
Amino acid composition of epilithic biofilm and benthic animals in a large Siberian river
Summary
- We studied amino acid (AA) composition of epilithic biofilms and zoobenthos near the shore at a middle section of the Yenisei River (Siberia, Russia). We hypothesised that there was an imbalance between the composition and content of amino acids in the biofilm and its consumers, the zoobenthos, as well as between those in the zoobenthos and fish.
- Based on monthly sampling from 2007 to 2010, there was seasonal variation in AA profiles in the epilithic biofilms, probably caused by the succession of microalgal and cyanobacterial species.
- Overall, there was an imbalance in the percentage of the essential amino acids (lysine and histidine) between benthic animals and their food (the epilithic biofilm), which suggests that benthic animals may be limited by food quality. Moreover, the zoobenthos had a significantly higher content of AA, relative to carbon, than the biofilm.
- Based on sampling in 2012, there was an imbalance between the AA profiles of zoobenthos and that of their main consumer, the Siberian grayling (Thymallus arcticus), particularly in the percentages of two essential amino acids, lysine and leucine.
- In terms of overall content of essential amino acids, the nutritional value to fish of gammarids, which have recently invaded the river, was significantly lower than that of indigenous taxa, trichopteran and chironomid larvae.
Introduction
The transfer of matter and energy between primary producers and primary consumers is known to be limited by profound differences in elemental and biochemical composition of plants and animals. In aquatic ecosystems, some studies of the effects of the composition of primary producers on primary consumers have focussed on elemental stoichiometry (C : N : P; e.g. Giani, 1991; Stelzer & Lamberti, 2002; Acharya, Kyle & Elser, 2004; Cross et al., 2005; Ventura et al., 2008), on essential polyunsaturated fatty acids (PUFAs) of the ω3 family (e.g. Brett & Muller-Navarra, 1997; Boersma, Schops & McCauley, 2001) and on sterols (Martin-Creuzburg, Wacker & Von Elert, 2005). In contrast, comparatively little is known about the amino acid (AA) content of microalgae relative to requirements of consumers (Sterner & Schulz, 1998).
Studies of AA composition of algae are sparse compared to those of PUFAs, because the AA composition of diverse algal taxa is regarded as very similar (Ahlgren, Gustafsson & Boberg, 1992; Brown et al., 1997; Muller-Navarra, 2008). Moreover, most studies of AA composition are devoted to pelagic ecosystems and to phyto- and zooplankton. Nevertheless, in fast-flowing rivers, benthic (periphytic) algae are the major primary producers (e.g. Fuller & Bucher, 1991; Uehlinger, 2006; Kolmakov et al., 2008). Unfortunately, detailed chemical analyses of the AA composition of periphytic algae are comparatively very sparse and are based primarily on laboratory cultures (Steinman & McIntire, 1987; Khatoon et al., 2009; but see Ylla et al., 2011). Little is known about the AA composition of natural periphytic communities, particularly in large rivers.
Studies of the biochemical composition and nutritional quality of periphyton (epilithic biofilms) are important because, in some streams and rivers, epilithic biofilms are the principal source of matter and energy for the benthic invertebrates (Power & Matthews, 1983; Hill & Knight, 1987; Newell et al., 1995; Muller-Feuga, 2000; Sushchik et al., 2003). In turn, benthic animals are the main prey of many river fish (Power & Matthews, 1983; Ronnestad, Thorsten & Finn, 1999; Conceição, Grasdalenb & Ronnestad, 2003). An imbalance between the AA composition of food (epilithic biofilms) and those of consumers (the zoobenthos) seems to influence many physiological processes negatively, including growth, immunity and reproduction of aquatic invertebrates and fish (Kleppel, Burkart & Houchin, 1998; Conceição et al., 2003; Aragão et al., 2004; Helland, Hatlen & Grisdale-Helland, 2010). Fish have a definite requirement for 10 essential amino acids (EAAs), of which lysine is of particular concern because it is the EAA found in the highest concentration in fish tissues (Wilson & Cowey, 1985; Wilson & Poe, 1985; Kim & Lall, 2000). Lysine is the most limiting AA in plant protein for herbivores and, indirectly, higher consumers (Fox, Lawrence & Li-Chan, 1995; Forster & Ogata, 1998). Besides its important role in protein synthesis, lysine (together with methionine) serves as a precursor for carnitine that is involved in the transportation of long-chain fatty acids into the mitochondria for beta oxidation (Walton, Cowey & Adron, 1984). In aquaculture, the concentrations of lysine and methionine in fish food are regarded as important indicators of nutritional value of the diet (Li et al., 2009b; Yang et al., 2010).
Therefore, a comparison of the possible differences in the AA content of the first and the second links in a river food web (epilithon and zoobenthos) is desirable. Moreover, stoichiometric theory, extended to micronutrients, predicts that the amino acid composition of primary producers also may be the limiting factor for primary consumers (Anderson, Boersma & Raubenheimer, 2004). Our purpose was to study the AA composition of natural populations of epilithic biofilms and benthic animals (macroinvertebrates) in the Yenisei River. We aimed to test the following hypotheses: (i) AA profiles of epilithic microalgae (biofilms) do not change seasonally, despite the succession of species making up the epilithic flora, and are similar to those of other microalgal species, and assemblages, reported in the literature; (ii) there is an imbalance between the composition and content of amino acids in epilithic biofilms and their consumers, the zoobenthos; (iii) there is an imbalance between the AA profiles of zoobenthos and of their consumers, fish. Finally, we aimed to compare the nutritional value to fish of invasive gammarids with that of trichopteran and chironomid larvae, which were dominant before the invasion (Gladyshev & Moskvicheva, 2002).
Methods
Study site and sampling
The study was carried out in the Yenisei River (the largest river in Russia; Fig. 1), which drains a catchment of 2 650 000 km2 and has an average total annual discharge of c. 600 km3. Detailed information about the ecological characteristics of the river is given elsewhere (Telang et al., 1991), although its main hydrochemical features are low turbidity (<100 mg L−1), high oxygen content (c. 100% saturation), a total (dissolved and particulate) organic carbon content of 7–10 mg L−1 and hardness (Ca2+) of 21–23 mg L−1. Nutrient concentrations of PO4-P and NH4-N are 0–0.1 and 0.3–1.6 mg L−1, respectively (Gladyshev, Gribovskaya & Adamovich, 1993).
The sampling site (55°58′ N and 92°43′ E) was situated near the shoreline of the main channel of the river about 30 km downstream of the dam of Krasnoyarsk Hydroelectric Power Station (constructed 1972) and 1 km upstream of the city of Krasnoyarsk (Fig. 1). Here, the river flows through taiga (coniferous forest), and the river banks are rocky; current velocity is up to 2 m s−1, and the channel is about 1 km wide and of considerable depth (c. 15 m) with a stony bed. There is no ice cover during winter, because of discharge of water from deep in the reservoir. Water temperature ranges between 5 and 10 °C in spring–summer and 0 and 5 °C in autumn and winter.
Detailed hydroecological descriptions of the study site are given elsewhere (Sushchik et al., 2006; Kolmakov et al., 2008; Anishchenko et al., 2010; Kalachova et al., 2011; Gladyshev et al., 2012a,b). Briefly, dominant species in the epilithon in spring and early summer are green algae in the genus Ulothrix. In summer, the biofilm is mostly composed of diatoms, where several dominant species of the genera Gomphonema, Didymosphaenia, Fragilaria, Aulacoseira and Cymbella replace each other. In the late autumn and winter, cyanobacteria of genera Chamaesiphon, Oscillatoria and Phormidium become dominant (Kolmakov et al., 2008; Anishchenko et al., 2010; Sushchik et al., 2010).
Among benthic macroinvertebrates, Eulimnogammarus (Philolimnogammarus) viridis is dominant (Gladyshev & Moskvicheva, 2002; Sushchik et al., 2006). It invaded the Yenisei River from Lake Baikal via the Angara River (Fig. 1) and, in the last 50 years, moved upstream from the inflow of the Angara River to the dam of Krasnoyarsk Hydroelectric Power Station, near the city of Krasnoyarsk (Gladyshev & Moskvicheva, 2002). Now E. viridis has largely replaced the indigenous dominant taxa, larvae of trichopterans and chironomids, and comprises up to 90% of the benthic biomass (Gladyshev & Moskvicheva, 2002; Sushchik et al., 2006).
Subdominant species are larvae of the caddisfly, Apatania crymophila, and of Chironomidae, with Prodiamesa olivacea dominant and Pseudodiamesa branickii, Cricotopus algarum and Orthocladius rhyacobius occurring frequently. Larvae of Ephemeroptera are represented by Ephemerella setigera, Ephemerella ignita and Ephemerella aurivillii (Sushchik et al., 2006, 2007; Kalachova et al., 2011). Other benthic taxa such as oligochaetes have a comparatively low biomass (Sushchik et al., 2007).
The site was sampled monthly from February 2007 to April 2010, in a shallow area (0.5 m) about 10 m from the shore. One sample of biofilm was collected on each occasion. Samples of zoobenthic taxa were not available so regularly because particular taxa were absent or extremely small at the time of sampling. Numbers of different kinds of samples are given in Table 1.
| Date | Biomass | Amino acids | Carbon |
|---|---|---|---|
| 18.01.2007 | b | NDa | ND |
| 28.02.2007 | b g t c | b g t | b |
| 28.03.2007 | b | b t | b |
| 18.04.2007 | b g t c | b | b |
| 17.05.2007 | b | b c | b |
| 21.06.2007 | b g t c | b g t c | b |
| 26.07.2007 | b g t c | b g t c | b |
| 30.08.2007 | b g t c | b g t c e | b |
| 27.09.2007 | b g t c | b g t c e | b |
| 30.10.2007 | b g t c | b g t | b |
| 29.11.2007 | b g t c | b g t | b |
| 27.12.2007 | b g t c | b g t | b |
| 24.01.2008 | b g t c | b g t | b |
| 21.02.2008 | b g t c | b g | b |
| 20.03.2008 | b g t c | b g | b g t c |
| 24.04.2008 | b g t c | b g t e | b g t |
| 29.05.2008 | b | b g c | b |
| 23.06.2008 | b | b g c | b g c |
| 24.07.2008 | b | b g t c | b |
| 25.08.2008 | b g t c | b g t c | b g c |
| 22.09.2008 | b g t c | b g t | b |
| 29.10.2008 | b g t c | b g c e | b g t c |
| 26.11.2008 | b | b g c | b g c |
| 24.12.2008 | b | b g c | b g c |
| 28.01.2009 | b | b g c | b g c |
| 25.02.2009 | b | b g | b g c |
| 24.03.2009 | b | b g t c | b g c |
| 22.04.2009 | b | ND | ND |
| 26.05.2009 | b | b | b |
| 23.06.2009 | b | b g c | b g c |
| 28.07.2009 | b | b g c | b g c |
| 25.08.2009 | b | b g t | b g t c |
| 23.09.2009 | b | b g t c | b g t c |
| 28.10.2009 | b | b g t | b g t |
| 18.11.2009 | b | b t | b g t |
| 22.12.2009 | b | b g t | b g t c |
| 24.02.2010 | b g t c | g t | g t |
| 24.03.2010 | b g t c | g t | g t |
| 21.04.2010 | b g t c | g | g t |
| 26.05.2010 | b | ND | ND |
| Number of samples | |||
| Biofilm | 40 | 34 | 34 |
| Gammaridea | 19 | 32 | 20 |
| Trichoptera | 19 | 22 | 11 |
| Chironomidae | 19 | 17 | 14 |
| Ephemeroptera | 0 | 4 | 0 |
- a ND, no data
Biofilm samples were collected from the surface of stones. The substratum comprised mostly cobbles (10–15 cm diameter) and pebbles (2–10 cm diameter). For biofilm sampling, a 10 × 10 cm frame was placed on the river bed and the cobbles and pebbles inside the frame were picked up. Biofilm was scraped from the upper surface of cobbles and pebbles with a toothbrush, suspended in a small fixed volume of river water and transported back to the laboratory in cool boxes. In the laboratory, the biofilm suspensions were actively shaken for several minutes and aliquots immediately subsampled for microscopic identification and counting of microalgal and cyanobacterial species, size estimation and biomass calculations and amino acid analysis.
Zoobenthos samples were collected using a Surber sampler (quadrate area 40 × 35 cm; mesh size: 0.25 mm). As a rule, two samples were taken per sampling date; one was used for species identification and biomass measurements, and the second for biochemical analyses of animals. The samples were immediately washed and transported fresh to the laboratory within an hour of collection.
For assessing a possible AA imbalance between zoobenthos and fish, five specimens of the dominant fish species, Siberian grayling, Thymallus arcticus Pallas [=Thymallus baicalensis (Weiss et al., 2007; Knizhin, 2011)], c. 20 cm in length, were obtained from local fishermen in August 2012. Siberian grayling in this area feed mainly on the zoobenthos, E. viridis, A. crymophila and chironomid larvae (Sushchik et al., 2006). Muscle tissues below the dorsal fin were taken as the samples (Sushchik et al., 2006) for AA analysis and treated in the same way as the zoobenthos samples.
Samples pre-treatment and biochemical analyses
In the laboratory, benthic animals were identified and sorted to four groups of taxa: gammarids (Gammaridea), trichopteran larvae (Trichoptera), chironomid larvae (Chironomidae) and ephemeropteran larvae (Ephemeroptera). Individuals were pooled to obtain an appropriate biomass for biochemical analysis. Immediately after sorting, the live animals were placed into beakers with filtered water of an appropriate temperature (i.e. close to the field) to empty their guts for 24 h. Then, the animals were gently blotted with filter paper, weighed and kept frozen (−20 °C) until analysis in tightly closed glass tubes.
Microscopic analysis showed that epilithic biofilms consisted primarily of eukaryotic and prokaryotic phototrophs, while bacteria and small ciliates were a minor component (c. 7–12% of total biovolume). Thus, although the community comprised complex assemblages including algae, bacteria and micro- and meiofauna, we considered it simply as epilithic microphototrophs (i.e. algae and cyanobacteria). Algae and cyanobacteria were counted and identified using a Fuchs–Rosenthal counting chamber (0.0032 mL volume) under a microscope at ×400 magnification. The sizes were measured using an ocular micrometer, and biovolume and wet mass of algae were calculated using appropriate geometrical shapes (or their combinations) (Hillebrand et al., 1999).
For biochemical analysis, the aliquots of suspended biofilm were centrifuged at 6000 g for 12 min and pellets were finally collected onto filters covered with a BaSO4 layer to facilitate further removal of the residue. The filters with the pellets were dried in a desiccator for 24 h and then were kept at −20 °C until further analysis. Details of amino acid analysis are given elsewhere (Kalachova et al., 2004). Briefly, biofilm pellets or pre-weighed benthic animals were put into a 12 × 120 mm thick-wall glass ampoules and 6 m solution of HCl was added to hydrolyse the sample. Animal samples whose total wet mass was <100 mg (w.m.) were put into a 10 × 60 mm thick-wall glass ampoules. The volume of HCl added varied from 3 to 30 mL, depending on the mass of material available. The ampoules were vacuum-sealed and the samples hydrolysed at 110 °C for 22 h. They were then cooled, filtered and put into an evaporating dish. Evaporation was conducted in a boiling water bath. The amino acid solution was then evaporated in a water bath until dry. The solids were dissolved in a buffer solution A0992-7 (Knauer, Berlin, Germany). The samples were cleaned prior to analysis by running through Diapak C1 cartridges (BioChimMak ST, Moscow, Russia) in order to eliminate hydrophobic substances that might contaminate the column and interfere with separation. A high-performance liquid chromatograph A0326V2 (Knauer) equipped with 125 × 3 mm column (A0992-13v1; Knauer), a ninhydrin reaction block and a UV detector 2500 operated at 570 nm was used for the determination of amino acids. The sensitivity of the instrument was 0.1 nmol. Content of each amino acid (mg g−1 w.m.) was calculated by comparing the sample peak areas with those of the amino acid calibration standard (Pickering, Mountain View, CA, U.S.A.). Sixteen AAs were determined in this way, among them the essential amino acids lysine (Lys), histidine (His), arginine (Arg), threonine (Thr), valine (Val), methionine (Met), isoleucine (Ile), leucine (Leu) and phenylalanine (Phe) (e.g. Lehninger, Nelson & Cox, 1993; Guisande, Maneiro & Riveiro, 1999; Aragão et al., 2004; Peres & Oliva-Teles, 2006), plus the non-essential aspartic acid (Asp), serine (Ser), glutamic acid (Glu), glycine (Gly), alanine (Ala) and tyrosine (Tyr). Glutamine and asparagine were detected as glutamic and aspartic acids.
The samples for total organic carbon, both of epilithic microphototrophs and of zoobenthos, were analysed using a Flash EA 1112 NC Soil/MAS 200 elemental analyser (ThermoQuest, Milan, Italy) (Gladyshev et al., 2007).
Note that, due to the sampling approach, quantities of AA in epilithic biofilms were directly measured as mass per unit area, mg m−2. The pool of AA in epilithic microphototrophs was calculated from the quantity of AA in the aliquots of samples collected from the known area of river bed. For animals, the quantities of AA were determined directly as mass per unit wet mass (mg g−1 w.m). The quantity (pool) of AA in the zoobenthos per unit area (mg m−2) was obtained on the basis of the measured contents of AA in the wet mass of organisms multiplied by the corresponding approximate biomass of benthic animals per unit area (g m−2). The content of AA in benthic animals as mass per unit dry mass of biomass (mg g−1 d.m.) was calculated from the values per wet mass using data on the moisture content of the benthic taxon in question (Sushchik et al., 2003, 2007). Based on these data, the carbon content of AA per unit total organic carbon (AA/C) was calculated for biofilm and animal taxa using the proportion of carbon in molecules of each amino acid weighted by the mean proportion of this acid out of the total AA in each group of organisms (0.46 for animals and 0.45 for biofilm).
Statistical analysis
For comparison of data that have considerable seasonal variation, such as biomass, the nonparametric Wilcoxon matched pairs W-test was used (Campbell, 1967). For normally distributed data, one-way anova was used together with Fisher's LSD post hoc test for comparison of means. We also tested by anova whether AA percentages had a seasonal pattern in most frequently occurred taxon, gammarids, where the seasons (winter, spring, summer and autumn) were applied as the testing factor.
To reveal putative differences in the composition of AA in biofilms and animals, multivariate discriminant analysis (MDA) was used. Discriminant analysis is a method of linear modelling to classify observations into a priori known groups. MDA firstly tests for differences in the predictor variables among the pre-defined groups (i.e. it is identical to anova for a single explanatory variable) and secondly finds the linear combinations (called discriminant functions) of the variables that best discriminate among the groups (Legendre & Legendre, 1998). Here, the pre-defined groups were biofilms and animals, while their AA percentages were the variables. During computations by a stepwise procedure, variables with insignificant values of Fisher's F-test were excluded from the model. Two canonical roots were taken into account, which represented two orthogonal discriminant (canonical) functions and provided the most overall discrimination between the groups. Means of canonical variables were used to see which groups were discriminated by the first and the second canonical roots. Factor structure coefficients, representing correlations between the variables and the discriminant functions, were used to see which variables discriminate best of all between the above groups.
To find correlations between microalgal species composition (independent variables) and AA composition (dependent variables) in the epilithic biofilms, canonical correlation analysis (CCA) was used. This analysis is intended to find canonical axes of maximum linear correlation between two data sets (tables) (Legendre & Legendre, 1998). Two successively extracted canonical roots were used, which provided maximal orthogonal correlations between the canonical variates. Factor loadings, that is, the correlations between the canonical variates and the variables in each set, were used to determine variables with the highest contribution to the overall correlations between the two sets. Total redundancy value gave an indication of the magnitude of the overall correlations between the two sets of variables relative to the variance of the variables.
All the above tests and analyses were carried out using STATISTICA software, version 9.0 (StatSoft Inc., Tulsa, OK, U.S.A.).
Results
Biomass and total AA
Biomass of epilithon per unit area, although rather variable temporally, was on average more than six times higher than biomass of each of the dominant animal taxa (Fig. 2). However, the pool of AA contained in the epilithic biofilms was on average only 1.7 times higher than that of each dominant animal taxon, and the difference between the pool of AA in biofilms and in gammarids was insignificant (Fig. 2). The trichopterans had significantly higher AA/C (carbon content of amino acids per total organic carbon) than the gammarids and the chironomids (Fig. 2). All benthic taxa contained significantly more amino acids per unit carbon than the epilithic phototrophs (Fig. 2).
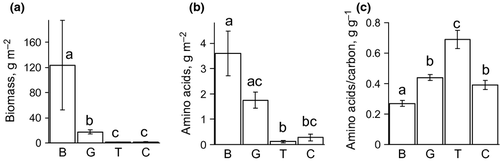
The total AA content per dry mass of trichopterans (613.5 ± 43.5 mg g−1 d.m.) was significantly higher than that of gammarids (369.2 ± 9.4 mg g−1) and chironomids (432.4 ± 24.1 mg g−1), according to the post hoc test (F = 24.15, P < 0.001). In turn, the chironomids contained significantly more amino acids per dry mass than the gammarids (P < 0.001).
AA composition
Percentages (% of the total AA) of Thr, Gly, Ser and Ala in the biofilms were significantly higher than those in each animal taxon (Fig. 3). There were two exceptions, in that the differences between the percentages of Thr and Ala in the biofilm and in ephemeropterans were not significant (Fig. 3). In contrast, percentages of Lys, His and Tyr in the benthic animals were higher than in the biofilms (Fig. 3).
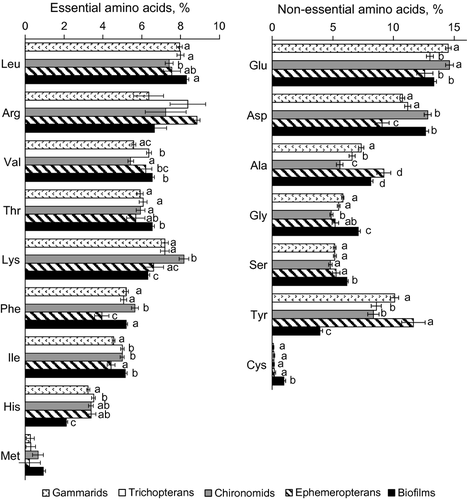
Among the zoobenthic taxa, chironomid larvae had the highest percentages of Lys, Phe and Asp, but the lowest percentages of Ala and Gly (Fig. 3). Ephemeropteran larvae had the highest percentage of Ala and the lowest percentages of Asp and Phe (Fig. 3).
According to multivariate discriminant analysis (MDA), there were significant differences in the AA compositions of the groups of organisms assessed. Thus, for epilithic microphototrophs and benthic animals, both discriminant functions (Root 1 and 2) were high and statistically significant (Table 2). The cumulative proportion of variance explained (discriminatory power) by the first two roots was 85.9%. Root 1 discriminated best the epilithic biofilms from all the animals (Fig. 4a; Table 2). Variables that gave the highest contribution to the first discriminant function (Root 1) were Tyr and His, on the one hand, and Gly, on the other (Table 2).
| Root 1 | Root 2 | |
|---|---|---|
| Canonical R | 0.941 | 0.847 |
| χ2 | 451 | 237 |
| d.f. | 52 | 36 |
| P | 0.000000 | 0.000000 |
| Means of canonical variables: | ||
| Biofilms | 3.991 | −0.295 |
| Chironomidae | −1.376 | 3.093 |
| Trichoptera | −1.532 | 0.453 |
| Gammaridea | −1.996 | −1.217 |
| Ephemeroptera | −3.684 | −3.390 |
| Factor structure coefficients: | ||
| Tyr | −0.555 | −0.149 |
| His | −0.469 | 0.170 |
| Ala | 0.163 | −0.458 |
| Gly | 0.392 | −0.318 |
| Glu | −0.080 | 0.080 |
| Phe | 0.023 | 0.225 |
| Val | 0.192 | −0.114 |
| Leu | 0.101 | −0.123 |
| Asp | 0.227 | 0.371 |
| Ser | 0.257 | −0.169 |
| Ile | 0.165 | 0.206 |
| Cys | 0.259 | −0.055 |
| Lys | −0.181 | 0.286 |
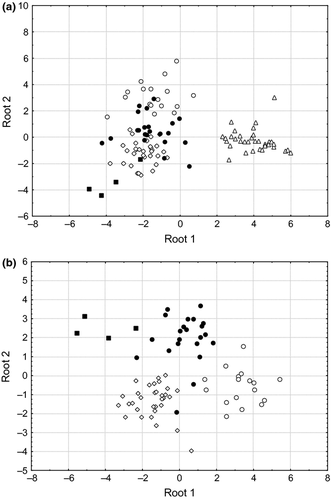
An additional MDA with only the benthic animal taxa revealed three distinct groups (chironomids, ephemeropterans and gammarids) with a distinctive AA composition according to their positioning on the plot and canonical values (Fig. 4b; Table 3). The cumulative proportion of variance explained (discriminatory power) by the two-first roots was 95.3%. Variables that made the highest contribution to the first discriminant function (Root 1) were Asp, on the one hand, and Ala, on the other (Table 3). Root 2 revealed some differences between ephemeropterans and gammarids, primarily due to the contributions of Val and Glu in the discriminant function (Table 3). Gammarids and trichopterans did not differ in their AA composition by either discriminant function, Root 1 or Root 2 (Fig. 4b).
| Root 1 | Root 2 | |
|---|---|---|
| Canonical R | 0.906 | 0.841 |
| χ2 | 223 | 110 |
| d.f. | 39 | 24 |
| P | 0.000000 | 0.000000 |
| Means of canonical variables: | ||
| Chironomidae | 3.310 | −0.590 |
| Trichoptera | 0.303 | 1.955 |
| Gammaridea | −1.440 | −1.337 |
| Ephemeroptera | −4.214 | 2.457 |
| Factor structure coefficients: | ||
| Asp | 0.395 | −0.092 |
| Gly | −0.259 | −0.129 |
| Val | −0.072 | 0.386 |
| Glu | 0.063 | −0.377 |
| Ile | 0.234 | 0.197 |
| Arg | 0.015 | 0.137 |
| Leu | −0.082 | 0.027 |
| Ala | −0.361 | 0.020 |
| Phe | 0.191 | −0.174 |
| Thr | 0.022 | 0.030 |
| His | 0.049 | 0.146 |
| Met | 0.063 | −0.019 |
| Ser | −0.107 | 0.047 |
Seasonal dynamics
It was impossible to separate different taxa in the epilithic biofilm. However, epilithic taxa fortunately showed a distinct seasonal pattern in the Yenisei River (Fig. 5). Therefore, to study possible differences in AA composition of the taxa, we compared seasonal dynamics of taxonomic composition of epilithic microphototrophs with the seasonal dynamics of amino acids in the biofilms, using a multivariate canonical correlation analysis. Cyanobacteria made up most of the biofilm biomass in winter (Fig. 5). Diatoms dominated during summer (about 100%), late winter–early spring and late autumn (Fig. 5). Green algae reached their maximum biomass (up to 100% of the total) in late spring and in late summer–early autumn (Fig. 5). The comparison of the seasonal dynamics by CCA revealed a strong correlation between the taxa and AA composition of the biofilms: both first and second canonical R were high and significant (Table 4). The highest contribution to the first canonical R was due to interactions between cyanobacteria and Ser, on the one hand, and between diatoms and Tyr, on the other (Table 4). The interactions between diatoms and Glu, and also between green algae and Phe, primarily contributed to the second canonical R (Table 4).
| Independent variables | Dependent variables | |
|---|---|---|
| No. of variables | 3 | 15 |
| Variance extracted (%) | 100 | 23.9 |
| Total redundancy (%) | 75.1 | 17.6 |
| Number of valid cases | 34 |
| Root 1 | Root 2 | |
|---|---|---|
| Canonical R | 0.941 | 0.815 |
| Eigenvalues | 0.885 | 0.664 |
| χ2 | 97.1 | 46.3 |
| d.f. | 45 | 28 |
| P | 0.000011 | 0.016187 |
| Factor loadings | ||
| Independent variables | ||
| Cyanobacteria | −0.841 | 0.356 |
| Diatoms | 0.680 | 0.653 |
| Green algae | −0.428 | −0.774 |
| Dependent variables | ||
| Asp | −0.497 | 0.332 |
| Thr | −0.281 | −0.285 |
| Ser | −0.681 | 0.021 |
| Glu | 0.174 | 0.431 |
| Gly | −0.068 | −0.023 |
| Ala | −0.304 | −0.065 |
| Val | −0.130 | −0.299 |
| Met | 0.305 | −0.113 |
| Ile | −0.212 | 0.032 |
| Leu | −0.409 | −0.138 |
| Tyr | 0.403 | 0.131 |
| Phe | −0.135 | −0.359 |
| His | −0.149 | 0.281 |
| Lys | −0.112 | 0.142 |
| Arg | 0.353 | −0.142 |
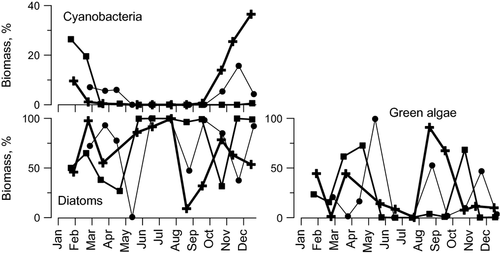
The number of samples of benthic animals was comparatively small (Table 1) and did not allow us to describe the seasonal dynamics of their AA composition.
Nutritional value of benthic animals for fish
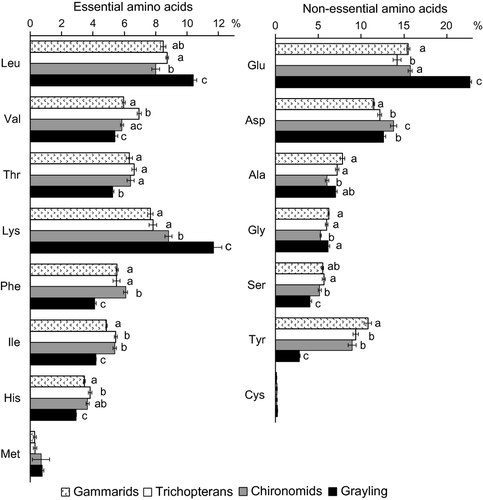
Comparison of the AA profiles of the Siberian grayling and its food, gammarids, trichopteran larvae and chironomid larvae, revealed an imbalance between them. The grayling had a significantly higher percentage of some essential amino acids (Leu and Lys; Fig. 6).
Important indicators of the nutritional value of the zoobenthos for fish (concentrations of Met and Lys and the sum of percentage of essential amino acids, EAAs) are given in Table 5. With respect to Met and Lys, the prey taxa were of similar nutritional value. The percentages of EAAs in trichopteran and chironomid larvae were significantly higher than in ephemeropteran larvae and gammarids (Table 5).
| Gammaridea | Trichoptera | Chironomidae | Ephemeroptera | F | P | |
|---|---|---|---|---|---|---|
| Met, mg g−1 | 0.26 ± 0.09 | 0.30 ± 0.11 | 0.80 ± 0.65 | 0.23 ± 0.16 | 0.7 | >0.05 |
| Lys, mg g−1 | 6.51 ± 0.19 | 7.25 ± 0.64 | 7.66 ± 0.36 | 5.33 ± 1.02 | 2.4 | >0.05 |
| EAAs,% | 46.24a ± 0.36 | 49.87b ± 0.66 | 48.87b ± 0.76 | 46.89a ± 0.94 | 9.1 | <0.01 |
Discussion
AA profiles of the epilithic biofilm
There have still been surprisingly few analyses of the AA composition of periphyton, with single studies of algal assemblages in a laboratory stream (Steinman & McIntire, 1987), in laboratory cultures of marine microalgae (Khatoon et al., 2009) and in epilithic biofilms from an intermittent stream (Ylla et al., 2011). We have supplemented this scant knowledge with data on the AA composition of natural assemblages of epilithic microphototrophs (biofilms) in a very large river. We have attributed the AA content and composition of the biofilms mainly to the microalgae and photoautotrophic bacteria, because biofilms in the Yenisei River consisted primarily of diatoms, green algae and cyanobacteria, while heterotrophic bacteria and small ciliates were a minor component. This observation agrees with previous data (Sushchik et al., 2007, 2010).
Indirect evidence that the AA composition of microalgae and cyanobacteria is taxon specific came from analyses of their seasonal dynamics. In general, the seasonal dynamics of epilithic biofilms in the Yenisei River was similar to that in a number of rivers (e.g. Potapova, 1996; Ostrofsky et al., 1998; Dodds, Biggs & Lowe, 1999; Kawecka & Sanecki, 2003; Soininen & Eloranta, 2004; Stewart, Lamoureux & Forbes, 2005). However, the pattern cannot be regarded as universal. We found high and significant correlations between cyanobacteria and Ser, on the one hand, and between diatoms and Tyr, on the other, as well as between diatoms and Glu and also between green algae and Phe. Thus, the AA composition of epilithic biofilms in this stretch of the Yenisei River, including the percentage of the essential amino acid Phe, was partly determined by the taxonomic composition of microalgae and cyanobacteria and by their seasonal succession.
As mentioned, the AA composition of various algal and cyanobacterial taxa is thought to be very similar, compared to fatty acids (Ahlgren et al., 1992; Brown et al., 1997; Muller-Navarra, 2008). Indeed, the distinct seasonal variation in species composition of the microalgae in the Yenisei River was accompanied by only modest variation in the AA composition of the biofilm. Moreover, our data on amino acids from the Yenisei River biofilms did not differ markedly from those from a laboratory channel (Steinman & McIntire, 1987) or an intermittent Mediterranean stream (Ylla et al., 2011) (Table 6). The percentages of Glu, Thr, Met, Leu and His in biofilms from a laboratory channel (Steinman & McIntire, 1987) and from the Yenisei River were similar (Table 6). The percentages of Ser, Ala, Val, Met and Ile in biofilms from an intermittent stream (Ylla et al., 2011) and from the Yenisei River were almost equal (Table 6). However, the percentages of Asp, Glu and Lys were substantially higher, although His and Arg lower, in biofilms from the Yenisei River than from the intermittent stream (Ylla et al., 2011) (Table 6).
| Group (taxa) | Epilithic biofilm | Epilithic biofilm | Algal assemblages | Scenedesmus quadricauda | Synechococcus sp. | Amphora sp. | Navicula sp. |
|---|---|---|---|---|---|---|---|
| Reference | Our data | Ylla et al. (2011) | Steinman & McIntire (1987) | Ahlgren & Hyenstrand (2003) | Khatoon et al. (2009) | ||
| Asp | 12.65 | 6.30 | 14.89 | 10.30 | 9.90 | 7.32 | 9.25 |
| Thr | 6.54 | 8.66 | 6.83 | 5.10 | 5.20 | 6.53 | 4.21 |
| Ser | 6.14 | 6.69 | 11.07 | 4.70 | 4.90 | 4.27 | 5.73 |
| Glu | 13.32 | 9.06 | 13.18 | 12.30 | 12.20 | 9.78 | 14.41 |
| Gly | 7.10 | 5.51 | 10.29 | 5.90 | 4.70 | 4.26 | 6.37 |
| Ala | 8.12 | 7.87 | 10.95 | 7.70 | 8.00 | 12.50 | 7.89 |
| Val | 6.55 | 7.48 | 7.86 | 6.00 | 6.20 | 3.97 | 3.06 |
| Cys | NDa | ND | ND | 1.50 | 0.90 | 1.41 | 2.70 |
| Met | 0.95 | 0.80 | 0.78 | 2.30 | 1.60 | 5.03 | 4.36 |
| Ile | 5.15 | 5.51 | 3.77 | 4.30 | 5.20 | 4.53 | 8.41 |
| Leu | 8.30 | 6.69 | 7.91 | 8.80 | 10.00 | 2.62 | 2.91 |
| Tyr | 3.95 | 4.72 | 0.63 | 4.30 | 4.80 | 7.09 | 6.88 |
| Phe | 5.19 | 7.48 | 3.50 | 5.70 | 5.80 | 6.29 | 5.20 |
| His | 2.12 | 7.48 | 1.97 | 2.20 | 1.70 | ND | ND |
| Lys | 6.32 | 3.94 | 3.06 | 7.80 | 5.10 | 8.03 | 8.45 |
| Arg | 6.66 | 11.81 | 3.31 | 6.20 | 7.90 | 11.18 | 8.17 |
| Pro | ND | ND | ND | 4.90 | 3.90 | 5.19 | 2.00 |
- a ND, no data.
The AA composition of the biofilm in the Yenisei River generally had many similarities with that of laboratory cultures of the green alga Scenedesmus quadricauda, the cyanobacterium Synechococcus sp. and the periphytic marine diatoms, Amphora and Navicula (Table 6). However, the percentages of Asp, Val and Leu were higher, but percentages of Cys, Met, Tyr, Lys and Arg lower, in the river biofilms than in the marine periphytic diatoms Amphora and Navicula (Table 6). Percentages of Met and Lys in the river biofilms were lower than those in the laboratory cultures of Scenedesmus and Synechococcus sp. and in the marine diatoms Amphora and Navicula (Table 6). Thus, in contrast to the conventional view (Ahlgren et al., 1992; Brown et al., 1997; Muller-Navarra, 2008), AA profiles of diverse microalgae have a number of differences.
AA imbalance between biofilm and the macroinvertebrates
Do amino acids in the food constrain the growth of the zoobenthos and fish? The nutritional value of food is commonly considered to be high if its composition of essential amino acids is close to that of the consumer (Brown et al., 1997; Conceição et al., 2003). Percentages of many essential amino acids in the biofilms were actually higher than in benthic animals. However, the mean percentages of essential AAs such as Lys and His in the biofilm were significantly lower than those in the zoobenthos. Evidently, to accumulate that higher percentage of Lys, the benthic animals need to consume species of algae with a comparatively high content of this essential amino acid. Alternatively, there might be differences in assimilation of the EAA by invertebrates.
In addition to qualitative composition (percentages), absolute amounts of AA in epilithic biofilms are evidently important in satisfying the nutritional requirements of consumers. Interestingly, our quantitative data showed two opposite patterns. Pools (standing stocks) of amino acids per unit area of river bed accounted for by the epilithic algae and cyanobacteria were higher, on average, than those in the dominant benthic animals, while the AA content per unit (carbon) biomass was higher in the zoobenthos than in the biofilm. Thus, in future, it would be worth comparing the production of AA by biofilms with the growth requirements of zoobenthos. In general, our present data indicate that both a qualitative and quantitative limitation of growth of the zoobenthos by AA composition and contents in the biofilms might occur.
AA composition of the zoobenthos
Variations in the specific nutrient content of herbivores, both between and within species, are generally smaller than the variation in autotrophs (Sterner & Hessen, 1994; Lauridsen et al., 2012). The AA composition of aquatic invertebrates is also considered to be relatively invariable (Cowey & Corner, 1963; Cowgill et al., 1986; Guisande, Riveiro & Maneiro, 2000). To check this latter proposition, we compared our data with the available literature on various aquatic invertebrates, primarily marine and freshwater zooplankton (Table 7). For instance, the percentages of Val, Ala, Glu, Phe, Gly, Ser, Asp, Ile, Leu and Lys in gammarids, trichopterans and ephemeropterans in the Yenisei River were very similar to those in the three species of freshwater cladocerans and copepods (Ventura & Catalan, 2010; Table 7). Percentages of Gly, Lys and Leu in marine copepods were similar to those in the zoobenthic taxa (Guisande et al., 1999; Table 7). Percentages of Phe in the Ephemeroptera larvae, Ile in the trichopteran larvae, Ser in the chironomid larvae and Val in the gammarids were equal to those in the marine copepods. Thus, many similarities between AA profiles of taxonomically and ecologically different aquatic invertebrates were found.
| Group (taxa) | Eulimnogammarus viridis | Apatania crymophila larvae | Chironomidae larvae | Ephemeroptera larvae | Euterpina acutifrons (Copepoda) | Cyclops abyssorum (Copepoda) | Daphnia pulicaria (Cladocera) | Diaptomus cyaneus (Copepoda) |
|---|---|---|---|---|---|---|---|---|
| Reference | Our data | Guisande et al.(1999) | Ventura & Catalan (2010) | |||||
| Asp | 10.73 | 11.18 | 12.82 | 9.06 | 8.7 | 9.4 | 10.3 | 9.8 |
| Thr | 5.91 | 6.07 | 5.94 | 5.7 | 4.9 | 5.2 | 6.4 | 4.9 |
| Ser | 5.15 | 5.16 | 4.79 | 5.27 | 4.7 | 4.4 | 5.5 | 4.7 |
| Glu | 14.51 | 12.99 | 14.59 | 12.57 | 13.7 | 13.0 | 13.1 | 13.8 |
| Gly | 5.82 | 5.47 | 4.88 | 5.2 | 6.3 | 6.1 | 5.3 | 5.2 |
| Ala | 7.34 | 6.59 | 5.57 | 9.2 | 7.6 | 8.1 | 6.9 | 8.6 |
| Val | 5.59 | 6.37 | 5.43 | 6.21 | 5.5 | 6.0 | 6.4 | 5.8 |
| Cys | 0.12 | 0.16 | 0.13 | 0.17 | NDa | 1.3 | 0.9 | 1.1 |
| Met | 0.27 | 0.29 | 0.66 | 0.23 | ND | 1.7 | 1.9 | 1.9 |
| Ile | 4.55 | 5.00 | 4.99 | 4.42 | 5.0 | 4.4 | 4.8 | 4.3 |
| Leu | 7.94 | 8.00 | 7.41 | 7.55 | 7.2 | 7.5 | 8.3 | 7.8 |
| Tyr | 10.08 | 8.58 | 8.33 | 11.64 | 7.8 | 7.6 | 4.9 | 6.7 |
| Phe | 5.19 | 5.07 | 5.64 | 3.94 | 3.6 | 4.4 | 5.1 | 4.2 |
| His | 3.24 | 3.52 | 3.39 | 3.39 | 2.7 | 2.0 | 2.0 | 2.2 |
| Lys | 7.19 | 7.18 | 8.17 | 6.61 | 7.4 | 6.7 | 6.1 | 7.5 |
| Arg | 6.36 | 8.37 | 7.23 | 8.83 | 9.1 | 6.8 | 6.8 | 6.1 |
- a ND, no data.
On the other hand, there is evidence of considerable variation in AA composition of aquatic invertebrates, attributable to various factors. For instance, reproductive status, gender and ontogenetic changes were important intraspecific sources of variability in AAs (Helland et al., 2003; Brucet et al., 2005). Some authors have suggested using the amino acid composition of a species as an indicator of its trophic niche and of the adaptations of the species to its abiotic environment (Guisande, 2006). Our data have also revealed some differences in AA profiles among the macroinvertebrates in the Yenisei River, that is, gammarids and several insect larvae. Besides phylogenetic factors, these differences might be caused by differences in diet (see below). Moreover, percentages of Asp, His, Thr, Tyr and Phe in all the benthic animals in this study were higher, and the percentage of Gly slightly lower, than those in marine copepods (Guisande et al., 1999; Table 7). Marked differences were found in the percentages of His, Tyr and Met between the river zoobenthos and freshwater zooplankton (Ventura & Catalan, 2010; Table 7). Thus, we conclude that phylogenetic and ecological factors might result in some variation in AA composition of the freshwater zoobenthos.
Larvae of the genus Apatania (Trichoptera) are scrapers that take periphytic microalgae (Becker, 1994). Indeed, using fatty acid biomarker analysis, we have found earlier that trichopterans in the Yenisei River primarily consumed diatoms and green algae (Sushchik et al., 2003). Previously research at our site of A. crymophila, using δ15N, showed that it was a primary consumer (Kalachova et al., 2011; Gladyshev et al., 2012a,b). Species of Eulimnogammarus (Philolimnogammarus) are known to be generalist omnivores (Morino et al., 2000). In previous studies, we found that in the Yenisei River, E. viridis consumed epilithic microalgae (Sushchik et al., 2003), but was also omnivorous (Kalachova et al., 2011; Gladyshev et al., 2012a,b). In contrast to the trichopteran larvae and gammarids, which were represented by single species, chironomid and ephemeropteran larvae each consisted of several species. However, the dominant species of chironomids, P. olivacea and P. branickii, as well as the dominant ephemeropterans, E. ignita and E. aurivillii, are thought to consume epilithic biofilm (Kalachova et al., 2011).
AA imbalance between the zoobenthos and the fish
Analysis of the amino acid content of the zoobenthos is important in determining the nutritional value of various prey species for fish. The successful culturing of invertebrates and fish has often been linked to dietary EAAs, with an emphasis on Lys and Met (e.g. Conceição et al., 2003; Li et al., 2009a; Helland et al., 2010). The mean percentage of Lys in the zoobenthos of the Yenisei River (7.4%) was practically the same as published values of various species of aquatic invertebrates: freshwater prawns Macrobrachium rosenbergii (7.8%) (Tidwell et al., 1998), mussels Mytilus coruscus (7.7%) (Li, Li & Li, 2010) and some other invertebrates (Table 7). The percentage of Lys in the dominant fish species of this stretch of the Yenisei (Siberian grayling) was significantly higher than that in zoobenthos. Thus, a conventional view (Conceição et al., 2003; Li et al., 2009a; Helland et al., 2010) would be that the grayling of the Yenisei River have a food imbalance for Lys. Moreover, there was an imbalance in the percentage of another essential AA, Leu, between the zoobenthos and the grayling (Fig. 6). In contrast, the percentage of Met in the zoobenthos of the Yenisei River (mean: 0.4%) was substantially lower than published data, which ranges between 1.7 and 3.9% (Tidwell et al., 1998; Aragão et al., 2004; Ventura & Catalan, 2010) with an extreme value of 7.3% (Li et al., 2010). Nevertheless, from our data, there was no significant imbalance in the percentage of Met between the zoobenthos and fish.
Nutritional value of the invading gammarids and indigenous prey
Over the last 50 years, the gammarid E. viridis (from Lake Baikal) has invaded this stretch of the Yenisei (Fig. 1) and replaced the indigenous chironomids and trichopterans as the dominant taxon in terms of biomass (Gladyshev & Moskvicheva, 2002). Our data suggest that invasion by the gammarids has resulted in a decline in the food quality for fish, particularly in terms of two essential AAs, Lys and Leu (Fig. 6). A high percentage of essential AAs in the food is known to be a key for fish growth (e.g. Peres & Oliva-Teles, 2006). The fraction of essential AAs in the trichopteran and the chironomid larvae was higher than in the gammarid. Thus, it is reasonable to suppose that the invasion of the gammarid resulted in an overall decrease in nutritional quality of the zoobenthos in this section of the river, regarding the essential AAs.
In conclusion, AA profiles of epilithic biofilms were not invariant during the season, but were dynamic, probably because of the succession of epilithic species. The importance of these seasonal variations in AA for consumers should be evaluated in future. In general, there was an imbalance in the percentage of the essential amino acids, Lys and His, between the biofilm and the zoobenthos. Moreover, the zoobenthos had significantly higher AA contents per unit carbon than the microalgae and cyanobacteria. Thus, our data demonstrate a potential constraint on invertebrate production due to food quality. We also found an imbalance in the percentages of two essential AAs, Lys and Leu, between the dominant fish species, Siberian grayling, and their food, gammarids and larval chironomids and trichopterans. The gammarids, E. viridis, which are recent invaders in this stretch of the river, were of a considerably lower nutritional value than the indigenous taxa.
Acknowledgments
This work was supported by the project B-15 of the Siberian Federal University carried out according to Federal tasks of Ministry of Education and Science of Russian Federation. We are sincerely grateful to four anonymous reviewers and to the Editor, Professor Alan G. Hildrew, for their very helpful friendly comments to significantly improve the manuscript.




