discussed in Heggenes et al., 1993) and, consequently, habitat selection. Both the rippled surface water in the shallow section and the instream cover in the deep section should have provided cover. Without ice cover, these two types of cover may have played a role, but in the presence of ice cover, they should have decreased in importance. Instead, by increasing foraging opportunities through a reduced need for vigilance, ice cover may have made the deep section more attractive for the fish, because slow water velocities are more suitable for drift foraging in cold water than fast velocities.
Acknowledgments
We are grateful to Johnny Norrgård for his assistance in collecting the fish. We would like to thank two anonymous referees for helpful comments and Professor Colin Townsend for editing the manuscript. Funding was provided by the Department of Environmental and Life Sciences, Karlstad University. The study was approved by the Animal Ethical Board of Sweden (reference 328-2011).




