
Long-term and contemporary environmental conditions as determinants of the species composition of bog organisms
Summary
- Environmental change alters ecosystem processes, minding biogeochemical cycles. These changes are accompanied by shifts in species composition. We predicted that long-term averages of environmental variables would explain more of the variability in species composition than short-term assessments, but that this difference would be weaker for short-lived organisms. We further predicted that short-lived organisms with rapid dispersal would reflect recent environmental change.
- In 51 plots of ombrotrophic summit bogs in two mountain ranges of Central Europe, we sampled the contemporary species composition of vascular plants, bryophytes, diatoms and testate amoebae. In the same plots, water chemistry (pH, conductivity, Ca, Mg, Na, K, Al, Fe, Pb, Hg, Zn, humic acids, ammonium, nitrates, nitrites, phosphates, sulphates and chlorides) and water level were monitored three times per year for 15 years. We tested species–environmental relationships using Mantel tests and canonical correspondence analyses with Monte Carlo permutation tests for overall, within-bog and between-bog effects.
- The species composition of all four taxa changed along a natural gradient of water level. Diatoms and testate amoebae also changed along a pH/calcium gradient, which has appeared recently in one of the study regions because of aerial liming of forests. Only diatoms reflected variations in humic acid concentration. Spot measurements of environmental factors were sufficient to describe the general pattern while significant effects of phosphates for diatoms and bryophytes, nitrites for vascular plants, potassium for vascular plants and testate amoebae, sodium for all groups except diatoms and sulphates for bryophytes and testate amoebae were evident only by using medium- or long-term averages.
- Although only long-term averages of some environmental variables were sufficient to explain species compositions, major patterns were revealed by single (spot) samples. The explanatory power of long- and short-term environmental conditions did not differ substantially between micro- and macroorganisms, although short-lived and well-dispersed microorganisms reflected the new pH/calcium gradient.
Introduction
Ombrotrophic bogs, which are naturally nutrient-poor and unproductive wetlands, belong to the most threatened ecosystems in Europe. Unlike lowland and submontane fens that have been drained and changed by human activity over the last centuries, ombrotrophic bogs have persisted widely until now, although they are exposed to high atmospheric deposition and other pollution (Hájková et al., 2011a; Jiroušek, Hájek & Bragazza, 2011; Poulíčková et al., 2013). In addition to losses in biodiversity, environmental change modifies ecosystem processes in bogs, with important consequences for the global carbon cycle (Bragazza et al., 2006, 2012; Dise, 2009).
Recognising the crucial role of bogs in global biogeochemical cycles has led to a recent boom in short-term experimental studies on carbon and nutrient cycling and their relationships to the cover and productivity of dominant species and major functional groups (Bragazza et al., 2012; Straková et al., 2012). Nevertheless, the functioning of a bog ecosystem and its modification by ongoing global change may be mediated by changes in species composition (Malmer et al., 2005; Hájková et al., 2011a), and we need to understand the relationships between species composition and environmental conditions in bogs. While many studies have dealt with the relationships between environmental factors and species composition of vascular plants and bryophytes (Bragazza, Gerdol & Rydin, 2003; Proctor et al., 2009), testate amoebae (Mitchell et al., 2000; Lamentowicz et al., 2010; Sullivan & Booth, 2011) or algae (Falasco & Bona, 2011; Štěpánková et al., 2012), different groups of organisms have not often been compared in a single study of the same sites. The parallel investigation of several taxa, including micro- as well as macroorganisms, may lead to a more complete understanding of progressive changes in species composition.
Bog vascular plants, the group most commonly investigated, are predominantly long-lived clonal organisms, dispersing to new sites by seeds, and thus, the establishment of new local populations may be limited by dispersal (Hájek et al., 2011); therefore, they may indicate past rather than present environmental conditions. Bryophytes and microorganisms (e.g. testate amoebae and diatoms) disperse by microscopic propagules, allowing them readily to reach new sites (Hájek et al., 2011). Bog bryophytes are long-lived, sessile organisms forming dense carpets, while testate amoebae and diatoms are short-lived with extremely high population sizes. The suitability of long-lived organisms as bioindicators is limited by their long-term persistence in suboptimal conditions (Kuussaari et al., 2009). Similarly, the use of dispersal-limited organisms may be confounded by the spatial configuration of study sites (Cottenie, 2005; Hájek et al., 2011), and the use of well-dispersed and short-lived organisms may be confounded by strong spatial mass effects causing frequent ephemeral and stochastic occurrences of the species in non-optimal conditions (Ng, Carr & Cottenie, 2009). Particular groups of organisms also differ in nutrition (e.g. autotrophic diatoms versus mostly heterotrophic testate amoebae), which may result in different determinants of species composition (Hájková et al., 2011b). Although vegetation and testate amoebae have been analysed jointly in some studies (Mitchell et al., 2000; Lamentowicz et al., 2010), we are not aware of any paper analysing vegetation and testate amoebae in bogs together with diatoms.
Most studies on species–environment relationships in bogs have used either single (‘spot’) or short-term measurements of water chemistry variables. This could be a crucial shortcoming because bog water chemistry fluctuates considerably within and among year(s) (Bragazza, Alber & Gerdol, 1998; Proctor, 2006; Hájková et al., 2011a). No study of bogs has compared the explanatory power of contemporary environmental data with that measured over many previous years. Although intuitively we might expect more compositional variability to be explained by long-term averaged water chemistry, a study of spring fens (Hájek & Hekera, 2004) found that spot values may be sufficient in many cases. In addition, using long-term means may be less advantageous in the case of short-lived organisms, such as diatoms or testate amoebae, as compared to long-lived vascular plants.
Here, we incorporated two neglected approaches, first comparing directly among different groups of short- and long-lived organisms and second using long-term environmental averages to find out: (i) which variables determined the species composition of bog assemblages under ongoing environmental change, (ii) how particular organisms differed in their ability to indicate environmental conditions and (iii) for which environmental variables long-term averages fit species compositions better. Bogs on the Sudetes Mountains in the Czech Republic represent a unique opportunity to test the effects of long-term water chemistry because, in the early 1990s, a network of monitoring plots was established here, in which complete water chemistry has been analysed annually (Hájková et al., 2011a). Apart from water level, particular plots differ in pH and concentrations of phosphorus and calcium. Sites have been diversified with respect to these factors over recent decades as a consequence of aerial liming (a forestry amelioration practice), which has affected the bogs unevenly. Entirely new calcium and phosphate gradients have appeared, especially in the Jeseníky Mountains (Hájková et al., 2011a; Poulíčková et al., 2013). In sites where long-term environmental data are available, we compiled a novel data set of four contrasting taxa (vascular plants, bryophytes, diatoms and testate amoebae). We hypothesised that long-term averages of environmental variables would explain more variation in species composition than would contemporary data and that this effect would be stronger for long- than for short-lived organisms. We further hypothesised that determinants of species composition would differ among particular groups of organisms, with the newly established pH/calcium gradient already affecting species composition of microorganisms.
Methods
Study area
Sampling was carried out in two ranges (150 km apart) of the Sudetes Mountains in the northern part of the Czech Republic. The western Jizera Mountains (N 50°50′, E 15°15′) are generally characterised by a suboceanic climate with higher air temperatures and slightly higher precipitation, which is relatively balanced during the year. The eastern Jeseníky Mountains (N 50°05′, E 17°10′) are colder, with precipitation maxima in the summer. In both areas, bog hummocks are characterised by Sphagnum magellanicum, S. russowii and Andromeda polifolia, while hollows by S. cuspidatum, Warnstorfia fluitans and Carex limosa. In the Jizera Mountains, species characterising suboceanic bog vegetation types (Trichophorum caespitosum, Sphagnum papillosum, S. tenellum, Erica tetralix), as well as dwarf pine bogs (with Pinus mugo), occur.
Both regions are highly polluted by atmospheric deposition. In the Jizera Mountains, N deposition is among the highest in Europe (Jiroušek et al., 2011). Mountain bogs in the Czech Republic are generally surrounded by Norway spruce (Picea abies) forests that are planted commercially. Acid rain, caused mostly by sulphur dioxide emissions, damaged many mountain forests in the 1970s and 1980s. Aerial liming has been broadly applied as a forestry amelioration practice since the 1980s (e.g. Bošeľa & Šebeň, 2010). It increased Ca and Mg concentrations in some bogs in the Jeseníky Mountains (Poulíčková et al., 2013).
Water chemistry
Pore water for chemical analyses was taken annually from 59 permanent plots in ombrotrophic hummocks, hollows, lawns and pools, which were established by K. Rybníček between 1991 and 1993 (Rybníček, 1997, 2000). The monitoring plots in bog pools with no bryophyte or vascular plants have not been included in this study. Altogether, 25 plots in five bogs were studied in the Jizera Mountains, and 26 plots in seven bogs were studied in the Jeseníky Mountains. Permanent sampling points for water chemistry (a small artificially dug holes, always being cleaned up before sampling) were located close to the corner of each vegetation plot. At least one sample per year was taken in each plot, but three seasonal samples (May, July and September) were collected in most years. The water was filtered through a 20-μm nylon mesh and transported in plastic bottles to the laboratory. The depth to water table (referred to as ‘water level’) was measured as a distance between water table and the bog surface directly in the field. Bog water pH and electrical conductivity (both standardised at 20 °C) were measured in the laboratory using a multimeter (HACH WTW 340i). Conductivity due to H+ ions was subtracted according to the formula of Sjörs (1952). We used only corrected conductivity in this study. Concentrations of calcium (Ca), magnesium (Mg), aluminium (Al), iron (Fe) and zinc (Zn) were measured using atomic absorption spectrometry. Sodium (Na) and potassium (K) were measured by a flame atomic emission spectrometry. Humic acids were analysed using a spectrophotometer at a wavelength of 420 nm. Ammonium ions (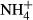 ) were determined by spectrometry by a method based on the Berthelot's reaction; salicylate and hypochlorite ions reacted with
) were determined by spectrometry by a method based on the Berthelot's reaction; salicylate and hypochlorite ions reacted with 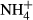 ions in the presence of the catalyst sodium nitroprusside. Nitrites (
ions in the presence of the catalyst sodium nitroprusside. Nitrites (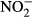 ) were measured using a photometric method with sulphanyl acid and N-(1 naphthyl) ethylenediamine dihydrochloride. Phosphates (
) were measured using a photometric method with sulphanyl acid and N-(1 naphthyl) ethylenediamine dihydrochloride. Phosphates (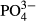 ) were detected spectrophotometrically as phosphomolybdenum blue. Nitrates (
) were detected spectrophotometrically as phosphomolybdenum blue. Nitrates (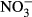 ) were measured using an isotachophoretic (ITP) analyser (until summer 1999), a spectrometer (autumn 1999–summer 2006) and capillary zone electrophoresis (2007–2008). Sulphates (
) were measured using an isotachophoretic (ITP) analyser (until summer 1999), a spectrometer (autumn 1999–summer 2006) and capillary zone electrophoresis (2007–2008). Sulphates (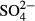 ) were detected using the ITP analyser and chlorides (Cl−) using a potentiometric titration with a silver nitrate solution.
) were detected using the ITP analyser and chlorides (Cl−) using a potentiometric titration with a silver nitrate solution.
Recording species
The vegetation plots were sampled in the Jizera Mountains in 2005 and in the Jeseníky Mountains in 2008. Their size varied from 2 m2 in hollows up to 25 m2 in patches with dwarf pine present (Hájková et al., 2011a). All vegetation plots were sampled in terms of total species composition (vascular plants, bryophytes), and species cover was estimated using a seven-grade scale (Braun-Blanquet, 1964). Taxonomic nomenclature of vascular plants follows Danihelka, Chrtek & Kaplan (2012), and that of bryophytes follows Kučera, Váňa & Hradílek (2012).
Diatoms and testate amoebae were collected in the sample plots by squeezing bryophyte tufts. Diatoms were sampled in July 2008 and testate amoebae in September 2008 in the Jizera Mountains or in September 2009 in the Jeseníky Mountains. After sampling, they were concentrated by sedimentation in a laboratory for 24 h. For testate amoebae, the total number of individuals in 0.1 mL of sedimented sample was counted. Identification was to species levels; the nomenclature follows Aescht & Foissner (1989). Diatom samples were cleaned with a mixture of concentrated sulphuric and nitric acids and mounted in Naphrax; 400 valves were counted per permanent slide. Diatoms were identified according to Krammer & Lange-Bertalot (1986–1991). The total list of recorded species of all taxonomic groups is given in Supporting Information (see Checklist S1).
Data analysis
The measurements between 1991 and 2008 were used, except for nitrates (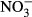 ) where only measurements between 1999 and 2008 were available. To describe differences in water chemistry between the regions, the means, standard errors and coefficients of variation were calculated separately for each permanent plot and then averaged for the Jizera Mountains and the Jeseníky Mountains, respectively. Differences were tested by Mann–Whitney U-test in Statistica (StatSoft Inc).
) where only measurements between 1999 and 2008 were available. To describe differences in water chemistry between the regions, the means, standard errors and coefficients of variation were calculated separately for each permanent plot and then averaged for the Jizera Mountains and the Jeseníky Mountains, respectively. Differences were tested by Mann–Whitney U-test in Statistica (StatSoft Inc).
The values of some water chemistry variables were transformed to an ordinal scale because the ion concentrations were below detection limits in many cases. This problem was frequent in the case of 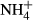 ,
, 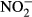 ,
, 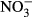 ,
, 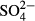 ,
, 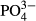 , Cl−, K, Hg and Pb. Exponential categories were used, with the value below the detection limit (0.05 in this example) equal to 1, values between 0.05 and 0.1 mg L−1 equal to 2, values between 0.1 and 0.2 mg L−1 equal to 3, values between 0.2 and 0.4 mg L−1 equal to 4, values between 0.4 and 0.8 mg L−1 equal to 5, etc. In the case of other water chemistry variables, samples below the detection limit were rare but were occasionally frequent in one sampling day. To avoid a variance close to zero, we replaced these measurements by a random number between zero and a value of the detection limit (Table S1). We repeated the generating routine of the random number and subsequent analyses and found that changes in a set of random numbers did not alter the results. Finally, the water chemistry data were rearranged into five different data sets of environmental variables: spot values taken at the time of species sampling, and three-, five-, 10- and 15-year averages of the measured variables.
, Cl−, K, Hg and Pb. Exponential categories were used, with the value below the detection limit (0.05 in this example) equal to 1, values between 0.05 and 0.1 mg L−1 equal to 2, values between 0.1 and 0.2 mg L−1 equal to 3, values between 0.2 and 0.4 mg L−1 equal to 4, values between 0.4 and 0.8 mg L−1 equal to 5, etc. In the case of other water chemistry variables, samples below the detection limit were rare but were occasionally frequent in one sampling day. To avoid a variance close to zero, we replaced these measurements by a random number between zero and a value of the detection limit (Table S1). We repeated the generating routine of the random number and subsequent analyses and found that changes in a set of random numbers did not alter the results. Finally, the water chemistry data were rearranged into five different data sets of environmental variables: spot values taken at the time of species sampling, and three-, five-, 10- and 15-year averages of the measured variables.
To illustrate the explanatory power of the particular environmental variables, marginal effects on the species composition of particular taxonomic groups were calculated by canonical correspondence analysis (CCA) and tested by Monte Carlo unrestricted permutation tests (999 permutations) using Canoco for Windows 4.5 (ter Braak & Šmilauer, 2002). Hill's scaling, logarithmic transformation of species data and down-weighting of rare species were used. The CCA was chosen because the gradient lengths in individual detrended correspondence analyses ranged from 2.26 up to 4.13 S.D. units and because unimodal responses of many species were expected (e.g. lawn species avoiding hollows as well as dry hummocks or P. mugo stands).
To select important environmental variables, the CCA with Monte Carlo permutation tests (999 permutations) and the forward selection procedure were used. The analyses were performed separately for the two regions and each taxonomic group with the five sets of environmental data (a total of 40 combinations). For each combination, three different selection procedures were performed following the experimental design with five (Jizera Mountains) and seven (Jeseníky Mountains) independent bogs and two to seven vegetation plots in each bog. As a first step, the overall CCA with an unrestricted Monte Carlo permutation test (ter Braak & Šmilauer, 2002) was applied. The principal aim of this analysis was to reveal percentage variances explained by spot, short-term and long-term environmental data. Because the statistical significance of this unrestricted Monte Carlo permutation test may be affected by spatial autocorrelation of samples (i.e. pseudoreplications within a single bog), we performed two further permutation tests aimed at testing either within- or among-bog variation. First, environmental variables describing the variability within bogs were tested using the forward selection in the CCA and permutations restricted to a bog, so that plots were permutated within a single bog from where they originated. The variable with the best explanatory statistic (‘pseudo-F’ statistic) and a small probability of the type I error (P ≤ 0.01) was chosen. Second, the environmental variables describing the variability among bogs were tested using permutations restricted to blocks. Because the number of plots was not equal for all of the bogs, an additional selection of two and three plots was made for the bogs in the Jeseníky and Jizera Mountains, respectively. Forward selection in the CCA was performed, and the couples/trinities of plots were permutated among bogs. The variable with the best explanatory statistic (‘pseudo-F’ statistic) and a small probability of the type I error (P ≤ 0.05) was chosen and recorded. The higher probability of the type I error was used because fewer plots entered the analysis and the permutation test was less powerful. If no tested variable in the analysis had a P-value ≤0.05, then a virtual variable – ‘none’ – was recorded. After repeating this selection 1000 times, the most frequent of the ‘best’ variables occurring more than 250 times in the register was chosen for the CCA model and the next step of the forward selection followed. These analyses were performed in R software (R Development Core Team, 2012) using the package VEGAN (Oksanen et al., 2008).
To describe more effectively the patterns in community similarity among particular taxonomic groups and their relationships to some environmental parameters, we calculated Pearson's correlation coefficients between pairs of Euclidean dissimilarity matrices and tested their significance using the Mantel permutation test (999 permutations) with the R-package VEGAN (Oksanen et al., 2008). We used 15-year averages of water level and pH and a more general expression of environmental distance using standardised values of 15-year averages of water level, pH, 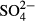 ,
, 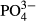 and Na (the most important and interpretable variables in the CCAs).
and Na (the most important and interpretable variables in the CCAs).
Results
Conductivity and concentrations of Ca, 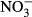 , Mg,
, Mg, 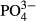 and humic acids were higher in the Jeseníky than in the Jizera Mountains, whereas concentrations of Al, Na,
and humic acids were higher in the Jeseníky than in the Jizera Mountains, whereas concentrations of Al, Na, 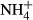 and
and 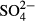 were lower. Mean water level and pH were similar in the two regions (Table 1). The explanatory power of particular environmental variables, calculated as marginal effects on species composition of particular taxonomic groups in the CCAs, is given in Table 2. Using the forward selection procedures in the overall CCA models, we found that single measurements of water level (all groups), pH (diatoms and testate amoebae in the Jeseníky Mountains) and humic acids (diatoms) were sufficient to explain the species data variation (Table 2). On the other hand, the effects of some variables have been revealed only using long-term averages (the past 5–15 years):
were lower. Mean water level and pH were similar in the two regions (Table 1). The explanatory power of particular environmental variables, calculated as marginal effects on species composition of particular taxonomic groups in the CCAs, is given in Table 2. Using the forward selection procedures in the overall CCA models, we found that single measurements of water level (all groups), pH (diatoms and testate amoebae in the Jeseníky Mountains) and humic acids (diatoms) were sufficient to explain the species data variation (Table 2). On the other hand, the effects of some variables have been revealed only using long-term averages (the past 5–15 years): 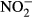 for vascular plants, K for vascular plants and testate amoebae, Na for all groups except diatoms,
for vascular plants, K for vascular plants and testate amoebae, Na for all groups except diatoms, 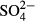 for bryophytes and testate amoebae and Zn for testate amoebae. Only medium-term averages (the past 3–5 years) were significant in the case of diatoms and
for bryophytes and testate amoebae and Zn for testate amoebae. Only medium-term averages (the past 3–5 years) were significant in the case of diatoms and 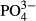 in the western region, for Na and testate amoebae also in the western region and for bryophytes and conductivity, as well as
in the western region, for Na and testate amoebae also in the western region and for bryophytes and conductivity, as well as 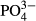 , in the eastern region.
, in the eastern region.
| Jizera Mts | Jeseníky Mts | Mann–Whitney U-test | |||
|---|---|---|---|---|---|
| Mean ± SE | CV | Mean ± SE | CV | Z-values | |
| WL (cm) | −11.77 ± 1.352 | n.d. | −11.37 ± 1.541 | n.d. | −0.08 (n.s.) |
| pH | 4.39 ± 0.046 | 6.2 | 4.64 ± 0.076 | 9.3 | −0.71 (n.s.) |
| cond. (μS cm−1) | 24.07 ± 1.916 | 48.1 | 34.18 ± 2.129 | 35.6 | −4.74*** |
| Ca (mg L−1) | 1.70 ± 0.216 | 76.5 | 2.66 ± 0.370 | 81.5 | −3.23** |
| Mg (mg L−1) | 0.49 ± 0.058 | 73.4 | 1.12 ± 0.123 | 66.8 | −2.38* |
| K (mg L−1) | 0.32 ± 0.047 | 90.5 | 0.30 ± 0.062 | 117.9 | 0.91 (n.s.) |
| Na (mg L−1) | 0.69 ± 0.068 | 60.5 | 0.46 ± 0.070 | 85.9 | 5.34*** |
| Cl− (mg L−1) | 0.97 ± 0.162 | 100.9 | 0.85 ± 0.185 | 126.5 | 2.18* |
| NH4 (mg L−1) | 0.23 ± 0.068 | 193.4 | 0.09 ± 0.028 | 175.5 | 4.68*** |
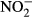 (mg L−1) (mg L−1) |
0.01 ± 0.002 | 140.0 | 0.01 ± 0.003 | 132.0 | −1.63 (n.s.) |
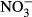 (mg L−1) (mg L−1) |
0.64 ± 0.119 | 86.9 | 0.78 ± 0.164 | 88.4 | −2.80** |
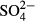 (mg L−1) (mg L−1) |
3.88 ± 0.591 | 92.2 | 2.03 ± 0.353 | 94.8 | 5.12*** |
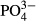 (mg L−1) (mg L−1) |
0.03 ± 0.009 | 153.3 | 0.05 ± 0.014 | 165.0 | −3.57*** |
| Humic (mg L−1) | 28.23 ± 4.255 | 82.3 | 51.68 ± 7.227 | 72.8 | −4.98*** |
| Al (mg L−1) | 0.45 ± 0.042 | 44.4 | 0.18 ± 0.038 | 81.9 | 5.29*** |
| Fe (mg L−1) | 0.66 ± 0.070 | 60.7 | 0.75 ± 0.106 | 113.7 | 1.25 (n.s.) |
| Pb (μg L−1) | 4.11 ± 0.649 | 71.2 | 5.09 ± 1.606 | 114.8 | −1.82 (n.s.) |
| Hg (μg L−1) | 0.12 ± 0.060 | 189.0 | 0.15 ± 0.073 | 158.9 | −1.82 (n.s.) |
| Zn (μg L−1) | 31.32 ± 5.681 | 79.9 | 34.86 ± 6.038 | 72.2 | −1.74 (n.s.) |
- WL, water level; cond., corrected conductivity; humic, humic acids; n.d., not defined.
- Z-values indicate significant differences between regions based on the Mann–Whitney U-test (*P < 0.05, **P < 0.01, ***P < 0.001; n.s., not significant).
| Jizera Mts | Jeseníky Mts | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| s.s. | 3y | 5y | 10y | 15y | s.s. | 3y | 5y | 10y | 15y | |
| Vascular plants | ||||||||||
| WL | 13.5 w,a | 8.7w,a | 12.0 w | 12.5 w | 11.5 w | 11.5 w,a | 7.7a | 8.7 w,a | 13.7 w,a | 14.8 w,a |
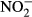
|
8.7 | 7.7 | 10.6 | 15.4 w,a | 15.9 w,a | 2.7 | 3.8 | 3.3 | 3.3 | 3.8 |
| K | 6.3 | 5.3 | 7.2 | 8.2w | 9.1 | 6.0 | 3.8 | 2.7 | 2.7 | 4.9 |
| Na | 5.8 | 6.3 | 6.7 | 7.2 | 7.7w | 6.0 | 4.9 | 4.4 | 4.9 | 3.8 |
| Ca | 6.3 | 3.8 | 5.3 | 4.3 | 6.3 | 3.8 | 6.0 | 6.0 | 8.7a | 8.7 |
| Mg | 6.3 | 4.8 | 5.8 | 7.2 | 7.2 | 7.7 | 7.1 | 7.1 | 7.1 | 7.7 |
| pH | 7.2 | 4.8 | 3.8 | 1.9 | 3.4 | 5.5 | 6.0 | 6.0 | 7.1 | 7.1 |
| cond. | 7.7 | 3.4 | 3.8 | 3.8 | 5.3 | 2.2 | 3.3 | 3.8 | 6.6 | 7.1 |
| Bryophytes | ||||||||||
| WL | 18.2 w,a | 17.4 w,a | 18.6 w,a | 18.2 w,a | 18.2 w,a | 23.8 w,a | 18.0 w,a | 20.0 w,a | 24.5 w,a | 25.1 w,a |
| Na | 8.3 | 10.7 | 11.1 | 12.6 | 12.6 a | 5.2 | 6.4 | 3.9 | 5.2 | 4.5 |
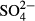
|
n.a. | 6.3 | 11.1 a | 13.4 a | 12.3 | 0.0 | 4.5 | 4.5 | 5.2w | 5.8w |
| Mg | 6.7 | 7.5 | 9.1 | 11.9 | 11.1 | 7.7 | 7.1 | 7.1 | 6.4 | 5.8 |
| cond. | 9.1 | 4.3 | 7.1 | 7.1 | 6.7 | 3.2 | 2.6 | 6.4w,a | 5.2 | 3.2 |
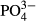
|
0.0 | 3.2 | 2.8 | 4.0 | 5.5 | 3.2 | 4.5 | 5.8a | 3.2 | 5.2 |
| Ca | 7.1 | 5.9 | 7.5 | 8.3 | 9.1 | 3.2 | 5.8 | 6.4 | 6.4 | 5.8 |
| K | 6.3 | 4.3 | 6.7 | 7.9 | 7.9 | 4.5 | 3.2 | 2.6 | 3.2 | 3.9 |
| pH | 9.5 | 6.7 | 4.0 | 2.8 | 2.4 | 4.5 | 5.8 | 6.4 | 7.1 | 7.1 |
| Diatoms | ||||||||||
| WL | 9.2w | 10.4 w | 11.5 w | 12.7 w | 12.7 w | 12.6 w,a | 8.2 | 8.7 w | 9.7 w | 10.7 a |
| Humic | 10.4w | 10.9 w,a | 10.4 w,a | 6.9 | 6.9 | 9.2 | 6.3 | 5.8 | 8.7 | 10.7 |
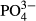
|
4.0 | 9.8 a | 9.8 | 8.6 | 7.5 | 3.9 | 5.8 | 4.9 | 6.3 | 7.3 |
| pH | 6.9 | 8.6 | 7.5 | 5.8 | 4.6 | 15.0 a | 16.5 w,a | 16.5 w,a | 16.0 w,a | 15.5 a |
| Ca | 6.3 | 2.9 | 5.2 | 5.8 | 6.3 | 10.2 | 14.1 | 14.1 | 13.1 w | 13.1 a |
| Mg | 6.9 | 6.9 | 6.3 | 6.9 | 6.3 | 14.1 | 15.0 | 15.0 | 15.5 | 14.1 |
| cond. | 6.9 | 4.6 | 4.0 | 6.9 | 6.3 | 3.9 | 4.9 | 7.3w | 10.7 | 11.2 |
| Al | 3.5 | 6.3 | 6.9 | 7.5 | 7.5 | 9.2 w | 5.3 | 5.8 | 6.8 | 8.2 w |
| Na | 5.2 | 3.5 | 5.8 | 5.2 | 6.3 | 7.3 | 5.3 | 5.3 | 6.3 | 5.3 |
| K | 5.2 | 2.9 | 2.9 | 1.7 | 2.3 | 8.2 | 4.9 | 3.4 | 2.4 | 4.4 |
| Testate amoebae | ||||||||||
| WL | 9.0 w,a | 11.1 w,a | 11.1 w,a | 11.1 w,a | 12.2 w,a | 18.3 w,a | 18.3 w,a | 17.6 w,a | 19.0 w,a | 19.8 w,a |
| pH | 3.2 | 4.8 | 5.3 | 3.7 | 2.6 | 11.0 a | 12.5 a | 13.2 a | 13.2 a | 13.2 |
| Mg | 4.2 | 4.8 | 4.8 | 5.3 | 5.3 | 11.7 | 12.5 | 11.7 | 12.5 | 13.2 |
| Ca | 3.2 | 3.7 | 4.2 | 5.3 | 5.8 | 4.4 | 8.1 | 10.3 | 11.7 | 13.2 a |
| cond. | 2.6 | 3.7 | 3.7 | 3.7 | 4.2 | 5.1a | 3.7 | 5.1a | 10.3 a | 11.7 |
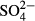
|
5.3 | 6.4 | 6.4 | 5.8 | 6.4 | 4.4 | 5.1w | 5.1w | 5.1w | 5.1 |
| Na | 6.4 | 6.9a | 5.8 | 5.3 | 5.3 | 6.6 | 6.6 | 5.1 | 4.4 | 3.7 |
| K | 3.2 | 4.8 | 4.2 | 7.4w | 6.9w | 2.9 | 3.7 | 2.9 | 3.7 | 3.7 |
| Zn | 4.8 | 5.8 | 5.3 | 6.9a | 3.2 | 2.9 | 2.9 | 3.7 | 3.7 | 7.3 |
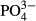
|
6.9 | 6.4 | 6.4 | 6.4 | 6.4 | 3.7 | 3.7 | 4.4 | 4.4 | 3.7 |
- s.s., single sampling; 3y-15y, averages for past 3-15 years; WL, water level; cond., corrected conductivity.
- The variables account for variation in species composition (P < 0.01) in at least one of the four study groups of organisms. Statistical significance was assessed using the following Monte Carlo permutation tests: the overall test permuting all plots (significant effects are in boldface); a test restricted to the within-bog variation (among vegetation types present within a single bog; significant effects are indicated by ‘w’); and a test restricted to the among-bog variation (significant effects are indicated by uppercase ‘a’). Conditional effects revealed by forward selections are shown for all cases; for other insignificant marginal effects, see Table S2.
Species composition within bogs
The first permutation scheme tested the effect of particular environmental factors on the variation in species compositions within bogs (i.e. predominantly among particular habitat types defined by hummock–hollow microtopography). An effect of water level was significant for all taxonomic groups (Table 2). The variation accounted for by particular chemical variables differed among taxonomic groups and between regions. Apart from water level, long-term averages of 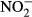 (the past 10 years), K (the past 10 years) and Na (the past 15 years) accounted for the variation in composition of vascular plant species in the Jizera Mountains (where the bog vegetation is more diverse). Bryophyte species composition was accounted for by water level in both regions and by long-term averages of
(the past 10 years), K (the past 10 years) and Na (the past 15 years) accounted for the variation in composition of vascular plant species in the Jizera Mountains (where the bog vegetation is more diverse). Bryophyte species composition was accounted for by water level in both regions and by long-term averages of 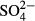 and corrected conductivity in the Jeseníky Mountains (Table 2).
and corrected conductivity in the Jeseníky Mountains (Table 2).
For diatoms, the determinants of species data variation differed considerably between the regions and depending on whether short- or long-term means were used (Table 2). In both regions, diatom species composition was significantly explained by water level, although this effect was weaker than that of pH in the Jeseníky Mountains. In addition, diatom species composition in the Jizera Mountains was accounted for by short- or medium-term means of humic acid concentration. The species composition of testate amoebae was best accounted for by water level. Apart from water level, K was a statistically significant determinant of species composition in the Jizera Mountains, while 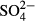 and conductivity together determined species composition of amoebae within bogs of the Jeseníky Mountains (Table 2).
and conductivity together determined species composition of amoebae within bogs of the Jeseníky Mountains (Table 2).
Species composition among bogs
The second permutation scheme tested the effect of particular environmental factors on the variation in species compositions among bogs (Table 2). In the Jizera Mountains, variation in vascular plants was accounted for by the long-term mean of NO2 (the past 5–15 years). In the same region, variation in bryophyte species was accounted for by long-term 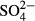 (the past 5–15 years) and Na concentrations (the past 3–15 years). In the Jeseníky Mountains, 10-year averages of Ca concentration were significant determinants of vascular plants, while the 5-year averages of corrected conductivity and
(the past 5–15 years) and Na concentrations (the past 3–15 years). In the Jeseníky Mountains, 10-year averages of Ca concentration were significant determinants of vascular plants, while the 5-year averages of corrected conductivity and 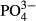 concentrations were significant determinants of bryophytes. In the Jizera Mountains, only diatoms were affected significantly by
concentrations were significant determinants of bryophytes. In the Jizera Mountains, only diatoms were affected significantly by 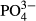 and humic acid concentrations. In the same region, Na and Zn concentrations accounted for variation in testate amoebae species composition. In the Jeseníky Mountains, where some bogs have been affected by liming, pH appeared to be an important determinant of diatoms and testate amoebae (Table 2). Visualisation of the CCA results illustrates the relationships between species occurrences and significant environmental variables (see Figs S1–S4 in Supporting Information).
and humic acid concentrations. In the same region, Na and Zn concentrations accounted for variation in testate amoebae species composition. In the Jeseníky Mountains, where some bogs have been affected by liming, pH appeared to be an important determinant of diatoms and testate amoebae (Table 2). Visualisation of the CCA results illustrates the relationships between species occurrences and significant environmental variables (see Figs S1–S4 in Supporting Information).
Assemblages of long-lived plants did not coincide significantly with the pH/Ca gradient in the eastern region, except for the significant effect of the 10-year average of Ca concentration, in the case of vascular plants, and the 5-year average of conductivity, for bryophytes.
Concordance between particular assemblages
The coefficients of dissimilarities in community compositions among the taxa studied were significantly correlated, with the exception of vascular plants and diatoms in the Jizera Mountains (Table 3). Correlations between species and environmental dissimilarities mirrored the results of CCAs. In the Jeseníky Mountains, the correlation between species and environmental dissimilarities was generally higher.
| Bryophytes | Diatoms | Testate amoebae | Water level | Water pH | Environmental distance | |
|---|---|---|---|---|---|---|
| Jizera Mts | ||||||
| Vasc. pl. | 0.64*** | 0.11 (n.s.) | 0.29*** | 0.35** | 0.02 (n.s.) | 0.21* |
| Bryophytes | 0.21* | 0.30*** | 0.33*** | −0.08 (n.s.) | 0.22* | |
| Diatoms | 0.21* | 0.27** | −0.11 (n.s.) | 0.12 (n.s.) | ||
| Testate am. | 0.29** | −0.07 (n.s.) | 0.09 (n.s.) | |||
| Jeseníky Mts | ||||||
| Vasc. pl. | 0.48*** | 0.44*** | 0.44*** | 0.55*** | 0.13 (n.s.) | 0.30** |
| Bryophytes | 0.57*** | 0.59*** | 0.72*** | 0.10 (n.s.) | 0.31*** | |
| Diatoms | 0.60*** | 0.44*** | 0.53*** | 0.34*** | ||
| Testate am. | 0.58*** | 0.39*** | 0.44*** | |||
- CCA, canonical correspondence analysis.
Discussion
Do long-term averages explain more variability in species data?
Single measurements of environmental factors, or their short-term averages, are frequently used in correlative studies of species and environment. We demonstrated that, for temporally stable and generally important variables such as water level and pH, this approach yields a sufficient description of species–environment relationships. On the other hand, for other factors, the use of short-term averages is insufficient. An analogous result was obtained from spring fens (Hájek & Hekera, 2004), where single measurements of stable variables such as pH accounted for much more variability in plant species data than those of unstable Fe and 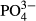 ; both the latter, however, appeared to be important ecological factors in follow-up studies focussing on nutrient stoichiometry in plants (Rozbrojová & Hájek, 2008).
; both the latter, however, appeared to be important ecological factors in follow-up studies focussing on nutrient stoichiometry in plants (Rozbrojová & Hájek, 2008).
Sulphates represent a specific case, where the insufficiency of a single recent sampling is attributed to strongly decreasing 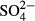 concentrations over the last 20 years (Hájková et al., 2011a). In the western region (formerly heavily polluted), present-day species composition reflects past rather than present patterns in
concentrations over the last 20 years (Hájková et al., 2011a). In the western region (formerly heavily polluted), present-day species composition reflects past rather than present patterns in 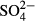 concentration. A group of peat moss species (S. cuspidatum, S. majus, S. papillosum) disappeared here between 1960 and 1970, and a metal-tolerant liverwort Gymnocolea inflata has occupied vacant niches (Rybníček, 2000; Hájková et al., 2011a). Other taxonomic groups have either not been affected, or their species composition has already been restored. The latter explanation is plausible for the two groups of microorganisms (diatoms and testate amoebae) because of their sensitivity to sulphates (e.g. Payne, Charman & Gauci, 2010) and short generation time (e.g. Zeeb et al., 1994).
concentration. A group of peat moss species (S. cuspidatum, S. majus, S. papillosum) disappeared here between 1960 and 1970, and a metal-tolerant liverwort Gymnocolea inflata has occupied vacant niches (Rybníček, 2000; Hájková et al., 2011a). Other taxonomic groups have either not been affected, or their species composition has already been restored. The latter explanation is plausible for the two groups of microorganisms (diatoms and testate amoebae) because of their sensitivity to sulphates (e.g. Payne, Charman & Gauci, 2010) and short generation time (e.g. Zeeb et al., 1994).
In general, our results call into question the results of observational studies comparing the explanatory power of particular water chemistry variables that differ in temporal stability. In several cases, the different variables accounted for variation in species composition of all the assemblages studied when spot, medium-term and long-term averages of environmental data were used (Table 2; Table S2). On the other hand, when only spot measurements were used, the main patterns (that water level affected all groups in both regions and pH affected microorganisms in the Jeseníky Mountains) were clearly revealed. While pH was stable temporally, water level varied through time (Table 1). However, the water-level differences among microhabitats usually remained consistent during hydrological fluctuations because of the hummock–hollow microtopography.
Do the predictors of species composition differ among taxonomic groups?
Our initial hypothesis about differences between short- and long-lived organisms in their correlation with short- and long-term environmental conditions was not generally supported. Nevertheless, determinants of species composition differed substantially among the various groups of organisms, with the exception of water level. The crucial role of water level for all taxa coincides with clear and well-known hummock–hollow topography within bogs formed by different species of peat mosses. In addition to bryophytes and vascular plants, microorganisms are also known to reflect this microtopographical pattern in peatlands (Poulíčková et al., 2004; Sullivan & Booth, 2011). Testate amoebae, in particular, are considered excellent indicators of moisture conditions in bogs, and their fossil assemblages are widely used for the reconstruction of past moisture conditions (van der Knaap et al., 2011; Elliott, Roe & Patterson, 2012; Dudová et al., 2013).
The two groups of microorganisms differed in the environmental determinants of their assemblages, and some species–environment relationships agreed well with published data. The negative relationship between the occurrence of Euglypha rotunda and Trinema lineare and Zn concentration found by Nguyen-Viet et al. (2007) was supported here (Fig. S4). A significant relationship between Na concentration and species composition of testate amoebae was also found by Mitchell et al. (2000), Mitchell, Bragazza & Gerdol (2004) and Lamentowicz et al. (2011). Sodium concentration is probably related not only to nutrition or toxicity, but it may covary with some causally important variables such as substratum structure (Hájková et al., 2011b) or silicon availability (Bhattacharyya & Volcani, 1980).
In the Jizera Mountains, short- and medium-term averages of 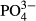 and humic acids determined the species composition of diatoms. The significant effect of phosphorus is not surprising because diatoms are frequently used as bioindicators of overall eutrophication, which is evaluated using total phosphorus annual averages (Zeeb et al., 1994; Bradshaw & Anderson, 2001; Schönfelder et al., 2002). The significant relationship between diatom species composition and
and humic acids determined the species composition of diatoms. The significant effect of phosphorus is not surprising because diatoms are frequently used as bioindicators of overall eutrophication, which is evaluated using total phosphorus annual averages (Zeeb et al., 1994; Bradshaw & Anderson, 2001; Schönfelder et al., 2002). The significant relationship between diatom species composition and 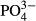 was largely driven by an increasing representation of euryvalent species or species complexes with increasing P concentrations. As did Miettinen (2003), we found a positive link between Hantzschia amphioxys and a reduced P-concentration and a negative link between Brachysira serians and an enhanced P-concentration. In addition to diatoms, we found a significant effect of
was largely driven by an increasing representation of euryvalent species or species complexes with increasing P concentrations. As did Miettinen (2003), we found a positive link between Hantzschia amphioxys and a reduced P-concentration and a negative link between Brachysira serians and an enhanced P-concentration. In addition to diatoms, we found a significant effect of 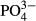 for bryophytes in the Jeseníky Mountains, where an enhanced
for bryophytes in the Jeseníky Mountains, where an enhanced 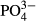 concentration was associated with generalist bryophyte species such as Calypogeia azurea, Dicranum scoparium, Pleurozium schreberi and Plagiothecium laetum (Fig. S2). The surprising lack of coincidence between
concentration was associated with generalist bryophyte species such as Calypogeia azurea, Dicranum scoparium, Pleurozium schreberi and Plagiothecium laetum (Fig. S2). The surprising lack of coincidence between 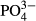 and the species composition of testate amoebae species contrasts with the results of studies from minerotrophic habitats (Mitchell, 2004; Hájková et al., 2011b). Extremely low productivity of ombrotrophic bogs could be an explanation, because the response of testate amoebae to phosphorus is mediated by food availability (Mitchell, 2004).
and the species composition of testate amoebae species contrasts with the results of studies from minerotrophic habitats (Mitchell, 2004; Hájková et al., 2011b). Extremely low productivity of ombrotrophic bogs could be an explanation, because the response of testate amoebae to phosphorus is mediated by food availability (Mitchell, 2004).
Diatoms responded significantly as a single group of organisms to varying Al concentrations. Vrieling et al. (1999) found that Al affects silica polymerisation and thus ultimately frustule formation and that this effect is species specific. Aluminium further affects phosphorus uptake (Exley et al., 1993) and ameliorates Cu toxicity in diatoms (Stauber & Florence, 1987); these processes may be important in our polluted bogs. Diatoms differed further from the other organisms by the significant effect of humic acid concentration. Humic acids affect diatoms more than other groups because dissolved organic carbon influences light availability and may be also important for nutrition of submerged autotrophic organisms (Znachor & Nedoma, 2010).
Bryophytes differed from the other groups by their significant coincidence with the among-bog variation in 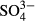 and Na in the western Jizera Mountains. Although the effect of
and Na in the western Jizera Mountains. Although the effect of 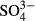 may be caused by past pollution, the effects of Na reflect natural differences among the bogs. The species composition of some bogs in the Jizera Mountains resembles oceanic bogs, with the presence of S. papillosum, S. tenellum and E. tetralix. We found that this vegetation type is associated with enhanced concentrations of Mg and Na, although this was evident only when long-term means were used. In truly oceanic ombrotrophic bogs, Na and Mg concentrations and Ca : Mg and Na : Mg ratios are higher than in continental bogs, because of the influence of sea water (Proctor, 2006; Proctor et al., 2009). Differences in Mg and Na concentration may thus causally influence species distribution patterns of particular species in the Jizera Mountains, although the physiological background is unknown.
may be caused by past pollution, the effects of Na reflect natural differences among the bogs. The species composition of some bogs in the Jizera Mountains resembles oceanic bogs, with the presence of S. papillosum, S. tenellum and E. tetralix. We found that this vegetation type is associated with enhanced concentrations of Mg and Na, although this was evident only when long-term means were used. In truly oceanic ombrotrophic bogs, Na and Mg concentrations and Ca : Mg and Na : Mg ratios are higher than in continental bogs, because of the influence of sea water (Proctor, 2006; Proctor et al., 2009). Differences in Mg and Na concentration may thus causally influence species distribution patterns of particular species in the Jizera Mountains, although the physiological background is unknown.
Do microorganisms respond faster to newly created gradients?
The most important difference in the determinants of species composition determinants between long- and short-lived organisms was noted in the case of pH, Mg and Ca; the effects of these factors were strong for short-lived organisms in the Jeseníky Mountains, where the pH gradient developed due to heavy pollution of some localities by Ca and Mg due to imprecise aerial liming of the surrounding forest. This result was the same for two contrasting groups of microorganisms (diatoms and testate amoebae). In addition, Štěpánková et al. (2012) found a significant effect of Ca on desmids in the same bogs of the same region.
Testate amoebae particularly associated with enhanced pH and enhanced Ca and Mg concentrations were Quadrurella symmetrica, Centropyxis spinosa and T. lineare, which are reliable indicators of calcium-rich fens (Lamentowicz & Mitchell, 2005; Lamentowicz et al., 2011). In the case of diatoms, limed bogs were characterised by the species of neutral or slightly acidic conditions (Cymbella amphicephala, Gomphonema gracile, Eunotia steineckei; van Dam, Mertens & Sinkeldam, 1994), in contrast to less affected bogs where acidobiontic species dominated (Eunotia paludosa, Kobaiashiella parasubtilissima). Strongly acidic conditions in non-limed bogs (pH ~4) represent extremely unfavourable conditions for most diatoms (Poulíčková et al., 2004); only 19 taxa are considered true inhabitants of highly acidic waters (DeNicola, 2000).
The species composition of macroscopic plants correlated much less clearly with the pH/Ca gradient. The enhanced Ca concentration in bog water was followed by the colonisation of mesic vascular plants, including tree seedlings (Picea and Salix) or generalist grasses (Avenella flexuosa, Calamagrostis villosa and Molinia caerulea). In the case of bryophytes, Bryum pseudotriquetrum, Cephalozia bicuspidata and Straminergon stramineum were recorded in limed bogs. We interpret the stronger effects of pH and calcium in microorganisms in terms of a faster response to newly created gradients. Indeed, Poulíčková et al. (2013) found that calcicole diatoms appeared in our study bogs as recently as after the liming event in 1993. Microorganisms generally disperse more effectively than macroorganisms (Finlay & Clarke, 1999; Hájek et al., 2011), which allows them to colonise island-like habitats more effectively and makes them suitable bioindicators that are not greatly affected by spatial constraints. Vascular plants limited by dispersal (Hájek et al., 2011) are usually unable to colonise habitat islands as fast as microorganisms. Horsák et al. (2012) found that the number of vascular plant specialists in an island-like habitat depends strongly on habitat age, even on a millennial scale. Bryophytes, however, may disperse as effectively as microorganisms (Hájek et al., 2011), although their colonisation may be slower because of their longer lifespan and greater competition pressure in dense moss carpets (Gunnarsson & Söderström, 2007). Bogs are generally characterised by a strong dominance of long-living peat mosses that persist at a site for hundreds of years (van der Knaap et al., 2011; Dudová et al., 2013). Even though calcium-rich water may kill Sphagnum mosses (Granath, Strengbom & Rydin, 2010), they can recover after the water level decreases and can limit the establishment of more calcium-tolerant species (Paal et al., 2010). The great difference in response rate between peat mosses and microorganisms was supported by the high-resolution palaeoecological study of van der Knaap et al. (2011), who found a great fluctuation in testacean assemblages even if the substratum was still formed by the peat moss Sphagnum fuscum. In addition, the dispersal ability of bryophytes is probably rather low at the time scale considered because many rare bryophytes spread mainly vegetatively. The low dispersal ability and long lifespan of vascular plants and bryophytes correspond to very small, even statistically insignificant, species compositional changes in permanent plots established in limed bogs (Hájková et al., 2011a).
Overall, our results demonstrate that the magnitude of the most important factors that determine the variation in bog biotic assemblages (water level, pH, Ca and humic acids) can be sufficiently assessed by spot or short-term sampling. On the other hand, the importance of some other important variables, such as 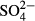 ,
, 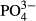 ,
, 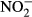 , Na or K, cannot reliably be assessed from single samples. Water level appeared to be a general determinant of species composition in all the taxa assessed. Macro- and microorganisms did not differ substantially with respect to whether they reflect contemporary or long-term water chemistry, but they differed in terms of the determinants of their species composition. Unlike bryophytes and vascular plants, the species composition of rapidly dispersing organisms with short lifespan (diatoms and testate amoebae) already clearly reflects the pH/calcium gradient that appeared only about 15 years before our samples of species composition were taken. We can therefore conclude that species composition responses to ongoing environmental change may depend on dispersal and life-history traits of particular organisms.
, Na or K, cannot reliably be assessed from single samples. Water level appeared to be a general determinant of species composition in all the taxa assessed. Macro- and microorganisms did not differ substantially with respect to whether they reflect contemporary or long-term water chemistry, but they differed in terms of the determinants of their species composition. Unlike bryophytes and vascular plants, the species composition of rapidly dispersing organisms with short lifespan (diatoms and testate amoebae) already clearly reflects the pH/calcium gradient that appeared only about 15 years before our samples of species composition were taken. We can therefore conclude that species composition responses to ongoing environmental change may depend on dispersal and life-history traits of particular organisms.
Acknowledgments
The research was supported by the Czech Science Foundation (project no. GA 206/08/0389, all authors) and by student's grant projects IGA PrF – 2012 – 001 (Palacký University; K.B., R.H.) and GD 526/09/H025 (Masaryk University; M.J.). We thank Alan Hildrew and two anonymous referees for their valuable comments.




