
Population-level thermal performance of a cold-water ectotherm is linked to ontogeny and local environmental heterogeneity
Summary
- Negative effects of global warming are predicted to be most severe for species that occupy a narrow range of temperatures, have limited dispersal abilities or have long generation times. These are characteristics typical of many species that occupy small, cold streams.
- Habitat use, vulnerabilities and mechanisms for coping with local conditions can differ among populations and ontogenetically within populations, potentially affecting species-level responses to climate change. However, we still have little knowledge of mean thermal performance for many vertebrates, let alone variation in performance among populations. Assessment of these sources of variation in thermal performance is critical for projecting the effects of climate change on species and for identifying management strategies to ameliorate its effects.
- To gauge how populations of the Rocky Mountain tailed frog (Ascaphus montanus) might respond to long-term effects of climate change, we measured the ability of tadpoles from six populations in Glacier National Park (Montana, U.S.A.) to acclimate to a range of temperatures. We compared survival among populations according to tadpole age (1 year or 2 years) and according to the mean and variance of late-summer temperatures in natal streams.
- The ability of tadpoles to acclimate to warm temperatures increased with age and with variance in late-summer temperature of natal streams. Moreover, performance differed among populations from the same catchment.
- Our experiments with a cold-water species show that population-level performance varies across small geographic scales and is linked to local environmental heterogeneity. This variation could influence the rate and mode of species-level responses to climate change, both by facilitating local persistence in the face of changes in thermal conditions and by providing thermally tolerant colonists to neighbouring populations.
Introduction
Increases in temperature driven by climate change are likely to have especially large negative effects on species that are specialised to occupy narrow thermal ranges, acclimate poorly or possess limited dispersal abilities (Janzen, 1967; Deutsch et al., 2008). However, habitat use, vulnerabilities and mechanisms for coping with local conditions can differ among populations and by age and developmental stage within populations, potentially affecting species-level responses to climate change (Kingsolver et al., 2011; Radchuk, Turlure & Schtickzelle, 2013). For many species, there is still limited knowledge of thermal performance, including these basic sources of intraspecific variation. Yet understanding this variation is critical to projecting how climate change will affect populations, species’ distributions and ecosystem function. Understanding this variation is also a key step in identifying management strategies to prevent population losses.
Freshwater ectotherms limited to cold environments are especially vulnerable to climate warming because compared with species that evolved in environments with greater thermal variation, they have narrow thermal breadths and smaller thermal safety margins. In the northern Rocky Mountains (U.S.A.), increased stream temperatures have already resulted in loss of thermally suitable habitat for some salmonid fish (Wenger et al., 2011; Isaak et al., 2012). These changes could have profound effects on species other than fish, including endemic families of amphibians adapted to small, cold streams. These species are frequently habitat specialists confined to headwater streams (e.g. 1st to 3rd order) and can have lower thermal optima than local fish (de Vlaming & Bury, 1970; Brown, 1975; Bury, 2008). For example, the upper limit of the realised thermal niches of the coastal tailed frog (Ascaphus truei) in northern California and Oregon (U.S.A.) were 13.6 °C and 15.1 °C, respectively, which were lower than those of stream fish in the same areas (Huff, Hubler & Borisenko, 2005; Welsh & Hodgson, 2008). In addition to low temperature tolerances, most stream amphibians have long generation times and are weak dispersers (de Vlaming & Bury, 1970; Corn, Bury & Hyde, 2003), which would limit their evolutionary and demographic resilience to change (Mills, 2007; Chevin, Lande & Mace, 2010).
Although thermal adaptation of aquatic-breeding vertebrates has typically been examined at the level of species, there is growing evidence of local adaptation despite extensive gene flow among populations (Friedenburg & Skelly, 2004; Sørensen et al., 2009; Phillimore et al., 2010; Eliason et al., 2011). Some temperate amphibian species might benefit from warming (Deutsch et al., 2008), but it is unclear whether cold-water stream amphibians have similar capacities to tolerate variable thermal conditions or adapt to warmer temperatures. In the southern Appalachian Mountains, two stream salamanders were not adapted to elevation or temperature clines, which suggested that low-elevation populations were already living near their thermal limit and that future temperature increases were likely to further fragment and isolate populations (Bernardo & Spotila, 2006). Experiments on the thermal performance or preference of A. truei and other stream amphibians in the Pacific Northwest have sourced animals from a single population (de Vlaming & Bury, 1970; Brown, 1975; Bury, 2008), precluding measurement of variation among populations. Without more knowledge of the thermal performance of stream amphibians, and of variation in performance among populations, we cannot begin to assess the threat posed by increased temperatures, or the potential efficacy of conservation measures that are often based around co-occurring species (e.g. economically important fish).
We used laboratory experiments to assess differences in thermal performance among populations of Rocky Mountain tailed frog (Ascaphus montanus) in Glacier National Park, Montana, U.S.A. Glacier National Park is an ideal place to investigate how species will be affected by climate change because the park contains strong climatic gradients that produce diverse habitats in close proximity (Carrara, 1989; Lowe & Hauer, 1999). Also, the region is warming at 2–3 times the global average (Pederson et al., 2010), increasing the relevance and urgency of climate-related research. Specifically, we compared the ability of tadpoles from six streams to acclimate to a range of laboratory temperatures and determined whether variation was linked to local environmental heterogeneity (mean and variance of stream temperature) or tadpole age. We expected resistance to higher temperatures would be associated with environmental heterogeneity (e.g. Lind & Johansson, 2007) and expected younger tadpoles to be more susceptible than older tadpoles to high temperatures (e.g. Dupré & Petranka, 1985).
Methods
Study system and species
Glacier National Park is a 4082-km2 reserve that straddles the Continental Divide along the U.S.A.–Canada border. The park is characterised by steep, U-shaped valleys that reflect extensive Pleistocene glaciation (Carrara, 1989). Elevations range from approximately 950 m to 3190 m. The climate is characterised by long, cold winters with large snow accumulations and warm, dry summers. The local hydrology is typical of snow-dominated systems in the region, with peak flows during snowmelt in May and June, then low flows during late summer and autumn. West of the Continental Divide, a moist Pacific maritime climate prevails. East of the Divide, continental-polar air masses result in a colder, drier and more variable climate (Carrara, 1989). This dramatic topographic and climatic variation produces strong gradients in floral communities over small spatial scales. For example, less than 40 km separates the eastern-most extension of moist, western red cedar (Thuja plicata)–western hemlock (Tsuga heterophylla) forest characteristic of the Pacific coast from the dry grasslands of the Great Plains along the eastern boundary of the park.
Along with frogs of the family Leiopelmatidae in New Zealand, tailed frogs (Ascaphus spp.) are the most ancient extant anurans (Frost et al., 2006). Rocky Mountain tailed frogs reach peak abundances in small, cold streams (i.e. ≤ 3rd order) with clean, coarse substrata, where tadpoles are the dominant grazers (Kiffney & Richardson, 2001; Werner et al., 2004). Permanent streams are required for viable populations because tadpoles take about 4 years to reach metamorphosis. This species is among the slowest of anurans to reach maturity (6–8 y after hatching), and females have low fecundity (<100 eggs biennially) (Nussbaum, Brodie & Storm, 1983; Corn et al., 2003). These life-history traits make the species susceptible to decline and probably limit its capacity for recovery and rapid evolution (Allendorf & Luikart, 2007; Mills, 2007). Furthermore, tailed frogs mate in the autumn but do not lay eggs until the following spring, and their eggs are rarely found in the wild (Karraker et al., 2009). These peculiar reproductive characteristics preclude genotype × environment experiments that can separate heritable and environmental influences on performance.
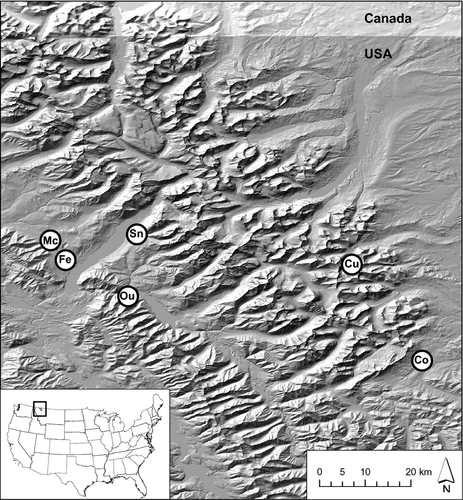
We sampled tadpoles from six 1st- and 2nd-order streams that represented a wide range of terrestrial habitat conditions (Fig. 1, Table 1). McGee, Fern and Ousel creeks are tributaries of the Flathead River on the west side of the Continental Divide. Ousel Creek is on U.S. Forest Service property just outside the national park. These streams flow through dense, mixed-conifer forests composed primarily of Douglas-fir (Pseudotsuga menziesii), lodgepole pine (Pinus contorta) and western larch (Larix occidentalis). The Fern Creek catchment burned in 2003, but tadpoles remain abundant (Hossack, Corn & Fagre, 2006). Snyder Creek is also a tributary to the Flathead River, but it flows through an old-growth western red cedar–western hemlock forest. Snyder Creek was the only stream that originated from a lake. Coonsa and Cutbank are unnamed tributaries to Coonsa and North Fork Cutbank creeks, respectively, in the Missouri River drainage east of the Continental Divide. The Coonsa tributary flows through an open forest of lodgepole pine and quaking aspen (Populus tremuloides) and is the eastern-most stream (c. 60 km from McGee Creek, the western-most stream we sampled). The Cutbank tributary was in a sparse, subalpine forest. In the Cutbank drainage and most other areas on the east side of the park, A. montanus is distributed patchily, whereas populations are more continuous on the west side of the park (B.R. Hossack and P.S. Corn, unpublished data). All streams had mean gradients >5% and had riffle–pool or step–pool morphologies that were dominated by cobble and boulder substrata.
| Stream | No. tadpoles | Tadpole weight (g) | Mean temperature (°C) | Temperature variance | Elevation (m) |
|---|---|---|---|---|---|
| Coonsa tributary | 42 |
0.36 (0.10) 0.99 (0.20) |
7.77 | 4.26 | 1647 |
| Cutbank tributary | 24 |
0.19 (0.04) 0.62 (0.10) |
6.63 | 5.32 | 1696 |
| Fern Creek | 48 |
0.35 (0.13) 1.28 (0.33) |
8.25 | 1.88 | 1226 |
| McGee Creek | 24 |
0.28 (0.10) 1.28 (0.14) |
7.16 | 0.65 | 1183 |
| Ousel Creek | 44 |
0.21 (0.11) 0.84 (0.25) |
7.98 | 1.46 | 1175 |
| Snyder Creek | 46 |
0.34 (0.13) 1.03 (0.34) |
9.76 | 1.99 | 1135 |
Thermal acclimation experiments
We collected an equal number of live tadpoles of each age class from streams during 16–18 August 2010. Tadpole age (1 year or 2 years) was assigned based on discrete size classes and developmental characteristics within each stream (Hossack et al., 2006). Tadpoles were placed in 3.8-L zip-top bags with stream water and kept on ice during transport to the field station, where they were stored in a refrigerator. Our goal was to have 48 tadpoles from each of five streams for the experiment; however, tadpoles were uncommon in the Cutbank drainage, so we collected only 24 in that stream and then added an additional stream on the west side of the park (McGee Creek) to collect the remaining 24 tadpoles for the experiments. A few tadpoles died during transport or in the laboratory before the experiment began, reducing the number of animals available for the experiment.
After collecting tadpoles, we installed HOBO Pendant® (Onset Computer Corporation, Bourne, MA, U.S.A.) temperature loggers (accuracy: ± 0.53 °C; resolution: 0.14 °C) in the lower and upper end of the stream reach that we sampled (≤1 km long). We used the averaged values from the two loggers to characterise the thermal conditions of each stream. Each logger was inside a flow-through polyvinyl chloride pipe enclosure that shielded it from solar radiation. We secured each enclosure to the bottom of the channel in a location that ensured adequate flow and mixing of the water column. Loggers recorded temperature hourly between 19 August and 20 September 2010, a period encompassing the warmest and most variable temperatures of the year and known to have important effects on vital rates of stream organisms in the region (Isaak et al., 2010; Jones et al., 2013). We installed the loggers in the same locations in 2011, but we focus primarily on data from 2010, the year we conducted the experiment.
We transported tadpoles to the U.S. Fish and Wildlife Service Bozeman Fish Technology Center in Bozeman, Montana, on 19 August 2010, where they were kept in quarantine tanks and slowly acclimated to 10 °C. Cobble and gravel were placed on the tank bottoms for cover. We transferred tadpoles to the thermal experiment after 11 days. Water for the experiment was taken from cold and warm springs and was disinfected by ultraviolet light and heated by three 40,000 BTU water heaters. Water was degassed in head columns before mixing. We monitored saturation of dissolved oxygen, total dissolved gases and nitrogen plus argon (N + Ar) with a Common Sensing Model TBO-DL6 meter (Common Sensing, Clark Fork, Idaho). Dissolved gases were within an acceptable range for aquatic species culture, with dissolved oxygen ranging from 84 to 90% saturation, total dissolved gases ranging from 96 to 101% saturation and N + Ar ranging from 96 to 101% saturation.
We used the acclimated chronic exposure (ACE) method to test survival of tadpoles at 10 °C, 14 °C, 18 °C and 22 °C in a fully replicated and randomised experiment. The ACE method uses gradual increases in temperature that allow animals to acclimate to a changing environment, after which they are maintained at a constant temperature (Zale & Gregory, 1989; Selong et al., 2001). We used test temperatures between 10 °C and 22 °C because this range encompassed the width of the estimated realised thermal niches of A. truei in northern California and Oregon (Huff et al., 2005; Welsh & Hodgson, 2008) and because A. montanus tadpoles have occasionally been documented in habitats warmer than 20 °C (Dunham et al., 2007). We also chose this temperature range because it is similar to that of cold-water fish in the northern Rocky Mountains (e.g. Selong et al., 2001), facilitating future comparisons of temperature sensitivities among species that share habitats.
We used 12 flow-through 75-L tanks to apply the four temperature treatments simultaneously, providing three replicates of each temperature. Within the tanks, we had five plastic aquaria (13 × 9 × 10 cm 2-in-1 Fish Hatchery, Aquatic Eco-systems Inc., Apoka, FL, U.S.A.). Slats in each end of each aquarium allowed water exchange and a centre divider created two chambers. The divided aquaria provided 10 chambers in each 75-L tank. In total, this design resulted in 30 chambers available for each of the 4 temperature treatments. Each chamber had cobble to provide cover and hosted one 1-year-old and one 2-year-old tadpole. We used mixed tadpole sizes so that we could track individuals without having to mark them. All tanks remained at 10 °C for the first 7 days, after which temperatures in the 14 °C, 18 °C and 22 °C tanks were increased up to 2 °C per day until they reached their target temperature. The experiment started after all tanks reached their target temperature and lasted for 79 days.
Tadpoles were fed a food formulated at the Bozeman Fish Technology Center. Because the Technology Center is biosecure, we could not bring periphyton-covered rocks from local streams to provide a food source. Instead, we formulated food by combining ground rice hulls (78.9 g), spirulina (10 g), wheat starch (5 g), guar gum (5 g), vitamins (1 g) and trace minerals (0.1 g). We added 8 ml of water to 1 g of dry food to make a slurry that we spread onto 3 × 3 cm square ceramic tiles (1 ml of food slurry per tile) and dried overnight in a convection oven at 60 °C. One tile with food was placed in each tadpole chamber and was replaced daily if all food was consumed. At the same time, any dead tadpoles were recorded and removed from the enclosure. If the tile was not cleared of food, we replaced it every other day. All chambers were examined daily and cleaned of excrement and excess food every Monday, Wednesday and Friday.
Disease testing
Before we conducted the experiment in a biosecure facility that breeds threatened and endangered species, we first had to demonstrate that source populations of wild animals were not likely to be diseased. The year before we conducted experiments, we collected 10 tadpoles from each stream to test for presence of ranavirus frog virus 3 (FV3; family Iridoviridae) via liver cultures conducted on fathead minnow (Pimephales promelas) cell lines following methods described by Green & Dodd (2007). Prior to tissue cultures at the Bozemen Fish Health Center, we tested each tadpole for the presence of amphibian chytrid fungus (Batrachochytrium dendrobatidis) via PCR at Pisces Molecular LLC (Boulder, CO, U.S.A.). Neither FV3 nor B. dendrobatidis was detected from the samples.
Statistical analysis
We compared differences in survival according to experimental temperature, population source (natal stream), tadpole age and mean and variance of stream temperatures recorded by the data loggers. We estimated the probability that an individual was dead at the end of the 79-day experiment with generalised linear mixed-effects models with a binomial link and maximum-likelihood methods (package lmer4 in program R v. 2.12.2; R Development Core Team, 2011). Tank, aquarium and chamber were included as nested random effects to account for the design of our experiment and to provide unbiased estimates of the treatment effects. We excluded data from the 22 °C treatment from the analysis because nearly all tadpoles at that temperature died. Including these data caused problems with fitting some models and was not informative for comparisons across tadpole ages or streams.
We fit eight models to determine which provided the best description of the data. Experimental temperature was modelled as a factor variable and was included in all models because it was fundamental to the experiment. The first model included only experimental temperature (10 °C, 14 °C and 18 °C). The second model included the additive effects of tadpole age (1 year or 2 years) and experimental temperature. This model was used to assess our hypothesis that younger tadpoles would be more susceptible than older tadpoles to high temperatures (Dupré & Petranka, 1985). The third model included the additive effect of population and experimental temperature and described our hypothesis that thermal sensitivity varied among populations. The fourth model included additive effects of experimental temperature, tadpole age and population.
In the remaining 4 models, we replaced the population term in the models above with covariates representing either the mean or variance of in situ stream temperatures. These models allowed us to assess the importance of environmental heterogeneity in the stream of origin for predicting thermal performance. We measured support for competing models based on differences in the Akaike information criterion (ΔAIC) and Akaike weights (wi), which represent the probability that a particular model is the best for the given set of models and data (Burnham & Anderson, 2002).
Results
Stream characteristics
Between 19 August and 20 September 2010, the mean and variance in temperature of the six streams we sampled ranged from 6.63 °C to 9.76 °C and 0.65 °C to 5.32 °C, respectively (Table 1). We expected mean and variance of stream temperatures to be positively correlated in these low-volume streams, but they were weakly, negatively correlated (r = −0.30). We suspect that this relationship was a consequence of the wide range of habitats we sampled. The coldest stream (Cutbank) was the most variable; it also flowed through a sparse subalpine forest that probably had more variable air temperature than forests around other streams. The warmest stream (Snyder Creek) flowed from a lake and through an old-growth red cedar–hemlock forest; both of these factors can moderate temperature fluctuations (Mellina et al., 2002).
Thermal performance
The laboratory experiments revealed significant variation in thermal performance that was linked to tadpole age and environmental heterogeneity in the natal stream. The model that included effects of experimental temperature, tadpole age and variance in field-measured stream temperatures received the most support (Table 2). Based on this model, the probability of death ranged from only 0.015 (SE = 0.017) in the 10 °C treatment to 0.643 (SE = 0.124) in the 18 °C treatment (Fig. 2). Mortality was higher for 1-year-old tadpoles compared with 2-year-old tadpoles (log-odds b = 1.043 [0.437]). Survival of 1-year-old tadpoles was approximately one-half that of 2-year-old tadpoles in the 10 °C and 14 °C treatments (Fig. 2). The disparity in probability of death between age classes was smaller at 18 °C, where 75% of 1-year-old tadpoles died compared with 53% of 2-year-old tadpoles. The log-odds of death for individuals was inversely related to variance in stream temperature (b = −0.413 [0.157]; Fig. 2), indicating that tadpoles from more variable environments were more tolerant of high temperatures.
| Model | k | -2log(L) | ΔAIC | w i |
|---|---|---|---|---|
| Experiment Temp. + Tadpole Age + Stream Temp. (σ2) | 8 | 133.82 | 0.00 | 0.64 |
| Experiment Temp. + Tadpole Age + Population | 12 | 128.52 | 2.70 | 0.17 |
| Experiment Temp. + Stream Temp. (σ2) | 7 | 139.80 | 3.98 | 0.09 |
| Experiment Temp. + Tadpole Age | 7 | 141.44 | 5.62 | 0.04 |
Experiment Temp. + Tadpole Age + Stream Temp. (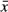 ) ) |
8 | 139.62 | 5.80 | 0.04 |
| Experiment Temp. + Population | 11 | 134.50 | 6.68 | 0.02 |
| Experiment Temp. | 6 | 147.30 | 9.48 | 0.01 |
Experiment Temp. + Stream Temp. (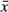 ) ) |
7 | 145.58 | 9.76 | 0.00 |
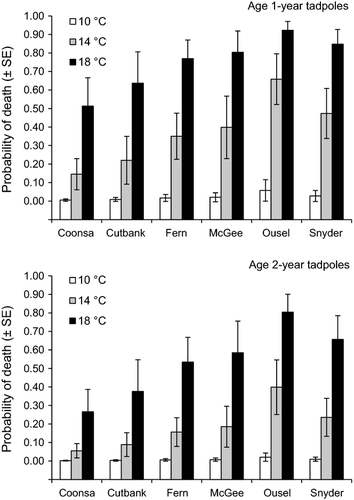
The model that included population rather than temperature variance received the second most support and provided estimated effects of experimental temperature and tadpole age similar to the top-ranked model (Table 2, Fig. 3). Estimates from this model revealed significant differences in survival of tadpoles among populations for the 14 °C and 18 °C treatments. This population-focused model actually described more variation in the response data than the variance-focused model (based on -2log[L]), but it provided a less parsimonious fit because of the four extra parameters in the model. The remaining models all received weak support (wi ≤ 0.09), including those that included mean stream temperature (Table 2).
Discussion
The consequences of climate warming are predicted to be most severe for species that experience a limited range of temperatures (Janzen, 1967; Deutsch et al., 2008). These predictions are typically applied over broad geographic scales (e.g., tropical v. temperate zones; Deutsch et al., 2008; Diamond et al., 2012), but our experiment with a cold-water species shows that population-level performance across a range of temperatures varies at small geographic scales and is related to tadpole ontogeny and to local environmental heterogeneity. Consistent with Janzen's (1967) hypothesis, we found thermal performance of A. montanus tadpoles increased with variance in water temperature in the natal stream, even among streams separated by less than 15 km.
The greater ability of tadpoles from more variable environments to acclimate to warm temperatures matches predictions for species with long generation times, where individuals experience more within-generation than between-generation variation (Angilletta, Niewiarowski & Navas, 2002; Gabriel, 2005; Meráková & Gvozdík, 2009). Variation in thermal regimes can affect performance through several mechanisms, including the modification of gene expression and cellular processes that enhance thermal tolerance (Podrabsky & Somero, 2004; Somero, 2010; Barshis et al., 2013). Ideally, we would have incorporated genotype × environment interactions as well as temperature variation into our experiment. Nevertheless, using the same method that is frequently used to assess thermal performance of fish in our region (e.g. Selong et al., 2001), we found large differences in thermal performance among populations and between tadpole ages. For example, model-estimated survival at 14 °C and 18 °C was three times higher for tadpoles from Coonsa Creek than from Ousel Creek. Ousel Creek had slightly warmer water temperatures; however, variance in temperature of Ousel Creek was only one-third of that in Coonsa Creek. Similarly, survival of tadpoles at these same temperatures was nearly two times higher in Fern Creek than in Ousel Creek. These streams are only about 13 km apart and in the same drainage, but Fern Creek also had more variable water temperatures.
Differences in acclimation indicate that some A. montanus populations have the capacity to persist in warmer water than they currently occupy, as evidenced by high survival (>95%) of tadpoles from all populations at 10 °C. Temperature variation in the streams we sampled in 2010 was strongly correlated with variation measured during the same time period in 2011 (r = 0.78), despite the 50% larger snowpack and later melt in 2011 (http://www.wcc.nrcs.usda.gov/nwcc/site?sitenum=482&state=mt), which suggests that the environmental cue is reliable. Moderate increases in temperature and stream productivity could even accelerate growth or development rates and increase population growth (Bury & Adams, 1999; Govindarajulu, Altwegg & Anholt, 2005). However, these potential benefits to population resilience may be offset by the inverse relationship between developmental stage and thermal performance of Ascaphus spp. and many other amphibians, as well as the apparent lack of variation in age at metamorphosis for A. montanus (de Vlaming & Bury, 1970; Brown, 1975; Dupré & Petranka, 1985).
Predicting the consequences of warming for species with complex life cycles is difficult without knowledge of mechanisms of change and of the sensitivity and response of each life stage to change (Kingsolver et al., 2011; Radchuk et al., 2013). Furthermore, laboratory-based thermal tolerances often exceed the thermal distribution of organisms in the wild, where biotic interactions can exacerbate the direct effects of reduced thermal performance (Welsh & Hodgson, 2008; Wenger et al., 2011; Treanor et al., 2013). In our experiment, high mean survival by all populations at temperatures similar to what tadpoles currently experience in the wild (10 °C) indicates that factors other than thermal performance are important determinants of the distribution and abundance of the species. For example, tadpoles from the Cutbank stream had the second best performance at 14 °C and 18 °C, but the species is less common in that area compared with streams on the west side of the park (Fig. 1). This inconsistency between performance and abundance may indicate that survival of adults in the terrestrial environment is the primary determinant of the species’ distribution.
How population-level differences in thermal performance will mediate species-level responses to warming depends on the specific mechanisms involved and their interactions. If differences in performance are primarily under genetic control, then local adaptations to current thermal conditions could be maladaptive under future climates (Visser, 2008). In this scenario, unless there is strong directional selection on genes affecting thermal performance (Hemmer-Hansen et al., 2007), populations with better high-temperature performance (i.e. those from thermally variable streams) would be more important to protect because they can provide colonists adapted to higher temperatures (Somero, 2010). Variation in thermal performance can instead result from epigenetic effects (e.g. maternal influences) or plasticity shaped by environmental influences early in the development (Mousseau & Fox, 1998; Meráková & Gvozdík, 2009). In the short term, at least, resilience should be higher if environmental influences shape performance because more populations will have the capacity to tolerate warming temperatures, although there is uncertainty whether reaction norms evolved under previous climates can keep pace with change (Visser, 2008; Chevin et al., 2010; Phillimore et al., 2010).
Concern over keeping pace with change is especially relevant in the northern Rocky Mountains, which are warming rapidly and where changes in precipitation have caused significant shifts in timing and amount of stream flow (Pederson et al., 2010). Importantly, however, the pattern of variation in performance we documented for A. montanus is at odds with the expected pattern of stream warming: individuals from more variable environments were better able to acclimate to warm temperatures, but these individuals were from high-elevation streams that are generally less threatened by warming than are low-elevation streams (Isaak et al., 2010). Regardless of whether the variation in thermal performance resulted from adaptive plasticity or genetic adaptation, this pattern suggests that low-elevation populations exposed to high mean and maximum temperatures and low thermal variability are most at risk of extinction under future climate scenarios.
Our study adds to a small set of examples that demonstrate local variation in thermal performance among populations (e.g. Skelly, 2004; Phillimore et al., 2010; Weber et al., 2012). Population-level variation in thermal performance clearly has important implications for forecasting responses to future climate scenarios compared with assuming a uniform, species-level response (Chevin et al., 2010; Eliason et al., 2011). To accurately predict responses at population and species levels, it will be critical to incorporate realistic variation into experiments and to link experimental results to vital rates. It will also be critical to isolate mechanisms of thermal adaptation and, where possible, take local management actions that slow the rate of warming and promote population connectivity and facilitate adaptation in a changing landscape.
Acknowledgments
We thank C. Fraser, J. Ilgen and M. Toner for help in the laboratory; K. Honeycutt, P. Scarr, N. Muhn and C. Doman field assistance; and D. Affleck and C. Hollimon for advice on the analysis. Comments by A. Woods, N. Nelson, C. Muhlfeld, R. Muth, two anonymous reviewers and C. Townsend improved the manuscript. This research was conducted in accordance with the Animal Welfare Act and its amendments and was funded by the USGS Amphibian Research and Monitoring Initiative (ARMI) and a USGS Park-Oriented Biological Support (POBS) grant. Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the U.S. Fish and Wildlife Service. This manuscript is ARMI contribution no. 451.




