
Patterns of density dependence in growth, reproduction and survival in the invasive freshwater snail Pomacea canaliculata in Japanese rice fields
Summary
- Patterns of density dependence in growth, reproduction and survival are important for predicting the population dynamics of a species. The patterns may change with environmental factors, such as the harshness of winter, but very little is known about such patterns and their mechanisms in unmanipulated natural populations of invasive animal species.
- We studied the extent of density dependence in the growth, reproduction and survival of an invasive freshwater snail, Pomacea canaliculata, in rice fields in Nara (cold district) and Kumamoto (warm district), Japan, over 2- and 1-year periods, respectively.
- In both areas, growth was negatively density dependent within the same generation, and the density of snails in the parental generation negatively affected the growth of offspring. The number of eggs per unit area was independent of adult density, suggesting eggs per adult female were few at high densities. Survival over the cold winter of 2005–2006 was independent of density in Nara. However, survival over the warm winter of 2006–2007 in both Nara and Kumamoto was negatively density dependent.
- Irrespective of the various negative density-dependent patterns, population density tended to show positive correlations with the density of the previous generation. This appears to reflect the substantial capacity of this snail to resist extremely low densities due to the various negative density-dependent patterns rather than indicating susceptibility to extinction at low densities.
Introduction
Negative density dependence, whereby individuals reduce growth, reproduction or survival at high densities, generally contributes to the stability of a population. This process has been documented in many animal populations, mainly in captivity or under controlled field conditions (e.g. fish, Amundsen, Knudsen & Klemetsen, 2007; insects, Greig & Mcintosh, 2008; crustaceans, Jenkins, Murua & Burrows, 2008; gastropods, Baur, 1988; Carlsson & Brönmark, 2006). In other cases, critical life-history traits, such as survival in harsh winters, may be independent of density. These density-dependent and density-independent processes interact to determine the population dynamics of a species (Lack, 1966; Courchamp, Clutton-Brock & Grenfell, 1999).
The major mechanisms of negative density dependence are competition for common resources and predation (Hixon & Carr, 1997; Amundsen et al., 2007). The relative importance of key factors is expected to vary under natural conditions; thus, the patterns of density dependence in critical life-history traits may vary according to factors such as winter temperature (Lack, 1966; Forchhammer et al., 1998) or population density (Imre, Grant & Cunjak, 2005). However, such patterns of density dependence and their underlying mechanisms are not well understood in field populations, except for some well-studied animals (mammals, Forchhammer et al., 1998; birds, Lack, 1966; Ekman, 1984; fish, Hixon & Carr, 1997; Lobon-Cervia, 2007; gastropods, Baur, 1988; Stoner & Ray-Culp, 2000) because of the difficulty in monitoring either many populations at a time or a few populations for a long time.
For invasive species, patterns of density dependence are important for predicting the establishment and maintenance of introduced populations (Courchamp et al., 1999; Taylor & Hastings, 2005). For instance, many highly invasive species have a substantial capacity to adjust their growth or reproduction (Catford, Jansson & Nilsson, 2009). However, very little is known about the pattern and process of density dependence and potential mechanisms in unmanipulated field populations of invasive animals.
The apple snail Pomacea canaliculata (Lamarck, 1822) (Caenogastropoda: Ampullariidae) is a freshwater snail indigenous to tropical, subtropical or temperate South America (Martín, Estebenet & Cazzaniga, 2001; Martín & Estebenet, 2002). This snail invaded many Asian countries in the 1980s and voraciously attacks rice seedlings and other aquatic plants, having a substantial impact on local ecosystems (Carlsson, Brönmark & Hansson, 2004; Joshi & Sebastian, 2006). The size of the snails at maturity differs greatly according to environmental factors such as food availability (Estoy et al., 2002a,b; Tamburi & Martín, 2009), meaning that density-dependent processes are likely to operate in this species. In fact, negative density-dependent growth and reproduction have been experimentally shown in P. canaliculata and other Pomacea species (Tanaka et al., 1999; Carlsson & Brönmark, 2006; Conner, Pomory & Darby, 2008). Additionally, low temperature is the major cause of mortality (Shobu et al., 2001; Yoshida et al., 2009), and winter mortality is size dependent, with mid-sized snails of 7.5–15 mm shell height surviving best (Yoshida et al., 2009). Thus, overwintering success may also be density dependent: the density during summer and autumn may affect the overwinter survival rate by affecting growth rates during the rice-growing season.
We studied the density-dependent processes of P. canaliculata under field conditions in rice fields without any experimental manipulation. Rice fields are suitable to study density-dependent processes of aquatic species because each field can be regarded as an almost independent replicate, at least in the short term. We monitored snail densities for 2 years in a cold district (Nara) and for 1 year in a warm district (Kumamoto).
Methods
Study sites
Populations of P. canaliculata were monitored in two districts, each within a 1-km2 area in Kashiwagi, Nara, Honshu Island (34°40′N, 135°47′E), and Shimo-suzurikawa, Kumamoto, Kyushu Island (32°51′N, 130°41′E). Although the annual lowest temperatures were similar in both districts (between −3 and −5 °C), the average winter temperature (from October to March) was lower in Nara than in Kumamoto: 7.7 °C in 2005–2006 and 9.3 °C in 2006–2007 in Nara and 12.2 °C in 2006–2007 in Kumamoto (Yoshida et al., 2009).
Most rice fields in the study areas are flooded from June to September, and the fields are dried once (mid-term drying) for 2–3 weeks from July to August. The entire schedule is 10–20 days earlier in Nara than in Kumamoto. In response to this seasonal schedule, a small number of young P. canaliculata survive over the winter season and begin reproducing in June–July. New hatchlings appear from July, and some grow to 20 mm or more by September (Yoshida et al., 2009). The hatchlings bury themselves in the soil until the following June, but the survival rate is rather low in the study areas (0.2% in 2005–2006 and 0.9% in 2006–2007 in Nara and 9.0% in 2006–2007 in Kumamoto; Yoshida et al., 2009). Almost no snails survive a second winter. Few predators of the apple snail are found in Japanese rice fields (Yusa, Sugiura & Wada, 2006; Yamanishi et al., 2012), and we rarely observed predators of the snail in our study fields, with the exception of some dragonfly larvae (mainly Pantala flavescens) after the mid-term drying.
Sampling methods
Our methods were fully explained in Yoshida et al. (2009). To reiterate them briefly, we selected 16 rice fields in Nara in 2005 and collected apple snails once or twice a month when the fields were flooded between June and September. From July 2006 to June 2007, we increased the sampling fields to 28 due to a low density of snails. Similarly, we sampled snails in 13 fields in Kumamoto, from July 2006 to June 2007 when the fields were flooded. Fields in Kumamoto (999–3006 m2) were larger than fields in Nara (308–1385 m2). We used the data collected in June, July and September in this study. We avoided choosing rice fields where many snails appeared to have been killed by pesticides.
Snail density was estimated using five 1 × 1 m quadrats set along the levee in each field, and all snails ≥5 mm in shell height were collected. When the density of snails was extremely high (more than 50 individuals m−2), we sampled small snails of 5–10 mm using five smaller quadrats of 0.2 × 0.5 m. The snails were released into the original fields after measurement. In addition, the maximum length of each egg mass found in the 1 × 1 m quadrats was measured, and the number of eggs was estimated using the following regression equation: EN = 166.4 EL – 404.5, where EN is the estimated egg number, and EL is the maximum length (mm) of the egg mass transformed to a natural logarithm (Yoshida et al., 2009).
We studied the plant biomass to assess the food availability for the apple snails in mid-July and mid-September 2006 in Nara. We also estimated the plant biomass in Kumamoto, but almost no plants were collected there due to the high snail densities. Hence, the plant data in Kumamoto were excluded from the analysis. We used 12 of the 16 rice fields that we observed from 2005 and where water was present at that time. Five quadrats of 0.5 × 0.5 m were set adjacent to five larger quadrats for snail sampling, and all the plants in the quadrats were collected (weeds and algae, except for the rice plants that were too large to be attacked by the snail). Most of the plants collected in this study are utilised by apple snails as food (e.g. Lemna paucicostata and Chara braunii). Whole plants were collected because the snail often eats the underground part (personal observations). The plants were washed carefully in the laboratory and dried for 48 h at 60 °C, and dry biomass was determined.
Data and statistical analysis
Snail density was estimated following Yoshida et al. (2009). To summarise, we used the average value of the five 1 × 1 m quadrats as the snail density estimate; when snail density was extremely high, we used data from five smaller (0.2 × 0.5 m) quadrats for snails smaller than 10 mm. We averaged the data from the two censuses just after the field was flooded (in June and July for 2005 and 2006, and in June for 2007) to represent snail density for each field because densities were very low (Watanabe et al., 2000) and a large sampling error was likely.
After the mid-term drying, some newly hatched snails became so large that they were indistinguishable from the snails in the overwintered generation. As survival during summer was high (more than 90%; Yoshida et al., 2009), we postulated that all the overwintered snails survived the summer. The shell heights of the offspring snails were calculated from the size–frequency relationship in September by excluding the overwintered snails, which were presumed to always be larger than the newly hatched snails. Negative density estimates due to sampling errors (for one field in 2005 and two fields in 2006) were excluded from analysis. Although we assumed that all the overwintered snails survived the summer season, few major results differed if we assumed none of the overwintered snails survived (Supporting Information Fig. S1).
To test the presence of density-dependent processes, we used structural equation modelling (Shipley, 2000), followed by a model selection procedure. The initial model (Fig. 1) included (i) the average density of overwintered snails in June and July; (ii) their shell height in July, a time when their growth almost ceased (Yoshida et al., 2009); (iii) the number of eggs m−2 in July, a time when egg density is highest (Yoshida et al., 2009); (iv) the density of newly hatched snails in September; (v) their shell height in September and (vi) snail density in the following June and July. The data were separately analysed for Nara and Kumamoto and for 2005 and 2006 in Nara. The best-fit model was selected based on the minimum Akaike information criteria (AIC) value (McCarthy, 2007).
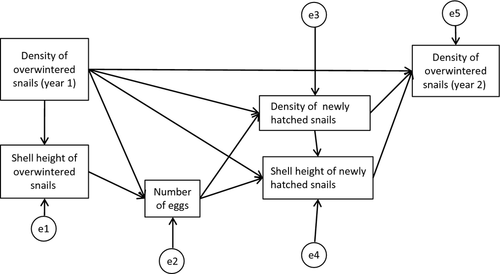
Additionally, to address the factors affecting these density-dependent patterns, the effect of plant biomass (in July and September 2006, Nara) on shell height of the snails was analysed using both simple and multiple regressions. In the multiple regressions, snail density in the parental generation and plant biomass recorded in September were used as the independent variables, and shell height of newly hatched snails was the dependent variable. The effect of snail density in autumn on overwinter survival was analysed using simple regression. The density data were converted to common logarithm (decimal logarithm) after adding 0.5 to the original values (Yamamura, 1999). Survival rate was calculated as the snail density after winter divided by the density before winter (both untransformed values) within the same field. All variables were standardised to calculate the standard regression coefficient. Statistical tests were conducted using AMOS 20 (IBM software, New York, NY, U.S.A.) or JMP version 8 (SAS Institute Inc., Cary, NC, U.S.A.).
Results
Patterns of density dependence
In the season of 2005–2006 in Nara, the best-fit model (AIC = 32.90) indicated that the density of overwintered snails in June–July 2005 negatively affected their own shell height in July (Fig. 2a; Fig. 3a). The shell height of the newly hatched snails in September was negatively affected by their own density and by the density of the overwintered snails (i.e. their parental generation). Unsurprisingly, the density of the overwintered snails in June–July 2006 was positively affected by that of the snails in September 2005 (within the same generation). Other relationships were not significant or selected in the final model.
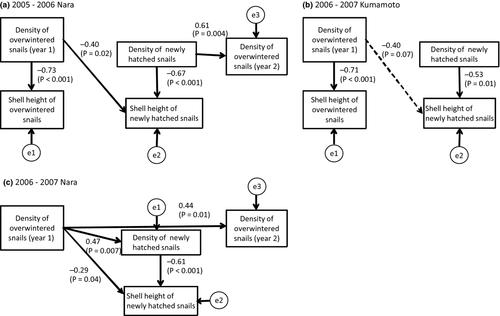

In the 2006–2007 season, the best-fit model (AIC = 26.27) indicated that shell in Nara height of the overwintered snails was not affected by their own density (Fig. 2b; Fig. 3b), whereas shell height of newly hatched snails was negatively affected by their own density and by the density of overwintered snails. In contrast to the first year, the density of the overwintered snails was not affected by their own density before winter. Moreover, the density of the parental snails (June–July 2006) positively affected the density of their offspring before overwintering (September 2006) (Fig. 2b), and the effects of the parents remained significant even after the winter season (June 2007) (Fig. 2b; N = 28, r = 0.443, P = 0.01).
Similar to the results for Nara in 2005, the best-fit model (AIC = 24.89) indicated that shell height of overwintered snails was negatively affected by their own density in Kumamoto in the 2006–2007 season (Fig. 2c; Fig. 3c). Shell height of the snails in the next generation was negatively affected by their own density. Although not significant, the density of the overwintered snails tended to suppress the shell height of the snails in the next generation. As for Nara in 2006–2007, density of the overwintered snails was not affected by their own density before winter. The density of the parental snails had no effects on the density of their offspring, either before or after winter (Fig. 2c).
Factors affecting the patterns of density dependence
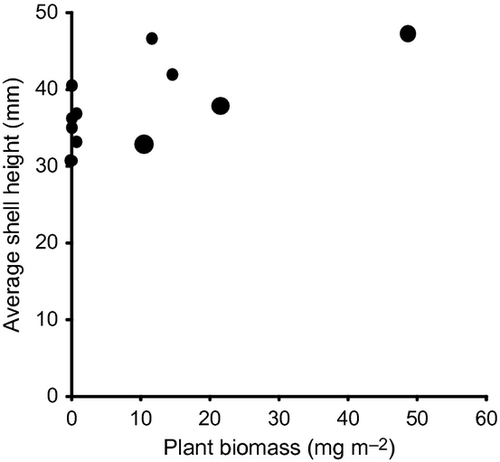
There was a positive effect of plant biomass on the size of the snails in the overwintered generation (N = 11, r = 0.620, P = 0.04; Fig. 4) but not in the newly hatched generation (N = 12, r = 0.168, P = 0.60). However, a multiple regression showed that both snail density of the overwintered generation and plant biomass affected the size of the newly hatched snails (N = 12, overwintered population density: F = 26.24, P < 0.001; plant biomass: F = 20.61, P = 0.001).
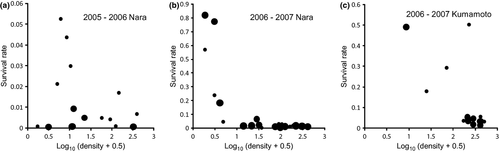
Snail density in September had a negative effect on overwinter survival rate in Nara and Kumamoto in 2006–2007, a mild winter (Fig. 5b, c; Nara: N = 26, r = −0.723, P < 0.001; Kumamoto: N = 11, r = −0.655, P = 0.03). No significant relationship was detected between snail density and overwinter survival rate in Nara for 2005–2006, a time when the temperature was low (Fig. 5a; N = 14, r = −0.239, P = 0.42). There was no correlation between the overwinter survival rates in each field between the 2 years (2005–2006 and 2006–2007) in Nara (N = 14, r = 0.210, P = 0.47).
Consequence of density dependence
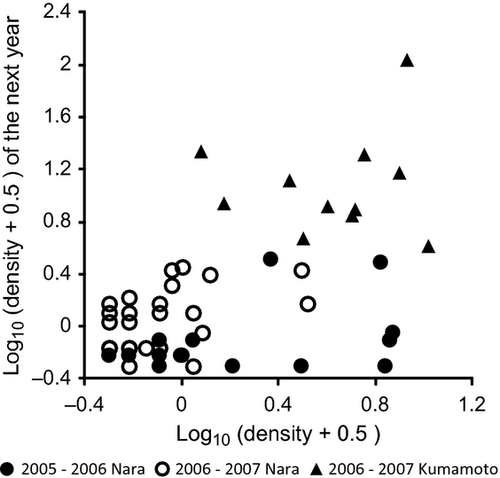
As explained above, there was a positive relationship between snail density in June–July 2006 and density in June–July 2007 in Nara (Fig. 2b; Fig. 6). No such relationship was detected between 2005 and 2006 in Nara (N = 15, r = 0.368, P = 0.18) or between 2006 and 2007 in Kumamoto (N = 11, r = 0.078, P = 0.82). However, when the data for Nara and Kumamoto were analysed together, snail density had a positive effect on density the next year (N = 54, F = 4.66, P = 0.03) as did the effect of study site/year (ancova; F = 43.21, P < 0.001; Fig. 6).
Discussion
This study demonstrated various density-dependent life-history traits of the invasive freshwater snail Pomacea canaliculata over 2 years in rice fields in two districts. The snails generally attained a large body size at low densities, although egg production per unit area was independent of snail density. Overwintering survival rate was also density dependent in warm winters but not in the cold winter of 2005–2006 in Nara. Overall, there was a positive relationship between after-winter population densities in two successive years. In the following discussion, we consider the possible mechanisms responsible for these patterns and the implications of these density-dependent processes for the population dynamics of this invasive species. As this study was conducted in rice fields typical in western Japan, care must be taken in applying these results to snail populations in ponds, creeks or other waterbodies, or even in rice fields with very different practices (e.g. growing two rice crops a year).
There was negative density-dependent growth both in the overwintered generation in June–July (2005 in Nara and 2006 in Kumamoto) and in the summer newly hatched generation in September (2005 and 2006 in Nara and 2006 in Kumamoto). Density-dependent growth in Pomacea snails has been documented in experimental populations (Tanaka et al., 1999; Conner et al., 2008). The present study confirms this and shows that such density-dependent growth occurs in multiple field populations over years. However, no density dependence was detected in 2006 in Nara, a period when density was remarkably low due to low overwinter survival (0.2%; Yoshida et al., 2009). Thus, growth is density dependent at intermediate or high densities but independent at low densities.
Most likely, such density dependence was due to food limitation. Significantly positive relationships were detected between plant biomass and shell height of the snails in the overwintered generation and also in the newly hatched generation in Nara in 2006. These results suggest that the plant biomass is a limiting factor for the growth of the apple snail in field populations. The lack of density dependence at low densities (when food limitation is not severe) supports this interpretation. Laboratory experiments also show that growth is limited by low food availability in this species (Estoy et al., 2002a,b; Tamburi & Martín, 2009).
The density of overwintered snails negatively affected the size of newly hatched snails in 2005 and 2006 in Nara. Carlsson & Brönmark (2006) reported that both adult and juvenile P. canaliculata utilise common food plants and that adults and juveniles compete with each other for food (although the growth of adults rather than juveniles was affected more in this case). The growth of juveniles of the congener P. paludosa is reduced when reared with adult conspecifics in the laboratory (Conner et al., 2008). In our study, food availability in the field in September was very low, as almost no plants were collected in most rice fields, either in Nara or in Kumamoto. However, in P. canaliculata, the size of eggs does not differ according to food availability (Estoy et al., 2002b; Tamburi & Martín, 2011). Thus, the negative effects of overwintered snails on the newly hatched ones are most likely due to competition for common food.
Although the effect of density of overwintered snails on the number of eggs per unit area was included in the initial model, this effect was not significant or retained in the best-fit model in either year (2005 or 2006) or district (Nara or Kumamoto). This means that total egg production per unit area was independent of snail density and therefore that egg production per individual female was low at high snail densities. Such density-dependent egg production has been reported in a field experiment involving this snail (Tanaka et al., 1999).
Over the winter of 2006–2007, survival rate was negatively density dependent both in Nara and in Kumamoto. However, survival did not depend on snail density in Nara in 2005–2006, a period when the temperature was low and few snails survived (0.2%; Yoshida et al., 2009). Thus, in mild winters, survival is density dependent, whereas it is density independent in harsh winters. Such shifts between density dependence and independence in overwinter survival rate have rarely been documented in field populations of animals, although Lack (1966) did suggest this scenario for birds.
Three factors may be responsible for negative density-dependent survival over the winter season. First, overwinter survival is size dependent in this snail, with highest survival in mid-sized snails (7.5–15 mm shell height; Yoshida et al., 2009). However, at high snail densities, most snails cannot grow to this size and die during winter. Second, food availability per individual is low at high densities, and snails might not retain enough energy for overwintering. Third, snails bury themselves in the soil when the fields are drained before winter, and they are often observed to gather in depressions and, thus, tend to be on top of one another in the soil. Accordingly, when snail density is high, many individuals might not be able to bury themselves deep enough to survive the winter. Further study is needed to determine the relative importance of these three factors. In contrast, the lack of negative density dependence in survival rate in Nara in 2005–2006 might be interpreted as a stochastic event: the low temperatures killed almost all snails in fields with low snail densities, resulting in 0 survival rates (i.e. <0.1 snails m−2) in these fields.
Irrespective of the many negative density-dependent processes, the effect of overwintered snail density on the density in the next year was positive between 2006 and 2007 in Nara and when all the data were analysed together. It is possible that the positive relationship was an apparent relationship caused by the presence of rice fields that are suitable for overwintering and those that are unsuitable for overwintering, which are constant over years. However, this interpretation is not supported in Nara because the overwintering survival rate in each field was not correlated between the 2 years, suggesting that overwintering success of snails was not a characteristic of the fields. Thus, the relationship represents a real positive relationship between densities in two successive generations. However, this should not be regarded as evidence for the Allee effect, in which individuals respond positively to an increase in density at low densities (Courchamp et al., 1999; Stephens, Sutherland & Freckleton, 1999; Taylor & Hastings, 2005; Jerde, Bampfylde & Lewis, 2009). Ironically, the positive relationship appears to be due to the considerable capacity of this snail to resist extremely low densities rather than the weakness of negative density dependence (Jerde et al., 2009). Due to the various negative density-dependent processes, the snails do not become extinct, even at very low densities (<0.1 overwintered individual m−2 compared with the highest density of 107 in this study). The density-dependent processes are strong enough to maintain the snail populations, even at extremely low densities, but they might not be strong enough to fully recover from extremely low population densities in one generation, resulting in the positive relationship in densities of successive years.
In summary, the apple snail responded to population density in various ways in terms of growth, reproduction, survival and population dynamics. The response in each life-history trait tended to be negatively density dependent, although such responses may differ among years or localities with different conditions. Such density-dependent patterns have implications for the control of this invasive species. Various negative density-dependent mechanisms stabilise the population dynamics of this species (Tanaka et al., 1999; Wada et al., 2004; Yoshida et al., 2009). However, the presence of positive relationship between densities in successive years suggests that the population increase is not enough to fully recover from the low densities. Although this is a promising result when considering control of this snail, care must be taken because the observations might have resulted from the snail's considerable resistance, rather than vulnerability, to extinction at extreme low densities.
Acknowledgments
We thank Keiji Wada, Masaya Matsumura, Keiichiro Matsukura, Yoko Yamanishi, Akiko Hara, Manami Yamada, Noriomi Fujimori and members of the Laboratory of Population and Community Ecology at Nara Women's University for their valuable discussions and assistance. We thank the editor and two anonymous referees for constructive comments.




