
Clear, crashing, turbid and back – long-term changes in macrophyte assemblages in a shallow lake
Summary
- During eutrophication, submerged macrophytes in temperate European shallow lakes are thought to undergo a sequence from seasonally ‘stable’ conditions characterised by high water clarity in spring and summer, through ‘crashing’ conditions where the water is clear in spring but dominated by phytoplankton in late summer, to ‘turbid’ conditions with year-round phytoplankton dominance. However, it is not known whether this sequence is reversed during re-oligotrophication and whether this contributes to the often observed delay in macrophyte recovery during lake restoration.
- We analysed long-term (100 years) data on macrophyte species presence, maximum colonisation depth, Secchi depth and seston concentration in shallow Lake Müggelsee during eutrophication from around 1900 and during re-oligotrophication that started in 1990. The current clonal diversity of the dominant species (Potamogeton pectinatus) was investigated to determine whether vegetative dispersal was predominant during its re-establishment.
- During eutrophication, Lake Müggelsee went through a crashing phase for c. 70 years with a gradual decline in macrophyte species diversity from c. 24 to 5 species. From around 1970, the lake became turbid and was dominated by phytoplankton for the next 20 years. Following a reduction in external nutrient loading by 50% from 1990, spring clear-water conditions immediately re-appeared, and P. pectinatus started to re-establish from a few stands that had survived in very shallow areas. By 2011, species diversity had increased to 25 species and maximum colonisation depth had reached 3.2 m. Despite a continuing dominance of P. pectinatus, seasonally persistent (Ceratophyllum demersum) and late-season associated (Najas marina) species re-appeared suggesting potential for seasonally stable macrophyte conditions in future.
- Based on microsatellite analyses, more recently established P. pectinatus stands had lower genotype diversity and were comprised of only a small subset of genotypes from shallower areas, suggesting that vegetative dispersal was more important than seed dispersal for plant re-establishment. We argue that this prevailing reproduction by tubers in combination with negative effects of herbivory and periphyton shading, shown for P. pectinatus in earlier studies in this lake, contributed to the long duration of macrophyte re-establishment.
Introduction
Submerged macrophytes are a key component of shallow lake communities, and changes in their abundance have significant consequences for these ecosystems (Carpenter & Lodge, 1986). Submerged macrophytes contribute to the stabilisation of clear-water conditions, and their disappearance is often associated with the transition to phytoplankton dominance (Scheffer et al., 1993). Before complete macrophyte disappearance in European shallow lakes, a common macrophyte successional pathway during eutrophication is the transition from a diverse community with broad-leaved Potamogeton, Myriophyllum and Utricularia species and often containing abundant Characeae, to a less diverse community containing species tolerant of low light (e.g. Rintanen, 1996; Sand-Jensen et al., 2000; Vestergaard & Sand-Jensen, 2000; Sayer, Davidson & Jones, 2010b; Sayer et al., 2010a).
Sayer et al. (2010b) showed that reductions in macrophyte species richness can be accompanied by a decline in the number of plant reproductive strategies present. According to their scheme, so-called ‘stable’ lakes were associated with a long period of macrophyte dominance (May–September), and seasonal changes in phytoplankton abundance were dampened with low chlorophyll-a (<10–15 μg L−1) over the entire summer. In contrast, so-called ‘crashing’ lakes were dominated by species from Potamogeton pectinatus, P. pusillus and Zannichellia palustris (PPZ), and consistently showed a mid-summer crash in the plant population and thus only a short (c. 2 months) macrophyte-covered period. Seasonality of nutrient concentrations and chlorophyll-a in the crashing lakes was similar to turbid lakes without submerged macrophytes. Sayer et al. (2010b) suggested that PPZ species are capable of compressing their life cycle into a short early summer phase as stresses associated with abundant phytoplankton and periphyton populations start to mount in eutrophic lakes. In contrast, C. demersum and a number of Chara species show less growing season fluctuation in biomass and thus promote a longer period of macrophyte production. Consequently, they hypothesised that a shift from Chara–Ceratophyllum to PPZ dominance may often be accompanied by a substantial reduction in the seasonal duration of plant dominance and a greater tendency for incursions by phytoplankton, thus placing further pressure on remaining macrophytes. Sayer et al. (2010b) described a slowly enacted (10–100 years) feedback loop in nutrient-enriched shallow lakes whereby increases in algal abundance are associated with gradual losses of macrophyte species and hence different plant seasonal strategies. However, much less is known about the reverse development from phytoplankton to plant dominance during the recovery of shallow lakes from eutrophication, and re-establishment of submerged macrophytes during re-oligotrophication has often failed, or no consistent pattern has been observed (Jeppesen et al., 2005; Hilt et al., 2006).
We hypothesised that the suggested sequence during nutrient enrichment, from a seasonally ‘stable’ clear lake with ever present dense macrophyte cover to a turbid state without submerged macrophytes, will occur in reverse order during re-oligotrophication of shallow European temperate lakes. We also hypothesised that P. pectinatus, the dominant species during re-establishment, mainly reproduces from tubers which contribute to a slow increase in the area colonised. To test the first hypothesis, we used historical records (from the 20th century) and recent (1993–2011) survey data for macrophyte species presence, abundance and maximum colonisation depth as well as long-term monitoring data for Secchi depth, seston and nutrient concentrations available from shallow temperate Lake Müggelsee, Germany. We tested the second hypothesis by assessing the genotypic diversity of P. pectinatus stands in shallow (older) and deeper (more recently established) water of Lake Müggelsee using microsatellite screening and comparing it to other lakes with a contrasting macrophyte colonisation history.
Methods
Lake Müggelsee
Lake Müggelsee is a shallow (mean depth 4.9 m), polymictic lake in Berlin (Germany), with a surface area of 750 ha. It is flushed by the River Spree and has a catchment area of 7000 km2 composed of 36% forestry, 42% agriculture and 22% urban land (with 720 000 inhabitants). The retention time of the lake is about 6–8 weeks and mean discharge declined by around 34% between the periods 1979–1990 and 1997–2003. Total phosphorus (TP) loading changed from 5.9 via 3.6 to 2.8 g P m−2 year−1 and total nitrogen (TN) loading from 138 via 74 to 45 g N m−2 year−1 in 1979–1990, 1991–1996 and 1997–2003, respectively (Köhler et al., 2005). The high nutrient loading to the lake in the 1970s and 1980s resulted in hypertrophic conditions (Behrendt & Nixdorf, 1993), and its submerged vegetation disappeared relatively abruptly in 1970 (Barthelmes, 1978). Following the reduced nutrient loading since 1990, macrophyte cover has been re-establishing.
Turbidity, nutrient concentrations and phytoplankton abundance
Lake Müggelsee has been sampled weekly (from spring to autumn) or biweekly (during winter) since 1979. Secchi depth has been recorded at the deepest point of the lake. From 1979 to 1986, water samples were taken from depths of 0.5, 4 and 7 m at the deepest point of the lake using a 5-L Friedinger sampler, and mixed samples were analysed. Since 1987, integrated volumetrically weighted samples have been taken, based on 21 subsamples from five stations (for a detailed description of sampling see Driescher et al., 1993). Phytoplankton biovolume was analysed using an inverted microscope (see Köhler & Hoeg, 2000). From 1931 to 1990, daily measurements of total seston content were carried out by pouring 100 L of lake water through a plankton net (30 μm) and measuring the fresh volume of the isolated seston in an Imhoff funnel (Behrendt & Nixdorf, 1993). Since 1991, seston dry weight was determined weekly by filtering 300–400 mL water through pre-weighed cellulose acetate filters (0.45 μm; Sartorius, Göttingen, Germany) and drying at 105 °C. Secchi depth data for the periods 1908 and 1954 were available from Behrendt & Nixdorf (1993). Unpublished biweekly Secchi depth measurements at the Friedrichshagen Water Works (on the Northern shore of Lake Müggelsee) from 1931 to 1939 were provided by Horst Behrendt. Concentrations of total phosphorus (TP) and total nitrogen (TN) were analysed according to Anonymous (2003).
Macrophyte species diversity and maximum colonisation depth
Historical records of submerged and floating-leaved macrophyte species presence in Lake Müggelsee are based on data from the literature, herbaria and macrophyte surveys carried out in 1993 and 1999 published in Körner (2001). Recent data were added from surveys conducted in 2006 along the entire lake littoral using an aquascope and a rake and in 2011 by Scuba diving along 25 transects. Surveys without diving were always carried out during the clear-water phase (Secchi depths > 3 m) and took more than a week as the entire littoral was included. Given the substantial survey effort associated with the 2006 survey, the change in methodology to scuba diving in 2011 is not expected to have significantly increased the accuracy of the data on species number or maximum colonisation depth. Determination of species abundance in different depth zones followed the German method developed for implementation of the EU Water Framework Directive (Schaumburg et al., 2004). It was applied to 5 transects in 2006 and 25 transects in 2011. Abundance of species was estimated in three depth zones: 0–1, 1–2 and 2–4 m based on a five degree scale (1: very rare; 2: rare; 3: common; 4: frequent; and 5: abundant). Macrophyte abundance data were transformed into plant quantity estimates using the function plant quantity = (abundance)³ to reflect the three-dimensional development of aquatic plants. Species determination followed Van de Weyer & Schmidt (2011). Maximum colonisation depths of submerged macrophytes were recorded in surveys for 1993, 1999, 2006 and 2011.
Clonal diversity of P. pectinatus
We sampled P. pectinatus plants from five different sites along the western (sites 1 and 2) and north-eastern (sites 3–5) littoral zones of Lake Müggelsee (Table 1). Plants were taken from 50 cm water depth at all sites and from 100 cm depth at a subset of three sites (sites 3–5), resulting in eight sampling locations in total (Table 1). The sites at 100 cm depth had no plants prior to 2000 (Körner, 2001), and our aim was to determine whether the plants now present there had originated from vegetative propagation. Plants were collected randomly within a rectangle of 40 m length parallel to the shore and 2 m width. Tissue was stored at −20 °C for later analysis. From each sampling location, between 6 and 40 individuals were genotyped at nine microsatellite loci designed for P. pectinatus (Nies & Reusch, 2004). For comparability, at least 35 individuals were genotyped in both the shallow (50 cm) and deep (100 cm) locations at sites 3 and 5, with fewer samples from the other sites (Table 1). Genomic DNA was extracted using DNeasy plant kits (Qiagen, Hilden, Germany) and PCR-amplified using fluorescently labelled forward primers in 3 multiplex reactions following Nies & Reusch (2004). Fragments were separated on a 3500 xl Genetic Analyser (Applied Biosystems – Life Technologies, Darmstadt, Germany) and scored using GENOTYPER (Applied Biosystems). As in the study by Nies & Reusch (2005), microsatellite loci were effectively diploid despite P. pectinatus being hexaploid. Genetic diversity (measured as heterozygosity, He, observed heterozygosity Hobs, and allelic diversity, A) and genetic differentiation among sampling localities (FST) were calculated using ARLEQUIN v. 3.5.1.3 (Excoffier & Lischer, 2010). Genotype diversity was measured using Simpson's index of diversity corrected for finite sample size (see Hangelbroek et al., 2002).
| Site | Depth (cm) | n | H e | K | Genotype | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||||
| 1 | 50 | 6 | 0.303 | 1 | 6 | ||||||
| 2 | 50 | 30 | 0.464 | 4 | 19 | 9 | 1 | 1 | |||
| 3 | 50 | 40 | 0.328 | 4 | 36 | 2 | 1 | 1 | |||
| 4 | 50 | 8 | 0.296 | 1 | 8 | ||||||
| 5 | 50 | 40 | 0.421 | 3 | 3 | 34 | 3 | ||||
| 3b | 100 | 35 | 0.281 | 1 | 35 | ||||||
| 4b | 100 | 8 | 0.296 | 1 | 8 | ||||||
| 5b | 100 | 39 | 0.417 | 2 | 29 | 10 | |||||
| Total | 206 | 0.406 | 144 | 34 | 12 | 9 | 5 | 1 | 1 | ||
- Sites 3–5 were sampled at two depths (50 cm and 100 cm), and the deeper samples are labelled ‘b’. Reported below are the number of individuals genotyped (n), heterozygosity (He), number of genotypes (K) and the frequency of genotypes at each site.
Results
Turbidity, nutrient concentrations and phytoplankton abundance
Available data indicate that Lake Müggelsee had summer Secchi depths of up to 2 m at the beginning of the 20th century, but these declined soon after 1910 (Fig. 1). Between 1900 and 1970, the lake was characterised by relatively clear water in spring and low Secchi depth with cyanobacteria blooms in summer (Figs. 1 & 2). In 1970, a shift occurred in the phytoplankton community as indicated by substantial increases in both spring and summer seston concentrations and phytoplankton biovolume (Fig. 2). Turbid conditions prevailed from spring to summer as evident in the Secchi data until 1989 (Figs. 1 & 2). After 1990, Oscillatoriales disappeared, and a spring clear-water phase re-appeared (Fig. 1).
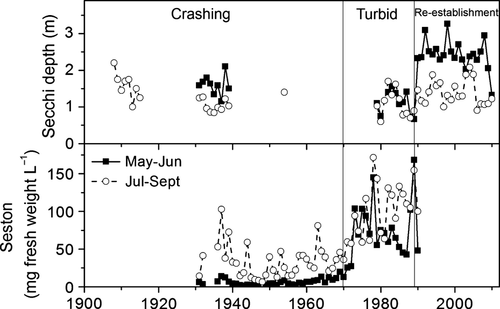
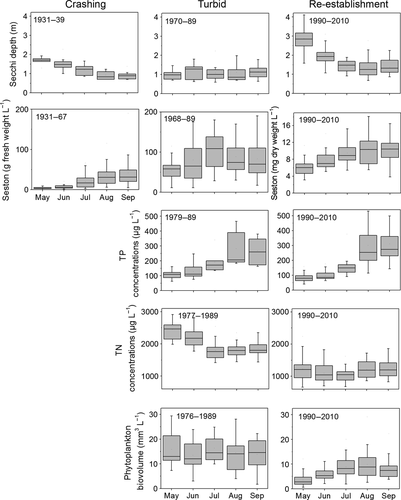
Nutrient concentrations were high during the hypertrophic phase (1970–1989) with TP concentrations of 110 (May) to 270 μg L−1 (Sept.) and TN concentrations between 2.5 and 1.9 mg L−1. Phytoplankton biovolumes were high from May to September (on average 15 mm3 L−1, Fig. 2). In response to reduced nutrient loadings, TP concentrations declined by about 25% in May and June between 1990 and 2010 (Fig. 2). However, due to enhanced P release from sediments, TP concentrations in August and September increased by 10% during this period. In contrast to TP, mean TN concentrations declined not only in May and June by on average 50% but also in summer by 58–66% (Fig. 2). Phytoplankton biovolumes were strongly reduced in May (77%) and June (56%), but also between July and September (40%) (Fig. 2).
Macrophyte species diversity and abundance
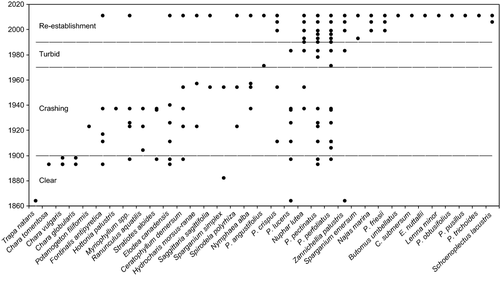
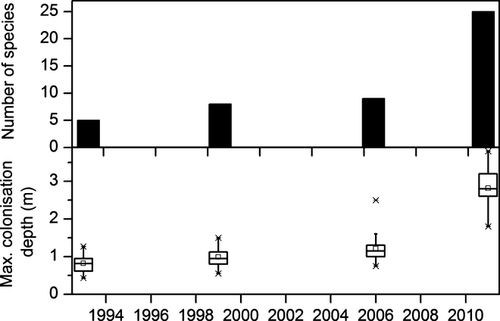
Total macrophyte species richness in Lake Müggelsee was difficult to assess for the period before 1990 due to a lack of surveys. However, assuming that all species recorded in the literature and from herbaria before 1990 occurred in the lake before 1900, a total of 24 species would have been present, including three species of Characeae (Fig. 3). Between 1900 and 1970, species diversity gradually declined, with Characeae disappearing first. Another 8 species were lost after 1940 including Fontinalis antipyretica, Myriophyllum spp. and Elodea canadensis. Potamogeton pectinatus, P. perfoliatus, Nuphar lutea and most probably Zannichellia palustris were the only species that survived the hypertrophic phase from 1970 to 1989 and expanded across the lake from 1990 (Fig. 3). In macrophyte surveys between 1993 and 2006, a maximum of seven species and low maximum colonisation depths between 1 m and 1.2 m were found (Fig. 4). In 2011, about 20 years after nutrient loading reduction, a sudden increase in maximum colonisation depth and species richness was evident with 25 submerged and floating-leaved macrophyte species recorded (Fig. 4). Four of these species had persisted throughout the turbid period, another 10 had been detected before macrophyte loss in 1970 and thus potentially re-occurred from propagule banks. Eleven species had not been recorded in the lake before (Fig. 3). Nine of 16 species that were detected in 2011 but were not present between 1990 and 2006 were found in shallow areas (0–1 m) indicating that the use of scuba diving was not responsible for their detection. Abundance data from 2006 and 2011 show that shallow areas were dominated by P. pectinatus, which did not change its relative abundance per transect. In contrast, the abundances of Najas marina spp. intermedia and C. demersum increased significantly, especially in deeper water at 2–4 m (Fig. 5).

Genetic diversity of P. pectinatus
Microsatellite screening identified 35 alleles (mean = 3.8 alleles per locus, SD = 1.9) and these were partitioned into seven genotypes in the 206 P. pectinatus individuals. Genotypes were separated by a mean of c. seven allelic differences (range 6–9), and thus, we are confident that these are not differences based on analytical error. Genotype 1 was found at all locations including nearly 70% of the samples (Table 1). All seven genotypes occurred in shallow (50 cm) locations whereas the deeper and thus more recently re-established locations (100 cm) contained only a subset: the predominant genotype 1 and, in one location, genotype 3 (Table 1). Total heterozygosity (mean He across populations and loci) was 0.406 (SD = 0.219). We observed a large number of heterozygous individuals in Lake Müggelsee (Hobs = 0.685). Allelic richness (A) was 2.15 (SD = 0.854) and genotype diversity (Simpson's index of diversity) was 0.522. Genetic differentiation among locations was low (FST < 0.05) with the exception of location 5a which was significantly different from all others (pairwise FST = 0.13–0.23, P < 0.01). This was also the only location that was not dominated by genotype 1 but was composed largely of genotype 2.
Discussion
Macrophyte development during eutrophication
Variation in summer Secchi depths between 1.5 and 2.3 m (Vorkastner, 1938) and the presence of Characeae and a diverse flora with 24 macrophyte species suggests seasonally ‘stable’ conditions sensu Sayer et al. (2010b) at the end of the 19th century. However, as in many other water bodies in the Berlin area, Characeae had largely disappeared from Lake Müggelsee by the end of the 19th century (see Körner, 2001). The available historical data indicate that subsequent eutrophication resulted in a typical seasonally ‘crashing’ scenario, with high Secchi depth in spring-early summer (May–June) and low Secchi depth in mid-late summer (July–September) beginning in the early 20th century and lasting for about 70 years. Since 1908, decreasing summer Secchi depths led to a gradual loss of macrophyte species, especially of seasonally persistent, potentially evergreen species. The aquatic moss Fontinalis antipyretica formed meadows down to 3–4 m depth in the early 20th century (Rehbronn, 1937) but declined at the end of the 1930s (Körner, 2001). Since 1931/32, the lake has been dominated by Potamogeton species which almost completely disappeared in 1970 (Barthelmes, 1978). The ‘crashing’ phase was followed by a rapid shift to a ‘turbid’ phase which persisted for around 20 years (c. 1969–89), when plants were largely absent; only a few stands of P. pectinatus, P. perfoliatus and N. lutea in very shallow littoral areas survived this era (Körner, 2001).
Macrophyte development during re-oligotrophication
The reduction in nutrient loading to Lake Müggelsee by 50% since 1989 resulted in lower TP concentrations during winter and spring, while during summer, P release from the sediments was favoured by reduced nitrate import. Therefore, the lake acted as a net P source for 6 years after the external load reduction despite a water retention time of only 0.1–0.16 years. Because of the likely limitation by P in spring and by N in summer, phytoplankton biovolume declined immediately after nutrient loading was reduced (Köhler et al., 2005). Spring water clarity became higher than during the ‘crashing’ phase, but the lake has remained turbid during summer. From 1990, submerged macrophytes started re-establishing, but species numbers were low, and 99% of the plant stands were dominated by P. pectinatus which occupied only shallow areas (<0.8 m) (Körner, 2001). Our data suggest that species characteristic of ‘crashing’ lakes, especially P. pectinatus, were the first to re-establish in Lake Müggelsee during re-oligotrophication. P. pectinatus also dominated in other previously turbid, temperate European lakes after phosphorus load reduction and no other internal measures (Germany: Kabus et al., 2007; Blüml et al., 2008; Hilt et al., 2010; Sweden: Blindow, 1992; Strand, 1999; The Netherlands: Scheffer, De Redelijkheid & Noppert, 1992; Van den Berg et al., 1999; Denmark: Schriver et al., 1995; U.K.: Phillips et al., 2005). This dominance may stem from its ability to survive turbid phases in very shallow areas, or from its ability to compress its whole life cycle into a few spring and early summer months.
The maximum colonisation depth only increased slowly in Lake Müggelsee and macrophyte biomass was low (Körner, 2001) indicating a hampering of macrophyte re-establishment. Herbivory by both waterfowl and fish in combination with periphyton shading have negatively affected P. pectinatus during its re-establishment (Körner & Dugdale, 2003; Roberts et al., 2003; Hilt, 2006). Prevention of re-establishment of P. pectinatus due to herbivory has also been shown for Danish Lakes Væng and Engelsholm (Lauridsen, Jeppesen & Andersen, 1993; Lauridsen, Sandsten & Hald Møller, 2003). Thus, P. pectinatus, one of the most common species in temperate European shallow lakes (Vestergaard & Sand-Jensen, 2000; Van Geest et al., 2005), may be especially prone to herbivory. It has a higher palatability (Dorenbosch & Bakker, 2011) and lower content of secondary metabolites (Hilt & Gross, 2008) than other submerged macrophyte species and waterfowl have been shown to graze selectively on P. pectinatus (Hidding et al., 2010). In addition, P. pectinatus does not produce allelopathically active substances that inhibit periphytic algae (Hilt & Gross, 2008), although studies of periphyton on different macrophyte species have not yet conclusively detected this interaction (e.g. Blindow, 1987; Gross, Feldbaum & Graf, 2003). Furthermore, Jones & Sayer (2003) did not find an effect of host plant species on the relationship between periphyton biomass and plant biomass.
The recent increase in species number and maximum colonisation depth along with the high abundance of N. marina spp. intermedia and C. demersum in deeper zones suggests a development towards a more seasonally ‘stable’ situation where multiple species permit persistence of plant stands over the growing season. In particular, C. demersum is known to overwinter in shallow lakes (Sayer et al., 2010b), and N. marina is typical of late summer conditions (Stansfield et al., 1997). The increasing abundance of species with a longer seasonal duration may eventually constrain phytoplankton development in the summer.
Genetic diversity of re-established P. pectinatus
Our data indicate that the genotype diversity of P. pectinatus in Lake Müggelsee is low. Simpson's index of diversity was lower than that for 12 German lakes (0.689) reported by Nies & Reusch (2005). Hangelbroek et al. (2002) estimated genetic diversity for 25-year old populations of P. pectinatus in Lake Lauwersmeer (Netherlands) over similar spatial scales (100s m) using RAPDs and found much higher genotype diversity (0.990). The genotype diversity values in Lake Müggelsee were more similar to those for P. pectinatus stands with prevailing tuber regrowth compared to stands with higher seedling recruitment (Triest et al., 2010). Total heterozygosity was similar to values reported from well-established ponds and newly founded populations in Belgium (Triest et al., 2010; He = 0.174–0.444) and was slightly lower than that of 12 lakes in northern Germany (He = 0.527; Nies & Reusch, 2005).
The low clonal diversity and the fact that more recently established (in 100 cm depth) stands of P. pectinatus were a subset of genotypes in shallower water suggest that re-establishment of deeper plant stands mainly occurred vegetatively by tubers from a few plants that had survived turbid conditions rather than from seeds generated through recombination. Hangelbroek et al. (2002) detected a similar pattern with water depth being negatively correlated to clonal diversity (samples from 30–70 cm depth; data taken from Nolet et al., 2001). Although Van Wijk (1988) proposed that successful establishment of P. pectinatus seeds within a population is scarce, Hangelbroek et al. (2002) found frequent and successful repeated seed recruitment in Lake Lauwersmeer. In Lake Müggelsee, seed formation by P. pectinatus during re-oligotrophication is assumed to have been prevented by the joint effects of herbivory and periphyton shading, as plants did not reach the water surface and did not flower until at least 2001 (Roberts et al., 2003; Hilt, 2006). Although axillary tubers may contribute to longer-distance vegetative dispersal of P. pectinatus (Van Wijk, 1989), seed dispersal would have been more efficient for the establishment of new stands. The role of dispersal of both vegetative and sexual propagules by wind, water and waterfowl as well as seed banks and remnant populations has been discussed (Bakker et al., 2013), but knowledge about the origin of recolonising macrophytes in restored shallow lakes remains scarce. We propose that a prevalence of re-establishment from tubers and a lack of local seed dispersal can contribute to slow re-establishment of P. pectinatus, despite its wide distribution across the northern Hemisphere which is commonly attributed to the generally high dispersal potential and high colonisation ability of freshwater plants (Santamaria, 2002).
In conclusion, our long-term data in Lake Müggelsee suggest that the pathway of macrophyte decline in European shallow lakes during eutrophication (clear→crashing→turbid) may occur in reverse order during re-oligotrophication and macrophyte re-establishment. A potentially long-lasting phase (>20 years) with low species diversity, low maximum colonisation depth and turbid water in late summer resembles the seasonally ‘crashing’ macrophyte phase observed during eutrophication. Following nutrient load reduction, re-establishing macrophyte stands may be dominated by species that survived turbid conditions in very shallow areas and compress their life cycle into a short clear-water period such as P. pectinatus. The increases in macrophyte species diversity and maximum colonisation depth in Lake Müggelsee about 20 years after the start of nutrient load reductions indicate that patience rather than other measures such as biomanipulation or sediment removal is needed to allow the few species of the re-establishment phase to pave the way for a diverse, seasonally persistent macrophyte community that may eventually stabilise clearer water conditions.
Acknowledgments
We thank the many staff and students of the Leibniz-Institute of Freshwater Ecology and Inland Fisheries who helped with sampling and nutrient analysis, especially Hans-Jürgen Exner, Jörg Gelbrecht, Marianne Graupe, Thomas Hintze, Reinhard Hölzel, Antje Lüder, Barbara Meinck, Thomas Rossoll, Bernd Schütze, Helgard Täuscher and Elke Zwirnmann. We acknowledge scientific advice and provision of data by Horst Behrendt, Andreas Nicklisch, Norbert Walz, Timm Kabus, Antje Köhler and Rüdiger Mauersberger and constructive comments by two anonymous reviewers and Roger I. Jones. Microsatellite genotyping was performed by Katrin Preuß, and we thank Sandro Schöning and Maria Alp for their assistance with data analysis. Klaus van de Weyer surveyed the macrophytes by Scuba diving in 2011 which was financed by the Senate of Berlin and the Federal Ministry of Education and Research (BMBF 033L041 A-G). S.H. was partly financially supported by the German Research Foundation (DFG Ni 366/5), and R.A. was supported by the EU-project LIMNOTIP funded under the FP7 ERA-Net Scheme (Biodiversa, 01LC1207A).




